Abstract
While circadian disruption is recognized as a potential driver of depression, its real-world impact is poorly understood. A critical step to addressing this is the noninvasive collection of physiological time-series data outside laboratory settings in large populations. Digital tools offer promise in this endeavor. Here, using wearable data, we first quantify the degrees of circadian disruption, both between different internal rhythms and between each internal rhythm and the sleep-wake cycle. Our analysis, based on over 50,000 days of data from over 800 first-year training physicians, reveals bidirectional links between digital markers of circadian disruption and mood both before and after they began shift work, while accounting for confounders such as demographic and geographic variables. We further validate this by finding clinically relevant changes in the 9-item Patient Health Questionnaire score. Our findings validate a scalable digital measure of circadian disruption that could serve as a marker for psychiatric intervention.
Similar content being viewed by others
Introduction
The advent of modern lifestyles has introduced a plethora of behaviors (e.g., shift work) that disrupt circadian rhythms. Such behavior-induced circadian disruption has been implicated in the global surge of mental health conditions, with about 20% of the world’s population encountering mood or anxiety disorders1. Notably, depression and anxiety are among the top 10 contributors to increased mortality, disability, and illness2. To tackle the prevalence of psychiatric disorders, it is critical to predict and understand mental health through modifiable behavioral, physiological, environmental, and social factors. Among these factors, circadian disruption induced by changes in behaviors is considered a particularly important contributor to mental health risks3,4,5.
Circadian disruption measures are typically classified into two main categories: 1) misalignment between internal rhythms (e.g., timing of central vs peripheral clocks), and 2) misalignment between internal rhythms (either timing of central clock or peripheral clock) and behavioral rhythms (e.g., sleep-wake cycle)6. These forms of disruption vary in their causes, how they are measured, and may vary in their relationships with mental health. Early research on circadian disruption has focused on absolute levels of misalignment between internal rhythms and behavioral rhythms, such as the sleep-wake cycle7,8,9,10. For example, a shorter phase angle between sleep and circadian biomarkers is correlated with greater depressive symptoms among major depressive disorder patients or even healthy individuals7,8. Researchers have expanded their scope on internal-behavioral rhythms to include circadian disruption measured between internal misalignment measures due to the discovery of the presence of molecular clocks existing across all peripheral organs11,12,13,14,15. One human study found evidence of an association of depressive severity with the phase angle between internal rhythms8. This progress has led to mounting interest in circadian rhythms as a potential prevention and treatment target for psychiatric disorders9.
However, although this knowledge about circadian disruption and its effect on mental health from laboratory studies is robust, there is little analogous data from real-world settings that are applicable to clinical and population health6. One main challenge of translating the laboratory findings into real-world clinical practice involves the continuous assessment of circadian rhythms and sleep over an extended period in real-world settings. A promising remedy is to passively create their digital analogs by integrating computational algorithms with physiological and behavioral data collected using wearable devices. Recent research has shown that simulating mathematical models of the circadian clock coupled with wearable activity or light data can predict circadian biomarkers16,17,18. Moreover, statistical approaches based on Markov chain Monte Carlo or nonlinear least squares methods have been proposed to analyze peripheral circadian rhythms in heart rate (HR) or body temperature from wearable data19,20. Notably, we recently developed a nonlinear Kalman filtering framework that enables simultaneous statistical inference of the time evolution of multiple circadian biomarkers from wearable data under highly noisy conditions20,21.
Another hurdle in exploring interconnections between circadian disruption and mental health involves collecting data on symptoms of psychiatric disorders in large-scale cohorts. To circumvent this, recent studies have developed protocols based on the use of mobile technology22,23,24,25,26. For instance, the Intern App, a mobile platform gathering passive data on mood, activity, and HR, was created and validated through a large-scale prospective cohort study, the Intern Health Study25,27,28,29. There have also been recent studies exploiting the data collected from this mobile technology to analyze the relationship between various sleep parameters and depression risks24,30. Notably, the Intern Health Study showed that medical trainees experienced reduced total sleep time and later bedtime, and a significant increase in day-to-day variability in both total sleep time and wake time after starting the internship24. These changes were associated with an increased risk of depression.
In this study, we derived statistical measures of circadian rhythms from wearable HR, activity, and sleep data by exploiting the recently developed and validated nonlinear state estimation approach described above20,21. That includes 1) the circadian rhythm in the central oscillator (CRCO) and 2) the circadian rhythm in the peripheral oscillator (CRPO). The wearable device also measures the behavioral rhythm that is represented by the sleep midpoint. Then, we used these measures to calculate three digital markers that represent circadian disruption: 1) the misalignment between the CRCO and the sleep midpoint (CRCO-sleep misalignment), i.e., the absolute phase difference between the CRCO and the sleep midpoint, 2) the misalignment between the CRPO and the sleep midpoint (CRPO-sleep misalignment), and 3) the internal misalignment between CRCO and CRPO. Importantly, these three digital markers capture distinct components of circadian disruption. Specifically, the CRCO-sleep and CRPO-sleep misalignment reflect misaligned timing of the sleep-wake cycle with the central and peripheral clocks, respectively, and often arise under shift work schedules or social jetlag conditions9. The central and peripheral circadian clocks respond differently to external perturbation31, and disruptions of central and peripheral oscillators with respect to behavioral rhythms may reflect different physiological dynamics. Compared to internal-behavioral misalignment, the misalignment between internal circadian rhythms is more directly linked to abnormal physiological events, such as disrupted metabolic patterns32. Thus, the relationship between mood and each disruption marker should be separately analyzed to achieve more specialized intervention strategies under different environmental conditions.
Therefore, the goal of our study was to explore the relationship between mood and the three wearable-based circadian disruption biomarkers in real-world settings. Specifically, we investigated the associations of these circadian disruption biomarkers with mood and depressive symptoms among medical interns in the Intern Health Study. This cohort provides a unique opportunity to study circadian disruption because interns are newly exposed to demanding work conditions with changing shift schedules over the year. We also investigated the effect of work conditions during the internship on the strength of association between each circadian disruption marker and mood to better understand the utility of each marker under different working conditions. Finally, we examined the association between circadian disruption and 9-item Patient Health Questionnaire (PHQ-9) scores to further validate the proposed circadian disruption measures and examine their relationship with depressive symptoms.
In this study, we found the CRCO-sleep misalignment had the most significant negative impact on the next day mood, whereas the mood exhibited the most pronounced impact on the internal misalignment between CRCO and CRPO. We also found clear associations of CRCO-sleep misalignment with specific depressive symptoms, such as sleep-related troubles, poor appetite, and overeating, which matches previous laboratory findings9,32,33,34. Our large-scale study suggests the potential of employing a digital approach based on wearable and mobile technologies to understand the interconnections between circadian disruption and mood in real-world settings.
Results
Assessment of circadian disruption and mood using wearable data and a mobile app
The study cohort consisted of 2077 interns whose HR, activity, and sleep data were collected using the Fitbit Charge 2TM over an average of 120.8 (standard deviation (SD) = 79.2) days. During the baseline period and intern year, an average of 21.8 (\(\pm\) 13.3) and 102.2 (\(\pm\) 78.8) days of wearable data were collected, respectively. After carefully applying the exclusion criteria (see Methods and Supplementary Fig. 1 for details), a total of 833 subjects were included in the analysis. Demographic and geographic information of these subjects is provided in Supplementary Table 1 and Supplementary Fig. 2.
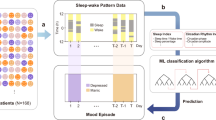
The wearable data were analyzed to estimate CRCO, CRPO, and sleep midpoint using previously proposed and validated statistical approaches18,19,20,21 (Fig. 1a and Supplementary Fig. 3; see Methods for details). Specifically, CRCO was estimated by using a state-of-the-art Kalman filtering approach, which incorporates indirect information about the CRCO from the CRPO into a probabilistic model of the central circadian clock20,21. Using the estimates of circadian and sleep parameters, three circadian disruption wearable measures were calculated. The first measure, denoted by CRCO-sleep misalignment, assesses the misalignment between the estimated CRCO and sleep-wake cycle. It was defined as the absolute difference between the time of the minimum in the central oscillation and sleep midpoint, taking into account that the circadian minimum of the model estimate occurs approximately 1 hour after sleep midpoint under normal conditions18,35,36,37. CRPO in the heart was estimated using a nonlinear least squares method recently proposed in ref. 20. The second measure, denoted by CRPO-sleep misalignment, was defined as the absolute difference between the time of the circadian HR minimum and sleep midpoint in hours. No reference angle is required here because the time of the circadian HR minimum and sleep midpoint align under normal conditions19. The third statistical measure assesses the internal misalignment between CRCO and CRPO. It was defined as the log-likelihood value of the absolute difference between the central and peripheral HR clock phases. Further details of the three measures are described in Methods.
a The wearable heart rate and activity data are analyzed using previously validated statistical methods (20,21; see Methods for details) to estimate the phase of the circadian rhythm in the central oscillator (CRCO; blue) and the phase of the circadian rhythm in the peripheral oscillator (CRPO; red), specifically from the cardiac pacemaker. The sleep midpoint (yellow) was measured from activity and sleep data. The shaded gray region denotes sleep episodes. b Distribution of circadian disruption measures. c Box-and-whisker plots of circadian disruption measures before and during the internship. d Interns were asked to evaluate their daily mood scores from 0 to 10, where a higher score corresponds to a better mood. e Interns also completed quarterly PHQ-9 questionnaires to evaluate their depression risk from 0 to 27, where a higher score corresponds to elevated depression risks. A two-tailed t-test was used to assess statistical significance. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001.
In Fig. 1b, we show the distributions of the three measures for 52,061 days of Fitbit data obtained from 833 interns in the Intern Health Study. After starting the internship, the interns experienced a significant increase in CRCO-sleep misalignment from a mean of 1.67 (SD = 1.58) to 2.19 (SD = 2.35) hours (p < 0.001; Fig. 1c left), and CRPO-sleep misalignment from a mean of 4.12 (SD = 2.90) to 4.62 (SD = 3.21) hours (p < 0.001; Fig. 1c middle). The internal misalignment also increased during the internship (5.62 [SD = 3.59] vs 5.74 [SD = 3.88]; p = 0.005; Fig. 1c right).
For the analysis of daily mood, interns used the Intern App to report their daily mood score from 0 to 10, where a higher score corresponds to better mood (Fig. 1d; see Methods for details). Because the overall distribution of mood scores was highly skewed (Fig. 1d top right), we standardized the raw mood scores within each subject using z-statistics, following previous studies38,39. After starting the internship, the subjective mood score significantly decreased (0.21\(\pm\) 0.99 vs −0.06 \(\pm\) 0.99; p < 0.001; Fig. 1d bottom). Moreover, the interns were instructed to complete a quarterly PHQ-9 questionnaire during the internship to evaluate their depression risk beyond their daily mood score. The average PHQ-9 score for each subject was obtained by averaging all the quarterly scores during which the subject’s wearable data was available. The average PHQ-9 scores among subjects significantly increased after starting the internship (2.57 \(\pm\) 2.77 vs 5.46 \(\pm\) 3.86; p < 0.001; Fig. 1e bottom).
Effects of circadian disruptions on mood in real-world settings
To investigate the effects of circadian disruptions on mood, we evaluated the relationship between the current day circadian disruption measures and the next day mood score. The correlation between current day CRCO-sleep misalignment and next-day mood score was not statistically significant before the internship (n = 10,387 days, p = 0.583; Fig. 2a). However, during the internship, the correlation became statistically significant (n = 41,084 days, \(\rho\) = −0.861, p < 0.001; Fig. 2b). Unlike CRCO-sleep misalignment, increased CRPO-sleep misalignment was significantly correlated with worsened next day mood both before (\(\rho\) = −0.604, p = 0.029; Fig. 2c) and during (\(\rho\) = −0.970, p < 0.001; Fig. 2d) the internship. However, the predictive ability of CRPO-sleep misalignment on next day mood during the internship was lower than that of CRCO-sleep misalignment, as represented by the slope of the line of best fit, i.e., the beta value (b = −0.022 \(\pm\) 0.008 vs b = −0.011 \(\pm\) 0.002; 95% confidence interval; Fig. 2b, d, respectively). Furthermore, the internal misalignment was negatively correlated with the next day mood both before and during the internship (\(\rho\) < −0.765, p < 0.001; Fig. 2e, f), although its overall predictive ability during the internship was also lower compared to the CRCO-sleep misalignment (b = −0.022 \(\pm\) 0.008 vs b = −0.007 \(\pm\) 0.003; 95% confidence interval; Fig. 2b, f). So far, we have investigated the association between circadian disruption and next day mood using misalignment measures binned at 1-hour intervals. Indeed, the association remained consistent even when the bin size changed (Supplementary Fig. 4a–c). Moreover, the statistical significance of this association persisted after adjusting for the error in each bin using weighted least squares regression40 (Supplementary Table 2). Consistent with our findings that CRCO-sleep misalignment had a higher predictive ability for daily mood compared to other circadian disruption measures during the internship, an increase in CRCO-sleep misalignment levels after the internship began was negatively correlated with a decrease in daily mood scores (Supplementary Fig. 5).
a, b The correlation between current day CRCO-sleep misalignment level and mood score on the following day before the internship (a), and during the internship (b). c, d The correlation between current day CRPO-sleep misalignment level and mood score on the following day before the internship (c) and during the internship (d). e, f The correlation between current day internal misalignment level and mood score on the following day before the internship (e) and during the internship (f). The next day mood scores were binned by each misalignment level. Bin edges were computed every 1 hour, and the mean and standard error of the mean of mood scores were plotted against the midpoint of each bin. The line and shaded region represent the best-fitted line and the corresponding 95% confidence band, respectively, obtained by applying linear least squares regression to the binned data. b, ρ, and p denote the beta estimate, Pearson’s correlation coefficient, and p-value, respectively. g, h The negative correlation between current day circadian disruption measures and mood score on the following day persisted even after adjustment for demographic and geographic variables using a generalized estimating equation analysis (g) and a linear mixed-effects modeling (h). The 95% confidence intervals of the estimated beta values and their respective p-values are indicated with error bars and asterisks. A two-tailed Wald t-test was used to compute p-values and assess statistical significance. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001.
We further investigated the relationship between the current day circadian disruption measures and the next day mood score by conducting a generalized estimating equation (GEE) analysis (see Methods for details). This method adjusts for repeated measures from the same subject and individual demographic and geographic factors, including sex, age, race, and time zone. Even after adjusting for these variables, our findings remained consistent (Fig. 2g and Table 1). We then validated these findings using a linear mixed-effects model (LME) that considers repeated measures from the same subject and demographic and geographic variables as subject-specific random effects (see Methods for details). This confirmed that the negative correlation between circadian disruption measures and daily mood scores persisted, even when accounting for individual differences in demographics and geography (Fig. 2h and Table 2). Indeed, all findings remained statistically significant after multiple test corrections using the two-stage linear step-up procedure (TSL)41. Notably, the negative correlation observed during the internship remained statistically significant after adjusting for autoregressive and synchronous correlation effects in mood and circadian disruption markers using a cross-lagged panel model (Supplementary Fig. 6 and Supplementary Table 3; see Methods for details). These results indicate that the CRPO-sleep misalignment can be attributed to the worsened next day mood regardless of stressful work conditions such as an internship. Conversely, it is only under stressful work conditions leading to a more irregular lifestyle that a more robust CRCO can become significantly misaligned with sleep patterns, and this results in a pronounced detrimental impact on mood on the following day. Indeed, CRCO-sleep misalignment continued to show the strongest association with worse next day mood among all three markers, even after adjusting for the effects of the other two markers (Supplementary Table 4).
We also investigated the effect of time awake and variability in sleep timing, represented by the shift in the sleep midpoint relative to the previous day, on the association between circadian disruption measures and daily mood (Supplementary Figs. 7 and 8). We found that the statistically significant negative correlation between CRPO-sleep misalignment and mood persists regardless of an individual’s total time awake and variability in sleep timing (Supplementary Figs. 7b and 8b). This supports the negative impact of the disruption in peripheral circadian rhythms on mood, independent of working conditions (Fig. 2c, d). In contrast, the time awake and variability in sleep timing affect the association of CRCO-sleep misalignment with mood, respectively (Supplementary Figs. 7a and 8a). For example, as an individual experienced greater variability in sleep timing relative to the previous day, the negative correlation between CRCO-sleep misalignment and mood became more pronounced (b = 0.010 \(\pm\) 0.016, \(\rho\) = 0.458, and p = 0.302 vs b = −0.025 \(\pm\) 0.010, \(\rho\) = −0.830, and p < 0.001; Supplementary Fig. 8a(i) and 8a(iv), respectively). This highlights the importance of carefully considering the negative impact of specific circadian disruptions on mood in conjunction with an individual’s lifestyle.
Effects of mood on circadian disruptions in real-world settings
To find a specific bidirectional relationship between circadian disruptions and mood, we next investigated the effects of mood on circadian disruptions by comparing the current day mood score to the next day circadian disruption measures. Similar to the effect of the current day CRCO-sleep misalignment on the next day mood, the correlation between the current day mood score and the next day CRCO-sleep misalignment level was not significant before the internship (n = 10,881 days, \(\rho\) = −0.252, p = 0.512; Fig. 3a), but it became significant during the internship (n = 41,180 days, \(\rho\) = −0.556, p = 0.048; Fig. 3b). Likewise, the current day mood score did not exhibit a significant negative correlation with the next day CRPO-sleep misalignment before the internship (\(\rho\) = −0.501, p = 0.170; Fig. 3c), but it became significant during the internship (\(\rho\) = −0.796, p = 0.001; Fig. 3d). The current day mood score was negatively correlated with the next day internal misalignment both before and during the internship (\(\rho\) < −0.820, p < 0.001; Fig. 3e, f). Notably, the overall predictive ability of mood on internal misalignment was greater than those on CRCO-sleep and CRPO-sleep misalignment during the internship (b = −0.142\(\pm\) 0.038 vs b = −0.038 \(\pm\) 0.034 and −0.064 \(\pm\) 0.029, respectively; 95% confidence interval; Fig. 3b, d, and f). These findings remained consistent both after changing the bin size (Supplementary Fig. 4d–f) and after accounting for the error in each bin using weighted least squares regression (Supplementary Table 2). We obtained similar results after adjusting for repeated measures from the same subject and demographic and geographic information using GEE (Fig. 3g and Table 1) and LME (Fig. 3h and Table 2). Additionally, the results remained consistent even after accounting for autoregressive and synchronous correlation effects in mood and circadian disruption markers using a cross-lagged panel model (Supplementary Fig. 6 and Supplementary Table 5). After multiple test corrections using TSL, the association between current day mood and next day circadian disruption measures assessed using the weighted least squares regression remained statistically significant during the internship, but not before the internship. However, all tests from GEE and LME remained statistically significant after multiple test corrections. These results suggest that a combination of worsened mood and extreme work conditions disrupts the alignment between circadian rhythms and sleep patterns. In contrast, a worsened mood strongly affects the synchronization of the circadian rhythms, regardless of stressful working conditions.
a, b The correlation between current day mood score and CRCO-sleep misalignment level on the following day before the internship (a) and during the internship (b). c, d The correlation between current day mood score and CRPO-sleep misalignment level on the following day before the internship (c) and during the internship (d). e, f The correlation between current day mood score and internal misalignment level on the following day before the internship (e), and during the internship (f). The next day circadian misalignment levels were binned by the corresponding mood score. Bin edges were computed every 0.5, and the mean and standard error of the mean of misalignment levels were plotted against the midpoint of each bin. The line and shaded region represent the best-fitted line and the corresponding 95% confidence band, respectively, obtained by applying linear least squares regression to the binned data. b, ρ, and p denote the beta estimate, Pearson’s correlation coefficient, and p-value, respectively. g, h The negative correlation between current day mood score and circadian disruption measures on the following day persisted even after adjustment for demographic and geographic variables using a generalized estimating equation analysis (g) and a linear mixed-effects modeling (h). The 95% confidence intervals of the estimated beta values and their respective p-values are indicated with error bars and asterisks. A two-tailed Wald t-test was used to compute p-values and assess statistical significance. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001.
Effects of circadian disruptions on longitudinal depression risks
Because daily mood scores only capture one symptom of mental health, we also examined the association of circadian disruption measures with PHQ-9, a well-validated measure of major depressive disorder that assesses levels of nine symptoms in the last two weeks42. PHQ-9 scores were assessed once before the internship began and then at the end of each quarter throughout the internship. A score was included when a subject had at least 10 days of valid wearable activity, HR, and sleep data during the two-week period assessed by the PHQ-9. The average PHQ-9 score for each subject during the internship was then calculated by averaging their quarterly PHQ-9 scores. Similarly, the circadian misalignment levels during the two weeks preceding the PHQ-9 assessment were averaged. After removing subjects who had insufficient days of wearable recording (see Methods and Supplementary Fig. 1 for details), a total of 465 and 671 subjects (18.6 \(\pm\) 7.6 and 59.6 \(\pm\) 53.3 days of recording) were included in the before and during internship analyses, respectively.
Average circadian misalignment levels were not associated with baseline PHQ-9 scores assessed before the internship (Supplementary Table 6). In contrast, during the internship, average CRCO-sleep misalignment was significantly positively correlated with the average PHQ-9 scores (b = 0.705, \(\rho\) = 0.879, p = 0.009; Fig. 4a), consistent with its negative correlation with the daily mood. This statistically significant positive correlation persisted even after accounting for random effects of demographic and geographic variables and the other two markers (Supplementary Tables 7 and 8). Notably, this statistically significant positive correlation is not evident if CRCO is estimated using conventional approaches that solely rely on mathematical model simulation18,43 (see Methods for details) but do not incorporate indirect information about the CRCO from other circadian biomarkers in predicting CRCO phase (Supplementary Fig. 9). This demonstrates the importance of considering circadian rhythms from multiple molecular clocks to obtain an optimal prediction of CRCO through a data assimilation method20,21. Unlike CRCO-sleep misalignment, we did not find a significant correlation between the average PHQ-9 scores and average CRPO-sleep and internal misalignments (Supplementary Fig. 10). This is consistent with our finding that CRCO-sleep misalignment has a stronger predictive ability for daily mood than other circadian disruption measures.
a The average CRCO-sleep misalignment level and average PHQ-9 score within each participant are positively correlated. b–g The average CRCO-sleep misalignment level was also positively correlated with the average scores of six subitems from PHQ-9 questionnaires. Here, the average misalignment was computed using data from two weeks before the completion of the questionnaire. The PHQ-9 score and scores of its six subitems were binned by the average CRCO-sleep misalignment level. Bin edges were computed every 30 min, and the mean and standard error of the mean of scores were plotted against the midpoint of each bin. The line and shaded region represent the best-fitted line and the corresponding 95% confidence band, respectively, obtained by applying linear least squares regression to the binned data. b, ρ, and p denote the beta estimate, Pearson’s correlation coefficient, and p-value, respectively. A two-tailed Wald t-test was used to compute p-values.
We next investigated the association between CRCO-sleep misalignment and the itemized score (from 0 to 3) of each nine symptoms within the PHQ-9 questionnaire to pinpoint particular aspects of depression that are linked with circadian disruptions. Interns with greater CRCO-sleep misalignment reported higher scores for the item “Trouble falling asleep or staying asleep, or sleeping too much” (b = 0.144; \(\rho\) = 0.964, p < 0.001; Fig. 4b). This supports the validity of our statistical measure of CRCO-sleep misalignment. Interns with higher CRCO-sleep misalignment also reported higher scores for the item “Poor appetite and overeating” (b = 0.084; \(\rho\) = 0.824, p = 0.023; Fig. 4c). This matches previous experimental studies demonstrating that circadian misalignment induces dysregulation of appetite-stimulating hormones and glucose metabolism, leading to changes in feeding behaviors9,34,44,45. Moreover, elevated average CRCO-sleep misalignment was associated with other potential indicators of increased fatigue level and worsened emotional state, such as “Feeling badly about yourself” (b = 0.09; \(\rho\) = 0.784, p = 0.037; Fig. 4d), “Trouble concentrating” (b = 0.09; \(\rho\) = 0.828, p = 0.021; Fig. 4e), “Moving too slow or restless” (b = 0.024; \(\rho\) = 0.765, p = 0.045; Fig. 4f), and “Thoughts of hurting yourself” (b = 0.042; \(\rho\) = 0.866, p = 0.012; Fig. 4g). The correlation between the average CRCO-sleep misalignment and the items “Trouble with sleeping”, “Poor appetite or overeating”, “Trouble concentrating”, and “Thoughts of hurting yourself” remained statistically significant after multiple test corrections using TSL. These associations between CRCO-sleep misalignment and the itemized scores of nine symptoms generally remained consistent in the LME analysis, although the statistical significance decreased (Supplementary Tables 7 and 8). Taken together, these results strongly suggest that CRCO-sleep misalignment plays a critical role in increased risks of various negative physiological and psychiatric outcomes.
Discussion
In this study, we derived digital biomarkers of circadian disruption by combining wearable and mobile technologies with our scalable statistical methods validated in previous work18,19,20,21,46. We applied these digital circadian biomarkers in a large-scale cohort study, the Intern Health Study, to assess their associations with mood and depressive symptoms. By analyzing longitudinal wearable and mobile app data collected from over 800 medical interns under real-world conditions, we identified that the three circadian disruption measures were all statistically significantly correlated with next day mood. Among the measures, greater misalignment between sleep midpoint and CRCO inferred from wearable data exhibited the strongest negative association with the next day mood, potentially suggesting its most pronounced detrimental effect (Fig. 2), while a worse current day mood had the most significant negative correlation with the next day internal misalignment (Fig. 3). We also showed that the CRCO-sleep misalignment was associated with both the overall PHQ-9 score and the scores of specific subitems from the PHQ-9 questionnaire (Fig. 4). Our findings remained consistent even when we adjusted for potential confounding variables and repeated measures from the same subject using GEE and LME (Tables 1 and 2 and Supplementary Table 7). These results suggest the potential use of digital circadian biomarkers to open a new window for personalized mental health care outside the laboratory or clinical settings.
The complementary findings from basic, preclinical, and clinical research indicate that circadian disruption is a potential underlying source of elevated mental health risks3,5. For example, previous experimental studies on night shifts and jet lag have identified some meaningful correlation between circadian disruption and increased depressive behaviors7,8,9,10,47. Despite this, there is still significant progress to be made in translating these findings into applications in clinical practice6. To address this gap, there have been recent efforts leveraging wearable and mobile technologies24,48,49. For instance, our previous work shed light on how increased day-to-day variability in sleep parameters contributes to depression risk in medical trainees during the internship, using wearable data from the same cohort of medical interns24. We also found that an increase in time awake nonlinearly amplifies the effect of circadian rhythm on mood39. Recently, the causal dynamics of mood and sleep/circadian phases, which are estimated from wearable activity and sleep data, was identified26. Here, we expanded this hypothesis by first developing wearable-based digital markers that measure the levels of three distinct types of circadian disruptions using wearable heart rate, activity, and sleep data. We assessed their effectiveness on a larger cohort compared to the previous study in real-world settings26. Moreover, while the previous study solely relied on subjective mood scores, we also utilized PHQ-9 and this allowed us to identify specific depressive symptoms that are linked with circadian disruptions. Our results suggest that circadian rhythms and sleep are inseparable processes that should be collectively considered for accurate assessment of mental health risks.
To extract circadian rhythms from wearable activity and heart rate data, we exploited previously proposed computational algorithms18,19,20,21,50. These methods were validated using both experimental and in-silico data. Specifically, our prior work showed that the CRCO estimate, obtained by simulating a mathematical model of the human circadian clock, can predict the experimental circadian marker, dim light melatonin onset, within approximately 1 hour18. Recently, we further refined the method by applying a Kalman filter, which optimally integrates model predictions with experimental observations, and showed that it outperforms the previous method using in-silico wearable data20,21. We also showed that properties of the estimated CRPO in heart closely match those of the experimentally measured circadian rhythm in HR19,20. These findings provide a rationale for using wearable-based circadian markers in this study. Despite these validations, there is still room for further validation and improvement. Since previous studies have focused on evaluating the predictive accuracy of the circadian estimate for experimental markers among younger working individuals, there is a need to assess their predictive capabilities in larger, more diverse cohorts, including elderly adults. Additionally, given the substantial interindividual differences in circadian parameters, such as light sensitivity and intrinsic period17,51,52, personalizing mathematical models—such as by employing a dual estimation approach—represents a promising avenue for future research. We should also note that obtaining large-scale data with DLMO—which, by itself, does not measure internal circadian misalignment—would be challenging.
Ubiquitous consumer-grade wrist-worn wearable devices, including the Fitbit Charge 2™ used in this study, are unable to record light levels. Therefore, it is necessary to accurately estimate CRCO with consumer-grade wearable devices only collecting activity and heart rate data. Recently, we proposed a mathematical approach using activity measurements that accurately estimates CRCO18,20,21. Indeed, this approach improved the estimation accuracy compared to methods that rely on light measurements18. A possible reason for this improvement includes activity being associated with both nonphotic circadian phase-shifting effects and light exposure18. Another reason is that light measurements may not accurately reflect true ocular light exposure due to factors such as individuals wearing long sleeves. Additionally, exercise is known to shift the circadian clock53,54, so higher levels of activity may capture these effects along with increased light exposure. Future research using data from various light sources (e.g., optical lighting) could help explain the superior performance of activity measurements.
There is increasing evidence suggesting that circadian disruption plays a critical role in the etiology and pathophysiology of depression via direct or indirect pathways55. Specifically, circadian disruptors (e.g., disrupted light-dark cycles and shift work) affect both the SCN clock and other oscillators in brain regions, such as the hippocampus, hypothalamus, and amygdala, which are involved in mood, anxiety, feeding, and metabolism3. This leads to reduced neurogenesis56,57, dysregulation of metabolic hormones58,59, and disruption of the melatonin rhythm60,61,62. In particular, disruption in the melatonin rhythm affects insulin secretion rhythm63,64 or alters the CLOCK gene expression rhythm, which is known for its genetic linkage to energetic disorders65. As a result, individuals experiencing circadian disruption can suffer from depressive symptoms, such as sleep-related troubles, poor appetite, and depressed mood55. Indeed, pharmacological intervention with melatonin has been shown to alleviate depressive symptoms in both animal and human studies66,67. In light of these previous findings, the melatonin rhythm appears to be an upstream factor of depressive symptoms. This explains why the disruption in the CRCO, which is directly related to the melatonin rhythm, was most strongly associated with a worse mood on the following day. This also provides an explanation for the association between disruption in the CRCO and the scores of specific questions related to sleep and feeding from the PHQ-9 questionnaire.
While we found a strong correlation between CRCO-sleep misalignment and PHQ-9 scores during the internship, no significant associations were observed between the other two markers and PHQ-9 scores. These findings may be explained by differences in the underlying physiology of the circadian markers. Previous work has shown that an altered phase of peripheral circadian rhythms does not necessarily imply a change in the phase of central circadian rhythms31,50. Moreover, clocks respond differently to circadian disruptors, suggesting that disruption of the peripheral heart clock does not imply disruption of brain clocks tightly associated with depressive symptoms. This indicates that a disrupted peripheral clock rhythm in HR does not always imply a disrupted central clock rhythm. Thus, disruption in the CRPO relative to sleep timing and CRCO may not necessarily indicate increases in CRCO-sleep misalignment correlated with depression severity. This could explain why the CRPO-sleep and internal misalignments did not exhibit a strong positive correlation with the PHQ-9 score.
Another notable finding is the association between CRCO-sleep misalignment and the score of specific question related to suicidal risks from the PHQ-9 questionnaire. Indeed, circadian disruption is increasingly being considered a potential risk factor for suicide. Rodents with circadian disruptions, such as those induced by alternating light exposure patterns, exhibited increased immobility during forced swim tests55,68. A recent meta-analysis further found that certain indicators of circadian or behavioral rhythmicity are associated with suicidal thoughts and behaviors in humans69. However, further research is needed to draw definitive conclusions. Nevertheless, our approach quantifying sleep and circadian rhythm disruptions using wearable data, which, for the first time, identified a real-world association between circadian misalignment and suicidal thoughts, provides a useful framework for future research.
Among our findings, of particular interest is the increasing magnitude of the error bars in Fig. 4 with higher levels of circadian misalignment, which indicates greater interindividual variation in the severity of depression symptoms among subjects with elevated circadian misalignment. Such interindividual differences were also observed in previous laboratory research70. Yet, the underlying molecular mechanisms driving these differences remain unknown. Previous studies have shown that local oscillators in brain regions associated with depressive symptoms are linked to disruptions in the suprachiasmatic nucleus, the central clock3. This suggests that there may be interindividual differences in downstream responses to temporal signals from the central clock, such as those involving monoamine signaling and hypothalamic-pituitary-adrenal axis hormone signaling55. Investigating these differences would be an intriguing avenue for future research.
We investigated the effect of shift work during the internship on the association between circadian disruption and mental health risks by separately analyzing data collected before and during the internship. Before the internship, the CRPO-sleep and internal misalignments, but not the CRCO-sleep misalignment were associated with a worse mood on the following day. This may be explained by the rapid phase-shifting responses of a peripheral oscillator in the heart to transient behavioral or environmental stimuli31,71, which are expected to lead to a short-term, though not as large, effects on mood. During the internship, CRCO-sleep misalignment was most strongly associated with the worse next day mood among the three measures. This suggests that if the rhythms in the central circadian clock, which are more robust than other peripheral rhythms36,72 are disrupted, mood significantly worsens. Additionally, we found that, before the internship, mood was associated with the next day internal misalignment, but not with the CRCO-sleep or CRPO-sleep misalignments. On the other hand, during the internship, mood was negatively correlated with all three measures. A possible explanation would be that worsened mood and tough working conditions (e.g., increase in work hours) synergistically deteriorate sleep regularity, thereby disrupting the synchrony between circadian rhythms and sleep during the internship. Conversely, worsened mood may in general alter the feeding timing regardless of working conditions. This shifts the peripheral oscillators away from synchrony73,74,75 and induces high internal misalignment32 both before and during the internship.
The cross-lagged panel analysis provided valuable insights into the reciprocal relationship between circadian disruption and daily mood (Supplementary Tables 3 and 5). To further explore their casual dynamics, it is necessary to carefully account for the complex and nonlinear dynamics of circadian rhythms and mood19,20,76,77. For example, the autoregressive relationship between circadian markers on the current day and the subsequent day is determined by the time propagation of nonlinear endogenous circadian rhythms, which are affected by exogenous factors such as physical activities19,20. Approximating this nonlinear relationship as linear may lead to inaccurate inferences about causal dynamics. To circumvent this, we can employ recently developed models that capture the day-to-day nonlinear dynamics of circadian rhythms and mood21,77, along with causality detection methods, such as Granger causality78, convergent cross mapping79, and transfer entropy analysis26.
Despite the strengths of our study, there are some limitations. For instance, the cohort lacked age and racial diversity, mainly consisting of younger and Caucasian individuals. Moreover, due to the nature of questionnaire-based data collection, there may be sampling biases, such as selection bias and non-response bias, which could lead to skewness of data. Therefore, further verification of our study’s external validity needs to be performed on other populations, such as the elderly population or clinically diagnosed mentally disordered patients from various backgrounds. However, the effectiveness of our data collection mobile app, the Intern App, has been validated across various socioeconomic statuses, cultures, and geographic regions using a cohort consisting of about 500 randomly selected healthcare workers in Nairobi, Kenya80, suggesting the potential generalizability of our approach to studying circadian disruption and mental health in real-life conditions. Moreover, our current study only focused on the phase misalignments and did not consider other important circadian parameters, such as the circadian amplitude or uncertainties in circadian rhythms. Considering these additional circadian measures, along with other sleep measures such as sleep onset latency and time spent in REM sleep using wearable-based sleep scoring algorithms81,82, would also be an interesting future direction. Another consideration is that subjects might experience worsened mood during the biological night, regardless of any circadian misalignment. To investigate this, it is necessary to separate the potential direct impact of night shift work from the effects of circadian misalignment. However, the current study designs, including ours, are not suitable for this83. Therefore, further studies are needed to address this issue. Finally, our analysis is retrospective. Thus, there still exist large gaps in translating our current analysis into real-time monitoring of mental health risks and development of potential intervention strategies. Extending our current retrospective analysis to real-time analysis, for instance, by exploiting real-time anomaly detection methods84, would be valuable future steps.
Methods
Data collection and exclusion criteria
All analyzed data were from the Intern Health Study, a multisite prospective cohort study of depression among training physicians (see refs. 27,85; https://www.internhealthstudy.org/ for more information). In this study, the consumer-grade wrist-worn Fitbit Charge 2™ was used to continuously collect behavioral and physiological signals from first-year training physicians. Specifically, activity levels and heart rate (Fig. 1a) were measured using the accelerometer and photoplethysmography sensor in the Fitbit Charge 2™. These measurements were then used to quantify sleep using Fitbit’s proprietary algorithms. Summary sleep and heart rate metrics were reported in refs. 24,19, respectively. Examples of changes in activity levels over time are shown as actograms in Supplementary Fig. 3. Note that the Fitbit Charge 2™ has been compared to laboratory polysomnography, showing a sensitivity of 0.96 (accuracy in detecting sleep) and a specificity of 0.61 (accuracy in detecting wake) in healthy adults86.
Using the Fitbit Charge 2™, activity, HR, and sleep data were anonymously collected from 2077 medical interns. Daily mood data were collected through the Intern App by sending a push notification to participants. Out of 2077 subjects, subjects with less than 20 days of complete heart rate, activity, and sleep data and daily mood score were removed from the analysis due to the necessity of having sufficiently long data records to perform the statistical analysis20,21. We also required the number of minute-by-minute heart rate, activity, and sleep data measurements to be greater than 20 counts per day, since sufficient data points are needed to ensure accurate analysis. Consequently, 833 subjects were included in the daily mood analysis. See Supplementary Fig. 1 for further details on subject exclusion criteria.
Depression data were collected with the PHQ-9, a self-administered tool used to help screen, diagnose, and measure the severity of depression. See24 for details of the collection of mood and depression data. For the analysis of depression data, 162 subjects who either did not complete the PHQ-9 questionnaire or had less than 10 days’ worth of valid heart rate, activity, and sleep data within the two weeks prior to the completion of the questionnaire were further excluded. A total of 671 subjects were included in the during-internship analysis of PHQ-9 data. Likewise, 206 additional subjects who did not complete the baseline PHQ-9 questionnaire before beginning the internship were removed from the before-internship analysis. A total of 465 subjects were included in the before-internship analysis of PHQ-9 data. This study was approved by the University of Michigan IRB and all subjects provided informed consent after receiving complete description of the study.
Estimation of circadian rhythms
We estimated the circadian biomarkers using three methods: (i) a regression approach to measure the phase of CRPO in HR from wearable heart rate, activity, and sleep data20, (ii) an approach to predict the phase of CRCO solely using a mathematical model of the circadian clock, with wearable activity and sleep data as inputs18, and (iii) an advanced Kalman filtering approach that assimilates the two phase estimates obtained using the previous two methods20,21. Note that the third method was employed to estimate the CRCO in all figures except for Supplementary Fig. 9. In Supplementary Fig. 9, the second method was used. Below, we provide detailed descriptions for each method.
A method for estimating the circadian rhythm in the peripheral oscillator in the heart (CRPO)
To estimate the CRPO, we adopted the nonlinear curve-fitting approach, which has been validated against a large-scale wearable dataset, from20. It assumes that heart rate (HR) consists of a 24 h periodic oscillation, plus a separate term to account for the effect of physical activities on HR. This yields a model for HR:
where \(a\) is the basal HR in beats per minute (bpm), \(b\) is the amplitude of a circadian oscillation in HR, HRmin is the time of the circadian HR minimum, \(d\) is the increase in HR per unit of activity, and \({v}_{t}\) is the model error. This error \({v}_{t}\) follows the first-order autoregressive noise process model (i.e., \({\left({v}_{t+1}=\alpha \cdot {v}_{t}+N\left(0,{\sigma }^{2}\right)\right)}\). That is, a fraction \(\alpha\) of the noise at time \(t\) is carried over at time \(t+1\), and \(\sigma\) is independent measurement noise or new external influences. The values for HR and activity come from the wearable dataset. For each individual, the model parameters, including HRmin, are fitted directly from the data by using an approximation-based nonlinear least-squares method20,87. We denote the estimated HRmin on day i as \({{CRPO}}_{i}\). The previous day’s fit is used as an initial condition to estimate the parameters on the successive day, and this enables the estimation of both mean and uncertainty for each parameter. More details of the method can be found in ref.20 and the codes to run this algorithm are openly available at https://github.com/daewookk/ALSM.
A method for predicting the circadian rhythm in the central oscillator (CRCO) solely using the limit-cycle oscillator model of the circadian clock
We adopted a limit-cycle oscillator model of the human central circadian clock, which has been validated against multiple laboratory and field studies, to estimate the CRCO18,35. Note that this method solely based on the mathematical model was used only for Supplementary Fig. 9, while the CRCO phase estimates in other figures were obtained from the recently developed data assimilation method described below. The limit-cycle oscillator model describes the effect of external factors, such as light or activity, on the human circadian pacemaker:
where \(\alpha \left(I\right)={\alpha }_{0}{\left(\frac{I}{{I}_{0}}\right)}^{P}\), \(B=\hat{B}\left(1-0.4x\right)\left(1-0.4{x}_{c}\right)\), \(\hat{B}=G\alpha \left(I\right)\left(1-n\right)\), \(\mu =0.23\), \({\tau }_{x}=24.2\), \(k=0.55\), \(\beta =0.013\), \({\alpha }_{0}=0.16\), \(P=0.6\), and \({I}_{0}=9500\). The first two equations explain a limit-cycle oscillator for \(x\) and \({x}_{c}\), where the variable \(x\) describes endogenous circadian rhythms and \({x}_{c}\) serves as a mathematically necessary complementary variable essential for achieving the limit cycle.
The method takes light or activity wearable data as the model input to simulate the differential equations. In this study, we used wearable activity data, as done in previous work18, to generate the results in Supplementary Fig. 9. For further details, see ref. 18. The codes for implementing the model are available at https://github.com/pepperhuang/predictCircadianRhythms.
A data assimilation method for estimating the CRCO
The method described earlier does not utilize information about the CRPO when predicting the CRCO. This might overlook the potential for more accurate estimation of the CRCO, as all circadian clocks are interconnected via hormonal and neuronal signals, suggesting that the CRPO holds indirect information about the CRCO. To tackle this issue, a statistical method based on a Kalman filter was recently proposed21. Specifically, this method captures the hierarchical nature of the circadian timekeeping system by integrating the CRCO phase estimates obtained from the mathematical model of the human circadian clock18,35 with the CRPO phase estimate20, as detailed in the previous two sections. This can be formulated as the filtering problem of a continuous-discrete system described as
where xt represents the state of the circadian clock at time t under the dynamics described by the drift velocity v; Wt is the standard n-dimensional Brownian motion; K denotes the diffusion matrix; zi represents the measured peripheral clock phase on day i; h is the measurement function relating the estimated phase of the central and peripheral circadian clocks based on previously proposed phase angle difference; and ϵi is the Gaussian noise describing the error in the measurement of the peripheral circadian clock phase.
We let the state variable xt and drift velocity v be defined as in the van der Pol type limit-cycle oscillator model as described in the previous section. In particular, we define
The detailed expressions for the differential equations are given as
where the parameter values are specified in the previous section.
Here, we set zi as the HRmin on day i (CRPOi). To obtain the CRPOi, we used the approximation-based nonlinear least-squares method described above. The measurement function h relates the CRPOi to the central clock state at the time of the circadian x minimum on day i, denoted by \({x}_{{{CRCO}}_{i}}\), as follows:
where CRCOi is the value such that \(E\left[{x}_{{{CRCO}}_{i}}\right]\le E\left[{x}_{t}\right]\) for all \(t\in \left[24\cdot \left(i-1\right),24\cdot i\right]\), and \({\phi }_{{ref}}=-1\) represents the average phase angle difference reported in previous work19,35,37. Note that the symbol \(E\left[{x}_{t}\right]\) denotes the expectation of the random variable \({x}_{t}\).
We solved the filtering problem by employing a recently introduced extension of Kalman filtering called the level-set Kalman filter88, which was also utilized in prior research21. This approach enabled us to derive the CRCO phase estimate, denoted by \({{CRCO}}_{{i|i}}\), using wearable heart rate, activity, and sleep data collected up to day \(i\). This estimate was used to produce the results in all figures except Supplementary Fig. 9. For further details about this data assimilation approach and its implementation, see refs. 20 and21. The codes for implementing the method are available at https://github.com/phillee62/LSKF_circadian.
Assessment of circadian disruption markers, daily mood, and depressive symptoms
The level of misalignment between a circadian rhythm and the sleep-wake cycle was defined as the difference between the circadian phase and the sleep midpoint, following previous work19. CRCO-sleep misalignment was computed as the absolute difference between the CRCO phase estimated by the data assimilation method20,21 and the sleep midpoint, taking into account that the CRCO phase occurs approximately 1 hour after the sleep midpoint for humans under normal conditions18,21,35. Specifically, based on this phase relation between CRCO phase and sleep midpoint, the CRCO-sleep misalignment on a given day was calculated as \(|({CRCO}+{\phi }_{{ref}})-{sleep\; midpoint|}\) hours where \({\phi }_{{ref}}=-1\) is the reference angle. The CRPO-sleep misalignment was similarly computed as \(|{CRPO}-{sleep\; midpoint}|\) hours based on previous results that CRPO phase and sleep midpoint occur around the same time under normal conditions19,20.
The internal misalignment is a statistical measure computed using the Kalman filtering approach20,21. Specifically, the internal misalignment on a given day \(i\), \({\xi }_{i}\), was defined as the absolute difference between the CRCO phase predicted using wearable data until day i - 1, CRCOi|i−1, and the actual CRPO phase on day \(i\), \({{CRPO}}_{i}\), scaled by the standard deviation σi|i−1 we would expect in this measurement, i.e., the log-likelihood value \({\xi }_{i}\) of the absolute difference between CRCOi|i−1 + ϕref and CRPOi. See89 for details.
Daily mood was evaluated and quantified by the response to the following question: “On a scale of 1 (lowest) to 10 (highest), how was your mood today?”90 Depressive symptoms were evaluated with the PHQ-9. For each of the nine depressive symptoms outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), study participants were asked about the frequency of these symptoms over the past two weeks, ranging from “not at all” to “nearly every day.” Each symptom corresponds to a score of 0–3, resulting in a total score ranging from 0 to 27. To assess the PHQ-9 scores, quarterly evaluations were performed.
Circadian disruption measures were estimated from wearable data collected throughout the day20,21, while mood was assessed every evening via a mobile app24. Thus, both circadian disruption measures and mood were sampled once daily. In Fig. 2, each measure estimated on day \(i\) was paired with the corresponding mood score assessed on day \(i+1\). The next day mood scores were binned by each circadian disruption measure, with the bin size of 1 hour. In Fig. 3, each mood score assessed on day \(i\) was paired with the corresponding circadian disruption measures estimated on day \(i+1\). The next day measures were binned by the corresponding mood score, with the bin size of 0.5.
Generalized estimating equation analyses
To investigate the association between current day circadian disruption measures and next day daily mood after adjusting for repeated measures from the same subject and demographic and geographic variables, we applied GEEs with an exchangeable correlation structure, a widely used statistical technique for analyzing longitudinal and correlated data91. Specifically, using data obtained either before or after the internship began, we conducted GEE analyses with the following generalized linear model:
where \({Moo}{d}_{i+1,j}\) represents the daily mood z-score on day \(i+1\) for subject \(j\), \({Disruptio}{n}_{i,j}\) represents the CRCO-sleep misalignment, CRPO-sleep misalignment, or internal misalignment on day \(i\) for subject \(j\), and \(\beta\)’s represent the regression coefficients. In these analyses, we assumed a Gaussian family distribution and that the measurements from different subjects were independent. GEEs provide robust standard errors, making them less sensitive to misspecification of the correlation structure92.
We next investigated the association between current day daily mood and next day circadian disruption markers by conducting GEE analyses using data obtained either before or after the internship began with the following generalized linear model:
The estimated values of β1, which represent the relationship between circadian disruption measures and daily mood, along with their 95% confidence intervals and respective p-values, are reported in Figs. 2g and 3g, and Table 1. All the GEE analyses were implemented using the Python ‘statsmodel’ package in Python 3.10.12.
Linear mixed-effects modeling analyses
To investigate the association between current day circadian disruption markers and next day daily mood after adjusting for random effects of demographic and geographic variables and repeated measures, we applied LMEs, a widely used statistical technique for analyzing data that are multilevel, longitudinal, or correlated93. Unlike in GEE analyses, where we modeled the demographic and geographic variables as fixed effects, we considered the demographic and geographic variables and repeated measures as random effects. Specifically, we conducted LME analyses using data obtained either before or after the internship began with the following crossed-random effect model:
where \({Moo}{d}_{i+1,j}\) represents the daily mood z-score on day \(i+1\) for subject \(j\), \({Disruptio}{n}_{i,j}\) represents the CRCO-sleep misalignment, CRPO-sleep misalignment, or internal misalignment on day \(i\) for subject \(j\), \(\beta\)’s and \(\gamma\)'s represent the fixed and random effects, respectively, and \({u}_{j}\) represents the random effect of repeated measures within subject \({j}\) (i.e., the subject-specific random effect). The association between current day mood and next day disruption was analogously investigated with the following model:
Similarly, we analyzed the association between circadian disruption and PHQ-9 scores before and during the internship using the following model:
where PHQj represents the PHQ-9 score for subject \({j}\), \({Disruptio}{n}_{j}\) represents the CRCO-sleep misalignment, CRPO-sleep misalignment, or internal misalignment for subject \({j}\) averaged over the two weeks preceding the PHQ-9 assessment. Note that PHQ-9 scores were evaluated only once before the internship, so there were no repeated measures at that time. Additionally, since we averaged each subject’s PHQ-9 scores across their individual quarterly questionnaire submissions throughout the internship, there were no repeated measures within that period either. Thus, the model for PHQ-9 scores did not include a term for the random effect of repeated measures. The same model was applied to investigate the association between scores of individual subitems from PHQ-9 questionnaires and circadian disruption. The estimated values of \(\beta\)’s, which represent the relationship between circadian disruption measures and daily mood and depressive severity, along with their 95% confidence intervals and respective p-values, are reported in Figs. 2h and 3h, Table 2, and Supplementary Table 7. All the LME analyses were implemented using the Python ‘statsmodel’ package in Python 3.10.12, with the lbfgs optimizer employed to maximize the log-likelihood of the parameter estimates.
Cross-lagged panel analyses that describe reciprocal relationships between circadian disruption measures and daily mood
We conducted regression analyses using a cross-lagged panel modeling approach that assesses the reciprocal relationship between circadian disruption and daily mood94. Specifically, we analyzed data collected either before or during the internship using a cross-lagged panel model that estimates fixed autoregressive, cross-lagged, and synchronous correlation effects of one variable on another, while adjusting for random effects due to demographic and geographic variables (Supplementary Fig. 6):
where \({Moo}{d}_{i,j}\) represents the daily mood z-score on day \(i\) for subject \(j\), \({Disruptio}{n}_{i,j}\) represents the CRCO-sleep misalignment, CRPO-sleep misalignment, or internal misalignment on day \(i\) for subject \(j\), \(\beta\)’s and \(\gamma\)'s represent the fixed and random effects, respectively, and u’s represent the random effect of repeated measures within subject \({j}\). Note that \({\beta }_{11}\) and \({\beta }_{21}\) represents autoregressive effects, \({\beta }_{12}\) and \({\beta }_{22}\) represents cross-lagged effects, and \({\beta }_{13}\) and \({\beta }_{23}\) represents synchronous correlation effects. The estimated values of \(\beta\)’s, along with their 95% confidence intervals and respective p-values, are reported in Supplementary Table 3 and 5. All the cross-lagged panel analyses were implemented using the Python ‘statsmodel’ package in Python 3.10.12.
Linear mixed-effects modeling analyses that account for all three circadian disruption measures
We conducted regression analyses using a linear mixed-effects modeling approach that accounts for correlations between circadian disruption measures, repeated measures from the same subject, and demographic and geographic variables while estimating the overall influence of the predictor of interest93. Specifically, we analyzed data obtained either before or during the internship using a linear mixed-effects model that estimates the fixed effect of one circadian disruption measure on next day mood across groups with varying levels of the other two circadian disruption measures, while adjusting for random effects from these measures to capture variability among groups:
where \({Moo}{d}_{i+1,j}\) represents the daily mood z-score on day \(i+1\) for subject \(j\), \({Disruptio}{n1}_{i,j}\) represents one circadian measure considered as an independent variable, \({Disruption}{2}_{i,j}\) and \({Disruption}{3}_{i,j}\) represent the other two measures considered as covariates, \(\beta\)’s and \(\gamma\)'s represent the fixed and random effects, respectively, and \({u}_{j}\) represents the random effect of repeated measures within subject \(j\) (i.e., the subject-specific random effect). For example, if CRCO-sleep misalignment is chosen as the independent variable, \({Disruptio}{n1}_{i,j}\), then CRPO-sleep and internal misalignments are considered as covariates, \({Disruption}{2}_{i,j}\) and \({Disruption}{3}_{i,j}\).
Similarly, we analyzed the association between CRCO-sleep misalignment and PHQ-9 scores before and during the internship using the following model:
where \({CRC}{O}_{j}\), \({CRP}{O}_{j}\), and \({Interna}{l}_{j}\) represent CRCO-sleep misalignment, CRPO-sleep misalignment, and internal misalignment for subject \({j}\) averaged over the two weeks preceding the PHQ-9 assessment, respectively. The estimated values of \({\beta }_{1}\), which represent the relationship between circadian disruption measures and daily mood and depressive severity, along with their 95% confidence intervals and respective p-values, are reported in Supplementary Table 4 and 8. All the LME analyses were implemented using the Python ‘statsmodel’ package in Python 3.10.12, with the lbfgs optimizer employed to maximize the log-likelihood of the parameter estimates.
Statistical analysis
Two-tailed t-test and linear least-square regression analysis were performed using the Python SciPy package to determine statistical significance. The beta value, Pearson’s correlation coefficient, and p-value were calculated as part of least-square regression. Detailed descriptions of these tests, along with the relevant parameters, can be found in the main text, figures, and figure legends.
Multiple test corrections were applied using the two-stage linear step-up procedure, implemented with Python’s MultiPy package41. In the daily mood analysis (Figs. 2 and 3), these corrections were applied to the p-values obtained by comparing CRCO-sleep, CRPO-sleep, and internal misalignment with the daily mood score. In the PHQ-9 analysis (Fig. 4), the corrections were applied to the p-values obtained by comparing CRCO-sleep misalignment with scores of the nine PHQ-9 subitems. A p-value < 0.05 was considered statistically significant. All data processing and statistical analysis were completed using Python 3.10.12.
Data availability
The de-identified data from Intern Health Study that supports our findings are available from the corresponding author upon reasonable request.
Code availability
Code for data processing and statistical analysis is available on (https://github.com/phillee62/Digitial_circadian_disruption_markers).
References
Saxena, S., Funk, M. & Chisholm, D. WHO’s Mental Health Action Plan 2013-2020: what can psychiatrists do to facilitate its implementation? World Psychiatry 13, 107 (2014).
Sartorius, N. The economic and social burden of depression. J. Clin. Psychiatry 62, 8–11 (2001).
Karatsoreos, I. N. Effects of circadian disruption on mental and physical health. Curr. Neurol. Neurosci. Rep. 12, 218–225 (2012).
Bedrosian, T. A. & Nelson, R. Timing of light exposure affects mood and brain circuits. Transl. psychiatry 7, e1017 (2017).
Walker, W. H., Walton, J. C., DeVries, A. C. & Nelson, R. J. Circadian rhythm disruption and mental health. Transl. psychiatry 10, 1–13 (2020).
Fishbein, A. B., Knutson, K. L. & Zee, P. C. Circadian disruption and human health. J. Clin. Investig. 131, e148286 (2021).
Emens, J., Lewy, A., Kinzie, J. M., Arntz, D. & Rough, J. Circadian misalignment in major depressive disorder. Psychiatry Res. 168, 259–261 (2009).
Hasler, B. P., Buysse, D. J., Kupfer, D. J. & Germain, A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 178, 205–207 (2010).
Baron, K. G. & Reid, K. J. Circadian misalignment and health. Int. Rev. psychiatry 26, 139–154 (2014).
Emens, J. S. & Lewy, A. J. Mood correlates with circadian alignment in healthy individuals. Sleep Health 10, S154–S156 (2024).
Sletten, T. L. et al. Health consequences of circadian disruption. Sleep 43, zsz194 (2020).
Arushanyan, E. & Popov, A. Influence of damage to the suprachiasmatic nuclei of the hypothalamus of rats on the dynamics of short-period fluctuations of normal and abnormal behavior. Neurosci. Behav. Physiol. 25, 290–295 (1995).
Tataroğlu, Ö., Aksoy, A., Yılmaz, A. & Canbeyli, R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 1001, 118–124 (2004).
Landgraf, D. et al. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. psychiatry 80, 827–835 (2016).
Vadnie, C. A. et al. The suprachiasmatic nucleus regulates anxiety-like behavior in mice. Front. Neurosci. 15, 765850 (2022).
Woelders, T., Beersma, D. G., Gordijn, M. C., Hut, R. A. & Wams, E. J. Daily light exposure patterns reveal phase and period of the human circadian clock. J. Biol. Rhythms 32, 274–286 (2017).
Stone, J. E. et al. The role of light sensitivity and intrinsic circadian period in predicting individual circadian timing. J. Biol. Rhythms 35, 628–640 (2020).
Huang, Y. et al. Predicting circadian phase across populations: a comparison of mathematical models and wearable devices. Sleep 44, zsab126 (2021).
Bowman, C. et al. A method for characterizing daily physiology from widely used wearables. Cell Rep. Methods 1, 100058 (2021).
Kim, D. W. et al. Efficient assessment of real-world dynamics of circadian rhythms in heart rate and body temperature from wearable data. J. R. Soc. Interface 20, 20230030 (2023).
Kim, D. W., Lee, M. P. & Forger, D. B. Wearable Data Assimilation to Estimate the Circadian Phase. SIAM J. Appl. Math. S452–S475 (2023).
Andone, I. et al. in proceedings of the 2016 ACM international joint conference on pervasive and ubiquitous computing: Adjunct. 624–629.
Caldeira, C. et al. In AMIA Annual Symposium Proceedings. 495 (American Medical Informatics Association).
Fang, Y., Forger, D. B., Frank, E., Sen, S. & Goldstein, C. Day-to-day variability in sleep parameters and depression risk: a prospective cohort study of training physicians. NPJ digital Med. 4, 28 (2021).
Fang, Y. et al. Trends in depressive symptoms and associated factors during residency, 2007 to 2019: a repeated annual cohort study. Ann. Intern. Med. 175, 56–64 (2022).
Song, Y. M. et al. Causal dynamics of sleep, circadian rhythm, and mood symptoms in patients with major depression and bipolar disorder: insights from longitudinal wearable device data. eBioMedicine 103, 105094 (2024).
Sen, S. et al. A prospective cohort study investigating factors associated with depression during medical internship. Arch. Gen. Psychiatry 67, 557–565 (2010).
Li, W. et al. Mental health of young physicians in China during the novel coronavirus disease 2019 outbreak. JAMA Netw. open 3, e2010705 (2020).
Chen, L. et al. Prevalence and risk factors for depression among training physicians in China and the United States. Sci. Rep. 12, 8170 (2022).
Kalmbach, D. A. et al. Effects of sleep, physical activity, and shift work on daily mood: a prospective mobile monitoring study of medical interns. J. Gen. Intern. Med. 33, 914–920 (2018).
Skene, D. J. et al. Separation of circadian-and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl Acad. Sci. 115, 7825–7830 (2018).
Chellappa, S. L. et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 7, eabg9910 (2021).
Karatsoreos, I. N. et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151, 2117–2127 (2010).
Chaput, J.-P. et al. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 19, 82–97 (2023).
Forger, D. B., Jewett, M. E. & Kronauer, R. E. A simpler model of the human circadian pacemaker. J. Biol. rhythms 14, 533–538 (1999).
Benloucif, S. et al. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J. Biol. rhythms 20, 178–188 (2005).
Wichniak, A. et al. Treatment guidelines for circadian rhythm sleep-wake disorders of the Polish Sleep Research Society and the Section of Biological Psychiatry of the Polish Psychiatric Association. Part I. Physiology, assessment and therapeutic methods. Psychiatr. Pol. 51, 793–814 (2017).
Boivin, D. B. et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry 54, 145–152 (1997).
Shapiro, B., Fang, Y., Sen, S. & Forger, D. Unraveling the interplay of circadian rhythm and sleep deprivation on mood: A Real-World Study on first-year physicians. PLOS Digital Health 3, e0000439 (2024).
Mardia, K. V., Kent, J. T. & Taylor, C. C. Multivariate analysis. 88 (John Wiley & Sons, 2024).
Benjamini, Y., Krieger, A. M. & Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507 (2006).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ‐9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Cheng, P. et al. Predicting circadian misalignment with wearable technology: validation of wrist-worn actigraphy and photometry in night shift workers. Sleep 44, zsaa180 (2021).
Hampton, S. et al. Postprandial hormone and metabolic responses in simulated shift work. J. Endocrinol. 151, 259–267 (1996).
Schoeller, D., Cella, L., Sinha, M. & Caro, J. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J. Clin. Investig. 100, 1882–1887 (1997).
Mayer, C. et al. Consumer-grade wearables identify changes in multiple physiological systems during COVID-19 disease progression. Cell Rep. Med. 3, 100601 (2022).
Lewy, A., Sack, R., Singer, C. & White, D. The phase shift hypothesis for bright light’s therapeutic mechanism of action: theoretical considerations and experimental evidence. Psychopharmacol. Bull. 23, 349–353 (1987).
Ali, F. Z., Parsey, R. V., Lin, S., Schwartz, J. & DeLorenzo, C. Circadian rhythm biomarker from wearable device data is related to concurrent antidepressant treatment response. NPJ Digital Med. 6, 81 (2023).
Cui, S. et al. CARE as a wearable derived feature linking circadian amplitude to human cognitive functions. NPJ Digital Med. 6, 123 (2023).
Huang, Y. et al. Distinct circadian assessments from wearable data reveal social distancing promoted internal desynchrony between circadian markers. Front. Digital Health 3, 727504 (2021).
Wright, K. P. Jr, Gronfier, C., Duffy, J. F. & Czeisler, C. A. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. rhythms 20, 168–177 (2005).
Phillips, A. J. et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl Acad. Sci. 116, 12019–12024 (2019).
Yamanaka, Y. et al. Effects of physical exercise on human circadian rhythms. Sleep. Biol. Rhythms 4, 199–206 (2006).
Youngstedt, S. D., Elliott, J. A. & Kripke, D. F. Human circadian phase–response curves for exercise. J. Physiol. 597, 2253–2268 (2019).
de Leeuw, M. et al. The role of the circadian system in the etiology of depression. Neurosci. Biobehav. Rev. 153, 105383 (2023).
Horsey, E. A. et al. Chronic jet lag simulation decreases hippocampal neurogenesis and enhances depressive behaviors and cognitive deficits in adult male rats. Front. Behav. Neurosci. 13, 272 (2020).
Wang, H. et al. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc. Natl Acad. Sci. 112, 9745–9750 (2015).
Mistlberger, R. E. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Rev. 49, 429–454 (2005).
Savalli, G., Diao, W., Schulz, S., Todtova, K. & Pollak, D. D. Diurnal oscillation of amygdala clock gene expression and loss of synchrony in a mouse model of depression. Int. J. Neuropsychopharmacol. 18, pyu095 (2015).
Srinivasan, V. et al. Melatonin in mood disorders. World J. Biol. Psychiatry 7, 138–151 (2006).
Parry, B. L. et al. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J. Clin. Endocrinol. Metab. 93, 54–60 (2008).
Crasson, M. et al. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology 29, 1–12 (2004).
Picinato, M. C., Haber, E. P., Carpinelli, A. R. & Cipolla‐Neto, J. Daily rhythm of glucose‐induced insulin secretion by isolated islets from intact and pinealectomized rat. J. Pineal Res. 33, 172–177 (2002).
Garaulet, M. et al. Melatonin effects on glucose metabolism: time to unlock the controversy. Trends Endocrinol. Metab. 31, 192–204 (2020).
Williams, S. R., Zies, D., Mullegama, S. V., Grotewiel, M. S. & Elsea, S. H. Smith-Magenis syndrome results in disruption of CLOCK gene transcription and reveals an integral role for RAI1 in the maintenance of circadian rhythmicity. Am. J. Hum. Genet. 90, 941–949 (2012).
Kunz, D., Mahlberg, R., Müller, C., Tilmann, A. & Bes, F. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J. Clin. Endocrinol. Metab. 89, 128–134 (2004).
Crupi, R. et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone‐treated mice. J. Pineal Res. 49, 123–129 (2010).
Mendoza, J. Circadian insights into the biology of depression: Symptoms, treatments and animal models. Behavioural brain Res. 376, 112186 (2019).
Walsh, R. F., Maddox, M. A., Smith, L. T., Liu, R. T. & Alloy, L. B. Social and circadian rhythm dysregulation and suicide: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 158, 105560 (2024).
Sletten, T. L., Segal, A. Y., Flynn-Evans, E. E., Lockley, S. W. & Rajaratnam, S. M. Inter-individual differences in neurobehavioural impairment following sleep restriction are associated with circadian rhythm phase. PloS one 10, e0128273 (2015).
Cuesta, M., Boudreau, P., Cermakian, N. & Boivin, D. B. Rapid resetting of human peripheral clocks by phototherapy during simulated night shift work. Sci. Rep. 7, 16310 (2017).
McHill, A. W. et al. Robust stability of melatonin circadian phase, sleep metrics, and chronotype across months in young adults living in real‐world settings. J. Pineal Res. 70, e12720 (2021).
Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N. & Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 12, 1574–1583 (2002).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Stephan, F. K. in Circadian clocks 223-246 (Springer, 2001).
Cochran, A. L., Schultz, A., McInnis, M. G. & Forger, D. B. Testing frameworks for personalizing bipolar disorder. Transl. Psychiatry 8, 36 (2018).
Cochran, A., McInnis, M. & Forger, D. Data-driven classification of bipolar I disorder from longitudinal course of mood. Transl. psychiatry 6, e912 (2016).
Granger, C. W. Investigating causal relations by econometric models and cross-spectral methods. Econometrica: J. Econometr. Soc. 37, 424–438 (1969).
Sugihara, G. et al. Detecting causality in complex ecosystems. science 338, 496–500 (2012).
Njoroge, W. et al. Use of mobile technology to identify behavioral mechanisms linked to mental health outcomes in Kenya: protocol for development and validation of a predictive model. BMC Res. Notes 16, 226 (2023).
Walch, O., Huang, Y., Forger, D. & Goldstein, C. Sleep stage prediction with raw acceleration and photoplethysmography heart rate data derived from a consumer wearable device. Sleep 42, zsz180 (2019).
Lee, M. P., Kim, D. W., Mayer, C., Walch, O. & Forger, D. B. The combination of topological data analysis and mathematical modeling improves sleep stage prediction from consumer-grade wearables. J. Biol. Rhythms https://doi.org/10.1177/07487304241288607 (2024).
Chellappa, S. L., Morris, C. J. & Scheer, F. A. Circadian misalignment increases mood vulnerability in simulated shift work. Sci. Rep. 10, 18614 (2020).
Alavi, A. et al. Real-time alerting system for COVID-19 and other stress events using wearable data. Nat. Med. 28, 175–184 (2022).
Guille, C. et al. Web-based cognitive behavioral therapy intervention for the prevention of suicidal ideation in medical interns: a randomized clinical trial. JAMA psychiatry 72, 1192–1198 (2015).
De Zambotti, M., Goldstone, A., Claudatos, S., Colrain, I. M. & Baker, F. C. A validation study of Fitbit Charge 2™ compared with polysomnography in adults. Chronobiol. Int. 35, 465–476 (2018).
Gavin, H. P. The Levenberg-Marquardt algorithm for nonlinear least squares curve-fitting problems. Duke Univ., Dep. Civ. Environ. Eng. 19 (2019).
Wang, N. & Forger, D. B. The level set kalman filter for state estimation of continuous-discrete systems. IEEE Trans. Signal Process. 70, 631–642 (2021).
Forger, D. B. Biological clocks, rhythms, and oscillations: the theory of biological timekeeping. (MIT Press, 2017).
Foreman, A. C., Hall, C., Bone, K., Cheng, J. & Kaplin, A. Just text me: using SMS technology for collaborative patient mood charting. J. Participatory Med. 3, e45 (2011).
Liang, K.-Y. & Zeger, S. L. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 (1986).
Hardin, J. W. & Hilbe, J. M. Generalized estimating equations. (chapman and hall/CRC, 2002).
Lindstrom, M. J. & Bates, D. M. Newton—Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J. Am. Stat. Assoc. 83, 1014–1022 (1988).
Finkel, S. E. Causal analysis with panel data. (Sage, 1995).
Acknowledgements
We thank the training physicians who participated in the Intern Health Study. We acknowledge funding from NIH grant R0101459, ARO MURI grant W911NF-22-1-0223, and NSF DMS grant 2052499. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.P.L. performed computational simulations, analyzed the data, contributed to data interpretation, and wrote the manuscript draft. D.W.K. was involved in study conception and design, analyzed the data, contributed to data interpretation, and wrote the manuscript draft. Y.F. extracted the data and contributed to data interpretation. R.K. curated the data and contributed to data interpretation. A.B. contributed to data interpretation. S.S. was involved in study conception and design and contributed to data interpretation. D.B.F. was involved in study conception and design and contributed to data interpretation. All authors revised the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.P.L., D.W.K., Y.F., R.K., A.B., and S.S. declare no competing interests to disclose. D.B.F. is the CSO of Arcascope, a company that makes circadian rhythms software. Both he and the University of Michigan own equality in Arcascope. The licensed intellectual property was not used in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, M.P., Kim, D.W., Fang, Y. et al. The real-world association between digital markers of circadian disruption and mental health risks. npj Digit. Med. 7, 355 (2024). https://doi.org/10.1038/s41746-024-01348-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-024-01348-6
This article is cited by
-
Circadian Rhythm Disorders: Focusing on Environmental Risk Factors, Interrelationship with Diseases, and Developing Chronotherapy
Reviews of Environmental Contamination and Toxicology (2026)
-
Seasonal timing and interindividual differences in shiftwork adaptation
npj Digital Medicine (2025)
-
Long term monitoring shows phase lagged behaviorally driven locomotor autonomic coupling
Scientific Reports (2025)
-
Association between circadian syndrome and periodontitis in U.S. adults: results from NHANES 2009–2014
Journal of Diabetes & Metabolic Disorders (2025)
-
Sleep duration moderates association between screen time and emotional and behavioural problems in young children
World Journal of Pediatrics (2025)