Abstract
Accurate assessment of lymph node metastasis (LNM) in T1/2-stage esophageal squamous cell carcinoma (ESCC) is critical for treatment planning but remains challenging due to diagnostic inaccuracies and unclear metastatic mechanisms. This study aimed to predict LNM in T1/2-stage ESCC using machine learning-based radiomics and elucidate its biological underpinnings. We retrospectively analyzed 374 surgically treated ESCC patients from two centers, employing six machine-learning algorithms to derive an optimal radiomics score. Key pathways and genes linked to LNM were investigated via bioinformatics and experimental validation. The decision tree (DT)-based radiomics model demonstrated superior predictive performance, with AUCs of 0.933 (training), 0.887 (validation), and 0.845 (test). Bioinformatics analysis implicated tumor-lymphatic invasion pathways, with EFNA1 emerging as a potential key regulator. These findings highlight the clinical utility of radiomics for LNM prediction in early-stage ESCC and provide insights into its molecular mechanisms.
Similar content being viewed by others
Introduction
Esophageal cancer, a prevalent global malignancy, predominantly manifests as distal esophageal adenocarcinoma (EAC) in Western populations and esophageal squamous cell carcinoma (ESCC) in Eastern populations1,2,3. These subtypes exhibit distinct pathogenesis, lymph node metastasis (LNM) patterns, and prognoses, suggesting they may represent biologically distinct diseases2,3. ESCC, in particular, is highly susceptible to LNM due to its rich submucosal lymphatic network and the absence of a serosal layer, contributing to poorer clinical outcomes4,5. The 5-year survival rate for ESCC patients without LNM ranges from 60% to 80%, but it drops significantly to 20% in cases with LNM6,7,8. Currently, clinical assessment of LNM primarily relies on the size, morphology, and enhancement characteristics of lymph nodes in imaging (CT-based) examinations2,8,9,10. However, this approach is inherently subjective and fails to account for site-specific lymph node characteristics. For example, subcarinal lymph nodes tend to be larger but have a lower metastatic rate, whereas paracardial and left gastric artery lymph nodes are typically smaller yet exhibit a higher propensity for metastasis, leading to reduced diagnostic sensitivity10,11. Although lymph node biopsy provides higher diagnostic accuracy, its invasive nature and sampling limitations restrict its routine application. As a result, current diagnostic methods remain suboptimal, with an overall accuracy of less than 70%, particularly for T1/2 ESCC patients, who are at a higher risk of misdiagnosis10,11,12. Furthermore, the presence or absence of LNM significantly influences treatment strategies for ESCC patients. For instance, T1 ESCC patients without LNM may benefit from minimally invasive endoscopic mucosal resection, which preserves organ function, whereas those with LNM require radical esophagectomy, extensive lymph node dissection, and adjuvant therapies13,14. Therefore, accurate diagnosis of LNM is critical for optimizing treatment planning and improving prognostic outcomes, especially in early-stage ESCC patients.
Radiomics is a technique for non-invasive quantitative analysis and information extraction of medical imaging11,15. By deconstructing and analyzing texture features in high-throughput medical images, it can extract a large number of radiomics parameters potentially related to tumor intrinsic bio-heterogeneity, invasiveness, and immunoreactivity, thus enabling the prediction of tumor biological behaviors and gene expression levels15,16. At the same time, it has been demonstrated that the radiomics features of the peripheral region of tumors also contain important information, which can not only reveal information about the subclinical lesion but also reflect information about the microenvironment and molecular regulation, thus assisting in the evaluation of biological behaviors16,17,18. In addition, the combination of radiomics and machine learning algorithms in recent years has brought great hope for early diagnosis, prognosis prediction, and efficacy assessment of cancer. The predictive value of machine learning-based radiomic biomarkers for ESCC lymph node metastasis has been demonstrated18,19,20. Huang19 found that LASSO-based radiomics model had the potential to predict LNM of the recurrent laryngeal nerve in esophageal cancer patients. Chen20 found that machine-learning-based radiomics model had the value of revealing the lymph node status of ESCC patients. Therefore, this study tries to explore the predictive value of machine learning models combining radiomics features of tumors and peritumors for LNM in patients with T1/2 stage esophageal cancer and its incremental value to existing diagnostic mode. Furthermore, in addition to exploring the predictive value of radiomics for ESCC biological behavior, this study also attempts to reveal its association with key genes of LNM to assist in the surveillance and intervention of T-early LNM in ESCC.
Results
Clinical data
A total of 374 patients were included in this study, 251 in center1 and 123 in center2. There were 276 males and 98 females, with a median age of 67 and a range of 47–83. There were 155 cases in T1 stage and 219 cases in T2 stage; 235 cases in N0 stage and 139 cases in N+ stage. General clinical data were not statistically different between Center1 and Center2 (Table 1).
Screening of radiomics parameters and construction of radiomics model
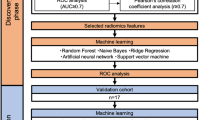
Highly robust parameters with intra- and inter- ICC > 0.75 were 897 in GTV and 856 in CTV. After removing parameters with P > 0.05 and correlations>0.8, the remaining 36 radiomics features in GTV and 27 in CTV were used for machine learning modeling. The DT-based radiomics model demonstrated the best predictive efficacy (Fig. 1), where tumor and peritumor radiomics showed complementarity. The area under the curve (AUC) of the combined model was 0.933, 0.887 and 0.845 in the training, validation and test sets, respectively (Table 2). The RQS21 and METRICS22 were used to assess the research methodology, where the RQS score was 22 out of 36 (Table S1) and the METRICS score was 67.9% (Table S2).
A–C show the comparison of AUC in six machine learning models based on tumor, peritumor, and tumor&peritumor radiomics parameters, in which the Decision Tree model showed the best overall performance; (D–F) show the ROC curves of machine learning models based on tumor&peritumor radiomics parameters in the training, validation, and test sets.
Incremental value of radiomics on existing indicators
To further analyze the incremental value of radiomics on existing imaging and clinical indicators, we combined the best Radscore with various clinical parameters such as imaging diagnosis, tumor markers and hematological indicators. The results showed that LNMr, T-stage, Squamous cell carcinoma antigen (SCC-Ag), Systemic inflammatory response index (SIRI), Prognostic nutritional index (PNI), and Radscore were significantly correlated with LNM in patients with T1/2 esophageal cancer, in which LNMr, PNI, and Radscore were independent prognostic factors (Fig. 2). The equation of nomogram is: Y = 3.341*LNMr - 0.095*PNI + 6.9126*Radscore + 0.716. The AUC of the combined model (nomogram) in the training, validation and test sets were 0.960, 0.937, and 0.928. The 1000 times ten-fold cross-validation suggested that the AUC fluctuated from 0.946 to 0.981, which indicated that the nomogram was stable. The Delong test shows that the prediction efficacy of the nomogram is significantly better (P < 0.05) than that of any single parameter and LNMr+PNI (Table 3). The NRI 38.3% (0.1744 ~ 0.5919) and the IDI 20.8% (0.1478 ~ 0.2679) suggest that the addition of Radscore significantly improves the overall prediction efficacy.
A shows the nomogram combined radiomics and clinical parameters; (B) shows the forest plot of nomogram; (C–E) show the calibration curves, decision curves, and clinical impact curves of nomogram, suggesting high model fit and clinical benefit; (F–H) show the ROC curves of nomogram in the training, validation and test sets, proving that the model is accurate, efficient and stable.
Radiomics-related differential genes
Grouping was based on Radscore, bounded by P < 0.05 and absolute LogFC value > 1. There were 1274 differential genes (up-regulated 580, down-regulated 694), functionally enriched in actin cytoskeleton, lymphocyte migration, T-cell extravasation, regulation of lymphocyte differentiation, and regulation of cell-cell adhesion (Fig. 3). ESTIMATE showed that there was no significant difference in immunological scores between the two groups (Figure S1). Cytoscape was used to pick out the TOP10 hub genes among the differential genes. The correlation between hub genes and radiomics features was analyzed, where EFNA1 showed a correlation with SALGLE (P < 0.001, rh0 = 0.866). In addition, The cancer genome atlas (TCGA) and Gene expression omnibus (GEO) databases further demonstrated the value of EFNA1 in predicting LNM and prognosis in T1/2 ESCC patients. Single-cell sequencing analysis suggested that EFNA1 was mainly expressed in esophageal cancer basal cells.
A shows the volcano map of differential genes stratified according to Radscore; (B) shows the GO&KEGG enrichment analysis of differential genes, with the functions mainly enriched to lymphatic migration-related pathways; (C) shows the top10 hub genes selected by the Cytoscape; (D) shows the correlation heatmap of hub genes with Radscore, where EFNA1, EPHA1, and EFNB2 were significantly correlated with Radscore (P < 0.05); (E) shows the correlation heatmap between hub genes and top10 radiomics features, in which the correlation coefficient of EFNA1 and SALGLE was greater than 0.75; (F, G) show the ROC curves of EFNA1 in TCGA and GEO for predicting lymph node metastasis in T1/2 ESCC patients, with AUCs of 0.712 and 0.721; (H, I) show the single-cell sequencing analysis, showing that EFNA1 was mainly expressed in basal cells of ESCC.
Functional verification of EFNA1
EFNA1 expression of KYSE150 (esophageal squamous carcinoma cells) was knocked down by siRNA. Quantitative polymerase chain reaction (qPCR) and Western Blot (WB) proved that gene knockdown was successful (Figure S2). Scratch and Transwell assay proved that knocking down EFNA1 reduced the migration and invasion ability of KYSE150 (Fig. 4). Cytoskeleton staining proved that the proportion of actin was lower when EFNA1 was lowly expressed. CCK8 and plate cloning assay proved that the knockdown of EFNA1 attenuated the proliferation level of KYSE150.
A)shows the scratch experiment, in which KYSE150 in the EFNA1 knockdown group had a weaker migration ability. B shows Transwell experiments, in which chambers with and without Matrigel demonstrated that KYSE150 with low EFNA1 had weakened invasion and migration ability. C shows the cytoskeleton staining, which demonstrated a lower actin ratio in KYSE150 with low EFNA1. D, E show CCK8 and plate cloning experiments, demonstrating that the knockdown of EFNA1 attenuated the proliferation level of KYSE150.
Discussion
In this study, CT-based radiomics parameters demonstrated the ability to predict LNM in patients with T1/2 stage ESCC, and the radiomics features of GTV and CTV were complementary. Meanwhile, the screening and combination of radiomics parameters were further optimized by multiple machine learning methods, which further improved the prediction accuracy of LNM. What’s more, the radiomics model demonstrated incremental value to the clinical indicators. The addition of radiomics led to a significant improvement in LNM prediction of the existing diagnostic model, with the Delong test, IDI, and NRI demonstrating statistical differences. The application of the combined model can facilitate early and accurate prediction of lymph node status in ESCC patients, thereby aiding clinical decision-making and improving patient outcomes. For instance, by utilizing the radiomics-based nomogram to assess the risk of LNM in ESCC patients, high-risk groups may benefit from more aggressive treatments, such as total esophagectomy combined with radiotherapy, while low-risk groups may be suitable for conservative strategies, such as endoscopic dissection or resection. In addition, we bridged the gap between radiomics and transcriptomics with the TCIA database. The possible molecular mechanisms of radiomics for predicting LNM and the intrinsic association between SALGLE and EFNA1 were revealed. Finally, cellular experiments were utilized to demonstrate the function and value of EFNA1 in promoting the proliferation and migration of ESCC, providing new ideas and targets for the study and treatment of LNM in esophageal cancer.
Radiomics can quantitatively characterize textural differences in images, revealing the micropathological and biological information behind macroscopic imaging15,16,17. Meanwhile, advances in artificial intelligence have made it possible to accurately screen and efficiently combine massive features in radiomics, providing a broader space for the application20. In this study, three-dimensional radiomics features were extracted from the primary lesions and the 5 cm region of the peritumor, which achieved the “virtual biopsy” of tumors and subclinical lesions noninvasively23. In addition, six machine learning methods were used to select the best combination of radiomics parameters, where DT model combining GTV and CTV radiomics features showed the best predictive efficacy. The combined model was significantly better than the single parameter and the conventional radiomics model (AUC of 0.93 VS 0.76)21. This suggests that the peritumor radiomics parameters of esophageal cancer also contain important biological information, which can compensate for the limited information about the tumor. Especially for early-stage tumors with shallow infiltration, the peritumor may contain more information about lymphovascular microfiltration, subclinical lesion invasion, and peripheral lymphangiectasia, which may be one of the reasons why the peritumor radiomics features have the value of predicting LNM in T1/2 stage ESCC22,24.
To further analyze the compatibility and complementarity of radiomics with existing diagnostic modalities and their incremental value. Multiple common clinical indicators were used to combine the best Radscore. The results showed that LNMr, PNI, and Radscore, complementing each other, showed independent predictive value in LNM in patients with stage T1/2 esophageal cancer. LNMr, as the most frequently used diagnostic index with the highest accuracy, is undoubtedly the cornerstone of LNM diagnosis. The predictive value of PNI for LNM has already been reported in other cancers. Chen25 found that PNI has the potential to reveal LNM in non-small cell lung cancer. Huang26 pointed out that PNI could be used as an effective predictive marker for LNM in gastric cancer. This study demonstrated the independent predictive value of PNI in the prediction of LNM in T-early ESCC. This may be because PNI, on the one hand, reveals the patient’s inflammatory, nutritional, and immune status, which is of great value in tumor progression and treatment; on the other hand, it reflects the process of angiogenesis-associated endothelial metabolism, which is conducive to the formation of a microenvironment for tumor tissue proliferation and dissemination25,26,27. The NRI and IDI demonstrated that the incorporation of Radscore improved the predictive accuracy of the model significantly. The predictive efficacy of the combined model was significantly better than any single-parameter and clinical model, with better accuracy (85.5–87.9%) than previous clinicopathological models (81%)28. This suggests that radiomics and clinical parameters are complementary, which can further improve the existing diagnostic model of LNM in T-early ESCC, and escort the clinical precision diagnosis and treatment.
Although radiomics can realize the prediction of clinical events, the biological significance of the findings cannot be directly known29. However, clarifying the radiomics features and the underlying mechanisms behind the model is important for understanding the development of the disease, exploring novel diagnostic and therapeutic modes, and promoting the clinical application of the model. Therefore, we tried to find the molecular information behind the radiomics by correlating radiomics and transcriptomics. As a result, EFNA1 was found to be significantly correlated with the SALGLE of tumor region, which measures the proportion in the image of the joint distribution of smaller-size zones with lower gray-level values. The association of SALGLE with EFNA1 may reveal the association of the grayscale differences and proportions within the tumors with the growth of actin, tumor migration, and extravasation of lymphocytes, which may enable the prediction of LNM by radiomics. The association of radiomics and transcriptomics implies that it may be possible, in the future, to achieve assessment and prediction of gene expression by noninvasive means.
EFNA1 has been shown to be involved in biological processes such as tumor proliferation, invasion, and angiogenesis30,31,32. In the study of Zhuo31, EFNA1 was shown to have an overlapping region with gastric cancer metastasis-associated GMAN gene, which was able to promote the invasive activity of tumors. Shi32 found that EFNA1 was closely related to the expression of EGFR and VEGF in esophageal cancers, and involved in the regulation of LNM. In our study, EFNA1 was demonstrated to have a role in promoting the increase of actin in ESCC, strengthening the ability of proliferation, invasion, and migration, which is also the underlying condition of LNM. Meanwhile, single-cell sequencing analysis suggested that EFNA1 was mainly expressed in basal cells of ESCC. While basal cells as an important barrier for tumor invasion and metastasis, once the function and integrity are damaged the tumor will directly contact the extracellular matrix, blood vessels, and lymphatic vessels, inducing metastasis33,34. This suggests that transcriptomic alterations in the germinal layer of esophageal cancer may play an important role in LNM. Detection and targeted intervention against basal cell EFNA1 may help in the diagnosis and treatment of LNM in esophageal cancer.
There are also many limitations in this study. Firstly, the TCGA cohort used in this study was small in number, which makes the association between radiomics and transcriptomics less reliable. Secondly, only arterial-phase images were analyzed in this research. In the future, we will analyze more sequences in multi-center, large-sample prospective studies for their applied and combined values. In addition, this study only analyzed the correlation between radiomics features and genes but didn’t prove its inevitability. Eventually, the basic experimental part of EFNA1 only did functional validation. The expression level of EFNA1 in basal cells and its intrinsic association with LNM still need to be verified by comprehensive experimental studies in the future.
In conclusion, radiomics features of GTV and CTV can, to some extent, reveal biological information and genotypes of tumors, which have great potential for application in predicting clinical events. The combined model based on radiomics and clinical indicators is outstanding in predicting LNM, which can provide objective indicators for lymph node status assessment and individualized treatment in patients with T1/2 ESCC.
Methods
Patients
The data of 852 esophageal cancer patients, who attended the First Affiliated Hospital of Anhui Medical University in Center1 and the First Affiliated Hospital of University of Science and Technology of China in Center2 from March 1, 2015, to March 1, 2023, were retrospectively collected. According to the inclusion and exclusion criteria, finally, 374 patients were enrolled in this study, of which 251 patients from center1, were randomly divided into training and validation sets by 7:3, and 123 patients from center2 were used as the test set (Fig. 5). All enrolled patients underwent esophageal cancer surgery and lymph node dissection at our two centers. General clinical data including age, maximum tumor diameter, Lymph node status (radiology and pathology), histologic type, and TNM staging were collected. Radiologically diagnosed LNM (LNMr): lymph nodes with a short axis ≥10 mm and uneven enhancement were recognized as metastasis. This study involving human participants was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived by the approving ethics committees due to the retrospective nature of this study: Ethics Committee of Anhui Provincial Cancer Hospital (Reference No: 2023-YXK-07); The First Affiliated Hospital of Anhui Medical University (Reference No: PJ2024-03-39). This study adhered to CLEAR and TRIPOD standards (Table S1–S4).
Inclusion criteria
(a) Esophageal cancer confirmed by pathology; (b) Underwent lymph node dissection; (c) Underwent CT two weeks before therapy; (d) Without history of tumor therapy.
Exclusion criteria
(a) T3 or T4 (n = 407); (b) Non-squamous cell carcinoma (n = 45); (c) Combination with other malignancies (n = 22); (d) Image quality failures (n = 4).
Protocol
Scanning protocols
Center1 was scanned using a Philips Big Core CT (Phillips Medical Systems, 96 Highland Heights, OH). Center2 was scanned using a LightSpeed Pro 16 CT (GE Medical Systems, Milwaukee).
Treatment protocols
Esophagectomy and radical lymphadenectomy. Surgical options include open and hybrid surgical approaches. Lymph node compartmentalization included the neck, mediastinum, and abdomen.
Image analysis
Pre-treatment arterial phase CT images were imported into 3D slicer software. Then the regions of interest (ROI) of tumor, defined as gross tumor volume (GTV), and the 5 cm region outside GTV, defined as clinical target volume (CTV), was outlined layer by layer by two radiologists. Then the ROI was fused into a volume of interest (VOI) to extract 3D radiomics features. Finally, 1037 radiomics parameters were extracted from each VOI by PyRadiomics (Reference to IBSI standards). Then, the Resample Image module (Linear) in 3D Slicer was used to resample images to a resolution of 1.0 mm × 1.0 mm × 1.0 mm. Z-score was used to standardize and normalize the radiomics parameters. ComBat was used to reduce the batch effect of the parameters. An overview of the image sketching and model construction is shown in Fig. 6.
Construction of radiomics model
T/U tests were performed on the radiomics parameters with intraclass correlation coefficient (ICC) > 0.75, where parameters with P < 0.05 were retained and analyzed by Spearman correlation to remove redundant parameters with correlations >0.80. Afterward, six machine learning methods, the least absolute shrinkage and selection operator (LASSO), Random Forest (RF), Decision Tree (DT), NaiveBayes (NB), Linear kernel, and Polynomial kernel Support Vector Machine (SVM), were used to screen the radiomics features and calculating radiomics scores (Radscore).
Union of radiomics and clinical indicators
To analyze the complementarity between radiomics and clinical indicators and to further improve the prediction model, we united multiple clinical markers with the best Radscore. A comprehensive nomogram was constructed based on the independent predictors in the multivariable Cox regression.
Finding relevant genes for radiomics model
The bridge between radiomics and transcriptomics was built by TCIA (5 of 16 patients met the inclusion and exclusion criteria). Patients were stratified according to the Radscore, differential genes were sought and enriched for analysis. The hub genes among the differential genes were solved by Cytoscape and analyzed for correlation with the top 10 ranked radiomics features using Spearman/Pearson. Eventually GSE43732 and GSE196756 datasets were used for validation of the predictive value of the differential genes.
Experimental validation of differential genes
The siRNA was used to knock down relevant genes. Functional experiments including transwell, scratch and cytoskeletal staining of Cytoskeleton staining assay for verifying the effect of genes on cell invasion and migration ability. Plate cloning and CCK8 assay for verifying the effect on cell proliferation ability.
Statistical analysis
The software of R4.3.2 was performed for statistical analysis: The ICC was used to test the robustness of radiomics parameters. LASSO, RF, DT, NB, and SVM models were used to construct the radiomics models. The best radiomics model and clinical indicators were used to construct a comprehensive nomogram. The 10-fold cross-validation, area under the curve (ROC), calibration curves, decision curves, clinical impact curves, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were used to assess the predictive efficacy and value of the combined model.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy restrictions but may be made available from the corresponding author upon reasonable request, subject to institutional review board approval.
Code availability
The custom code used for radiomics feature extraction and machine learning analysis in this study is publicly available at https://github.com/zhangyu199831/Radiomics.
References
Morgan, E. et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 163, 649–58.e2 (2022).
Gao, Y. et al. Machine learning-based automated sponge cytology for screening of oesophageal squamous cell carcinoma and adenocarcinoma of the oesophagogastric junction: a nationwide, multicohort, prospective study. Lancet Gastroenterol. Hepatol. 8, 432–445 (2023).
He, S. et al. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 11, 3379–3392 (2021).
Stein, H. J. et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann. Surg. 242, 566–573 (2005).
Dubecz, A. et al. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann. Thorac. Surg. 99, 1879–1885 (2015).
Okholm, C., Svendsen, L. B. & Achiam, M. P. Status and prognosis of lymph node metastasis in patients with cardia cancer - a systematic review. Surg. Oncol. 23, 140–146 (2014).
Cen, P. et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 112, 1020–1027 (2008).
Altorki, N. et al. Ten-Year Survival and Recurrence Patterns After Three-Field Lymph Node Dissection for Squamous Cell and Adenocarcinoma of the Esophagus. Ann. Surg. 278, e43–e50 (2023).
Urakawa, N. et al. Prognostic and Clinicopathological Significance of Lymph Node Metastasis in the Esophagogastric Junction Adenocarcinoma. Anticancer Res. 42, 1051–1057 (2022).
Li, Z. X. et al. Application value of CT examination of lymph node short diameter in evaluating cardia-left gastric lymph node metastasis in thoracic esophageal squamous cell carcinoma. Chin. J. Oncol. 45, 962–966 (2023).
Xie, C. et al. Prediction of Individual Lymph Node Metastatic Status in Esophageal Squamous Cell Carcinoma Using Routine Computed Tomography Imaging: Comparison of Size-Based Measurements and Radiomics-Based Models. Ann. Surg. Oncol. 29, 8117–8126 (2022).
Jiang, C. et al. Systematic review and meta-analysis of the accuracy of 18F-FDG PET/CT for detection of regional lymph node metastasis in esophageal squamous cell carcinoma. J. Thorac. Dis. 10, 6066–6076 (2018).
Yang, Y. M. et al. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target Ther. 5, 229 (2020).
van Rossum, P. S. N. et al. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 15, 235–249 (2018).
Shih, I. L. et al. PET/MRI in Cervical Cancer: Associations Between Imaging Biomarkers and Tumor Stage, Disease Progression, and Overall Survival. J. Magn. Reson Imaging 53, 305–318 (2021).
Xia, T. Y. et al. Predicting Microvascular Invasion in Hepatocellular Carcinoma Using CT-based Radiomics Model. Radiology 307, e222729 (2023).
Khorrami, M. et al. Distinguishing granulomas from adenocarcinomas by integrating stable and discriminating radiomic features on non-contrast computed tomography scans. Eur. J. Cancer 148, 146–158 (2021).
Hu, Y. et al. Assessment of Intratumoral and Peritumoral Computed Tomography Radiomics for Predicting Pathological Complete Response to Neoadjuvant Chemoradiation in Patients With Esophageal Squamous Cell Carcinoma. JAMA Netw. Open 3, e2015927 (2020).
Huang, Y. L. et al. The development of a nomogram model for predicting left recurrent laryngeal nerve lymph node metastasis in esophageal cancer based on radiomics and clinical factors. Ann. Transl. Med. 10, 1282 (2022).
Chen, L. et al. Radiomics Analysis of Lymph Nodes with Esophageal Squamous Cell Carcinoma Based on Deep Learning. J. Oncol. 2022, 8534262 (2022).
Lei, X. et al. Preoperative prediction of clinical and pathological stages for patients with esophageal cancer using PET/CT radiomics. Insights Imaging 14, 174 (2023).
Xu, Y. et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun. (Lond.) 40, 484–500 (2020).
Bera, K. et al. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19, 132–146 (2022).
Kwon, B. R. et al. Microcalcifications and Peritumoral Edema Predict Survival Outcome in Luminal Breast Cancer Treated with Neoadjuvant Chemotherapy. Radiology 304, 310–319 (2022).
Chen, M. et al. Nomogram based on prognostic nutrition index and Chest CT imaging signs predicts lymph node metastasis in NSCLC patients. J. Xray Sci. Technol. 30, 599–612 (2022).
Huang, C. et al. Clinical Significance of Serum CA125, CA19-9, CA72-4, and Fibrinogen-to-Lymphocyte Ratio in Gastric Cancer With Peritoneal Dissemination. Front Oncol. 9, 1159 (2019).
Okadome, K. et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 271, 693–700 (2020).
Yun, J. K. et al. Risk prediction of occult lymph node metastasis in patients with clinical T1 through T2 N0 esophageal squamous cell carcinoma. J. Thorac. Cardiovasc Surg. 164, 265–275.e5 (2022).
Hatt, M. et al. Radiomics: Data Are Also Images. J. Nucl. Med. 60, 38S–44S (2019).
Wu, R. et al. Comprehensive analysis of spatial architecture in primary liver cancer. Sci. Adv. 7, eabg3750 (2021).
Zhuo, W. et al. Long Noncoding RNA GMAN, Up-regulated in Gastric Cancer Tissues, Is Associated With Metastasis in Patients and Promotes Translation of Ephrin A1 by Competitively Binding GMAN-AS. Gastroenterology 156, 676–691.e11 (2019).
Shi, Z. Z. et al. The miR-1224-5p/TNS4/EGFR axis inhibits tumour progression in oesophageal squamous cell carcinoma. Cell Death Dis. 11, 597 (2020).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186, 1564–1579 (2023).
Mierke, C. T. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep. Prog. Phys. 82, 064602 (2019).
Acknowledgements
All authors sincerely thank two senior radiologists, Dr. Fei Gao and Dr. Chao Wei, for delineating lesions and image analysis for this study.
Author information
Authors and Affiliations
Contributions
All authors have had access to the data and all drafts of the manuscript. Specific contributions are as follows: study design: W.Q.B., W.X.; data collection: Z.Y., L.L., H.M.Y., L.L.R.; data management and analysis: Z.Y., L.L., H.M.Y., L.L.R.; manuscript drafting: Z.Y., L.L.; manuscript review: all. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Liu, L., Han, M. et al. Unraveling the power of radiomics: prediction and exploration of lymph node metastasis in stage T1/2 esophageal squamous cell carcinoma. npj Precis. Onc. 9, 176 (2025). https://doi.org/10.1038/s41698-025-00929-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-025-00929-2