Abstract
This phase II clinical trial aimed to evaluate the efficacy and safety of neoadjuvant long-course chemoradiotherapy combined with sintilimab in mismatch repair-proficient (pMMR)/microsatellite-stable (MSS) locally advanced rectal cancer (LARC) patients with a PD-L1 tumor proportion score (TPS) ≥1% or combined positive score (CPS) ≥1. The primary endpoint was pathological complete response (pCR), and safety was assessed. This trial was registered on ClinicalTrials.gov (identifying number: NCT04833387) with the registration date of April 4, 2021. Although the target pCR rate was not fully achieved, a notable improvement was observed, with 7/20 (35.0%) patients achieving pCR in the intention-to-treat analysis. A trend toward higher pCR rates was observed in patients with PD-L1 CPS ≥ 5 than in those with CPS < 5 (50.0% vs. 27.3%, P = 0.311). Treatment-related adverse events occurred in 12 patients (60.0%), with no grade 4 events. Biomarker analysis revealed that higher CD3 (P < 0.001) and CD8 (P = 0.018) expression, along with lower TIM-3 (P = 0.017) expression in the stroma, was associated with higher pCR rates.
Similar content being viewed by others
Introduction
The conventional treatment for patients with locally advanced rectal cancer (stages T3, T4, or N+) is neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) surgery, according to the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) guidelines at the time this trial was planned1,2. However, only 15–20% of patients can achieve pathological complete response (pCR), which is insufficient for meeting the needs of treatment and organ preservation3. As a result, researchers have explored innovative treatment modalities, such as total neoadjuvant chemoradiotherapy (TNT), to reduce the occurrence of distant metastasis and increase the likelihood of organ preservation. In the UNICANCER-PRODIGE 23 trial, 3-year disease-free survival was significantly better in the TNT group than in the conventional neoadjuvant chemoradiotherapy group (76% vs. 69%, P = 0.034)4. In addition, the STELLAR study demonstrated both better 3-year overall survival (86.5% vs. 75.1%; P = 0.033) and higher total rate of pCR and sustained cCR (21.8% vs. 12.3%, P = 0.002) in the TNT group, which consisted of short-course radiotherapy (5 Gy × 5) followed by four cycles of CAPOX and postoperative chemotherapy consisting of two cycles of CAPOX, than in the CRT group5. Total neoadjuvant therapy (TNT) has gradually gained widespread acceptance and has become one of the standard treatment approaches in this field. More effective treatment models are currently being explored to improve patient short-term and long-term outcomes.
Recently, immune checkpoint inhibitors (ICIs) have been proven to be highly effective for treating colorectal cancers (CRCs) with mismatch repair deficiency (dMMR)/high microsatellite instability (MSI-H), especially for rectal cancer6,7. A phase II trial conducted by Cercek et al. evaluating the antitumor activity of PD-1 inhibitors for dMMR rectal cancer revealed that all 12 patients enrolled achieved a complete clinical response and were successfully managed with a watch-and-wait approach8. Similar results were observed in the trial by Chen et al. and in our previous study9,10. However, most CRCs are characterized as proficient mismatch repair (pMMR)/microsatellite-stable (MSS) tumors and generally exhibit resistance to immunotherapy alone. This resistance is attributed primarily to insufficient lymphocytic infiltration within the tumor microenvironment11. Therefore, regulating this microenvironment might be a potential strategy to reverse immunotherapy resistance and enhance the efficacy in pMMR/MSS LARC patients.
Preclinical data have demonstrated that radiotherapy can reverse immunotherapy resistance by modulating the immunogenicity of tumor cells, enhancing antigen-specific CD8+ T-cell responses, and increasing PD-L1 expression on tumor and immune cells within the tumor microenvironment12,13,14,15. Recent trials, such as the PANDORA, AVERECTAL, AVANA, and VOLTAGE-A trials, have demonstrated that combinations of CRT and PD-1 inhibitors could offer promising clinical benefits with a manageable safety profile in patients with pMMR/MSS rectal cancer16,17,18,19. In these studies also performed biomarker analyses using pre-CRT biopsy samples were also performed to identify optimal candidates for treatment. Exploratory analysis in a phase II trial by Zhang et al. revealed that, although not statistically significant, patients with higher PD-L1 tumor proportion scores (TPSs) were more likely to achieve pCR (66.7% vs. 45.0%, P = 0.242)20. A similar result was observed in the VOLTAGE-A study, with a pCR rate of 75% in patients with a PD-L1 TPS ≥ 1%, with 17% in those with a TPS < 1%16. In addition to PD-L1 expression on tumor cells, PD-L1 expression in other cells within the tumor microenvironment is also an important biomarker for predicting response to immunotherapy21. The PD-L1 combined positive score (CPS) has been validated as a predictive biomarker in various cancers, including lung cancer and gastric cancer22,23. Although research on CPS in colorectal cancer is limited, it still represents a promising potential biomarker24. Based on these findings, we hypothesized that pMMR/MSS LARC patients with PD-L1 TPSs ≥ 1% or CPSs ≥ 1 are likely to benefit from CRT and PD-1 inhibitors.
In this study, we aimed to perform a prospective, single-arm, single-center, phase II trial to evaluate the clinical efficacy and safety of long-course CRT followed by the PD-1 inhibitor sintilimab in patients with PD-L1 TPS ≥ 1% or CPS ≥ 1 pMMR/MSS LARC. Additionally, we explored potential biomarkers that could predict treatment response.
Results
Baseline information
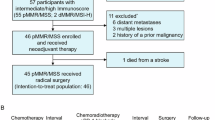
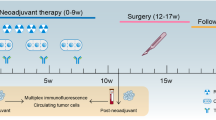
From March 3, 2021, to November 11, 2022, a total of 88 patients underwent PD-L1 TPS and CPS assessments, 33 (37.5%) of whom met the criteria of PD-L1 TPS > 1% (n = 9) or PD-L1 CPS > 1 (n = 33). Among the 33 screened patients, 13 were excluded due to unwillingness to participate. Ultimately, 20 patients were enrolled in the study. During the treatment period, all the patients completed standard CRT (50.4 Gy with 825 mg/m2 capecitabine twice daily for 5 weeks), followed by three cycles of 200 mg sintilimab for 3 weeks (Fig. 1). No patient received additional treatment or discontinuation
Baseline characteristics are presented in Table 1. The median age of the cohort was 56.5 years (IQR 47.5–66.0), and 4 (20.0%) patients were female. The majority had T3 disease (65.0%) and positive nodal disease (70.0%). Additionally, 55.0% of patients exhibited extramural vascular invasion (EMVI), and 70.0% had involvement of the mesorectal fascia (MRF). Twelve (60%) tumors were located within <5 cm from the anal verge.
Efficacy
A total of twenty patients were included in the efficacy analysis (Fig. 2A). A partial response was observed in 10 (50.0%) patients, with a median time to response of 6.0 weeks (IQR 5.2–12.8) from the first dose of sintilimab. The median time to response from the end of CRT was 10.1 weeks (IQR 8.8–12.2). Stable disease was observed in 7 (35.0%) patients. No cases of progressive disease were reported. cCR was observed in 3 (15.0%) patients, all of whom underwent surgery. Compared with the clinical stage before treatment, 7 (35.0%) patients had T downstaging and 8 (40.0%) had N downstaging (Fig. 2A).
Nineteen patients underwent surgery for the primary tumor (Table 2). Although not achieving cCR, another patient opted for organ preservation and declined surgery. At the time of manuscript submission (28 Mar 2025), this patient had not experienced local (primary tumor) progression or distant metastasis. Among those who received surgery, 18 patients received radical surgery, and another patient received transanal local resection. Among the patients who received radical surgery, 17 patients underwent anterior rectal resection (12 via laparoscopic surgery and 5 via robotic surgery), and one patient underwent laparoscopic abdominoperineal resection. The median interval from the completion of preoperative CRT to surgery was 12.0 weeks (IQR: 10.0–13.2), and that from the last dose of sintilimab to surgery was 4.4 weeks (IQR: 3.2–5.6) (Fig. 2B). The median degree of pathological regression was −90% (IQR −100 to −80). In the intention-to-treat analysis, a pCR was observed in 7/20 patients (35.0%) (Fig. 3). In the per-protocol analysis, the pCR rate was 36.8% (7/19) of the patients. Post hoc analysis revealed a trend toward higher pCR rates in patients with a PD-L1 CPS ≥ 5 than in those with a CPS < 5 (50% vs. 27.3%, P = 0.311).
The results of T2-weighted MRI of the rectum, endoscopic evaluations, and pathological results for two representative patients pre- and post-treatment are shown. A (Patient 4) shows a stable disease on endoscopic and imaging evaluations after treatment. B (Patient 5) shows an endoscopic complete response and a near‑complete response on T2‑weighted rectal MRI after treatment. Red arrows identify the tumor at each time point.
The median follow-up time from surgery to the data cutoff date (January 26, 2025) was 29.8 (IQR 21.0–32.8) months. At the time of data cutoff, the 1-year and 2-year DFS rates were 95.0% (95% CI, 84.6%–100.0%), with 1-year and 2-year OS rates of 100%. One DFS event was reported, in which a patient developed peritoneal metastasis and retroperitoneal lymph node metastasis 5 months after surgery.
Safety
Treatment-related adverse events of any grade occurred in 12 of the 20 patients (60.0%) (Table 3). The most common adverse events included lymphopenia (35.0%), hypothyroidism (10.0%), diarrhea (10.0%), and neutropenia (10.0%). Both diarrhea and neutropenia occurred during the CRT phase. Two patients experienced grade 3 adverse events (lymphopenia and diarrhea). No grade 4 events related to radiochemotherapy or sintilimab were observed.
Postoperative complications occurred in two patients. One patient (5.3%) experienced distal anastomotic stenosis and underwent endoscopic anastomotomy. The other patient experienced incomplete paralytic ileus and recovered after one week of conservative management. No additional surgery-related complications were reported at either 30 or 90 days following surgery.
Results of biomarker analysis
Multiplex immunohistochemistry analysis was carried out in 19 patients who underwent surgery for the primary tumor (Fig. 4A–D). The patients were divided into two groups according to response (pCR, n = 7) and non-response (non-pCR, n = 12). This exploratory biomarker analysis was conducted using samples obtained from primary tumors before treatment. The analysis revealed that higher CD3 (P < 0.001) and CD8 (P = 0.018) expression, or lower TIM-3 expression (P = 0.017) in the stroma, was associated with a higher pCR rate (Fig. 4E–G). No significant associations were found between the pCR rate and the expression levels of SETDB1, LAG-3, IDO, B7H4, B7H3, and VISTA in either tumor or stroma (Supplementary Fig. 1).
Discussion
To our knowledge, this is the first reported biomarker-directed prospective trial of neoadjuvant CRT followed by single-agent PD-1 inhibitors in pMMR/MSS LRAC patients. Although this study did not meet the primary endpoint, mainly owing to the limited sample size, our results showed that PD-L1 TPS ≥ 1% or CPS ≥ 1 may help identify patients who benefit from neoadjuvant long-course CRT followed by sintilimab. Furthermore, we identified several potential biomarkers that could also assist in selecting suitable patients for this treatment regimen.
Although immunotherapy has traditionally been ineffective in treating pMMR/MSS CRCs, preclinical studies have suggested that radiotherapy can reverse resistance to PD-1/PD-L1 blockade25,26. Our study demonstrated that the combination of long-course CRT followed by PD-1 inhibitors is a promising and feasible strategy. The efficacy of the combination of radiotherapy and chemotherapy with immunotherapy was also confirmed by the TORCH, Union, and POLARSTAR trials27,28,29. Previous studies have shown that total neoadjuvant chemoradiotherapy could considerably improve the pCR rate. On this basis, can combined immunotherapy further enhance the response? This result was also reported in a recent STELLER II study30. The pCR rate in the TNT combined with immunotherapy group was 45.5%, whereas it was 25.0% in the TNT-alone group (P = 0.002). However, the efficacy of each approach, whether long-course or short-course radiotherapy, concurrent/sequential PD-1 blockade, TNT, or chemoradiotherapy alone, warrants further investigation and direct comparison in future studies. Furthermore, whether biomarkers can be used to stratify patients and improve therapeutic efficacy remains to be explored.
The post-hoc analysis of previous clinical studies revealed that PD-L1 expression was significantly associated with the response to immunotherapy; however, its application in decision-making remains unclear. Therefore, in the present study, we selected pMMR/MSS LARC patients with PD-L1 TPSs ≥ 1% or CPSs ≥ 1 and treated them with long-course CRT followed by PD-1 inhibitors. Our data revealed a pCR rate of 35.0% among these patients, with the pCR rate nearly doubling compared with that following conventional long-course CRT. Although the trial did not meet its primary endpoint, the observed pCR rate was still higher than that reported in some studies using concurrent long-course CRT plus PD-1 inhibitors as the neoadjuvant approach. The VOLTAGE-A study reported a 30% pCR rate among patients receiving treatment with neoadjuvant long-course CRT and five subsequent cycles of nivolumab16. The AVANA study reported a 23% pCR rate after treatment with neoadjuvant long-course CRT with capecitabine and avelumab starting on Day 1. Although cross-study comparisons should be performed with caution, our data suggest that a PD-L1 TPS ≥ 1% or CPS ≥ 1 has the potential to be used to identify patients who are more likely to benefit from CRT followed by sintilimab31.
The expression of PD-L1 on tumor and immune cells has emerged as a critical factor in predicting the efficacy of PD-1 inhibitors across various types of cancers. In non-small cell lung cancer (NSCLC), the KEYNOTE-042 study has demonstrated that patients with a high PD-L1 TPS in their tumors tend to have better responses to pembrolizumab than those with a low PD-L1 TPS32. This has led to the use of PD-L1 immunohistochemistry as a standard diagnostic tool to guide treatment decisions in patients with NSCLC. Like PD-L1 expression on tumor cells, PD-L1 expression on immune cells also impacts the efficacy of immunotherapy as it induces immune tolerance. In our study, post-hoc analysis revealed a trend toward a higher pCR rate in patients with a PD-L1 CPS ≥ 5 than in those with a CPS < 5 (42.9% vs. 27.3%, P = 0.311), although the difference did not reach statistical significance due to sample size limitations. A previous study by Llosa et al. also supported this observation, showing that MSS metastatic CRC patients with high PD-L1 and PD-1 expression on CD8+ T cells had a tumor immune environment akin to that of dMMR/MSI-H patients, suggesting that these patients benefit from pembrolizumab treatment33. Further research is necessary to clarify the role of PD-L1 in guiding immunotherapy for pMMR/MSS LARC patients34.
The combination of CRT with PD-1 inhibitors was well tolerated, with 100% of patients completing all prescribed regimens. Only one patient experienced a grade 3 adverse event (diarrhea), which was attributed primarily to CRT. Compared with other trials, such as PANDORA and VOLTAGE-A, which combined immunotherapy with chemotherapy following radiotherapy, our study revealed a lower overall incidence of adverse events and better patient tolerance. This may be attributable to the fewer cycles of PD-1 inhibitors and the absence of combined chemotherapy with PD-1 inhibitors following CRT. Guided by PD-L1 expression, this approach may improve pCR rates while reducing treatment duration and adverse events.
We performed multiple immunofluorescence analyses using pretreatment biopsy samples to identify biological markers associated with tumor immunogenicity. Our findings suggest that patients with high expression levels of CD3 and CD8 or low expression levels of TIM3 in the stroma are more likely to benefit from this neoadjuvant strategy. This finding was echoed by the VOLTAGE-A study, which reported a promising pCR rate of 78% in patients with a high (≥2.5) CD8/eTreg ratio in TILs. Notably, patients with both positive PD-L1 expression and a high CD8/eTreg ratio presented the highest pCR rate (100%; 5/5)16. As PD-L1 expression is associated with tumor cell immunogenicity and CD8 is related to immune cell potential, the association of PD-L1 expression with CD8 expression might be a better predictive factor than PD-L1 expression alone. Additionally, TIM-3, a member of the TIM gene family, plays a crucial role in immune tolerance by negatively regulating immune cells such as CD8+ T cells or CD4+ Th1 cells35,36,37. Elevated expression of TIM-3 in gastric cancer, oral squamous cell carcinoma, and ovarian cancer has been associated with a poor prognosis38,39,40. Similarly, our data revealed increased expression of TIM-3 in non-pCR patients, suggesting that the addition of a TIM-3 inhibitor might help overcome resistance to the combined treatment with CRT and PD-1 inhibitors. Given the exploratory nature of this analysis and the small sample size, these findings should be interpreted with caution.
This trial represents an important step forward in exploring potential biomarkers for identifying patients who are sensitive to the combination of CRT with PD-1 inhibitors among pMMR/MSS LARC patients. However, several limitations in our study should be discussed in our study. First, this was a nonrandomized, single-arm, phase II study with a relatively small sample size. Moreover, this study did not meet the primary endpoint, mainly because of the limited sample size. Another possible reason is the complexity of the tumor microenvironment (TME) and the heterogeneous expression of PD-L1, which varies among cell types and fluctuates over time and in response to treatment. To further confirm the value of PD-L1 expression in predicting the efficacy of the combination of CRT with PD-1 inhibitors, large randomized trials in patients with high PD-L1 expression are needed. Finally, sintilimab is currently approved only in China, and the clinical data presented are based on a Chinese cohort. The results require further validation through international, multicenter studies.
In conclusion, although the target pCR rate was not fully achieved, we found that a PD-L1 TPS ≥ 1% or CPS ≥ 1 has the potential to be used to screen pMMR/MSS LARC patients who benefit from neoadjuvant CRT followed by sintilimab. These findings emphasize the importance of precision medicine in pMMR/MSS LARC patients. Further prospective, randomized, controlled, multicenter studies are necessary to confirm the benefit of CRT combined with immunotherapy and the value of PD-L1 in predicting treatment efficacy. Moreover, the expression of CD3, CD8, and TIM-3 has the potential to be used in conjunction with PD-L1 expression as a diagnostic tool to guide treatment decisions.
Methods
Ethics statement
This trial was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. B2019-092-Y02). All included patients provided written informed consent. This trial was performed in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. As a phase 2 trial experimenting on a commercially available drug (sintilimab), and the study drug is free for trial patients. In addition, our trial-specific commercial insurance covered all patients using the experimental drug.
Trial oversight
The protocol of this study was drafted by the first authors, is provided in the Supplementary Information. This trial was registered on ClinicalTrials.gov (identifying number: NCT04833387) with the registration date of April 4, 2021. As an investigator-initiated study, no funders participated in trial design, data collection, data analysis, data interpretation, or writing of the report. The study drug (sintilimab) was provided by Innovent Biologics. All authors had full access to the study data and vouch for its accuracy and completeness. The corresponding author had final responsibility for the decision to submit for publication.
Study design and participants
This study was a prospective, single-arm, open-label, phase II trial. The main inclusion criteria were as follows: histologically confirmed rectal cancer with pMMR and/or MSS status, locally advanced disease (cT3/4 or N+ for rectal cancer), tumors located within 12 cm of the anal verge, and tumors characterized as PD-L1 TPS ≥ 1% or CPS ≥ 1. For all staging, the American Joint Committee on Cancer, eighth edition, was used. Eligible patients were also required to be aged between 18 and 75 years and have an Eastern Cooperative Oncology Group performance score of 0–1, without severe hematologic, cardiac, pulmonary, hepatic, or renal functional abnormalities or immunodeficiency diseases. The key exclusion criteria included receiving prior anticancer treatment, metastatic disease, relapsed disease, and tumor-related complications requiring emergency surgery. The full list of inclusion and exclusion criteria is provided in the protocol (see Supplementary file).
Procedure
All eligible patients first received a baseline assessment, including information on demographics, medical history, disease characteristics before enrollment, a systematic physical examination, and relevant laboratory and imaging tests (chest computed tomography (CT), liver magnetic resonance imaging (MRI), and abdominal and pelvic CT or MRI).
All the tissue samples were obtained by using standard endoscopic biopsy forceps before treatment. Paraffin-embedded biopsy samples were stained with hematoxylin and eosin (H&E) and reviewed for each case. Moreover, a representative paraffin-embedded slide was selected for immunohistochemistry (IHC) analysis of PD-L1 expression.
After completion of the baseline assessment and PD-L1 expression evaluation, eligible patients received a standard CRT (50.4 Gy with 825 mg/m2 capecitabine twice daily for 5 weeks) followed by three cycles of 200 mg sintilimab every 3 weeks. Surgery was performed at 9–11 weeks after the end of CRT according to the principles of total mesorectal excision. Minimally invasive (laparoscopic and robotic) surgery was preferred. Low anterior resection was performed for middle and low rectal cancers with distal margins of more than 1 cm, and abdominoperineal resection was conducted by a surgeon for extremely low tumors. A trained pathologist (YHL) assessed all resected tumors for pathological regression using the 4-tier system recommended by the National Comprehensive Cancer Network (NCCN) guidelines41. For patients who achieved cCR, W&W were recommended after communication with the patient. The criteria for cCR were as follows: (1) no visible mass or only a small scar/erythematous ulcer/smooth narrowing on endoscopy, with negative biopsy; (2) no visible residual tumor on MRI, with no restricted diffusion on T2-weighted imaging and lymph nodes ≤5 mm; and (3) no palpable tumor or stiffness of rectal wall on digital the rectal examination.
For patients who underwent surgery, regular surveillance was done every 3 months in the first 2 years and every 6 months over the next 3–5 years. At each follow-up visit, a physical examination, imaging examination (CT or MRI), colonoscopy, and any laboratory monitoring were performed, as clinically indicated. For patients who underwent the watch-and-wait approach after achieving a clinical complete response, colonoscopy and radiological examination were performed every 3 months in the first 2 years and were repeated every 6 months over the next 3–5 years.
Adverse events were monitored and documented throughout treatment until 90 days after the last dose of sintilimab and were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). Postoperative complications were recorded for 30 and 90 days after surgery and scored according to the Clavien‒Dindo classification42.
PD-L1 expression upon immunohistochemistry analysis
IHC for PD-L1 was performed, and PD-L1 scoring was conducted by a trained technician and coauthor (RYS), following the manufacturer’s standard protocol. IHC for PD-L1 was performed using the PD-L1 IHC 22C3 pharmDx kit on the Autostainer Link 48 platform (Agilent Technologies, Cat# AS480) following the manufacturer’s protocol. The sections were incubated with the primary antibody at 4 °C overnight, followed by labeling with horseradish peroxidase (HRP) and chromogen development. Phosphate-buffered saline (PBS) was used in place of the primary antibody as a negative control. PD-L1 expression was evaluated across the entire tissue section, with scoring performed on all viable tumor cells present. Moreover, in accordance with established guidelines, only slides containing at least 100 viable tumor cells were considered adequate for PD-L1 assessment. The PD-L1 TPS was the percentage of tumor cells positive for PD-L1. The PD-L1 CPS was the number of PD-L1–positive cells (tumor cells, lymphocytes, macrophages) divided by the total number of tumor cells and multiplied by 100. PD-L1 positivity was defined as membranous staining at any intensity. All the IHC-stained samples from patients with melanoma were evaluated independently by two experienced pathologists. For slides with discrepant scores, the final score was determined after a review of the slides and discussion by the two pathologists.
Outcomes
The primary endpoint of this trial was the rate of pCR, defined as the absence of cancer cells in both the resected rectum and all sampled regional lymph nodes. The secondary endpoints included the rate of cCR, pathological tumor regression grade (TRG), and treatment-related toxicity. The tumor regression grade after preoperative treatment was evaluated semiquantitatively according to the National Comprehensive Cancer Network guidelines41. Additional secondary endpoints included 3-year disease-free survival (DFS, defined as the time from surgical intervention to (local or distant) disease relapse or death from any cause) and 3-year overall survival (OS, defined as the time from enrollment to death from any cause).
Evaluation of immunohistochemistry
An exploratory biomarker study was also conducted using samples obtained from patients before treatment. Formalin-fixed paraffin-embedded tumor tissue sections were also examined by multiplex immunohistochemistry analysis of SETDB143, CD344, LAG-345, CD846, IDO47, B7H448, B7H349, VISTA50, and TIM351. Multiplex immunohistochemistry was performed using an Opal Manual kit (TSA-RM-82758, Panovue, China) according to the manufacturer’s protocol. All analyses were centrally and independently performed by two pathologists who were blinded to the clinical outcomes.
Statistical analysis
Several studies have reported that the pCR rate after long-course CRT ranges from 15% to 20%3,52. For our study, we selected the median value of 18% as the baseline pCR rate. Previous studies have suggested that combining radiotherapy with PD-1 inhibitors can increase the pCR rate in patients with pMMR/MSS locally advanced rectal cancer, with reported rates ranging from 30% to 40%16,17. Among these, patients with high PD-L1 expression are likely to achieve even higher pCR rates, with rates reaching 70%16,17. However, this increase is relatively large and should be treated with caution. Therefore, we expected that the regimen of CRT followed by sintilimab could increase the pCR rate from 18% to 45%. A sample size of 18 patients was required to provide at least 80% power to detect this estimated improvement in a one-sided chi-square test with a significance level of 2.5% and an estimated 5% dropout rate, resulting in a total sample size of 20 patients planned for this study.
Categorical variables are presented as counts and percentages (n [%]), whereas continuous variables are presented as medians and interquartile ranges (IQRs). Associations between categorical variables were assessed using the chi-square test or Fisher’s exact test. Differences between groups in the exploratory biomarker analysis were tested using the Wilcoxon rank-sum test or Student’s t-test, depending on the outcome normality assessment. Statistical analyses were performed using R software (version 4.2.1; http://www.r-project.org/). A two-sided P < 0.05 was considered statistically significant.
Data availability
The datasets generated and/or analysed during the current study are available at the Research Data Deposit public platform at www.researchdata.org.cn.
Code availability
Not application
References
Glynne-Jones, R. et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv22–iv40 (2017).
Benson, A. B. et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 16, 874–901 (2018).
Kasi, A. et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw. Open 3, e2030097 (2020).
Conroy, T. et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 22, 702–715 (2021).
Jin, J., et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J. Clin. Oncol. 40, 1681–1692 (2022.
Hu, H. et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 38–48 (2022).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Cercek, A. et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Chen, G. et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol. Hepatol. 8, 422–431 (2023).
Yu, J. H. et al. Neoadjuvant camrelizumab plus apatinib for locally advanced microsatellite instability-high or mismatch repair-deficient colorectal cancer (NEOCAP): a single-arm, open-label, phase 2 study. Lancet Oncol. 25, 843–852 (2024).
Alsaafeen, B. H., Ali, B. R. & Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: updated insights. Mol. Cancer 24, 20 (2025).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Hecht, M. et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 65, 52–60 (2016).
Grapin, M. et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J. Immunother. Cancer 7, 160 (2019).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Bando, H. et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin. Cancer Res. 28, 1136–1146 (2022).
Grassi, E. et al. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial. ESMO Open 8, 101824 (2023).
Melissourgou-Syka, L. et al. A review of scheduling strategies for radiotherapy and immune checkpoint inhibition in locally advanced rectal cancer. J. Immunother. Precis. Oncol. 6, 187–197 (2023).
Shamseddine, A. et al. Short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma. BMC Cancer 20, 831 (2020).
Lin, Z. et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer. https://doi.org/10.1136/jitc-2021-003554 (2021).
Noguchi, T. et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol. Res. 5, 106–117 (2017).
Yamashita, K. et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer 23, 95–104 (2020).
Zhou, C. et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget 8, 58457–58468 (2017).
Fakih, M. et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 58, 101917 (2023).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 124, 687–695 (2014).
Demaria, S., Golden, E. B. & Formenti, S. C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 1, 1325–1332 (2015).
Xia, F. et al. Randomized phase II trial of immunotherapy-based total neoadjuvant therapy for proficient mismatch repair or microsatellite stable locally advanced rectal cancer (TORCH). J. Clin. Oncol. 42, 3308–3318 (2024).
Yang, Y. et al. Neoadjuvant chemoradiation with or without PD-1 blockade in locally advanced rectal cancer: a randomized phase 2 trial. Nat. Med. 31, 449–456 (2025).
Lin, Z. Y. et al. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann. Oncol. 35, 882–891 (2024).
Zhang, W. et al. Preoperative short-course radiotherapy followed by chemotherapy and PD-1 inhibitor administration for locally advanced rectal cancer: a study protocol of a randomized phase II/III trial (STELLAR II study). Colorectal Dis. 26, 1732–1740 (2024).
SalvatoreL, et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): the AVANA study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.15_suppl.3511 (2021).
Mok, T. S. K. et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393, 1819–1830 (2019).
Llosa, N. J. et al. Immunopathologic stratification of colorectal cancer for checkpoint blockade immunotherapy. Cancer Immunol. Res. 7, 1574–1579 (2019).
He, Y. et al. Soluble PD-L1: a potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J. Transl. Med. 21, 25 (2023).
Freeman, G. J., Casasnovas, J. M., Umetsu, D. T. & DeKruyff, R. H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 235, 172–189 (2010).
Das, M., Zhu, C. & Kuchroo, V. K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276, 97–111 (2017).
Wang, Z. et al. Application of bioinformatics in predicting the efficacy of digestive tumour immunotherapy target TIM-3 and its inhibitors. J. Cancer 15, 1954–1965 (2024).
Wang, H. et al. Altered expression of TIM-3, LAG-3, IDO, PD-L1, and CTLA-4 during nimotuzumab therapy correlates with responses and prognosis of oral squamous cell carcinoma patients. J. Oral. Pathol. Med. 48, 669–676 (2019).
Fucikova, J. et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin. Cancer Res.25, 4820–4831 (2019).
Jiang, J. et al. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS ONE 8, e81799 (2013).
Benson, A. B. et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 20, 1139–1167 (2022).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
Griffin, G. K. et al. Epigenetic silencing by SETDB1 suppresses tumour intrinsic immunogenicity. Nature 595, 309–314 (2021).
Wu, Z. et al. CD3(+)CD4(-)CD8(-) (double-negative) T Cells in Inflammation, Immune Disorders and Cancer. Front Immunol. 13, 816005 (2022).
Aggarwal, V., Workman, C. J. & Vignali, D. A. A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 24, 1415–1422 (2023).
Park, J., Hsueh, P. C., Li, Z. & Ho, P. C. Microenvironment-driven metabolic adaptations guiding CD8(+) T cell anti-tumor immunity. Immunity 56, 32–42 (2023).
Fujiwara, Y. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 110, 102461 (2022).
Wang, J. Y. & Wang, W. P. B7-H4, a promising target for immunotherapy. Cell Immunol. 347, 104008 (2020).
Kontos, F. et al. B7-H3: an attractive target for antibody-based immunotherapy. Clin. Cancer Res. 27, 1227–1235 (2021).
Martin, A. S. et al. VISTA expression and patient selection for immune-based anticancer therapy. Front. Immunol. 14, 1086102 (2023).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Maas, M. et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11, 835–844 (2010).
Acknowledgements
We thank all of the patients and their families who participated in this study. We are grateful to all coordinators and research staff at all study sites for their contribution. We thank Yan-Shuang Cheng for assistance in data collection and patient follow-up. The study drug was provided by Innovent Biologics (Suzhou, Jiangsu, China). We thank Jian Zhang, Ph.D., from Sun Yat-sen University Zhongshan Ophthalmic Center for evaluating statistics. This study was funded by the National Natural Science Foundation of China (grant number 82473395), Cancer Innovative Research Program of Sun Yat-sen University Cancer Center (CIRP-SYSUCC-0032), Guangzhou Science and Technology Key Research and Development Program (2024B03J0092) and Fostering Program for NSFC Young Applicants (Tulip Talent Training Program) of Sun Yat-sen University Cancer Center (TTP-SYSUCC-2024yfd12, TTP-SYSUCC-gzrpy-2021-12). The funder played no role in study design, data collection, analysis, and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
P.R.D., W.J., Z.Z.P., and Z.L.H. designed this study and wrote the protocol. P.R.D., W.W.X., K.H., D.D.L., and X.S.Z. recruited patients. L.E.L., Q.Q.S., Y.L., W.J.M., J.H.Y., R.Y.S., Z.G.H., and Q.C.C. collected data and did the statistical analysis. Z.L.H., L.E.L., W.J., and B.Y.X. verified all study data and wrote the initial manuscript. All authors reviewed and revised the manuscript and approved the final version of the manuscript. The corresponding author had full access to all the data in this study and had final responsibility for the decision to submit the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, Z., Liao, L., Xiao, W. et al. Neoadjuvant chemoradiotherapy plus sintilimab in pMMR/MSS rectal cancer patients with PD-L1 TPS ≥ 1% or CPS ≥ 1: an open-label, prospective, phase II study. npj Precis. Onc. 9, 237 (2025). https://doi.org/10.1038/s41698-025-01018-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01018-0