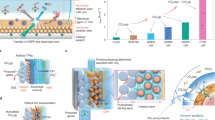
Abstract
Recent advancements in the CO2 reduction reaction (CO2RR) target multicarbon chemical production and scalable electrode designs for industrial applications. Here we introduce a zero-gap cell utilizing humidified gas-phase CO2 and circulated alkaline media, achieving a Faradaic efficiency of 66.9% for C3+ products and a current density of −1,100 mA cm−2. In situ spectroscopic analyses revealed formaldehyde as a key intermediate formed on copper oxide/hydroxide interfaces derived from a phosphorus-rich copper catalyst. Unlike conventional pathways based on dimerization of CO intermediates, our study selectively produces liquid-phase multicarbon products because of autonomous local pH variations under a weak alkaline microenvironment, with allyl alcohol as the dominant C3+ product. The high selectivity and efficiency for liquid products provide a substantial advantage for storage and transport, highlighting the scalability and practical feasibility of our approach, which offers a potential economically viable solution for CO2 utilization. This development encourages the adoption of CO2RR technologies in iron–steel and petrochemical industries to mitigate greenhouse gas emissions.

Similar content being viewed by others
Main
The urgent need to mitigate greenhouse gas emissions coupled with industrial demand for a broad range of chemicals necessitates innovative production methods1. This is particularly true for C3+ multicarbon products, defined as compounds with three or more carbon atoms, which are essential for various industrial applications. C3+ multicarbon products are traditionally produced through methods such as catalytic cracking, crude oil distillation and oligomerization2,3. However, these traditional methods rely heavily on fossil fuel feedstocks and require high pressures and temperatures, leading to substantial greenhouse gas emissions, environmental pollution and energy consumption4.
In contrast, by utilizing CO2, a notorious greenhouse gas, as the primary feedstock, electrochemical CO2 reduction offers the prospect of a more sustainable alternative, especially when powered by renewable electricity sources such as wind or solar power5,6,7,8. This method operates under ambient or near-ambient conditions, reducing energy demands compared with traditional methods. Despite these important advantages, electrochemical methods face challenges such as low Faradaic efficiencies (FEs), slow reaction rates (low current densities) and catalyst stability issues. Moreover, the variety of products formed during electrochemical CO2 reduction necessitates an energy-intensive separation process to achieve industrial purity standards for each hydrocarbon product.
Recent research has focused on addressing these challenges to enhance the industrial viability of the CO2 reduction reaction (CO2RR)8,9. For instance, the use of gas diffusion electrodes (GDEs) in electrolytic cells has been explored to establish triple-phase boundaries by incorporating gaseous CO2 inlets and liquid-phase alkaline catholytes into membrane electrode assembly (MEA) cells10,11,12,13. Despite considerable progress—for example, MEA cells have attained a heightened maximum current density (Jmax) of 1.4 A cm−2—obstacles remain, due in particular to the conversion of input CO2 reactant into bicarbonate, resulting in carbonate deposition within the GDEs2,13,14. Additionally, although some systems have demonstrated direct conversion of CO2 into C3+ hydrocarbons, their FEs remain below 20%, with current densities also typically falling below the industrially necessary level of 1 A cm−2 (refs. 15,16,17).
To overcome these limitations, we developed an approach that utilizes copper phosphide (CuP2) cathodes paired with non-precious-metal anode catalysts in a zero-gap MEA configuration for the CO2RR. With humidified CO2 as the source for CO2 electrolysis, we achieved successful operation at high current densities of up to −1.1 A cm−2 and a high partial current density of 735.4 mA cm−2 with a FE of 66.9% for C3+ products on a 9-cm2 electrode. Using a larger electrode, we obtained a 30-fold increase in yield rate (μmol cm−2 h−1) compared with previous studies2,10,11,15,16,18,19. Analytical techniques, including time-of-flight secondary ion mass spectrometry (ToF-SIM) and in situ Raman spectroscopy, revealed catalyst reconstruction involving copper oxide/hydroxide formation, demonstrating the potential of this approach to advance CO2 reduction technologies.
Results
CO2 electroreduction in zero-gap MEA configuration
The CO2 supply is a critical factor that markedly influences the electrochemical reduction of CO2 (ref. 20). Supplying humidified CO2 is more effective than using its dry gaseous form21. This is because water vapour in the humidified CO2 facilitates the conversion of CO2 into hydrocarbons by improving mass transport and ensuring an adequate supply of reactants at the electrode surface22. Additionally, humidification can enhance ion conductivity and proton availability within the cell, thereby promoting reaction kinetics23,24. This method reduces the thickness of the CO2 diffusion layers, thereby enabling the application of higher current densities. Utilizing humidified CO2 ensures a consistent and abundant supply, optimizes the conditions for efficient electrochemical reduction, and enhances the current density2,20. To minimize the CO2 diffusion layer on the surface of the electrode for high-current operation, humidified CO2 was supplied to the cathode, as depicted in Fig. 1a. This technique enhances CO2 transfer, leading to increased partial current density and FE even at low cell voltages. Moreover, this method makes it possible to achieve a considerably higher concentration of liquid multicarbon products by providing dissolved CO2 with minimal water content25.
a, Schematic illustration of humidified CO2 supply to CO2-to-C3+ systems, including the MEA with an anion-exchange membrane (AEM) and GDEs. The system uses a mass flow controller (MFC) to regulate the CO2 flow at 500 sccm, maintained at 5 °C in a water bath, with product analysis conducted via headspace GC–MS. The anolyte, 1-M KOH, is circulated using a glow pump. b, Chronopotentiometric cell voltage profile using platinum on carbon (Pt/C) as an anode on a 9-cm2 electrode, showing the voltage response to step changes in applied current density at 20, 40, 60, 80, 100, 120, 140, 180 and 200 mA cm−2, measured at room temperature every 10 min. c, Chronopotentiometric cell voltage profile using nickel–iron (NiFe) as an anode on a 9-cm2 electrode, showing the voltage response to step changes in applied current density at 20, 40, 60, 80, 100, 120, 140, 180 and 200 mA cm−2, measured at room temperature every 10 min.
Initially, a CuP2 catalyst airbrushed onto a gas diffusion layer was used, and various anode catalysts were compared (see Methods for detailed procedures)26. In this study, CO2 reduction experiments were conducted using a zero-gap MEA-type cell (Fig. 1a) with an electrode area of 9 cm2. We used chronopotentiometry with a humidified CO2 supply for CO2 reduction. CO2 electrolysis performance was evaluated by analysing the effluent gases and liquid products at room temperature and atmospheric pressure, specifically by calculating the FE values of the C3+ liquid products at various applied currents.
We first assessed the performance of CO2 reduction using the widely recognized Pt/C catalyst and evaluated the performance of the zero-gap MEA-type cell. An experiment was conducted to determine the extent of cell voltage reduction with the inclusion of Pt/C on the anode side (Fig. 1b). We applied current densities (J) of −20 mA cm−2 to −200 mA cm−2 at −20 mA cm−2 intervals, with each stage conducted for over 10 min. The cell voltage remained stable up to −140 mA cm−2; beyond this range, it showed considerable instability with pronounced fluctuations.
Given the low stability, high cell potential and the use of precious-metal Pt/C, numerous researchers are actively working on replacing oxygen evolution reaction (OER) catalysts with non-precious-metal alternatives27,28. Hence, we experimented with non-precious-metal NiFe catalysts, which demonstrated competitive performance compared with Pt/C. The cell voltage when using NiFe was substantially lower than that of Pt/C and remained stable even at −200 mA cm−2 (Fig. 1c). When the type of anode was varied, the cell potential was the highest for Pt/C, followed by NiFe and iridium black (11). Nevertheless, NiFe proved to be the most stable anode, maintaining a constant voltage for 2 h even at an increased current density of −200 mA cm−2.
During our investigation of the CO2RR, most of the products on our catalysts were liquid phase. Consequently, our analysis and subsequent discussions primarily concentrate on the performance of the CO2RR with respect to these liquid-phase products. Pt/C was used as the anode, and a current density of −100 mA cm−2 was applied for a duration of 2 h. Under these conditions, the FEs for the C2+ product spectrum revealed pronounced selectivity towards allyl alcohol, which comprised 48.7% of the total products (Supplementary Table 1). Within the array of C2+ products, the FEs for C3+ products were even more remarkable, exceeding 50% and reaching 56.0%.
When NiFe was used as the anode, the selectivity shifted towards C2 products such as acetaldehyde and ethanol, rather than C3+ products, such as propionaldehyde, propanol, allyl alcohol and butyraldehyde, as shown in Supplementary Table 1. This shift occurred even though the same current density was applied as used with the Pt/C anode. The cell voltage provided by NiFe was insufficient to achieve high selectivity for C3+ products, suggesting that the anode material substantially influences product distribution.
To further investigate this phenomenon, additional experiments using a three-electrode system were conducted to discern the effect of anode potential variations on the working electrode (Supplementary Fig. 2a,b). CuP2 was used as the working electrode, with Pt/C and NiFe as counter-electrodes. The results indicated that the choice of counter-electrode material influenced the reduction current at the working electrode. Specifically, when NiFe was used, the system exhibited a lower overpotential for the OER, leading to a lower cell voltage. This change in cell voltage affected the reduction potential at the cathode, thus altering the CO2RR selectivity. These findings underscore that the nature of the anode catalyst could impact the electrochemical environment at the cathode, subsequently affecting the product distribution.
All performance evaluations were conducted using a two-electrode system with chronopotentiometry, applying a constant current density and measuring the resulting cell voltage. The cell voltage reflects contributions from both the anode and cathode. The difference in OER potentials between Pt/C and NiFe alters the reduction potential at the cathode. NiFe exhibits a lower overpotential than Pt/C in alkaline media (Supplementary Fig. 2c), meaning the cell voltage required for the OER with Pt/C would be higher. This increased anode potential affects the reduction environment at the cathode, influencing the selectivity of the CO2 reduction products. Thus, the observed variation in product selectivity could be attributed to the different OER potentials of the anode catalysts, altering the electrochemical environment.
We also conducted linear sweep voltammetry (LSV) measurements in 0.5-M KHCO3 electrolyte under both argon and CO2 purging conditions, observing lower current densities under CO2 purging (Supplementary Fig. 2d). This suggests that the CO2RR dominate over the HER due to the formation of surface intermediates that inhibit the HER. Despite CuP2’s inherent activity for the HER, as evidenced by the free energy of hydrogen adsorption, the catalyst favours CO2RR in the presence of CO2, with intermediates blocking active sites for the HER and promoting C–C coupling reactions. These results underscore the catalyst’s selectivity towards multicarbon product formation under CO2RR conditions.
Reconstruction of CuP2 catalyst under CO2RR conditions
To delineate the alterations in the cathode catalyst due to CO2 electrolysis, we used various analytical techniques. A ToF-SIMS analysis of the as-prepared CuP2 catalyst (Fig. 2a,b) revealed that the CuP2 surface was predominantly coated with phosphate species26,29. Even in the unreacted catalyst, the high oxygen affinity of phosphorus allows oxygen to permeate, resulting in a superficial phosphate layer.
a,c, ToF-SIMS negative-ion depth profile and IL-SEM of a CuP2 cathode sample before the CO2RR (a) and after the CO2RR (c). All ions (Cu−, P−, O2−, PO− and P2−) are indicated on the profile. b,d, ToF-SIM 3D plots of each ion before the CO2RR (b) and after the CO2RR (d).
A complementary line-profiled scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) analysis, shown in Supplementary Fig. 3a, corroborates the presence of a phosphate layer on the surface. After the CO2RR experiments, a discernible reconstruction of the surface, manifesting as a compositional shift, was observed. The initially thin phosphate layer progressively diminished, and was supplanted by an emerging oxide layer, as shown in Fig. 2d,e by ToF-SIMS analysis. This transition was parallel to the line-profiled SEM-EDS pattern (Supplementary Fig. 3), where oxides became predominant at sites formerly occupied by phosphates. This CO2RR process suggests that the high oxygen affinity of phosphorus catalyses the surface reconstruction of the catalyst.
For a more detailed analysis, we used identical-location scanning electron microscopy (IL-SEM) (Fig. 2c,f). This technique makes it possible to observe the same particles before and after the reactions. A marked increase in the oxygen wt% and a corresponding decrease in the phosphorus wt% postreaction, aligning with earlier findings, substantiated the inferred catalyst modifications and confirmed the occurrence of prominent catalytic changes (Supplementary Table 2).
X-ray photoelectron spectroscopy (XPS) was performed to investigate the chemical bonding on the catalyst surface (Supplementary Fig. 4). The phosphorus 2p spectrum of the as-prepared CuP2 electrode revealed two distinct doublets. The peak at 134.3 eV corresponds to oxidized phosphorus (phosphate), indicating a hydrated phosphate layer on the surface, consistent with previous findings. After the CO2RR, a hydroxylated phosphate layer formed. The peak at 129.7 eV, lower than red phosphorus at 130.2 eV, suggests charge transfer between copper and phosphorus, with phosphorus being negatively charged. Comparing spectra before and after the CO2RR, the intensity of the phosphorus 2p3/2 peak increased, indicating the persistence of phosphide postreaction. Additionally, the oxygen 1s spectra showed a decrease in the P–O interaction peak intensity and an increase in the Cu–O interaction peak intensity after the CO2RR. These changes highlight notable surface chemistry modifications due to the reaction.
Although previous analyses were performed ex situ, leading to concerns about potential surface oxidation postreaction due to air exposure, we investigated surface changes during the CO2RR using in situ Raman spectroscopy. A custom-built cell designed for a three-electrode H-cell configuration (Supplementary Fig. 5) was used for these experiments30. A constant potential of −0.6 VRHE (RHE, reversible hydrogen electrode) was applied for 2 h, and the spectra were sequentially acquired to monitor temporal changes under a CO2-saturated atmosphere.
The initial observations at an open-circuit potential indicated the presence of a phosphate layer on the electrode surface, as shown in Fig. 3. Upon application of the potential, we observed a gradual decrease in the intensity of the peaks associated with Cu3(PO4)2 at specific wavenumbers (1,361, 1,595 cm−1), suggesting the depletion of the phosphate species. Concurrently, peaks corresponding to CuO and CuOx/(OH)y (389–394 cm−1) emerged, indicating the formation of copper oxide and hydroxide species31. This transition in the Raman spectra signifies the ongoing reconstruction of the catalyst surface during the CO2RR. To ensure the reliability of our findings and confirm the reproducibility of the observed surface transformations, Raman spectra were collected from multiple locations (≥3) across all samples and under each potential investigated in this study.
a, Copper oxide region of the reconstructed CuP2 electrode. b, Copper phosphate/phosphide region of the reconstructed CuP2 electrode. The measurements were conducted under a constant potential of −0.6 VRHE, with a total charge of −3.44 C applied for 45 min.
We investigated the electrochemical stability of CuP2 across various pH levels and potential conditions, focusing on the relevant operational range for CO2 reduction reactions—specifically, the stability region for CuP2 within a pH range from neutral to slightly alkaline, which corresponds to the local conditions during the CO2RR as OH− ions are generated, shifting the local pH towards more alkaline conditions. Within this defined stability region, CuP2 remains robust, avoiding considerable degradation or dissolution32. This is corroborated by the formation of stable CuP2 and other protective species, such as phosphate layers, which help mitigate corrosion and maintain the catalyst’s integrity.
Based on our results, we observed that CuP2 initially maintains a stable structure under CO2RR conditions but undergoes surface reconstruction as the reaction progresses. It is important to distinguish between reconstruction and degradation. The high oxophilicity of CuP2 leads to strong interactions with oxygen species, resulting in a gradual transformation of the surface to CuxPyOz (copper phosphate) phases, eventually progressing to copper oxide and finally to metallic copper. Despite this reduction to copper, phosphate groups remain on the surface, actively contributing to the catalyst’s performance and aiding in multicarbon product formation. This surface reconstruction aligns with the behaviour of Cu2O-derived copper catalysts, where the formation of active copper metal sites is beneficial for C2+ product selectivity.
Furthermore, the stability test conducted at a high current density of 500 mA cm−2 for 60 h demonstrated a consistent cell voltage with minimal fluctuations (Supplementary Fig. 6). This consistent voltage profile indicates that the postreconstruction catalyst maintains a robust structure without signs of marked degradation, as any degradation would have led to noticeable voltage instability. The reconstructed, CuP2-derived catalyst, featuring surface-bound phosphate groups, provides a stable and optimal environment for CO2 reduction, differentiating it from conventional copper catalysts that start in their metallic state. This unique reconstruction pathway enhances the catalyst’s ability to promote multicarbon coupling reactions, highlighting the critical role of phosphate groups in stabilizing the surface and maintaining catalytic activity.
Proposed CO2RR pathway for multicarbon product generation
Drawing from the detailed analysis of the conversion ratios in Fig. 5e, and reinforcing the mechanisms discussed in our previous work, we highlight a distinct pathway in CO2 electrolysis, leading to multicarbon products that do not primarily rely on CO intermediates and C–C dimerization. Diverging from the traditional focus on CO dimerization, our findings emphasize a formaldehyde-driven condensation mechanism as the initial step in C–C coupling26,29,33.
To elucidate the role of formaldehyde in the CO2RR, we carried out additional experiments comparing the reduction of CO, CO2 and formaldehyde under similar conditions. These experiments were performed using a microfluidic GDE cell (Supplementary Fig. 7) at a potential of −0.6 V versus RHE. For the reduction of CO and formaldehyde, we used a phosphate buffer solution saturated with argon to exclude CO2 participation. However, for CO2 reduction, a 0.5-M KHCO3 solution saturated with CO2 was used.
As shown in Supplementary Fig. 8, CO reduction did not yield any carbon products, whereas CO2 reduction produced formate, acetaldehyde and butanol, consistent with a previous report26. Formaldehyde reduction led to a slight increase in hydrogen production and a substantial increase in selectivity towards C2+ compounds, specifically acetaldehyde and butanol. Although the product distribution might differ from that in an MEA-type cell, the enhanced selectivity for multicarbon compounds during formaldehyde reduction underscores its crucial role in the C–C coupling process during CO2 reduction. These findings support the hypothesis that formaldehyde is a key intermediate in forming multicarbon products, highlighting its importance in the overall reaction mechanism.
To further explore our proposed mechanism, we outline several tentative steps, detailed in Supplementary Fig. 10: surface protonation, CO2 adsorption, rearrangement of CO2 and formation of formate, formate coverage at the CuP2 surface, and formaldehyde formation and aldehyde formation. The literature suggests that the Gibbs free energy (ΔG) values for these steps may be negative, indicating potential thermodynamic favourability, although this is based on preliminary evidence from previous studies29,34,35,36. At this stage, our working hypothesis for the mechanism is tentatively supported by these observations, but this will require additional experimental and computational investigations to confirm its validity.
As proposed in Supplementary Fig. 11a, in the initial step for CO2− binding, carbon-binding leads to CO formation, which can further go through CO dimerization to produce multicarbon products such as ethylene, ethanol, 1-propanol, etc. Oxygen binding, however, leads to the formation of formate, which is widely accepted as an end product. In the presence of Lewis acid catalysts, formaldehyde self-condensation can be catalysed to form the C2 compound known as glycolaldehyde. Some non-copper catalysts with Lewis acid sites have been proposed for the self-condensation of formaldehyde as a C2+ formation mechanism.
To further investigate the differences in C–C coupling mechanisms and validate the reaction pathways illustrated in Supplementary Fig. 11a, and to demonstrate the related intermediates of formaldehyde condensation shown in Supplementary Fig. 9, we conducted in situ surface-enhanced infrared absorption spectroscopy (SEIRAS) experiments. These experiments aimed to investigate the adsorption behaviour of CO on metallic copper and CuP2 surfaces under applied reduction potentials. As shown in Supplementary Fig. 11c,d, metallic copper exhibited a clear CO adsorption peak (∗COad) under reduction conditions, confirming its role in CO dimerization pathways that lead to multicarbon products. In contrast, CuP2 showed no detectable CO adsorption peaks across the entire range of applied potentials (0.3 VRHE to −1.8 VRHE), indicating a fundamentally different reaction mechanism. These results validate the proposed reaction pathways, where CuP2 avoids the CO-mediated C–C coupling, and instead support alternative routes, such as the formate pathway, for multicarbon product formation. The majority of formate-producing catalysts that require high overpotentials are known to form a curved structure of CO2 during the reaction, which is bound to oxygen atoms on the surface of the catalyst through a proton-coupled electron transfer process. This mechanism is consistent with our previous XPS results, which demonstrate that charge transfer takes place between copper and phosphorus. This suggests that copper has a positive charge and could potentially act as a hydride acceptor in the hydride transfer mechanism (CO2 + H− → HCOO−) producing formate, which is confirmed in Fig. 4a for HCOOad. After HCOOad adsorption, the reaction can proceed through HCOad to form formaldehyde (Fig. 4b). Under mild conditions, it is energetically favourable for two molecules of formaldehyde to undergo self-condensation and form a C–C bond. This reaction has a standard Gibbs free energy of −25 kJ mol−1. During the formaldehyde condensation reaction, OCH3ad (Fig. 4c) appears after the carbon atoms are coupled, and eventually the OC2H5ad (Fig. 4d) intermediate is formed, which is a primary intermediate for acetaldehyde. The onset of the OC2H5ad intermediate starts slightly behind the other intermediates which means it appears after the C–C coupling reaction.
a–d, HCOOad (a), HCOad (b), OCH3ad (c) and OC2H5ad (d) intermediates identified by in situ SEIRAS, related to the formaldehyde condensation reaction as a C–C coupling process. e, Proposed reaction pathways to various products, with colour coding for carbon chain length: C1 (purple/red), C2 (blue) C3 (green), C4 (orange).
In this refined pathway (Fig. 4e), the decreased amount of C2 intermediates steers the reaction towards formaldehyde condensation, yielding acetaldehyde37,38,39,40. This compound, together with formaldehyde, undergoes an aldol condensation reaction facilitated by specialized catalysts to form acrolein, a key intermediate of C3 products. This pathway bifurcates with the hydrogenation of acrolein, resulting in the formation of allyl alcohols and propionaldehydes. Subsequent hydrogenation leads to the synthesis of 1-propanol41,42.
Moreover, in line with the reaction schemes outlined in our earlier studies, the aldol condensation of two acetaldehyde molecules led to the production of butyraldehyde, a C4 compound. This clearly illustrates that the electrochemical conversion of CO2 to multicarbon chemicals involves a complex interplay of reactions, highlighting formaldehyde condensation as a pivotal step in the formation of higher-order carbon products.
By emphasizing the role of formaldehyde in this study, we aimed to refine the understanding of multicarbon product synthesis from CO2 and provide insights that can lead to the development of more efficient catalytic systems for CO2 utilization.
High current density electrochemical CO2 reduction performance
Following on from the electrolysis system described in Fig. 1a, we conducted a series of experiments under current densities of −100 mA cm−2 to −1.1 A cm−2. We monitored the full-cell voltage under a constant current density for 4 h using non-precious-metal catalysts on both the cathode and anode, as illustrated in Fig. 5a. The cell potential remained stable across all current densities, maintaining −3.4 V at −1.1 A cm−2 without any iR correction, as further detailed in Supplementary Fig. 12. Hydrogen was the predominant gaseous product, accompanied by a spectrum of CO2RR liquid products (Fig. 5b and Supplementary Table 3). Notably, allyl alcohol maintained the highest FE of 51.81% at a current density of −1 A cm−2, underscoring its selectivity in our system. Concurrently, we detected the formation of C1 compounds, such as formates, and C2 compounds, such as acetaldehyde and ethanol. The trend for C3 compound formation, specifically propionaldehyde and propanol, was equally promising, and butyraldehyde confirmed the presence of C4 products.
a, Stability of full-cell voltage over time across a range of current densities from −100 mA cm−2 to −1.1 A cm−2. b, FEs for different products at specified current densities. c, Partial current densities for C3 products (allyl alcohol, 1-propanol and propionaldehyde) plotted against total applied current density. d, Selectivity comparison for C1–4, C2 and C3+ products relative to CO2 reduction at different current densities. e, Conversion rate of CO2 into C1–C4 products at varying current densities. f, Comparison of full-cell voltage under no iR correction conditions across different studies including this work, for various current densities. Cal-Cu, cation-augmenting layer modified copper. g, FE of C3+ products against total current density in comparison to other studies. h, Product yield function against electrode area, demonstrating the scalability of the methodology in this work and other studies (detailed in Supplementary Table 4). The FE values are means, and error bars represent the s.d. from three independent measurements (n = 3).
To gain further insights into the cathodic environment and polarization potential at the CuP2 working electrode under these operating conditions, we designed a modified zero-gap three-electrode cell. This set-up incorporated a reference electrode insertion point to directly measure the cathode polarization potential at various applied current densities. Chronopotentiometric experiments revealed a strong correlation between the cathode polarization potential and CO2RR product selectivity. As shown in Supplementary Table 3 and Supplementary Fig. 13, the cathode polarization potential progressively shifted from −0.82 V versus RHE at −100 mA cm−2 to −1.12 V versus RHE at −1.1 A cm−2. This shift in potential directly influenced product distributions, with lower potentials favouring C1 and C2 products and higher potentials promoting C3 and C4 product formation.
A closer analysis of the C3 compounds revealed an escalating partial current density for allyl alcohol, 1-propanol and propionaldehyde, with the current density increasing up to −1.0 A cm−2, as depicted in Fig. 5c. However, a subtle decrease in the quantity of allyl alcohol was observed upon increasing the current density to −1.1 A cm−2. The selectivity trends for the C2 and C3+ products relative to the total CO2 reduction indicated a decline in C2 and an enhancement in C3+ selectivity with increasing current density, as shown in Fig. 5d. This trend suggests a transitional pathway in which C3+ products are derived through a coupling reaction, such as aldol condensation, from C2 intermediates.
Figure 5e shows the relationship between the C2, C3 and C4 products, indicating that as the current density increases, discernible shifts occur from C2 to C3 and from C2 to C4. However, the transition from C3 to C4 (Supplementary Fig. 14) did not exhibit a consistent trend across varying current densities, indirectly substantiating that C3 compounds originate from the coupling of C2 and C1, while C4 compounds arise from C2 coupling reactions rather than from C3 and C1. To further investigate the selectivity of CuP2 in formaldehyde reduction and its role as an intermediate in C–C coupling, isotopic labelling experiments were performed using 13C-labelled formaldehyde in an argon-saturated phosphate buffer solution (Supplementary Fig. 15). 13C NMR analysis revealed distinct peaks corresponding to various C1 and C2+ products, confirming the formation of methanol, acetaldehyde, propionaldehyde, ethanol, butyraldehyde and butanol. Notably, methanol was observed, which is attributed to the spontaneous conversion of formaldehyde under reaction conditions. This analysis provides direct evidence for the role of formaldehyde as a crucial intermediate in multicarbon product formation during the reduction process.
A comparative analysis of the product distributions obtained from formaldehyde reduction (Supplementary Fig. 16a) and the CO2RR (Supplementary Fig. 16b) experiments highlights pronounced differences driven by the reaction microenvironment and variations in local pH influenced by the cell configuration. In the CO2RR experiment using a MEA at high current density, products such as formate, acetaldehyde, ethanol, propionaldehyde, 1-propanol, allyl alcohol and butyraldehyde were detected. In contrast, the formaldehyde reduction experiment conducted at −0.9 V versus RHE in a flow cell predominantly yielded methanol, acetaldehyde, propionaldehyde, ethanol, butyraldehyde and butanol. The confined reaction zone in the MEA cell, with limited electrolyte presence, leads to rapid proton consumption and substantial changes in local pH near the catalyst surface. This environment favours C–C coupling reactions and facilitates the formation of multicarbon products. Conversely, the flow cell set-up, with a spacer creating a thicker electrolyte layer, results in a different local pH profile that stabilizes formaldehyde and promotes its reduction to methanol.
The combined use of headspace gas chromatography–mass spectrometry GC–MS and 13C NMR analysis was crucial in identifying the complete product distribution. Headspace GC–MS (Supplementary Figs. 16a and 17), with its high sensitivity to volatile organic compounds, detected trace-level allyl alcohol, which was not observed in the 13C NMR spectrum due to the latter’s higher detection limit. This highlights the complementary nature of these analytical techniques and underscores the importance of using multiple methods to fully characterize the product profile, especially for trace-level products.
The observed differences in product selectivity emphasize the role of formaldehyde as an active intermediate in C–C coupling reactions. During the CO2RR, formaldehyde is probably formed transiently on the catalyst surface but is rapidly consumed, facilitating the formation of higher-order products such as propionaldehyde and butyraldehyde. In the formaldehyde reduction experiment, however, the initial presence of labelled formaldehyde provided direct evidence of its nucleophilic activity, leading to methanol formation and enabling further C–C coupling to produce multicarbon alcohols. This demonstrates the high selectivity of CuP2 for formaldehyde condensation, validating the proposed reaction mechanism and the catalyst’s effectiveness in promoting C–C bond formation.
These findings illustrate the critical role of the reaction microenvironment and local pH variations influenced by cell configuration in determining product selectivity. The MEA cell configuration, with its confined reaction environment, promotes rapid C–C coupling and favours the formation of higher-order aldehydes and alcohols. In contrast, the flow cell set-up, with a different local pH profile, stabilizes formaldehyde as a key intermediate, promoting its direct reduction to methanol and subsequent multicarbon synthesis. This underscores the importance of formaldehyde in multicarbon product formation and highlights its pivotal role in C–C coupling pathways during CO2RRs.
To address concerns about electro-osmosis at current densities exceeding −1.0 A cm−2, we conducted additional experiments to analyse the anolyte for crossover products postelectrolysis. No products were detected in the anolyte, as detailed in Supplementary Fig. 18, indicating no notable electro-osmosis. Moreover, we monitored cell voltage over time while applying constant currents above −1.0 A cm−2. Figure 5a shows no considerable voltage changes, confirming the absence of oxidation reactions due to crossover products. This stability confirms that our system effectively prevents product loss from electro-osmosis, ensuring reliable CO2 reduction results.
To address concerns about potential anode material contamination affecting CO2RR activity, we conducted several analyses and additional experiments. SEM energy-dispersive X-ray analysis on the cathode postreaction showed no deposition of nickel or iron (Supplementary Fig. 19). Additionally, inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of the catholyte in CO2RR experiments detected no nickel or iron ions (Supplementary Table 5).
To further validate our findings, we performed a model electrolysis experiment using the same NiFe anode and the same anion-exchange membrane, paired with a Pt/C cathode. We applied constant current densities of 1.1 A cm−2 and 3.0 A cm−2 for 4 h, periodically sampling the catholyte for any potential nickel or iron contamination (Supplementary Table 6).
The results of SEM energy-dispersive X-ray and ICP-OES analyses consistently showed no evidence of nickel or iron contamination on the cathode or in the catholyte. These findings confirm that the observed differences in CO2RR activity are attributed to intrinsic catalytic properties and reaction conditions, rather than anode material contamination. This supports our claim that different anode materials result in different cell voltages, influencing the overall CO2RR activity without cross-contamination effects.
As delineated in Fig. 5f, our findings, particularly at a high current density of −1.1 A cm−2, showcased the lowest observed cell potential, outperforming even the Pt/C catalysts at equivalent current densities. Furthermore, when juxtaposed with other studies focusing on C3+ product yields10,11,15,16,18 (Fig. 5g), our system exhibited remarkable selectivity for C3+ products, showing an impressive FE of 66.9%.
Theoretically, an increased electrode area should proportionally increase the yield of conversion products. However, in practice, upscaling the electrode area can introduce challenges such as increased surface resistance. Nonetheless, our research demonstrates that the strategic enlargement of the electrode area can substantially enhance the yield rate, as evidenced by the production volume exceeding that of other studies by more than 30 times (Fig. 5h). This reinforces the notion that optimizing the electrode microenvironment for CO2 participation can markedly improve the efficiency of CO2RRs.
Conclusions
Our comprehensive study illuminates a series of pathways for the electrochemical reduction of CO2 to multicarbon compounds, leveraging non-precious-metal catalysts to optimize efficiency and selectivity. The experiments conducted not only demonstrated a stable cell potential across a wide range of current densities but also highlighted the robust selectivity for valuable C3+ compounds, particularly allyl alcohol, which achieved a FE exceeding 50%. These results, situated at the forefront of CO2RR research, mark a step towards the sustainable production of chemicals.
In situ Raman spectroscopy revealed the dynamic surface transformations of the catalyst during the CO2RR, offering a deeper understanding of the mechanisms at play. The emergence of copper oxides and hydroxides during the reaction provided clear evidence of the structural evolution of the catalyst, essential for guiding future catalyst designs. Our findings also shed light on the crucial intermediate role of C2 compounds and the importance of aldol condensation reactions in the formation of higher-order alcohols and aldehydes. This insight underlines the complexity of the CO2RR process and its potential to be harnessed for the targeted synthesis of specific products.
Additionally, in situ SEIRAS experiments were conducted to observe the intermediate species and support the reaction pathway. The SEIRAS results showed the formation of formate and subsequent formaldehyde on the catalyst surface, consistent with our proposed mechanism and highlighting the importance of these intermediates in the C–C coupling process.
Furthermore, our study challenges the traditional constraints associated with electrode area scaling. By strategically enhancing the electrode area, we achieved a remarkable increase in the product yield rate, outperforming existing benchmarks by approximately 30-fold in terms of yield rate (μmol cm−2 h−1), as detailed in Supplementary Table 4, which compares our results with previous studies2,10,11,15,16,18,19.This not only confirms the viability of scaling up the CO2RR but also emphasizes the importance of optimizing the electrode design to maximize reactant participation. This study marks an advancement in the electrochemical conversion of CO2, providing a viable route to high-value multicarbon compounds with high selectivity and efficiency. Our methodology provides guidance for the development of scalable, sustainable and economically feasible CO2RR technologies. The approach achieved a partial current density of 735.4 mA cm−2 and a FE of 66.9% for C3+ products, notably improving yield rates by approximately 30-fold compared with existing benchmarks (Supplementary Table 4), demonstrating a robust step forward in CO2 reduction technologies.
Methods
Electrode preparation
The cathode catalysts were synthesized according to the method described in our previous study26. The catalyst ink was composed of 27 mg of the synthesized catalyst, 27 μl of an ionomer solution (PiperION Dispersion; 5 wt%) and 7,860 mg of isopropyl alcohol. The ink was thoroughly dispersed, and then, using N2 as the carrier gas, was airbrushed onto a 3 cm × 3 cm gas diffusion layer (22BB, Sigracet). For the anode catalyst, nickel nitrate (Ni(NO3)2·6H2O; 99.999%; Sigma-Aldrich) and iron chloride (FeCl2·4H2O; 99.99%; Sigma-Aldrich) were utilized in their as-received forms for the electrodeposition process. A 0.1-M solution of metal precursors was prepared by dissolving these salts in distilled water. The Ni2+ and Fe2+ compositions of the electrolytes were maintained at an optimized ratio of 9:1. The working electrode, consisting of stainless steel fibre paper (Bekipor ST 40BL3, Bekaert), was subjected to a constant current of −2.5 mA cm−2. A carbon cloth was used as the counter-electrode. The deposition time was set to 7,200 s to achieve optimal performance.
Surface analysis methods
We analysed the catalyst surface by field emission SEM (Verios 5 UC, Thermo Fisher) to observe the morphological characteristics. The distribution of each element in the individual particles was analysed by EDS (Oxford Ultim Max 65, Oxford Instruments) for element line profile and mapping. We utilized ICP-OES (Optima 4300 DV, PerkinElmer) to measure metal ions leached into the electrolyte. XPS (NEXSA, Thermo Fisher Scientific) was used to analyse changes in the electrode before and after the experiments. For IL-SEM, catalyst was drop-cast onto gold transmission electron microscopy grids for use as a working electrode.
Raman spectroscopy measurements were conducted using a Horiba Xplora microscope situated within a custom-built PEEK cell, as depicted in Fig. 5b. Observations were made using a 60× water immersion lens (LUMPLFLN60XW). Calibration of the Raman frequency was achieved with a silicon wafer reference (520.6 cm−1). The experimental set-up included a 532-nm laser and an 1,800 lines mm−1 grating monochromator for light dispersion. Signal detection was performed with a 10-s integration time over 10 cumulative scans. Electrochemical measurements utilized a platinum coil as the counter-electrode and featured Ag/AgCl as the reference and catalyst-coated gold substrates as the working electrodes.
Electrochemical experiments
All electrochemical CO2RR experiments were performed in a zero-gap cell. An anode and cathode with geometric areas of 9 cm2 were placed between two current collectors; CuP2 was used as the cathode catalyst, and Pt/C (1 mg cm−2, 46.7%, TKK) airbrushed on a gas diffusion layer (MGL190, AvCarb) was used as the anode. An anion-exchange membrane (PiperION 20 nm, Versogen) was used after activation in 1-M KOH for 24 h. Gold-coated titanium single serpentine flow fields were used as the cathode and anode. To prevent leakage of the electrolyte and minimize the resistance in the MEA, a rubber gasket (630 μm) was utilized. All the components were assembled within an electrolyser, with a torque wrench being used to secure the screws to a tightness of 60 N·m. The anode was supplied with 1-M KOH electrolyte (at room temperature) at a flow rate of 18 ml min−1, and humidified CO2 (60 °C, with 100% relative humidity) was supplied to the cathode at a flow rate of 500 sccm. A chiller, circulating water at 5 °C, was connected to the outlet of the cathode to liquefy the vaporized products. We applied constant currents of −100 mA cm−2 to −1.1 A cm−2 for 4 h using a potentiostat/galvanostat (PGSTAT30, Autolab), for the CO2 reduction experiments. The liquid-phase products were quantified using headspace GC‒MS (Agilent Technologies). Subsequently, the FE values of each product were calculated.
Data availability
Data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Daehn, K. et al. Innovations to decarbonize materials industries. Nat. Rev. Mater. 7, 275–294 (2021).
Qi, K. et al. Unlocking direct CO2 electrolysis to C3 products via electrolyte supersaturation. Nat. Catal. 6, 319–331 (2023).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Alabdullah, M. et al. One-step conversion of crude oil to light olefins using a multi-zone reactor. Nat. Catal. 4, 233–241 (2021).
Lee, S., Choi, M. & Lee, J. Looking back and looking ahead in electrochemical reduction of CO2. Chem. Rec. 20, 89–101 (2020).
Park, G., Hong, S., Choi, M., Lee, S. & Lee, J. Au on highly hydrophobic carbon substrate for improved selective CO production from CO2 in gas-phase electrolytic cell. Catal. Today 355, 340–346 (2020).
Choi, M. et al. Syngas production for Fischer–Tropsch process via co-electrolytic processes of CO2 reduction and NH3 oxidation. Chem. Eng. J. https://doi.org/10.1016/j.cej.2021.132563 (2022).
Creel, E. B. & McCloskey, B. D. Scalable CO2-to-oxygenate production. Nat. Catal. 1, 6–7 (2018).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Garcia D. A. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science https://doi.org/10.1126/science.aay4217 (2020).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Chen, Y. et al. Efficient multicarbon formation in acidic CO2 reduction via tandem electrocatalysis. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01543-8 (2023).
Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Wen, G. et al. Continuous CO2 electrolysis using a CO2 exsolution-induced flow cell. Nat. Energy 7, 978–988 (2022).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Miao, R. K. et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule 5, 2742–2753 (2021).
Lee, M. G. et al. Selective synthesis of butane from carbon monoxide using cascade electrolysis and thermocatalysis at ambient conditions. Nat. Catal. 6, 310–318 (2023).
Xu, Y. et al. Self-cleaning CO2 reduction systems: unsteady electrochemical forcing enables stability. ACS Energy Lett. 6, 809–815 (2021).
Zhang, R. et al. Synthesis of n-propanol from CO2 electroreduction on bicontinuous Cu2O/Cu nanodomains. Angew. Chem. Int. Ed. 63, e202405733 (2024).
She, X. et al. Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A. Nat. Energy 9, 81–91 (2024).
Lee, W., Kim, Y. E., Youn, M. H., Jeong, S. K. & Park, K. T. Catholyte-free electrocatalytic CO2 reduction to formate. Angew. Chem. Int. Ed. 57, 6883–6887 (2018).
Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 12, 136 (2021).
Vennekötter, J.-B., Scheuermann, T., Sengpiel, R. & Wessling, M. The electrolyte matters: stable systems for high rate electrochemical CO2 reduction. J. CO2 Util. 32, 202–213 (2019).
Lee, J. et al. Catholyte-free electroreduction of CO2 for sustainable production of CO: concept, process development, techno-economic analysis, and CO2 reduction assessment. Green Chem. 23, 2397–2410 (2021).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Choi, M., Bong, S., Kim, J. W. & Lee, J. Formation of 1-butanol from CO2 without *CO dimerization on a phosphorus-rich copper cathode. ACS Energy Lett. 6, 2090–2095 (2021).
Seger, B., Robert, M. & Jiao, F. Best practices for electrochemical reduction of carbon dioxide. Nat. Sustain. 6, 236–238 (2023).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Calvinho, K. U. D. et al. Selective CO2 reduction to C3 and C4 oxyhydrocarbons on nickel phosphides at overpotentials as low as 10 mV. Energy Environ. Sci. 11, 2550–2559 (2018).
Kang, S. et al. Durable nickel–iron (Oxy)hydroxide oxygen evolution electrocatalysts through surface functionalization with tetraphenylporphyrin. Angew. Chem. Int. Ed. 61, e202214541 (2022).
Cheng, C. et al. Amorphous Sn(HPO4)2-derived phosphorus-modified Sn/SnO core/shell catalyst for efficient CO2 electroreduction to formate. J. Energy Chem. 81, 125–131 (2023).
Bale, C. W. et al. FactSage thermochemical software and databases, 2010–2016. Calphad 54, 35–53 (2016).
Delmo, E. P. et al. The role of glyoxal as an intermediate in the electrochemical CO2 reduction reaction on copper. J. Phys. Chem. C 127, 4496–4510 (2023).
David, A. K. et al. Combined computational and experimental investigation on the mechanism of CO2 hydrogenation to methanol with Mn-PNP-pincer catalysts. ACS Catal. 12, 15310–15322 (2022).
Shyama, C. M. et al. A computational study on ligand assisted vs. ligand participation mechanisms for CO2 hydrogenation: importance of bifunctional ligand based catalysts. Phys. Chem. Chem. Phys. 21, 3932–3941 (2019).
Bhaskar, M. et al. Toward rational design of 3d transition metal catalysts for CO2 hydrogenation based on insights into hydricity-controlled rate-determining steps. Inorg. Chem. 55, 5438–5444 (2016).
Tang, M. T., Peng, H.-J., Stenlid, J. H. & Abild-Pedersen, F. Exploring trends on coupling mechanisms toward C3 product formation in CO2R. J. Phys. Chem. C 125, 26437–26447 (2021).
Cronin, S. P. et al. Direct synthesis of 1-butanol with high Faradaic efficiency from CO2 utilizing cascade catalysis at a Ni-enhanced (Cr2O3)3Ga2O3 electrocatalyst. J. Am. Chem. Soc. 145, 6762–6772 (2023).
Li, Z. & Wu, J. Co-electrolysis of CO2 and formaldehyde to ethanol: an inspiring asymmetric C–C coupling pathway. Chem Catal. 2, 2808–2810 (2022).
Yan, T. et al. Multiscale CO2 electrocatalysis to C2+ products: reaction mechanisms, catalyst design, and device fabrication. Chem. Rev. 123, 10530–10583 (2023).
Liu, L., Ye, X. P. & Bozell, J. J. A comparative review of petroleum-based and bio-based acrolein production. ChemSusChem 5, 1162–1180 (2012).
Lan, X., Wang, T., Li, X., Huang, N. & Wang, J. Pt@SnOx/SiO2 catalysts with enhanced selectivity to allyl alcohol for acrolein hydrogenation. Catal. Sci. Tech. 6, 7703–7707 (2016).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021K1A4A8A01079455). This research was also supported by the NRF, funded by MSIT (RS-2021-NR060081).
Author information
Authors and Affiliations
Contributions
J.L. supervised the project. M.C. conceived the overall idea and structure of the study, designed and performed critical experiments (cathode electrocatalyst fabrication, activity/stability evaluation, in situ Raman and IR spectroscopy, isotope-labelling experiments), interpreted the data regarding mechanistic origins, and wrote the manuscript. S.B. created the experimental design of the zero-gap full-cell configuration with the reference electrode, provided comparable polarization curves between the half-cell and the full cell, and contributed NMR/XPS/ICP-OES analysis. Y.K. provided homemade the NiFe anode and carried out ICP-OES analysis. Y.L. performed near zero-gap cell experiments and carried out headspace analysis of liquid products. M.C. fabricated the CuP2 cathode electrocatalyst. S.K. contributed to the experimental design of the NiFe anode and to in situ Raman spectroscopy. All authors discussed experimental observations throughout the entire submission process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–19 and Tables 1–5.
Source data
Source Data Fig. 1–5
A single file containing all source data, with clearly named tabs for each Figure/Extended Data Figure item.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, M., Bae, S., Kim, Y. et al. Selective formaldehyde condensation on phosphorus-rich copper catalyst to produce liquid C3+ chemicals in electrocatalytic CO2 reduction. Nat Catal 8, 476–486 (2025). https://doi.org/10.1038/s41929-025-01341-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01341-6
This article is cited by
-
A formaldehyde condensation pathway in CO2 electrolysis
Nature Catalysis (2025)