Abstract
Critical findings in radiology reports are life threatening conditions that need to be communicated promptly to physicians for timely management of patients. Although challenging, advancements in natural language processing (NLP), particularly large language models (LLMs), now enable the automated identification of key findings from verbose reports. Given the scarcity of labeled critical findings data, we implemented a two-phase, weakly supervised fine-tuning approach on 15,000 unlabeled Mayo Clinic reports. This fine-tuned model then automatically extracted critical terms on internal (Mayo Clinic, n = 80) and external (MIMIC-III, n = 123) test datasets, validated against expert annotations. Model performance was further assessed on 5000 MIMIC-IV reports using LLM-aided metrics, G-eval and Prometheus. Both manual and LLM-based evaluations showed improved task alignment with weak supervision. The pipeline and model, publicly available under an academic license, can aid in critical finding extraction for research and clinical use (https://github.com/dasavisha/CriticalFindings_Extract).
Similar content being viewed by others
Introduction
Radiology reports contain substantial information about a patient including a radiologist’s observations and diagnosis from images as well as critical and incidental findings1. Critical findings are observations or results that require immediate communication (typically within hours) with the patient’s healthcare provider, since a delay in reporting such findings could cause a serious impact on the patient’s health and well-being2,3. Moreover, these findings must be communicated to the treating clinicians who must keep track in order to render necessary medical care in a timely manner4.
The extraction of critical findings from radiology reports presents opportunities for quality improvement and workflow optimization. Radiology reports are lengthy and suffer from inconsistencies in structure and format, which can make retrospective analysis and quality assurance challenging3. Each report can vary significantly in terms of the terminology used, the order in which the observations and findings are presented, and the level of detail provided5. This variability complicates systematic review and analysis of report content for quality assurance, research, and process improvement purposes4. While acute communication of critical findings occurs through direct channels like phone or chat, automated extraction tools can support downstream applications such as quality monitoring, compliance tracking, and institutional dashboards. These challenges highlight the need for reliable automated extraction of key findings from radiology reports to support these important secondary use cases.
Previous researchers have shown that artificial intelligence (AI)-based methods are useful in efficiently mining information from radiology reports and images6. Lakhani et al.7 and Heilbrun et al.8 propose systems that focus on a small dataset of chest CT reports. They propose rule-based text-mining approaches to automatically identify radiology reports containing critical findings. Mabotuwan et al.9 proposed an automated rule-based framework to extract critical findings from 1.2M radiology exams with an approximately 90% accuracy. This work uses a pre-defined list of ten specific critical finding terms and only reports that contain one or more of these terms are identified as critical. However, such rule-based models are limited in scope and approach, and lack generalization towards external data.
In subsequent years, studies have proposed clinical BERT-based models to identify reports that contain critical findings4,10. But the proposed models are limited in their scope of application, focusing only on Chest CT reports. Moreover, these studies can only classify whether a report contains any critical finding(s) or not, without extracting the specific category or type of findings.
With the recent advancements in natural language processing (NLP), large language models (LLMs) can be leveraged to automatically understand and retrieve answers to queries from related textual content11,12,13,14. Furthermore, with domain-specific fine-tuning, LLMs can efficiently detect critical information, showing state-of-the-art performance15,16 on retrieval tasks like medical question-answering, biomedical entity recognition, retrieval of related knowledge and clinical guidelines17,18,19. LLMs like GPT-4 have been used in20,21,22 for identification and interpretation of findings from radiology reports, largely reducing the manual effort required to parse such documents.
Use of general domain LLMs seem promising. But pre-trained AI-based systems must be aligned to domain-specific and task-specific data for more efficient performance and increased understanding of underlying clinical objectives6. Alignment of general domain LLMs relies on such domain and task specific data; but there exists a dearth of annotated data when it comes to the identification of critical findings in radiology reports4,9. Furthermore, although many academic centers maintain databases of tagged critical exams, they often lack detailed definitions and annotations of these findings. This gap makes it impractical and challenging to collect training data for extracting critical findings directly.
We propose an end-to-end pipeline to automatically identify and categorically extract critical findings from radiology reports. The major contributions of this work are as follows:
-
To address the issue of scarce labeled data for model fine-tuning4, we implement a novel weakly supervised and task-specific instruction-based training setup to align the Mistral-based LLMs for retrieval of critical and incidental findings from radiology reports across a wide variety of modalities and sites.
-
We manually curate a list of 210 critical finding-based terms that denote critical findings across different anatomical sites and reports. We expand upon an initial list by the Actionable Reporting Work Group (ACR)23 using ontological term-based expansion to create the first comprehensive list of terms to identify critical findings from a report.
-
We perform a novel evaluation, comparing the efficiency of LLM-aided evaluation to human-aided metrics to empirically analyze the usefulness and feasibility of using LLM-based oracle models like GPT-4, LLaMa, for NLP extraction tasks from radiology reports at scale. To verify the model’s performance and generalizability, we evaluate the system on both internal Mayo Clinic and external publicly available MIMIC-III24 and MIMIC-IV25 reports.
To the best of our knowledge, this is the first proposed system that can successfully identify and extract critical findings from radiology reports, irrespective of type and category.
Results
Dataset
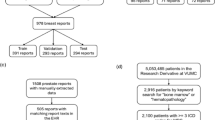
We collected a private dataset of radiology reports of various modalities (MR, CT, Radiograph, US) and anatomy (chest, abdomen, head, extremities) from four Mayo Clinic sites (Arizona, Rochester, Florida and Mid-West). For finetuning the model, we randomly selected 15,000 reports documented between 2015 and 2021 at Mayo Clinic and created a held-out dataset of randomly selected 80 reports for our internal model evaluation (see Table 1). To measure the models’ generalizability, we created an expert-annotated smaller dataset of 123 reports of varying modalities randomly selected from the publicly available MIMIC-III corpus24. The inter-annotator agreement for both manually annotated datasets was assessed using Cohen’s Kappa score (κ). For the MIMIC-III dataset, the Kappa score was κ = 0.738, while for the Mayo Clinic dataset, it was κ = 0.798. Additionally, to evaluate the model performance on a large-scale dataset, we randomly selected 5000 radiology reports from the publicly available MIMIC-IV25 (see Table 1).
Quantitative performance
We evaluate the proposed pipeline performance using two smaller manually annotated test datasets - (a) internal hold-out test set of randomly selected 80 radiology reports from Mayo Clinic, and (b) external test set of 123 radiology reports from MIMIC-III, and a large-scale test dataset of 5000 randomly selected radiology reports from MIMIC-IV. We compare the performance of the weakly finetuned models (WFT) with the baseline pre-trained versions of the model (PT). We provide more details on the task template and prompts for zero-shot and few-shot-based weak label generation, as well as the examples used in few-shot prompting in the Supplementary Sections A and B respectively. Figure 1 demonstrates the model performance for two human-based metrics (ROUGE-2, BLEU) and two LLM-based metrics (G-Eval, Prometheus). Model performance on the large-scale dataset is evaluated using LLM-based metrics, as shown in Fig. 2. The performance of the models are also evaluated using the classification-based metrics like F1-score, precision and recall, calculated on the Mayo Clinic and MIMIC-III datasets as shown in Table 2. For qualitative evaluation and further error analysis, we present examples of the extracted critical finding terms in Table 3. A larger set of human-based and LLM-based metrics report a detailed overview of the model performance on the small-scale human annotated internal and external datasets and large-scale MIMIC dataset respectively in the Supplementary Tables 1 and 2 (Supplementary Section 1).
a This column shows the performance on the Internal hold-out (Mayo Clinic) test set; b This column shows the performance on the External (MIMIC-III) test set. Each row corresponds to the pre-trained (PT) and weakly fine-tuned (WFT) models. For each model, we plot the histogram bars for Rouge-1 (blue), BLEU (yellow), G-Eval (green), and Prometheus (red). For each model, we consider two prompting techniques: Zero-shot (ZS) and Few-shot (FS) for weak label generation. The score for each metric (normalized between 0 and 1) is added to the top of its corresponding bar plot. Error bars denote standard deviations. The scores for models trained using weak labels generated by FS-based prompting are shown with  .
.
LLM-aided large-scale evaluation on a Pre-trained models and b Weakly Fine-tuned models. For each model, we plot the histogram bars for G-Eval (blue) and Prometheus (yellow). For each model, we consider two prompting techniques: Zero-shot (ZS) and Few-shot (FS) for weak label generation. The score for each metric (normalized between 0 and 1) is added to the top of its corresponding bar plot. Error bars denote standard deviations. The scores for models trained using weak labels generated by FS-based prompting are shown with  .
.
The quantitative results on the small-scale internal and external validation datasets (Fig. 1) show that with weakly supervised fine-tuning, the Mistral and BioMistral models perform consistently better than the pre-trained baseline. Moreover, the fine-tuned Mistral model performance is better than the BioMistral LLM. The models that were weakly fine-tuned with labels generated using few-shot prompting in Phase I, consistently performed better on the quantitative metrics. On the internal Mayo test set, the weakly fine-tuned Mistral model achieves a 48% Rouge-2 score, performing better than the pre-trained Mistral and BioMistral models. Similarly, the fine-tuned BioMistral model comes a close second, achieving 41% Rouge-2 score on the internal test data. On the external MIMIC-III test reports we observe a similar trend, with the weakly fine-tuned Mistral and BioMistral models performing better than their pre-trained unsupervised baselines with Rouge-2 scores of 59% and 51% respectively.
This observation is also supported by the F1-score results of weakly fine-tuned BioMistral (BioMistral-WFT) model, especially when the fine-tuning was done with labels generated using few-shot prompting. The weakly fine-tuned model consistently delivers the best performance across both the internal hold-out dataset from Mayo Clinic and the external MIMIC-III dataset and achieves the highest F1-scores, reaching 0.57 on both datasets, suggesting that combining weak supervision with domain-specific pre-training can significantly improve performance in clinical language processing tasks.
We also compared the LLM-aided metrics against the human annotator. Figures 1 and 2 show that though these metrics are not ideal for scoring overall LLM performance, the LLM-aided metrics consistently evaluated human annotation with higher score (referred as ‘Human’ in Supplementary Table D1) than neural model extraction, an observation consistent with human-based evaluation metrics. Figure 2 (and Supplementary Table 2) demonstrates the results of the models for the LLM-aided metrics on the large-scale dataset from MIMIC-IV. While the models perform moderately on the LLM-based scoring metrics, the scores are similar to the overlap-based measures on the human-annotated small-scale test dataset. This shows that while not completely perfect, with proper domain-knowledge on critical findings, these automated LLM-based scoring algorithms can be a promising alternative to human-aided metrics for large-scale validation task. Within the scope of both human-based (Rouge-2, BLEU, etc.) and LLM-based evaluation (G-eval, Prometheus), we observe that the weakly supervised models (WFT) perform better than the pre-trained LLMs (PT) on both internal and external validation datasets. We also observe that the general domain Mistral LLMs perform better than the biomedical domain BioMistral models.
Error analysis - To understand and visualize the performance and quality of the extraction by the weakly supervised models, we examine correctly predicted and falsely predicted examples as shown in Table 3. The examples show that the model completely and/or partly extracts critical terms from the reports where they are clearly demarcated or indicated (Table 3-Examples 1 and 2). These indications could be presence of phrases that indicate urgency or the need to communicate to physicians for further follow-ups, like usage of ‘concerning’, ‘new’. However, where the models clearly fail, are the reports with sentences that indicate the chronic critical findings along with the mention of the critical term, e.g. ‘No significant interval change..’ (Table 3-Example 3). The instruction-tuned LLMs are unsupervised in nature, and therefore have no prior exposure to complicated chronic instances. A chronic finding may not always be critical, it depends on the textual context of the finding - so the model must be trained to carefully discern between new, known and expected critical findings before raising a false alarm. Moreover, Table 3-Example 4 presents a report might only contain a single sentence that reports the absence of the critical finding(s), i.e. a non-critical report. In such an instance, an extractive LLM cannot process the negative sentences and extracts the critical findings terms, thus marking the radiology report as a false positive.
Discussion
We proposed an end-to-end LLM-based pipeline for extracting critical findings from a wide-variety of radiology reports, in terms of modalities and anatomical regions. Given the complexity of the critical finding extraction task and wide-range of potential categorization, the extraction is formulated as a weakly supervised problem without the requirement for manually labeled data for model training. We leverage the task-following generative properties of the instruction-tuned Mistral-class of models and follow a systematic prompt engineering process to create task-specific and comprehensive instructions for the best generative and extractive performance. We selected the Mistral class of models for this extraction task since Mistral models are optimized for inference speed and task performance. Additionally in Supplementary Section 2, we present the quantitative and qualitative results of LLaMa-13B13 models (pre-trained and weakly fine-tuned) on the human-annotated test datasets in Supplementary Tables 3 and 4. The performance of LLaMa-13B model is similar to the Mistral-7B model performance. We evaluated both generic Mistral-7B and domain-specific BioMistral-7B weakly supervised fine-tuned models with zero-shot and few-shot and compared the performance against baseline pre-trained models. We also perform an in-depth analysis of model performance using varying strategies, as shown in examples (A) through (D) in Supplementary Section 3.
We observe that the weakly supervised models (WFT) perform better than the pre-trained LLMs (PT) on both internal and external validation datasets. However, we do observe a difference in performance between the general domain Mistral and the biomedical BioMistral models, with the BioMistral models scoring consistently lower on the evaluation metrics. BioMistral model is trained only on biomedical literature26 which is different from the textual content and structure of radiology reports. Therefore, BioMistral model has limited knowledge about radiology reporting as well as the terms that denote critical or incidental findings are limited in the model vocabulary, thus limiting model performance. On the contrary, Mistral models have been trained on web-scraped textual content and may contain partial knowledge about the radiology reports available on the web14. Using few-shot prompting technique for label generation helps guide and align these instruction-based Mistral models using task-specific examples, leading to comparatively better extractive performance27.
We see that the models comparatively have higher performance scores on the external MIMIC-III dataset, than the internal Mayo Clinic dataset which is primarily because of the nature of the dataset - the average report length of Mayo Clinic dataset is 476.3 words, which is much higher than the length of MIMIC-III reports (53.5). Greater textual content is essentially not always beneficial for aligning an instruction-tuned model, and may cause the model to hallucinate or generate misinformation12. We have performed a stratified analysis of the models’ performance across varying report lengths, as shown in Supplementary Section 4. The quantitative results supporting this observation are shown in Supplementary Tables 5 (external MIMIC-III test data) and 6 (internal Mayo test data). Furthermore, the qualitative examples showing the model performance for short-length, medium-length, and long-length documents also shown in the Supplementary Tables 7, 8, and 9 respectively. Moreover, the reports from the Mayo Clinic dataset, tend to have a higher frequency of negative sentences or text that indicate the absence of critical findings, as shown in Table 3-Example 3. Therefore, the model picks up these sentences as false positive signals, leading to erroneous predictions.
Compared to the current literature4,7,8,9,10 which is primarily focused on developing supervised machine learning or rule-based model for critical finding extraction with narrower scope (e.g. single modality or specific anatomical region), we propose a weakly supervised pipeline for automated extraction of critical findings irrespective of the report type and category of the findings. In Supplementary Section 5, we perform a subgroup analysis of the models’ performance under varying anatomical sites to demonstrate difference in their critical term extraction capabilities. The quantitative results and qualitative examples are included in Supplementary Tables 10 and 11 respectively. The proposed pipeline was weakly-supervised with LLM generated labels and able to extract a wide variety of critical findings from both internal and external test sets without manual curation of labeled data. Due to the wide and unknown/rare category for potential critical findings, we can only present string overlapping metrics for performance which evaluates exact string matching, often without evaluating semantic context. Even with weakly supervised training, the proposed framework was able to achieve moderate overlap with human annotation. Such automated extraction of critical findings from radiology reports may reduce risk of human error regarding flagging and allows for timely communication of serious conditions such as tumors, fractures, or internal bleeding, leading to prompt and appropriate treatment4,9. Furthermore, our system holds promise for retrospective data analysis, ensuring that significant health issues are not overlooked in radiology reports. An additional future direction involves adapting the system to facilitate timely communication of critical findings to referring physicians. This could lead to more effective follow-up based on current guidelines, better management of conditions, and ultimately improved patient outcomes.
The study has several limitations. We view this work primarily as a proof-of-concept demonstrating that weakly supervised approaches can improve performance in scenarios where labeled data is scarce. We are aware that despite showing an improved performance, the approach when compared to existing technology, still remains mediocre and cannot be adopted as an immediately deployable clinical solution. The performance metrics, while showing clear improvement over baselines, indicate that additional refinement is needed before these models would be suitable for routine clinical use. Even after additional curation of critical finding term list from prior publications, ACR lists and ontology-based expansion, there may exist rare critical findings that are not captured by our curated list and thus lead to missed critical findings. In the absence of critical terms, the weakly supervised LLMs do not retrieve any findings, thus flagging an otherwise critical radiology report as non-threatening. Another reason for moderate performance in the term matching metrics is not considering partial term matching, e.g. ‘spinal injury’ vs. ‘injury of spine’. We also plan on extending our model to add the ability to extract critical findings with an increased granularity, where the model can be trained to identify new, known or expected and uncertain or unexpected findings. Finally, we plan to include a vision language model to directly analyze images to identify critical findings with currently trained LLM model as language encoding backbone.
The results of our study demonstrate that the proposed weakly supervised pipeline not only performs better than baseline pre-trained models but also has the potential to improve patient outcomes. By automating the extraction of critical findings from radiology reports, the pipeline reduces the risk of human oversight, ensuring that critical findings such as intracranial haemorrhage, spinal injury, pulmonary embolism, etc. are identified and communicated promptly. This can improve long-term treatment outcomes, and ultimately enhance overall patient care. This underscores the practical utility of our approach in clinical settings, where timely and accurate information can make a significant difference in patient outcomes.
In summary, we highlight the importance of promptly communicating critical findings from radiology reports to physicians for timely patient management. We leverage instruction-tuned Mistral-based large language models (LLMs) to identify and extract these critical findings from radiology reports. To address the lack of labeled datasets due to the rarity of such events, we propose a two-phase weakly supervised fine-tuning approach on Mistral models using unlabeled radiology reports. The weakly fine-tuned model was tested on internal and external datasets and evaluated with both human annotated-based and LLM-based metrics, showing that weakly supervised fine-tuning improves model performance significantly.
Methods
Given the complexity of critical finding extraction and wide-range of potential categorization, we formulated this as a weakly-supervised model learning problem where we leverage weak labels generated by LLM. We leverage the task-following generative properties of the instruction-tuned Mistral-class of models14,26. We also follow a systematic prompt engineering process to create task-specific and comprehensive instructions for the best generative and extractive performance15,16. Figure 3 demonstrates the overall pipeline of the proposed two-phase pipeline - (a) Phase I: The unsupervised instruction-tuned weak label generation; and (b) Phase II: Weakly supervised training framework for automated critical finding extraction. We further validate pipeline performance on small-scale manually annotated held-out internal and external datasets, along with a large-scale external dataset. The internal dataset was extracted from radiology reports from four Mayo Clinic sites (Arizona, Rochester, Florida, and Mid-West) after ethical review by the Mayo Clinic Institutional Review Board (IRB 21-009503) The external datasets came from MIMIC-III (small-scale annotated test) and MIMIC-IV (large-scale test). In the following sections, we provide a detailed description of our framework and evaluation methods.
The complete pipeline is composed of the following modules: a Phase I : This module generates the weak labels using unsupervised instruction-tuned Mistral class of LLMs; b Phase II: This module demonstrates the weakly supervised fine-tuning of the Mistral LLMs using the weak labels generated in Phase I; c This is module shows the expansion of critical findings terms and keywords. This expanded list is used for the key-based critical finding extraction; d This final pipeline demonstrates the model performance validation using internal and external datasets.
Weakly supervised training pipeline
To tackle the challenge regarding lack of annotated database for critical findings, we propose a two-phase weakly supervised training approach to align generic Large Language Models (LLMs) for extracting critical findings efficiently from radiology reports which are documented in a domain-specific clinical language.
In Phase I (Generating Weak Labels) of the pipeline, we leverage the instruction-tuned pre-trained Mistral-based large language models (LLMs), Mistral-7B and BioMistral-7B14,26 to extract the critical findings in the input reports in an unsupervised manner. We rely on the Mistral class of models14 since Mistral model architecture is an efficient version of Meta’s LLaMa model13 that leverages grouped-query attention to significantly accelerate model inference, thus reducing computational complexity while keeping model performance consistent. For understanding the effect of clinical domain-specific training, we leverage two pre-trained versions of the Mistral model in parallel - (a) Mistral-7B-Instruct28, a general domain model with 7B parameter size; and (b) BioMistral-7B29, a version of the Mistral model trained on 1.47M biomedical literature documents (about 3B tokens) from PubMed Central26. We use these instruction-tuned LLMs as an initial step to retrieve the critical findings from radiology reports as weak labels.
Using task-based prompt engineering, the instruction-tuned LLM can be aligned and guided to follow well-defined rules while generating or retrieving textual content12. Quality and usability of the model output is largely dependent on the nature of specific input prompt used to align the model during inference15. The prompt definitions and task instruction are shown in Table 4. For the task of extracting critical findings, the prompts are engineered to instruct the model to identify and retrieve the findings that appear as critical from the given reports based on linguistic style of reporting, order of documentation as well as definition of the findings30. In Phase I, the experimental setup is unsupervised and involves prompt-specific generation. We consider two prompting techniques - zero-shot (no task-specific examples) and few-shot (five task-specific examples). Given the fact that extraction of critical finding is a highly complex task and needs proper understanding of the severity of the findings, each prompt consists of the definitions of ‘critical’ and ‘incidental’ findings in a report, followed by the description of the task to be performed. While zero-shot method of prompting just includes the task definitions and instructions in the query body, few-shot prompting strategy includes examples showing the task input and desired output, to specifically align for in-context task-based learning16. The prompt templates and the few-shot examples used in prompting are shown in Supplementary Sections 6 and 7 respectively.
Mistral-based LLMs are generative transformer models and during inference outputs are descriptive textual content. Hence, the proposed pipeline incorporates a module for the automated extraction of key terms or phrases that represent the critical findings in the radiology reports, using a list-based term matching algorithm. To map the extracted findings from the reports to the actual terms, we first curate a manually verified and comprehensive list of critical findings keywords and phrases. The list of terms was collected from different academic institutions (Stanford, UCLA, Yale, Emory), to which we appended additional terms and phrases from a list developed by American College of Radiology’s Actionable Reporting Work Group (ACR)23. But radiologists often use abbreviations to refer to findings, for example ‘PE’ instead of ‘Pulmonary Embolism’, or there are spelling errors due to dictation (e.g., ‘hemorrhage’ or ‘haemorrhage’). To incorporate additional keywords that share the same ontological relations with the curated manual list of critical key terms and phrases, we follow an ontology-based expansion of critical findings keywords. We used the ‘PyMedTermino2’ Python library31 to parse NCBO32 ontologies such as SNOMED_CT, ICD10, and UMLS, and include synonym-based searches. We specifically used the SNOMED_CT ontology for our approach and starting with a reference list of 102 terms, expanded the list to 210 terms. Table 5 has a list of some sample terms and their corresponding expansions. We have included the complete list of terms in the Supplementary Section 8 (Supplementary Tables 12 and 13).
In our pipeline, we check for the mentions of the terms/phrases, from the curated list, in the output text containing the extracted findings generated by the Mistral LLMs. We use the Python library “fuzzywuzzy”33 to extract the keywords from the text using exact term-matching algorithms. We do not consider partial or relaxed term-matching to avoid incorrect flagging if part of a critical finding term appears in an otherwise non-critical radiology report. For a given unlabeled radiology report, the output of the proposed Phase I pipeline, is a set of terms and/or phrases that represent the critical findings in the radiology report and if there is no mention of any critical findings the extracted term is null. These are then augmented to the report as weak labels for fine-tuning the weakly supervised model during Phase II of the pipeline.
The main objective for Phase II (Weakly Supervised Fine-tuning) is to fine-tune the Mistral-based models using weakly supervised labels for an improved automated extraction of specific critical findings from radiology reports. Fine-tuning the pretrained LLM in a weakly supervised setting helps to refine the model’s understanding and enhance its ability to discern critical information in the required format12,34.
We first combine the ‘weak labels’ generated in Phase I with the unlabeled reports. For each report that has one or more key terms representing critical findings, the label is represented by those extracted keywords as a list. For a non-critical report, the label is an empty list. During training, we further append the task-specific instruction to the report text and its weak label list to emulate the instruction-tuning setup used to train the Mistral class of models. The main goal is to train an instruction-tuned generative LLM that can automatically identify and retrieve critical findings terms from radiology reports.
We use the standard fine-tuning configurations along with LoRA-specific hyperparameters35 for faster and computationally efficient training of the Mistral and BioMistral models. The LoRA parameter values used were - attention dimension = 16, scaling rate α = 4, dropout rate = 0.1. We also used weighted Adam optimizer with a constant learning rate scheduler, with an initial learning rate of 0.0002 and weight decay of 0.001. We ran the fine-tuning on four NVIDIA RTX A5000 GPUs for 20 epochs for a total of 3 hours.
Evaluation strategy
We describe the setup for the manual and automated metrics used to evaluate the performance of the models during both unsupervised Phase I and weakly supervised Phase II. To measure overall performance of the models and validate the quality and correctness of the extracted critical findings, we evaluate on both internal (held-out reports from Mayo Clinic) and external (reports from publicly available MIMIC-III) datasets. We evaluate the performance of two types of Mistral-based models - Mistral-7B14 and BioMistral-7B26. The instruction-tuned versions of these models (referred as PT) are considered the baseline models, for comparison with the weakly fine-tuned LLMs (referred as WFT). Two separate prompting methods (zero-shot and few-shot) were used to generate the weak labels (Phase I). We evaluate the models using two strategies - Human-based (small-scale) and automated LLM-based (large-scale).
Human-based evaluation
compares the critical findings automatically extracted by the LLMs with the manually extracted critical findings mentions in the radiology reports. This is the preferred method of evaluating generative models and consists of assessing the quality and correctness of the generated content by comparing with human annotated labels as the ground truth. Given the large variety of potential findings, standard accuracy-based metrics are not suitable for this evaluation. Therefore, we report the models’ performance score using the lexical-similarity metrics like BLEU36, ROUGE37 (-1 and -2), and METEOR38. These metrics quantify the overlap between the human-generated and LLM-generated mentions of critical findings. Additionally, we also look at the semantic similarity between the model generated labels and the manual labels using RadBERTScore. This metric uses BERTScore-based39 evaluation algorithm but replaces the vanilla BERT model with RadBERT40, a pre-trained RoBERTa-based model specifically fine-tuned on 4M radiology reports41. However, manual evaluation can be time-consuming and costly, and can only be performed on a smaller scale due to required effort.
LLM-based evaluation
leverage the causal reasoning and extensive knowledge of pre-trained LLMs, like GPT-411 and LLaMa13, to automatically rate quality of the extracted textual content, while achieving results comparable to a human annotator17,42. The main usefulness of LLM-based metrics lies in evaluating the performance of the proposed models on large-scale datasets of radiology reports, where manual annotation is challenging and time-consuming. So in addition to evaluating the smaller human-annotated internal (Mayo Clinic) and external (MIMIC-III) datasets with LLM-based scorers, we additionally use these metrics to evaluate the extracted critical findings from a large-scale dataset of 5000 random radiology reports from MIMIC-IV.
We report the model performance using two LLM-aided evaluation metrics - G-Eval42 and Prometheus43. G-Eval44 uses task-specific chain-of-thought prompting45 to instruct OpenAI’s GPT-411 model to evaluate the text. Prometheus46 also follows an approach similar to G-Eval, but instead of GPT-4, uses a pre-trained LLaMa-13B model as the scoring algorithm. These LLM-based scoring algorithms are instruction-tuned and require an evaluation metric definition and task-based prompt to rate model performance. We use the correctness metric to score the quality of the generated output from the models. The score is a continuous value between 0 to 1, with a higher value denoting an output closer to the ground-truth or gold-standard annotations, indicating better model performance. Based on the nature of the test dataset, we have two separate definitions of the correctness evaluation task.
For the human-annotated smaller test datasets, we have the human or ground truth labels. We provide the following task definition to the scoring LLM - ‘Given the human label, determine if the model output is correct. Consider partial matches.’ We use the LLM-based scorers to compare the human annotations with the model-extracted critical findings; we do not provide additional information like the report metadata, impressions, findings, etc. However, since we do not have human annotated labels for the large-scale dataset, we slightly change the definition and objective of the evaluation task. Since MIMIC-IV is a publicly available dataset, we provide the report text and the extracted critical findings by the models. We also provide the definition of critical finding, along with the correctness definition in the evaluation task prompt. The evaluation task definition given to the scoring algorithm is - ‘Given the report and the definition of critical findings, determine if the model output is correct. Consider partial matches.’ The examples of the prompts used in LLM-aided automated evaluation have been included in the Supplementary Section 9. In the absence of human labels, our main objective here is to leverage the prior knowledge of these LLM-based scoring algorithms for rating the quality of automated large-scale dataset annotation using weakly supervised LLMs.
Data availability
The Mayo Clinic data supporting the findings of this study are private and not available publicly. Corresponding author can be reached for data usage agreement for approving the access for the private data. The MIMIC-III and MIMIC IV data sets are publicly available at https://physionet.org/content/mimiciii/1.4/ and https://physionet.org/content/mimiciv/2.2/ respectively.
Code availability
Our code and trained models are publicly shared under open-source academic license at https://github.com/dasavisha/CriticalFindings_Extract. In our implementation, we used Python 3.8.10, and the following open-source libraries: torch = 1.13.1, tqdm = 4.66.1, pandas = 2.7.1, numpy = 1.21.0, transformers = 4.35.2, huggingface-hub = 0.19.4, accelerate = 0.25.0, and fuzzywuzzy = 0.18.0.
References
Cronin, P. & Rawson, J. V. Review of research reporting guidelines for radiology researchers. Acad. Radiol. 23, 537–558 (2016).
Berlin, L. Communicating findings of radiologic examinations: whither goest the radiologist’s duty? Am. J. Roentgenol. 178, 809–815 (2002).
Sistrom, C. L. & Langlotz, C. P. A framework for improving radiology reporting. J. Am. Coll. Radiol. 2, 159–167 (2005).
Banerjee, I. et al. Natural language processing model for identifying critical findings-a multi-institutional study. J. Digital Imaging 36, 105–113 (2023).
Clinger, N. J., Hunter, T. B. & Hillman, B. J. Radiology reporting: attitudes of referring physicians. Radiology 169, 825–826 (1988).
Van Leeuwen, K. G., de Rooij, M., Schalekamp, S., van Ginneken, B. & Rutten, M. J. How does artificial intelligence in radiology improve efficiency and health outcomes? Pediatric Radiol. 52, 2087–2093 (2022).
Lakhani, P., Kim, W. & Langlotz, C. P. Automated detection of critical results in radiology reports. J. Digital Imaging 25, 30–36 (2012).
Heilbrun, M. E., Chapman, B. E., Narasimhan, E., Patel, N. & Mowery, D. Feasibility of natural language processing–assisted auditing of critical findings in chest radiology. J. Am. Coll. Radiol. 16, 1299–1304 (2019).
Mabotuwana, T., Hall, C. S. & Cross, N. Framework for extracting critical findings in radiology reports. J. Digital Imaging 33, 988–995 (2020).
Jiang, Z. et al. Learning to summarize chinese radiology findings with a pre-trained encoder. IEEE Trans. Biomed. Eng. 70, 3277–3287 (2023).
Achiam, J. et al. GPT-4 technical report. https://arxiv.org/abs/2303.08774 (2023).
Ouyang, L. et al. Training language models to follow instructions with human feedback. Adv. neural Inf. Process. Syst. 35, 27730–27744 (2022).
Touvron, H. et al. Llama: Open and efficient foundation language models. arXiv preprint arXiv:2302.13971 (2023).
Jiang, A. Q. et al. Mistral 7b. Preprint at https://arxiv.org/abs/2302.13971 (2023).
Wei, J. et al. Finetuned language models are zero-shot learners. Preprint at https://arxiv.org/abs/2109.01652 (2021).
Brown, T. et al. Language models are few-shot learners. Adv. Neural Inf. Process. Syst. 33, 1877–1901 (2020).
Liu, L. et al. A survey on medical large language models: Technology, application, trustworthiness, and future directions. Preprint at https://arxiv.org/abs/2406.03712 (2024).
Wang, B. et al. Pre-trained language models in biomedical domain: A systematic survey. ACM Comput. Surv. 56, 1–52 (2023).
Wang, J., Yang, Z., Yao, Z. & Yu, H. JMLR: Joint medical llm and retrieval training for enhancing reasoning and professional question answering capability. Preprint at https://arxiv.org/abs/2402.17887 (2024).
Woo, K.-m. C. et al. Evaluation of GPT-4 ability to identify and generate patient instructions for actionable incidental radiology findings. J. Am. Med. Informatics Assoc. 31, 1983–1993 (2024).
Bhayana, R. et al. Use of GPT-4 with single-shot learning to identify incidental findings in radiology reports. Am. J. Roentgenol. 222, e2330651 (2024).
Kim, S. H. et al. Boosting LLM-assisted diagnosis: 10-minute llm tutorial elevates radiology residents’ performance in brain mri interpretation. medRxiv, https://www.medrxiv.org/content/10.1101/2024.07.03.24309779v1.full.pdf (2024).
Larson, P. A., Berland, L. L., Griffith, B., Kahn Jr, C. E. & Liebscher, L. A. Actionable findings and the role of it support: report of the acr actionable reporting work group. J. Am. Coll. Radiol. 11, 552–558 (2014).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci. Data 3, 1–9 (2016).
Johnson, A. E. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1 (2023).
Labrak, Y. et al. Biomistral: A collection of open-source pretrained large language models for medical domains. Preprint at https://arxiv.org/abs/2402.10373 (2024).
Zambrano Chaves, J. et al. Rales: a benchmark for radiology language evaluations. Adv. Neural Inf. Process. Syst. 36, 74429–74454 (2023).
Mistral-7B huggingface model card. https://huggingface.co/mistralai/Mistral-7B-Instruct-v0.2 (2023). Accessed: 2024-09-30.
BioMistral-7B huggingface model card. https://huggingface.co/BioMistral/BioMistral-7B (2024). Accessed: 2024-09-30.
Gramopadhye, O. et al. Few shot chain-of-thought driven reasoning to prompt llms for open ended medical question answering. Findings of the Association for Computational Linguistics: EMNLP 2024 (2024).
PyMedTermino toolkit. https://owlready2.readthedocs.io/en/latest/pymedtermino2.html (2024). Accessed: 2024-09-30.
NCBO ontologies. https://bioportal.bioontology.org/ (2011). Accessed: 2024-09-30.
FuzzyWuzzy python library. https://pypi.org/project/fuzzywuzzy/ (2020). Accessed: 2024-09-30.
Wei, J. et al. Emergent abilities of large language models. Transact. Mach. Learn. Res. https://doi.org/10.48550/arXiv.2206.07682 (2022).
Hu, E. J. et al. Lora: Low-rank adaptation of large language models. In Proceedings of The Tenth International Conference on Learning Representations (ICLR 2022).
Papineni, K., Roukos, S., Ward, T. & Zhu, W.-J. Bleu: a method for automatic evaluation of machine translation. In: Proceedings of the 40th annual meeting of the Association for Computational Linguistics 311–318 (ACM, 2002).
Lin, C.-Y. Rouge: A package for automatic evaluation of summaries. Text summarization branches out 74–81 (Association for Computational Linguistics, 2004).
Banerjee, S. & Lavie, A. METEOR: An automatic metric for mt evaluation with improved correlation with human judgments. In: Proceedings of the ACL workshop on intrinsic and extrinsic evaluation measures for machine translation and/or summarization 65–72 (ACL, 2005).
Zhang, T., Kishore, V., Wu, F., Weinberger, K. Q. & Artzi, Y. Bertscore: Evaluating text generation with bert. Proceedings of International Conference on Learning Representations (2019).
RadBERT model. https://github.com/zzxslp/RadBERT (2024). Accessed: 2024-09-30.
Yan, A. et al. RadBERT: Adapting transformer-based language models to radiology. Radiology 4, e210258 (2022).
Liu, Y. et al. G-Eval: NLG Evaluation using GPT-4 with Better Human Alignment. Proceedings of the 2023 Conference on Empirical Methods in Natural Language Processing (2023)
Kim, S. et al. Prometheus: Inducing fine-grained evaluation capability in language models. In: The Twelfth International Conference on Learning Representations (2023), OpenReview.net.
G-Eval model. https://github.com/nlpyang/geval (2023). Accessed: 2024-09-30.
Wei, J. et al. Chain-of-thought prompting elicits reasoning in large language models. Adv. Neural Inf. Process. Syst. 35, 24824–24837 (2022).
Prometheus model. https://github.com/prometheus-eval/prometheus (2023). Accessed: 2024-09-30.
Acknowledgements
The work is supported in part by a grant from Phillips Healthcare.
Author information
Authors and Affiliations
Contributions
A.D., I.T., D.R., and I.B. analyzed and interpreted the data used to train and evaluate the automated critical findings extraction pipeline. A.D. developed the pipeline, performed the empirical evaluation of the pipeline, and was a major contributor in writing the manuscript. I.T., D.R. and J.M.Z.C. provided the definitions and few-shot examples used in prompting setup. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Das, A., Talati, I.A., Chaves, J.M.Z. et al. Weakly supervised language models for automated extraction of critical findings from radiology reports. npj Digit. Med. 8, 257 (2025). https://doi.org/10.1038/s41746-025-01522-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-025-01522-4