Abstract
Immunotherapy has substantially changed the landscape of cancer. We present five patients with advanced cancer who had failed standard-of-care therapies, participated in clinical trials with anti-CTLA4/anti-PD1 therapy, and had prolonged progression-free survival (PFS): Pt1: microsatellite stable (MSS) rectal adenocarcinoma (34+ months); Pt2: high grade neuroendocrine carcinoma (68 months); Pt3: MSS colon adenocarcinoma (38+ months); Pt4: deficient mismatch repair (dMMR) prostate adenocarcinoma (68+ months); Pt5: high-grade neuroendocrine carcinoma (69+ months). Grade 3 immune-related adverse events (irAEs; n = 4) were treated successfully, allowing further treatment with the anti-PD1 therapy. Positron-emission tomography (PET)/computed tomography (CT) scans demonstrated complete response in 3 of 4 patients whose CT scans had shown partial response. These cases highlight the exceptional outcomes of selected patients with advanced cancer treated with immunotherapy and exemplify the value of personalized patient monitoring/management of irAEs while assessing response to immunotherapy. Ongoing trials evaluate biomarkers that predict response and toxicity associated with immunotherapy.
Similar content being viewed by others
Introduction
Immunotherapy has changed the landscape of cancer management by leading to deep and durable responses in a substantial number of patients, even those with stage IV disease1. Combination therapy using PD1/PD-L1 and CTLA4 blockade has demonstrated stronger antitumor activity compared to either monotherapy, and it is now approved for the treatment of several tumor types2,3. In addition to developing effective therapeutic agents, researchers have been investigating biomarkers that can predict response or resistance to immunotherapy.
To date, three biomarkers have been established for selecting patients for immunotherapy: PD-L1 expression, deficient mismatch repair/microsatellite instability (dMMR/MSI)-high status, and high tumor mutational burden (TMB)4. While the presence of these biomarkers increases the likelihood of response to anti-PD1/anti-CTLA4 immunotherapy, their absence does not necessarily predict a lack of response. Therefore, some investigators believe that these biomarkers should be used for patient inclusion, rather than exclusion, in determining who may benefit from immunotherapy5. In this article, we present five patients with advanced metastatic cancer who had failed standard treatments, participated in clinical trials with anti-CTLA4 and anti-PD1 combination therapy, and experienced prolonged response to immunotherapy (Table 1).
Results
Case 1
A woman in her 20s was diagnosed with rectal adenocarcinoma and underwent low anterior resection followed by six cycles of FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin). Ten months later, she developed metastatic disease to the lung and underwent a lung wedge resection. Subsequently, she was treated with capecitabine and bevacizumab. Imaging studies after 4 cycles demonstrated a small disease burden, and the patient elected to have a chemotherapy break.
Eight months later, the patient developed biopsy-proven progressive metastatic colorectal cancer to the lung and pleural effusion, and she presented to the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center for treatment options. Her Eastern Cooperative Oncology Group (ECOG) performance status was 0, and she had normal organ function. Genomic testing showed FBXW7 G423R, KRAS G12D, TP53 R282W, APC N942fs, and APC E1309* mutations. Immunohistochemistry (IHC) testing was negative for HER2 and PD-L1, and microsatellite status was stable (MSS).
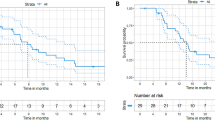
The patient elected to participate in a clinical trial with a combination of anti-CTLA4 and anti-PD1 monoclonal antibodies administered every 3 weeks (NCT03860272). At the end of the first cycle, she experienced febrile episodes that required a temporary treatment interruption. Workup for infection and autoimmune disorders was negative, and the anti-CTLA4 and anti-PD1 monoclonal antibodies were resumed. The first restaging computed tomography (CT) scans, after 2 cycles of treatment, demonstrated a partial response (PR; 86% decrease in tumor measurements per Response Evaluation Criteria in Solid Tumors [RECIST 1.1]6). Four months after starting treatment, the patient’s imaging studies with positron emission tomography/CT (PET/CT) demonstrated a complete response (CR). There was some nonspecific, scattered, and light fluorodeoxyglucose (FDG) uptake in the patient’s lymph nodes, with a maximum standardized uptake value (SUV) of 4.2 both above and below the diaphragm, suggesting an ongoing infectious/inflammatory process. Core needle biopsy of one enlarged lymph node demonstrated small non-necrotizing epithelioid granulomas with giant cells in a background population of reactive lymphoid cells, highlighted by CD3/CD20 dual immunostaining, which was consistent with granulomatous lymphadenitis. One year after starting treatment, the patient developed a metastatic brain lesion (16 mm) that was treated with stereotactic radiation therapy (Gamma Knife). She continued systemic therapy with the PD1 monoclonal antibody for a total of 18 cycles, with no evidence of disease on imaging studies. Treatment was discontinued per protocol after 2 years of therapy. Since then, the patient has been monitored with PET/CT scans every 3 months, and there has been no evidence of disease progression for over 10 months (Fig. 1).
Case 2
A man in his 50s was diagnosed with high-grade neuroendocrine carcinoma of colorectal origin. He initially presented with abdominal pain, and workup included laboratory tests and imaging studies. The patient underwent a colonoscopy and was found to have a large, friable, polypoid, circumferential mass in the distal ascending colon at the hepatic flexure. Biopsies of this area showed only evidence of a tubulovillous adenoma. Subsequent ultrasound-guided biopsy of a liver lesion showed high-grade neuroendocrine carcinoma. The assumption was that the pathology from the colonic lesion represented a sampling error rather than this being truly only a tubulovillous adenoma. The diagnosis was made at an outside institution. Slides from the right colon mass and liver metastasis biopsies were reviewed at our institution, and the diagnosis of high-grade neuroendocrine carcinoma was confirmed. The patient was treated with carboplatin and etoposide for 6 cycles. Subsequently, he underwent right hemicolectomy, which showed a small invasive carcinoma within a tubulovillous adenoma, suggesting a potential response of the neuroendocrine component to prior therapy. Three months later, imaging studies with CT scans showed progressive disease in the liver. Core biopsy of the liver lesion confirmed high-grade neuroendocrine carcinoma, and the patient was treated with second-line FOLFIRINOX for 12 cycles. His best RECIST response to FOLFIRINOX was a partial response (PR).
Seven months later, CT scans demonstrated progressive disease (PD) in the liver and portocaval lymph nodes. The patient was referred to the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for treatment options. His performance status was 0, and he had normal bone marrow and liver function, with a mildly elevated creatinine level. Tumor molecular profiling showed KRAS G12D and RAD50 E995fs*2 mutations. IHC was negative for HER2. TMB was 2.6 mutations per megabase, and the tumor was MSS.
The patient was treated with a combination of anti-CTLA4 and anti-PD1 monoclonal antibodies every 3 weeks (NCT03651271). His tumor response to treatment was PR by RECIST, showing a 51% decrease in tumor measurements after 2 cycles and a 72% decrease after 4 cycles. After 4 cycles, he developed nausea, vomiting, diarrhea, and fever, prompting a workup that included an upper gastrointestinal endoscopy and colonoscopy. The upper endoscopy demonstrated friable mucosa without active bleeding in the second portion of the duodenum, as well as diffuse, moderately erythematous mucosa throughout the entire examined stomach. The colonoscopy was unremarkable. Pathological analysis demonstrated possible autoimmune gastritis and duodenitis. Biopsy of liver lesions demonstrated normal liver tissue with fibrosis and lymphohistiocytic inflammatory infiltrate. No viable tumor was identified with an anti-cytokeratin cocktail (AE1/AE3, MNF116, Zym5.2, and Cam5.2). The patient was treated with steroids to manage the immune-related gastritis and duodenitis, and his symptoms completely resolved. He was monitored with laboratory tests and imaging studies every 3 months for the first 2 years, followed by monitoring every 6 months thereafter. One year after treatment interruption, PET/CT scans were negative for metastatic disease, and the patient remained disease-free approximately 5.5 years after his last dose of therapy (Fig. 2).
Case 3
A woman in her 30s presented to the local emergency room with progressive abdominal pain. Imaging studies revealed an abdominal mass, and she underwent ileocecal resection with excision of a 3.7 cm cecal mass. Pathology showed invasive well-differentiated adenocarcinoma of the cecum with no lymphovascular or perineural invasion and 0 out of 20 lymph nodes positive for malignancy. Six months later, a colonoscopy revealed multiple polyps in the remaining colon. She then underwent total colectomy, which revealed moderately differentiated adenocarcinoma. The patient received adjuvant FOLFOX (folinic acid, fluorouracil, and oxaliplatin) for 6 months and was monitored thereafter.
Four and a half years after the initial surgery, imaging studies demonstrated an abdominal mass. The mass was biopsied and confirmed to be adenocarcinoma, consistent with the primary colorectal cancer (positive immunostaining for CDX2). The patient underwent surgical resection of the tumor, followed by 6 cycles of FOLFOX plus bevacizumab. Restaging scans showed disease progression, and treatment was switched to FOLFIRI (folinic acid, fluorouracil, and irinotecan) plus bevacizumab, which she received for 10 cycles. She then underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) and continued with maintenance irinotecan for an additional 6 cycles. Restaging scans showed a new metastasis in the spleen, and the patient underwent exploratory laparotomy with splenectomy and secondary cytoreduction followed by HIPEC as salvage therapy. One year later, restaging scans showed disease progression, and the patient was treated with FOLFIRI plus bevacizumab for 18 cycles. However, subsequent CT scans demonstrated further disease progression, with an increase in upper midline retroperitoneal and intra-peritoneal adenopathy (the largest lymph node measuring 33 × 22 mm).
The patient presented to our department at MD Anderson Cancer Center to explore clinical trial options. Tissue molecular testing of the previously resected abdominal mass showed KRAS G12D, PIK3CA E545K, and CHEK2 T367fs*15 mutations. TMB was 3.2 mutations per megabase. IHC was negative for HER2 and PD-L1, and the disease was MSS. The patient participated in a clinical trial with anti-Globo H monoclonal antibody (OBI-888; received for 4 cycles)7, followed by a second clinical trial with a 4-1BB agonist with cetuximab and irinotecan for 8 cycles. Treatment was discontinued owing to disease progression. CT imaging studies demonstrated abdominal lymphadenopathy and peritoneal metastases, with no liver involvement. Subsequently, the patient was enrolled in a clinical trial with anti-CTLA4 and anti-PD1 monoclonal antibodies every 3 weeks (NCT03860272).
After 3 cycles of treatment, the patient had stable disease (SD) per imaging with a 3.7% increase from baseline according to RECIST 1.1. At this time, however, she developed abdominal cramping, and a colonoscopy showed an inflammatory stricture, for which she underwent serial balloon dilatation. Two months after the last treatment, a PET/CT scan showed no increased uptake, except in a portacaval lymph node that had a max SUV of 4.1. A biopsy of the concerning portacaval lymph node was negative for malignancy. Treatment was placed on hold, and the patient was monitored with physical examination, laboratory tests, and routine imaging studies every 1–3 months. Her best RECIST response by CT imaging was a PR, achieved 11 months after treatment initiation, and PET/CT scans demonstrated a complete metabolic response 7 months after the initiation of treatment. The patient remains in complete metabolic response, 2.5 years after the last dose of treatment (Fig. 3).
Case 4
A man in his 70s was diagnosed with metastatic prostate cancer and initially treated with androgen deprivation therapy, followed by sipuleucel-T (for ~1 month), abiraterone acetate/apalutamide/prednisone (as part of a clinical trial for approximately 2 years), and cabazitaxel/carboplatin (as part of a clinical trial for approximately 3 months). Four years after his initial diagnosis, he was referred to our department for participation in a clinical trial with anti-CTLA4 and anti-PD1 monoclonal antibodies (NCT03651271). At baseline, CT scans showed adenopathy in the posterior right external iliac and superficial right external iliac lymph nodes, as well as the prostate primary tumor. Tumor molecular testing showed a PIK3CA N1044K mutation, and IHC testing demonstrated dMMR. After 1 cycle of treatment, the patient developed grade 3 transaminitis and treatment was placed on hold. His PSA level decreased from 19.1 at baseline to <0.1 1 month after treatment initiation. Restaging CT scans of the chest, abdomen, and pelvis, 2 months after treatment started, showed a PR with a 38% decrease in tumor measurements compared to baseline. The patient was monitored with CT scans of the chest, abdomen, and pelvis, a bone scan, and PSA level measurements every 3 months for 2 years and then every 6 months. These tests have shown no evidence of disease progression for >5 years since he received one dose of the anti-CTLA4 and anti-PD1 monoclonal antibodies (Fig. 4).
Case 5
A woman in her 50s was diagnosed with a high-grade malignant neoplasm consistent with primitive neuroectodermal tumor of the uterus. She underwent total abdominal hysterectomy/bilateral salpingo-oophrectomy, node dissection, and appendectomy. Sixteen months later, metastatic disease was found in the lungs and bones. She was treated with vincristine and doxorubicin with concurrent radiation therapy to the bone lesions, which induced a CR. The patient was monitored with routine CT scans for 7 years, when a soft tissue mass in the left upper abdomen was identified. A biopsy of this lesion was performed, and pathologic examination demonstrated small tumor cells with scant amounts of acidophilic cytoplasm. These cells exhibited diffuse and strong positive staining for vimentin, synaptophysin, CD56, and cytokeratin. A very small number of cells also stained positive for desmin (dot-like). Staining for CD99 and TTF-1 was negative, and the Ki-67 proliferation index was 40%. These findings were consistent with a metastatic small cell malignancy related to the patient’s known neuroendocrine carcinoma.
The patient was treated with carboplatin and etoposide for 3 cycles, temozolomide for 1 cycle, and everolimus for 3 cycles, but the tumor progressed after each treatment. Subsequently, she was treated with an investigational peptide-drug conjugate for 2 cycles and had disease progression. Rechallenge with carboplatin and etoposide, combined with radiation therapy to a mediastinal/lung mass was attempted, but the disease continued to progress. Eleven years after the initial diagnosis, the patient was referred to our Department for investigational therapy. She participated in a clinical trial and was treated with anti-CTLA4 and anti-PD1 monoclonal antibodies (NCT03651271). Baseline scans showed multiple enlarged lymph nodes with adjacent lung and bronchus involvement. Tumor molecular profiling showed a PIK3CA Y1021H mutation. Her best response by RECIST after 4 doses of the anti-CTLA4 and anti-PD1 monoclonal antibodies was a PR with a 43% decrease in tumor measurements. According to the study design, the patient continued treatment with anti-PD1 monotherapy for an additional 35 cycles. She developed persistently elevated creatinine levels, and a kidney biopsy showed chronic hypertensive nephrosclerosis with moderate arterial and arteriolar sclerosis, unrelated to immunotherapy. CT scans at that time demonstrated no evidence of cancer, and the treatment was discontinued. A PET/CT performed 6 months after treatment discontinuation revealed a metabolic complete response. The patient has been off treatment for approximately 3 years, is being monitored regularly, and continues to have no evidence of cancer (Fig. 5).
Discussion
Herein, we present five cases of patients with advanced cancer who had failed standard-of-care therapies and subsequently achieved prolonged tumor responses to anti-CTLA4/anti-PD1 combination therapy. These patients experienced immune-related adverse events (irAEs), which were managed promptly through close monitoring and adherence to institutional and standard guidelines for irAE management.
There are several lessons to be learned from these case studies. First, it is established that patients receiving anti-CTLA4/anti-PD1 combination therapy can develop serious irAEs, which can be effectively managed. Although anti-CTLA4 monoclonal antibodies are not typically administered after grade 3 irAEs, such as colitis or elevated liver enzymes, patients can be monitored without treatment and/or continue with anti-PD1 monotherapy safely for an extended period of time. Second, patients may develop central nervous system metastases, which can be treated effectively with radiation therapy to the brain or spine without needing to discontinue immunotherapy (e.g., case 1). This underscores the importance of multimodal therapeutic approaches.
An intriguing observation is that CT scans may not accurately demonstrate patients’ disease status after treatment with immunotherapy. This was evidenced in some cases where PET/CT scans demonstrated no evidence of disease while CT scans indicated a PR. These findings suggest that the RECIST 1.1 tumor response assessment guidelines have limitations in assessing response to immunotherapy. Based on our experience, we recommend that patients treated with immunotherapy undergo periodic PET/CT scans to better assess disease activity. Additionally, immunotherapy is associated with atypical response patterns, including pseudo-progression and delayed response to treatment8.
An important question is the duration of treatment with immunotherapy. Typically, patients may continue treatment if they tolerate it well and have no evidence of confirmed disease progression. Given the nature of phase 1 studies and advanced disease status, the optimal duration of immunotherapy cannot be easily assessed in a randomized study. However, treatment de-escalation should be considered, especially in patients who develop serious irAEs, with close monitoring of disease activity. Some investigators have reported the safe discontinuation of immunotherapy without compromising the durability of response, especially in patients who achieved deep responses9,10. This observation has implications for developing strategies to optimize the antitumor effect, while minimizing irAEs. For instance, a retrospective analysis of a large cohort of patients with non-small cell lung cancer demonstrated that patients who discontinued immunotherapy after 2 years of progression-free survival did not have inferior survival to patients who continued immunotherapy indefinitely10. Additionally, a review of published data on immunotherapy discontinuation suggested that discontinuation at 1 year is also safe and reasonable, with an acceptable risk of disease progression9.
Response to immune checkpoint blockade in this case series may have been associated with various factors. Only one patient had an established biomarker predicting sensitivity to immunotherapy (Case 4, dMMR). However, patients had additional biomarkers that have been previously proposed to predict antitumor activity of immune checkpoint inhibitors. For example, 3 patients had mutations in PIK3CA, which aligns with published data suggesting clinical benefit from immunotherapy in patients with PIK3CA-mutated head and neck or colorectal cancer, even in the setting of MSS disease11,12. Other investigators suggested that activation of the PTEN/PI3K axis is associated with immunosuppressive effects13,14. Additionally, 3 patients in our series had a KRAS G12D mutation. The role of KRAS mutations in modulating the immune response and their correlation with the efficacy of immunotherapeutic agents remains controversial15. In some studies, KRAS mutations have been associated with a benefit from immune checkpoint inhibitors16,17,18,19,20, while in other studies KRAS G12D mutations have been linked with immunosuppression and primary resistance to anti-PD1 therapy21. Other notable alterations of interest include CHEK2 and RAD50 mutations. A role for DNA damage repair alterations, including CHEK2, in the immune function has been previously described22,23,24.
Another characteristic of improved outcomes after immune checkpoint inhibition is the absence of enlarging hepatic metastases (e.g., cases 1, 3, 4 and 5). This differential response in patients without hepatic metastases may be attributed to the unique microenvironment of the liver, as reported in several studies25,26,27,28,29. An important consideration for patients with advanced metastatic cancer is that they often have large metastatic tumors harboring multiple molecular alterations, and the complexity of their tumor biology cannot be addressed with a single targeted agent. Immunotherapy offers a unique advantage by harnessing the patients’ immune mechanisms to effectively overcome this complexity. The unique pattern of response to immunotherapy, including prolonged responses as noted in our patients, is not typically seen with other classes of drugs, such as small molecules or antibody-drug conjugates.
The limitations of this case series, as with similar reports, include the lack of ability to generalize, risk of over-interpretation, publication bias, and the retrospective nature of the report.
In conclusion, these cases highlight the exceptional and prolonged response achieved with anti-CTLA4 and anti-PD1 monoclonal antibodies in patients with advanced metastatic cancer. They also exemplify the role of personalized patient care, including close monitoring, managing irAEs, and carefully assessing patient response to immunotherapy. Ongoing clinical trials and prospective studies focus on identifying biomarkers associated with response to immunotherapy and the risk of toxicity. The results of these studies will help identify patients who may have exceptional, prolonged response to immunotherapy, ultimately improving clinical outcomes of patients with cancer.
Methods
Patients treated on two clinical trials with anti-CTLA4 and anti-PD1 monoclonal antibodies (NCT03651271 and NCT03860272) were reviewed, and all those who had prolonged response to treatment were included in this case series30,31. Patients were treated in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center. Prior to treatment, patients signed an informed consent document stating that they were aware of the investigational nature of the study. Clinical data, including demographics, disease characteristics, prior treatment history, radiologic findings, and outcomes, were collected retrospectively from electronic medical records. Imaging studies were reviewed by board-certified radiologists. Response to therapy was assessed based on RECIST v1.1 criteria6. Tumor tissue or liquid biopsy specimens were analyzed using next-generation sequencing platforms targeting cancer-relevant genes. Genomic alterations, TMB, MSI status, and gene fusions were reported in accordance with the American College of Genetics and Genomics/Association for Molecular Pathology guidelines. Patients were monitored clinically and radiographically in accordance with the clinical trial requirements and standard institutional protocols.
Data availability
All data are presented in the current paper.
References
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 38, 255 (2019).
Chae, Y. K. et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer 6, 39 (2018).
Catalano, M. et al. Immunotherapy-related biomarkers: confirmations and uncertainties. Crit. Rev. Oncol. Hematol. 192, 104135 (2023).
Pan, Y. et al. The key to immunotherapy: how to choose better therapeutic biomarkers for patients with non-small cell lung cancer. Biomark. Res. 10, 9 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Knisely, A. et al. Phase 1/2 trial of avelumab combined with utomilumab (4-1BB agonist), PF-04518600 (OX40 agonist), or radiotherapy in patients with advanced gynecologic malignancies. Cancer 130, 400–409 (2024).
Tsimberidou, A. M. et al. Trial reporting in immuno-oncology (TRIO): an American society of clinical oncology-society for immunotherapy of cancer statement. J. Clin. Oncol. 37, 72–80 (2019).
Jansen, Y., van der Veldt, A. A. M., Awada, G. & Neyns, B. Anti-PD-1: when to stop treatment. Curr. Oncol. Rep. 24, 905–915 (2022).
Sun, L. et al. Association between duration of immunotherapy and overall survival in advanced non-small cell lung cancer. JAMA Oncol. 9, 1075–1082 (2023).
Nusrat, M. et al. Association of PIK3CA mutations (mut) with immune engagement and clinical benefit from immunotherapy in microsatellite stable (MSS) colorectal cancer (CRC) patients (pts). J. Clin. Oncol. 37, 3604–3604 (2019).
Sun, Z., Zhang, Q., Duan, Q., Tan, Y. & Chen, D. 873P Identification of PIK3CA mutation as a novel predictor of efficacious immunotherapy in head and neck cancer. Ann. Oncol. 34, S563–S564 (2023).
Collins, N. B. et al. PI3K activation allows immune evasion by promoting an inhibitory myeloid tumor microenvironment. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-003402 (2022).
Vidotto, T. et al. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 122, 1732–1743 (2020).
Linette, G. P., Bear, A. S. & Carreno, B. M. Facts and hopes in immunotherapy strategies targeting antigens derived from KRAS mutations. Clin. Cancer Res. 30, 2017–2024 (2024).
Watterson, A. & Coelho, M. A. Cancer immune evasion through KRAS and PD-L1 and potential therapeutic interventions. Cell Commun. Signal 21, 45 (2023).
Xu, M., Zhao, X., Wen, T. & Qu, X. Unveiling the role of KRAS in tumor immune microenvironment. Biomed. Pharmacother. 171, 116058 (2024).
Peng, L. et al. Efficacy of immunotherapy in KRAS-mutant advanced NSCLC: a real-world study in a Chinese population. Front Oncol. 12, 1070761 (2022).
Chmielewska, I. et al. Breaking the ‘Undruggable’ Barrier: anti-PD-1/PD-L1 immunotherapy for non-small cell lung cancer patients with KRAS mutations—a comprehensive review and description of single site experience. Cancers https://doi.org/10.3390/cancers15143732 (2023).
Lauko, A. et al. Impact of KRAS mutation status on the efficacy of immunotherapy in lung cancer brain metastases. Sci. Rep. 11, 18174 (2021).
Liu, C. et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun. 42, 828–847 (2022).
Xu, P. et al. CHEK2 deficiency increase the response to PD-1 inhibitors by affecting the tumor immune microenvironment. Cancer Lett. 588, 216595 (2024).
Wu, Q., Fang, C., Wang, X., Huang, S. & Weng, G. CHEK2 is a potential prognostic biomarker associated with immune infiltration in clear cell renal cell carcinoma. Sci. Rep. 13, 21928 (2023).
Shi, C. et al. The role of DNA damage repair (DDR) system in response to immune checkpoint inhibitor (ICI) therapy. J. Exp. Clin. Cancer Res. 41, 268 (2022).
Fakih, M. et al. Immunotherapy response in microsatellite stable metastatic colorectal cancer is influenced by site of metastases. Eur. J. Cancer 196, 113437 (2024).
Liu, J. et al. Case report: MSS colorectal extrahepatic (non-liver) metastases as the dominant population for immunotherapy combined with multi-target tyrosine kinase inhibitors. Front. Oncol. 13, 1091669 (2023).
Conway, J. W. et al. The effect of organ-specific tumor microenvironments on response patterns to immunotherapy. Front Immunol. 13, 1030147 (2022).
Fakih, M., Sandhu, J., Li, X. & Wang, C. Abstract 4406: Impact of site of metastases on response to immunotherapy in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). Cancer Res. 83, 4406–4406 (2023).
Wang, C. et al. Clinical response to immunotherapy targeting programmed cell death receptor 1/programmed cell death ligand 1 in patients with treatment-resistant microsatellite stable colorectal cancer with and without liver metastases. JAMA Netw. Open 4, e2118416 (2021).
Bullock, A. J. et al. Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial. Nat. Med. 30, 2558–2567 (2024).
Tsimberidou, A. M. et al. Immunologic signatures of response and resistance to nivolumab with ipilimumab in advanced metastatic cancer. J. Exp. Med. https://doi.org/10.1084/jem.20240152 (2024).
Acknowledgements
This work was supported by Mr. and Mrs. Steven Mckenzie's Endowment, Katherine Russell Dixie’s Distinguished Professorship Endowment, and donor funds from Jamie's Hope and Mrs. and Mr. James Ritter for Dr. Tsimberidou's Personalized Medicine Program. This work was in part also supported by the National Institutes of Health/National Cancer Institute award number P30 CA016672 (University of Texas MD Anderson Cancer Center).
Author information
Authors and Affiliations
Contributions
C.B.X., M.A.G. and A.M.T. wrote the main manuscript text. C.B.X. prepared figures. C.B.X. and M.A.G. prepared the Table. A.M.T treated the patients. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Apostolia M. Tsimberidou declares receipt of Clinical Trial Research Funding (received through the institution): Immatics (Inst), OBI Pharma (Inst), Tempus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Agenus (Inst), Novocure (Inst), Tvardi Therapeutics (Inst), Orionis (Inst), Tachyon (Inst), Vividion Therapeutics (Inst), AbbVie (Inst), Macrogenics (Inst), Anaveon (Inst); and has consulting/advisory roles for Nex-I, BrYet, OBI Pharma. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xavier, C.B., Gouda, M.A. & Tsimberidou, A.M. Prolonged response to dual immune checkpoint blockade in patients with advanced solid tumors: a case series. npj Precis. Onc. 9, 322 (2025). https://doi.org/10.1038/s41698-025-01115-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-025-01115-0