Abstract
Preclinical and clinical studies suggest that lipid-induced hepatic insulin resistance is a primary defect that predisposes to dysfunction in islets, implicating a perturbed liver-pancreas axis underlying the comorbidity of T2DM and MASLD. To investigate this hypothesis, we developed a human biomimetic microphysiological system (MPS) coupling our vascularized liver acinus MPS (vLAMPS) with pancreatic islet MPS (PANIS) enabling MASLD progression and islet dysfunction to be assessed. The modular design of this system (vLAMPS-PANIS) allows intra-organ and inter-organ dysregulation to be deconvoluted. When compared to normal fasting (NF) conditions, under early metabolic syndrome (EMS) conditions, the standalone vLAMPS exhibited characteristics of early stage MASLD, while no significant differences were observed in the standalone PANIS. In contrast, with EMS, the coupled vLAMPS-PANIS exhibited a perturbed islet-specific secretome and a significantly dysregulated glucose stimulated insulin secretion response implicating direct signaling from the dysregulated liver acinus to the islets. Correlations between several pairs of a vLAMPS-derived and a PANIS-derived factors were significantly altered under EMS, as compared to NF conditions, mechanistically connecting MASLD and T2DM associated hepatic-factors with islet-derived GLP-1 synthesis and regulation. Since vLAMPS-PANIS is compatible with patient-specific iPSCs, this platform represents an important step towards addressing patient heterogeneity, identifying disease mechanisms, and advancing precision medicine.
Similar content being viewed by others
Introduction
Over 30 million Americans have type 2 diabetes mellitus (T2DM), constituting about 9.4% of the adult population, and about 84 million additional adult Americans have pre-diabetes1, amounting to an economic cost of $322 billion annually 2. Therefore, there is an unmet need for a better mechanistic understanding of T2DM pathophysiology coupled to the development of efficacious and safe treatments to improve patient outcomes. The underlying cause of all forms of diabetes is an inadequate insulin secretion relative to the metabolic needs. The pathogenesis of T2DM is complex and heterogeneous involving multiple tissues with preceding insulin resistance, in the liver, white adipose tissue, skeletal muscle and pancreatic β-cells3. Evidence from human and other model organism studies have demonstrated the critical importance of organ crosstalk including the role of myokines4,5,6,7,8,9, adipokines9,10,11,12,13,14, hepatokines15,16,17,18,19 and cytokines from immune cells20,21. In parallel with the increase in the incidence of T2DM, there is over a 70% comorbidity between T2DM and metabolic-dysfunction associated steatotic liver disease (MASLD), and it has been determined that patients with MASLD exhibit a higher risk of T2DM than patients without MASLD, suggesting a causal relationship between MASLD and T2DM in some patients22,23,24. While there are several approved and moderately valuable therapies for T2DM, currently the Food and Drug Administration (FDA) has approved only one drug (resmetirom; RezdiffraTM) for the treatment of MASLD25. However, resmetirom and many previously failed MASLD drugs have exhibited only up to 25% efficacy in general patient populations25. It has been suggested that this is due to the complex pathogenesis of T2DM and MASLD involving factors including genetics, environment, and lifestyle resulting in inter-patient heterogeneity 24,26. Therefore, a precision medicine approach is required for addressing MASLD-T2DM complexity and heterogeneity.
T2DM is characterized by fasting hyperglycemia and an inability to clear the glucose load that stems from an inadequate insulin release from the pancreatic islet β-cells and inappropriately high glucagon release from the pancreatic islet α-cells. This leads to an increase in gluconeogenesis and de novo lipogenesis from the increasingly insulin resistant and steatotic hepatocytes in the liver. The progression of MASLD leads to increased production of several hepatokines, that worsen insulin resistance27,28,29,30 and, along with the resulting hyperglycemia and increased serum free fatty acids (FFA), cause glucolipotoxicity that worsens β-cell function, in a vicious cycle. Thus, crosstalk between the liver and the islets is critical to the onset and perpetuation of T2DM.
MASLD and T2DM patients exhibit extensive heterogeneity in genotypes and phenotypes that includes the expression of genes that are linked to accelerated disease progression and severity, as well as environment and lifestyle characteristics that also impact disease progression and severity 24. To date, many rodent models have been developed and tested but do not reflect the heterogeneity of either MASLD or T2DM, or their comorbidity31,32,33,34. Simple human cell in vitro models do not recapitulate the complex disease due to the absence of important cell-cell and cell-matrix interactions35 and limit translatability from model organism to humans. Therefore, it is necessary to implement more complex in vitro systems to study human physiology and pathophysiology, as well as disease progression that allow for the study of organ crosstalk and interaction. The development of human liver36,37,38,39,40,41,42 pancreatic islets43,44,45,46, and coupled47,48,49,50 biomimetic microphysiology systems (MPS) that can reflect the genomic, as well as environment and lifestyle heterogeneity offer an important opportunity to recapitulate the heterogeneity of disease.
The use of a combined vascularized liver acinus MPS (vLAMPS)36,51 and pancreatic islet MPS (PANIS)46 could have an enormous impact on recapitulating many aspects of the liver-pancreatic islet axis. In addition to known insulin resistant liver-derived effectors that can induce β-cell hyperplasia26,30,52, we hypothesize that there exists a complementary set of factors derived from the progression to an insulin resistant liver that can potentiate β-cell dysfunction in the context of hyperglycemia and hyperinsulinemia. In this study, we tested our hypothesis by comparing results between stand-alone and physically coupled primary cell-based vLAMPS and PANIS to demonstrate the role of liver-factors influencing pancreatic islets dysfunction. We have harnessed cells from patient-derived cryopreserved cell lots for the liver and cadaver pancreatic islets. We recapitulated the environment and lifestyle phenotypes by progressing the models with media designed to mimic clinical blood chemistries starting with normal fasting (NF) and early metabolic syndrome (EMS) to drive early stage MASLD that can be an optimal starting point for testing therapeutics for early stage. The use of primary cells is important to first establish an adult model system. In parallel, we are developing a patient-derived model based on iPSCs that are differentiated into key liver and pancreatic islets cells that will be used in applications of precision medicine. We further demonstrated the importance of quantifying the reproducibility of the model system so that clinically relevant variations (heterogeneity) can be properly identified, including responses to therapeutics53,54,55,56.
Results
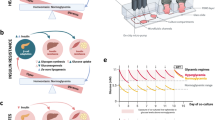
To address the hypothesis that the early stage MASLD liver has the potential to directly induce pancreatic islet dysfunction, a modular human structured biomimetic of the liver-pancreas axis was designed and implemented (Fig. 1). The overall approach involves a comparative functional characterization of the standalone hepatic (vLAMPS)36 and islet (PANIS)46 modules in relation to the physically coupled MPS (vLAMPS-PANIS) under both normal fasting (NF) and early metabolic syndrome (EMS) conditions (Table S1) using several diverse clinically relevant and reproducible metrics57(Table 1).
A Hypothesis: early metabolic syndrome (EMS) medium-induced MASLD secretome directly promotes early islet dysfunction. B Dynamic metabolic dyshomeostasis of the liver and pancreatic-islets associated with disease progression. C Schematic (see Figure S1c for actual photograph) depicting the modular two-organ coupled vLAMPS-PANIS chip. The hepatic and islet modules can also be used in an uncoupled standalone configuration (Figure S1A, B) to enable both intra- and inter-organ functional characterization to be deconvolved under normal (i.e., NF) and disease conditions. The hepatic influx medium composition determines a normal fasting (NF) or EMS state and its continuous flow among the self-assembled multiple cell types (see Methods and Results) can be monitored for biomarkers that complement in situ measurements.
Characterization of standalone vLAMPS and PANIS modules
The architecture of the vLAMPS is composed of a hepatic channel (chamber) and a vascular channel separated by a porous membrane, that allows communication between these channels. The hepatic chamber is assembled with primary human hepatocytes and human cell lines for stellate and Kupffer-like cells seeded in a biomimetic arrangement on the apical side of the middle layer oval. The basolateral side of the middle layer membrane is seeded with liver sinusoidal endothelial cells (LSECs). All together recapitulates the 3D structure of the liver acinus36 (Fig. S1A). Structural features within the vLAMPS that could impact the composition of the hepatic secretome include the space of Disse that mediates non autonomous cell signaling involving in part the liver extracellular matrix (LECM)36,58 (Fig. S1A) and the continuous oxygen zonation ranging from 17%-6% over three zones (Fig. S2A-G) that modulates oxidative phosphorylation, metabolism, and gene expression59. The modeling of the oxygen tension of the vLAMPS determined a continuous oxygen tension of 1.7 × 10−1 mol m−3 from inlet to outlet at the hepatic chamber, and oxygen gradient from 1.7 × 10−1 to 6 × 10−2 mol m−3 at the hepatocytes level (Fig. S2A–C). The oxygen gradient at hepatocytes level was experimentally validated with oxygen sensitive beads36 (Fig. S2D–G) in the context of a uniform distribution of hepatocytes and LSECs (Fig. S2H and S2I) whose oxygen consumption creates the gradient. In addition, we show that steatosis increases across the zones, consistent with the physiologically elevated de novo lipogenesis in zone 357,60 (Fig. S2J and S2K). Basic hepatocyte function assessed by albumin and urea synthesis and secretion is evident under both NF and EMS conditions (Fig. S3A, B) over the 8-day time course, and no appreciable progressive plasma membrane damage is observed as determined by a lactate dehydrogenase (LDH) release assay (Fig. S3C). The NF and EMS media conditions were previously shown to recapitulate normal fasting and early stage MASLD in LAMPS (liver acinus microphysiology system) using a panel of clinically relevant MASLD progression metrics that includes hepatic steatosis, immune activation, stellate cell activation, and the production of pro-fibrotic markers57. We now show in vLAMPS that EMS induces early stage MASLD using a more comprehensive panel of metrics. Relative to perfusion under NF conditions, perfusion with EMS in the standalone vLAMPS showed an increase in steatosis (Fig. 2A, B) and a decrease in insulin receptor substrate 2 (IRS-2) expression (Fig. 2C, D) on day 8, indicative of impaired insulin signaling61. However, no significant difference in α-SMA expression (Fig. 2E, F) by immunofluorescence image analysis was observed suggesting that activation of quiescent stellate cells to proliferative fibrogenic myofibroblasts was not fully induced by EMS perfusion. This is consistent with no significant increase in interstitial collagen (COL1A1) in the secretome (Fig. 2F), although the overall trend in COL1A1 secretion shows an increasing trend in EMS medium (Fig. S4). Interleukin 6 (IL-6) (Fig. 2B) and Granulocyte-macrophage colony-stimulating factor (GM-CSF) (Fig. 2F) showed significant increases under EMS perfusion compared to NF perfusion, indicating progression towards the pro-inflammatory state. As expected, the islet-specific secreted factors, amylin, C-peptide, glucagon, GLP-1, and pancreatic polypeptide (PP), were not detected in the standalone vLAMPS secretome (Fig. S4). Together these results suggest that an eight-day EMS perfusion in the standalone vLAMPS results in steatosis, impaired insulin signaling, and an altered proinflammatory secretome with no appreciable fibrogenesis, consistent with the early stages of MASLD progression. These metrics were reproducible across independent studies (Table 1) enabling biological differences at different times and/or under different perfusion conditions to be discerned.
A Representative immunofluorescence images of lipid accumulation (LipidTOX) in hepatocytes under NF and EMS conditions and B LipidTOX quantification in conjunction with secreted proinflammatory cytokines and chemokines. C Representative immunofluorescence images of Insulin receptor susbtrate-2 (IRS-2) in hepatocytes under NF and EMS conditions and D IRS-2 quantification in conjunction with an increase in hepatokine secretion. E Representative immunofluorescence images of stellate cell activation (α-SMA) under NF and EMS conditions and F α-SMA quantification in conjunction with secreted fibrotic and proinflammatory factors. Scale bar, 100 µm. Scatter plot graphs display the mean and standard error (SEM) of the EMS values normalized to NF for each of the metrics (n = 10 MPS). (see Fig. S4). Points on the graph represent individual chips. Statistical analysis by Wilcoxon signed-rank test of normalized EMS values to their corresponding NF study.
The seeding of cadaver-derived islets onto the porous PET membrane (Figs. 1C and S1B) and encapsulation within polymerized alginate hydrogel to maintain their 3-dimensional structure and function over a 2-week period in the assembled PANIS have been described46 and detailed in the Methods. We compared the ability of the chemically defined NF in PANIS to maintain islet phenotype and function with the proprietary Prodo Labs medium PIM(R) (Fig. S5). No significant difference between viability of C-peptide or glucagon expressing cells was observed (Fig. S5D). The ratio of insulin secreted per islet at 16 mM to 3 mM glucose is the insulin stimulation index (ISI), which is a critical test of islet function. The amount of insulin secreted per islet was higher in NF versus PIM(R) under both low and high glucose conditions (Fig. S5E), however no significant difference in the insulin stimulation index between the two media (Fig. S5F) was apparent. We then compared the structure and function of the islets in the standalone PANIS under NF and EMS conditions (Fig. 3). There was no significant difference in cell viability between NF and EMS media (Fig. 3A, B). Consistent with this result, immunofluorescence imaging showed no difference between the two conditions in the relative fraction of C-peptide expressing β-cells to glucagon-expressing α-cells (Fig. 3C, D). Clinically, reactive oxygen species (ROS) are associated pathophysiologically with oxidative stress and physiologically with homeostatic regulation of metabolic processes that include insulin secretion in islets62,63. DCFDA-based fluorescence staining for ROS showed no significant difference between the two perfusion conditions (Fig. 3E, F). Furthermore, no significant differences in the secretion of islet-specific factors were observed between the two conditions (Figs. 3G and S6). To evaluate islet insulin response, islets were retrieved on day 8 and analyzed for glucose stimulated insulin secretion (GSIS) under 3 mM and 16 mM glucose. Under both NF and EMS perfusion conditions, there was a healthy GSIS response (Fig. 3H) with only a trend towards a lower insulin stimulation index under EMS versus NF conditions (Fig. 3I and Table S2). Thus, in contrast to changes induced by EMS compared to NF in the standalone vLAMPS, significant media induced changes in the standalone PANIS were not evident in EMS indicating that perfusion with this medium alone for 8 days was not sufficient to drive islet dysfunction. We next addressed whether the EMS-induced changes in vLAMPS steatosis, insulin signaling and its secretome could promote islet dysfunction in the physically coupled vLAMPS-PANIS.
A Representative live/dead assay image for islets cultured in NF and EMS media. B Percentage of live cell shows no significant difference between NF and EMS (n = 10 islets and n = 7 islets). C Immunofluorescence labeling for C-peptide expressing β-cells and glucagon expressing α-cells for islets cultured in NF and EMS. D The fraction of quantified C-peptide and glucagon positive cells shows no significant difference (n = 8 islets and n = 10 islets). E Representative images showing mitochondrial associated ROS immunofluorescence labeling of islets. F Percentage of ROS intensity quantification with respect to a positive control (50 µM TBHP) for islets cultured in NF and EMS media (see Methods) demonstrates no significant difference between NF and EMS (n = 8 islets and n = 8 islets). G Standalone PANIS efflux analysis showed the change in islet-derived factors in EMS normalized to NF (see Fig. S6), with no significant difference between them (n = 5 MPS). H Glucose stimulated insulin secretion (GSIS) assay, where islet samples were subjected to low (3 mM), and subsequent high (16 mM) glucose concentration and the resulting secreted insulin was measured and showed no significant difference at either glucose concentration in islets that were maintained in either NF or EMS media (n = 4 islets and n = 3 islets). I Comparison of insulin stimulation index secretion for islets cultured in NF and EMS showing no statistically significant difference. Scatter plot graphs display the mean and standard error (SEM) with p-values shown. For graphs -B, D, F, H, and I- statistical analysis was done by two-tail t test with Welch’s correction. For graph -G- Statistical analysis was done by Wilcoxon signed-rank test of normalized EMS values to their corresponding NF study. All scale bar, 50 µm.
Characterization of the coupled vLAMPS-PANIS
The physical coupling of vLAMPS and PANIS is described in the Methods and configured with the hepatic chamber efflux of the vLAMPS serving as the influx for the PANIS (Figs. 1C, S1C). Similar to the standalone vLAMPS, the basic hepatocyte function of albumin and urea synthesis and secretion is evident under both NF and EMS conditions (Fig. S7A and S7B). Over the 8-day time course, no appreciable progressive plasma membrane damage is observed as determined by an LDH release assay (Fig. S7C). Analysis of diverse secretome measurements across independent studies for both NF and EMS conditions showed overall acceptable to excellent reproducibility of the metrics (Table 1) enabling biological differences at different times and/or under different perfusion conditions to be discerned. Consistent with the results of the image quantification from the standalone vLAMPS, an increase in steatosis (LipidTOX) and a decrease in IRS-2 expression by EMS relative to perfusion with NF are seen (Fig. 4A, B), with the latter indicative of impaired insulin signaling being induced in the vLAMPS module. Consistent with Fig. 2, no significant increase in vLAMPS α-SMA quantification was evident; however, an EMS induced increase in secreted COL1A1 in the coupled efflux suggested a trend towards systemic fibrogenesis, consistent with the early stages of MASLD progression (Fig. 4A, B). Analogous to the standalone PANIS, the viability quantification of the islet cells remained constant between NF and EMS and no significant change in the relative distribution of β- to α-cells suggesting that in the coupled vLAMPS-PANIS the islets were structurally intact with viable cells under both perfusion conditions (Fig. 4C–E and Table S2). By carrying out the coupling from the oxygen-rich hepatic chamber efflux (Supplementary Fig. S2B, C), we ensure that the oxygen tension of the coupled PANIS was comparable to standalone. Although there was no significant difference in the calculated ROS levels between NF and EMS conditions (Fig. 4F), as observed in the standalone PANIS (Fig. 3F), the average levels of ROS were 2-fold higher in the coupled system suggesting that hepatic factors from the vLAMPS module increased ROS levels63,64,65 in the PANIS module (Fig. S8 and Table S2). Coupling potentiated an EMS-dependent significant increase in the levels of the islet-specific secreted factors, amylin and pancreatic polypeptide when normalized to NF (Fig. 4G). The EMS to NF ratio of the same factors is higher in the coupled system compared to the standalone (Table S2). Under coupled NF conditions, a relatively lower secretion of insulin per islet was observed at 3 mM glucose compared to EMS, and under EMS conditions a trend toward lower insulin was observed at 16 mM glucose compared to NF (Fig. 4H and Table S2). In contrast to the standalone PANIS (Fig. 3H, I), the coupled system exhibited a highly significant reduction in glucose stimulated insulin secretion index under EMS versus NF perfusion conditions (Fig. 4I and Table S2). These results are consistent with the hypothesis that a healthy liver under NF conditions may impart homeostatic islet regulation to prevent hypoglycemia whereas a steatotic MASLD liver with impaired insulin signaling may predispose the islets towards a diminished capacity to secrete insulin at higher glucose. Corroborating these observations under NF conditions and consistent with this hypothesis, C-peptide secretion in the coupled vLAMPS-PANIS under NF conditions was two-fold lower than its secretion in the standalone PANIS while no difference due to coupling under EMS conditions was observed (compare Figs. S6 and S9).
A Representative images demonstrating the effect of EMS medium on vLAMPS indicated by markers of steatosis (LipidTOX), insulin signaling (IRS-2), and fibrosis (α-SMA) in hepatocytes. Scale bar, 100 µm. B Corresponding quantitation of hepatic EMS induced steatosis, decreased insulin signaling, and increasing fibrosis (red). Along with vLAMPS-derived EMS secreted factors (from the coupled efflux) normalized to complementary NF chips (n = 12 MPS). (reproducible, Table 1). C Representative images of the PANIS live/dead assay, immunofluorescence labeling for C-peptide (green) expressing β-cells and glucagon (red) expressing α-cells, and ROS oxidative stress for coupled islets cultured in NF and EMS (n = 10 islets and n = 7 islets). All scale bar, 50 µm. D Percentage of live cell of the islets shows no statical difference between conditions. E No statistically significant difference between the fraction of quantified C-peptide and glucagon positive cells in the islets (n = 7 islets and n = 10 islets). F Percentage of ROS intensity quantification with respect to a positive control for coupled islets cultured in NF and EMS (see Methods) demonstrated no significant differences between islets cultured in either NF or EMS media (n = 12 islets and n = 11 islets). G islet-derived factors (from the coupled efflux) in EMS normalized to NF (reproducible, Table 1) demonstrated significant increases in the secretion of both amylin and pancreatic polypeptide in EMS medium (n = 12 MPS). H The islets from the coupled vLAMPS-PANIS in EMS exhibit significant abnormal insulin response to low glucose in islets that were maintained in either NF or EMS media (n = 10 islets and n = 6 islets), which was not observed in the standalone PANIS islets (see Fig. 3H). I The same significance is observed in the insulin stimulation index response of islets that were coupled with NF medium compared to EMS medium. (see Methods). Scatter plot graphs display the mean and standard error (SEM) with p-values shown. For graphs -D, E, F, H, and I- statistical analysis was done by two-tail t test with Welch’s correction. For graph -B and G- statistical analysis was done by Wilcoxon signed-rank test of normalized EMS values to their corresponding NF study.
Correlation analysis supports functional response in coupled vLAMPS-PANIS
To gain further evidence and insights for communication between the two modules in the coupled vLAMPS-PANIS and test the hypothesis that liver derived factors can modulate islet derived factors important for T2DM progression, we assessed pairwise correlations between secreted factors selectively derived from the vLAMPS module and factors selectively derived from the PANIS module under NF and EMS conditions (defined as a pair). The reproducibility for the measurement of each secreted factor is presented in Table 155. Correlation analysis was restricted to hepatic and islet factors with reproducibility ANOVA p-values ≥ 0.01 (see Table 1). Statistically significant correlation coefficients were observed in at least one medium condition for 14 of the 23 reproducible pairs (correlation coefficient p-value ≤ 0.05, Table 2). Six of these 14 pairs (marked with an asterisk in Table 2) were between hepatic and islet factors with a more stringent reproducibility ANOVA p-value ≥ 0.05 in both NF and EMS conditions. As expected, the 3 statistically significant correlation coefficients derived from these six pairs (Table 2) were also identified in the less stringent analysis along with 13 other statistically significant correlation coefficients derived from the total of 14 pairs (as discussed below). This result indicated that amid potentially lower reproducibility of single measurements using the less stringent reproducibility criterion, statistically significant covariances could still be identified.
Using the p > 0.01 reproducibility threshold, 23 secreted factors pairs (one derived primarily from the vLAMPS and the other from PANIS) were identified and their correlation coefficients determined under both NF and EMS conditions (Table 2). Focusing exclusively on the statistically significant correlations (p ≤ 0.05) in at least one medium condition there were 8 pairs that had at least a 20% change between two conditions (highlighted in blue), and 6 that did not change (shown in gray) (Table 2). Seven of these 8 pairs showed a significant correlation only under NF conditions. Conversely, one correlation involving RBP4 and pancreatic polypepetide was only significant under EMS conditions. Particularly striking was the involvement of C-peptide (a direct marker of newly processed and secreted insulin) in 4 of these 7 correlations and 1 more involving the vLAMPS-derived cytokine, GM-CSF, and islet-derived GLP-1, that promotes insulin secretion. The loss of these 5 positive correlations under EMS conditions is consistent with the EMS-induced dysregulation of GSIS (Fig. 4I). In addition to their positive correlations with C-peptide under NF conditions, Fetuin A and RBP4 showed a strong trend toward a negative correlation with GLP-1 under EMS conditions (Table 2). This observation is complementary to the aforementioned positive correlations and further supports a sensitive condition-dependent liver-pancreas signaling network. These statistical based correlations can guide us toward a mechanistic model supported by the literature and are discussed below.
Discussion
The results presented in this study show that vLAMPS-PANIS can serve as a human biomimetic recapitulating several critical aspects of the early stages of MASLD development, including the development of insulin resistance, leading to pancreatic β-cell dysfunction. Its modular design can be used to distinguish inter-organ from intra-organ signaling and help to deconvolve the pleiotropic effects of secreted molecules. Its implementation here of an upstream vLAMPS and downstream PANIS has provided evidence supporting the hypothesis that an early stage MASLD liver can induce β-cell dysfunction through a hepatic-pancreatic axis that at least in part underlies the high comorbidity among MASLD and T2DM patients23. The diverse panel of metrics that is reproducible across independent experiments has (1) enabled pairwise correlations between a vLAMPS and a PANIS secreted factor to be identified and (2) their differential dependence between normal fasting and early metabolic syndrome conditions to be determined, lending further support for this working hypothesis.
These correlations and their dependencies on media-induced conditions provide not only statistical-based support for the liver-pancreas axis but in conjunction with clinical, in vitro, and animal model data give rise to mechanistic models and causal hypotheses. Fetuin A, a hepatokine associated with MASLD and T2DM66,67,68, is exclusively secreted from the vLAMPS module of vLAMPS-PANIS and under NF conditions is positively correlated with C-peptide, the direct and specific marker of newly processed and secreted insulin. A similar relationship between Fetuin A and insulin has been observed in animal models involving Fetuin A knockout studies and its exogenous administration69,70 and in patients71, and results from Fetuin A binding to hepatic insulin receptor, inhibiting its receptor tyrosine kinase (RTK) activity to induce a homeostatically regulated rise in insulin levels (i.e., C-peptide)69,72. Under EMS conditions a positive correlation between Fetuin A and C-peptide is no longer observed in the vLAMPS-PANIS. Mechanistically, circulating Fetuin A in the context of free fatty acids can bind directly to β-cells through the Toll-like receptor 4 (TLR-4), promoting a Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) dependent inflammatory response that disrupts glucose stimulated insulin secretion (GSIS)28,73. Consistent with this mechanism, a negative correlation between Fetuin A and GLP-1, the hormone that promotes GSIS, was observed only under EMS conditions that create the MASLD disease state in the liver. In studies involving mouse islets, Fetuin A in the context of free fatty acids and working through the TLR-4-NF-kB pathway was shown to enhance the expression of Dipeptidyl peptidase-4 (DPP-IV), the dipeptidyl peptidase responsible for degrading GLP-174. A complementary mechanism for the observed negative correlation between Fetuin A and GLP-1 likely involves the fetuin-dependent diminished capacity of inflamed β-cells to conduct the bidirectional paracrine signaling with α-cells that is required for GLP-1 processing and secretion75,76. By analogy, the retinol binding protein RBP4 that is associated with MASLD and T2DM77 and is secreted predominantly from vLAMPS under EMS conditions, has a negative correlation with GLP-1 under these conditions. As a complex with retinol, it has been shown in mouse systems to induce direct β-cell dysfunction through the RBP4/STRA6/JAK2/STAT1/insulin gene enhancer protein 1 to lower GSIS and insulin synthesis78,79. Together, this mechanistic analysis of the statistical correlations derived from vLAMPS-PANIS under EMS and NF conditions supports a model in which clinically relevant factors secreted from early stage MASLD liver can directly interact with islet β-cells to perturb the finely tuned paracrine signaling critical for GLP-1 regulation of GSIS75 (Supplementary Fig. S11). These liver secreted factors, as exemplified by Fetuin A, are multifaceted. Under NF conditions they can act in an autocrine or paracrine manner as normal homeostatic components of glucose regulation by modulating hepatic insulin signaling. Under EMS conditions these factors either become overexpressed or in the presence of FFAs assume a pathophysiological endocrine role to dysregulate intra-islet signaling. The functional, statistical and mechanistic analysis of this vLAMPS-PANIS study support the commonality of this mechanism to engender MASLD-T2DM comorbidity.
This current two-organ modular version of vLAMPS-PANIS does not include intestine-derived GLP-180 indicating the potential importance of pancreatic islet-derived GLP-1 in normal homeostasis and its dysregulation in early metabolic syndrome disease. Although this study focused on early-stage metabolic syndrome disease, positive correlations between the later stage liver stellate cell-derived fibrogenesis marker, COL1A1, and islet derived amylin and pancreatic polypeptide were observed under EMS conditions. This may represent the beginning of centrally mediated compensatory mechanisms involving amylin and pancreatic polypeptide that regulate satiety and gastric emptying when GSIS is compromised81. The correlation between stellate cell derived COL1A1 and islet F-cell derived pancreatic polypepetide82 indicates the capability of vLAMPS-PANIS to detect indirect but potentially important signaling interactions between relatively rare cell populations. Furthermore, in addition to the correlations made for NF and EMS media, we have also previously demonstrated the use of late metabolic syndrome (LMS) medium in liver MPS57. This medium contains pro-fibrotic molecular drivers including Transforming growth factor beta (TGF-β) and lipopolysaccharide (LPS), which recapitulate features in late stage MASLD57. Although not performed in the current study, correlations between hepatic and pancreatic factors using this medium will provide additional mechanistic insights in the future. However, here we focused on the earlier stage in the MASLD-T2D progression as a reasonable starting point to explore mechanisms and therapeutic strategies.
To determine the clinical relevance of vLAMPS-PANIS, well-established markers implicated in MASLD and T2DM were employed. Although this supervised approach enabled this objective and provided evidence supporting the underlying role of the liver-pancreas axis in MASLD and T2DM comorbidity, it nevertheless limited the full potential of vLAMPS-PANIS to provide a holistic understanding of physiological and pathophysiological mechanisms. We anticipate that future studies employing vLAMPS-PANIS will take a more comprehensive unbiased approach that utilize larger panels of metrics. These panels will include multiple ‘omics’ analyzed at different time points complemented by real-time imaging under media conditions that mimic both early and late-stage metabolic syndrome disease. Since MASLD and T2DM involve multiple organs in addition to the liver and the pancreas, we expect that future studies will exploit the modular design of vLAMPS-PANIS to include the coupling and recirculation of human microphysiology systems comprising adipose tissue, the intestine, and/or gut microbiome. As demonstrated with the coupled vLAMPS-PANIS in this study, the capability of systematically constructing and deconstructing complex disease biomimetic models provides a powerful approach for deciphering pathophysiology and identifying targetable mechanisms.
The use of primary cells was important to establish an MPS disease model that reflected fully matured cells. This will be critical in interpreting the use of patient-based, induced pluripotent stem cell (iPSC)-derived cells for the liver and pancreatic islets. However, the use of primary cells has the limitations of supply of the same cells for multiple studies over time and the ability to generate patient-derived or patient-specific MPS to define heterogeneity. The use of optimally matured, patient-derived iPSCs and synthetic biology in the disease MPS addresses the limitations of primary cells and allows the application of precision medicine to understand inter-patient heterogeneity that will inform optimal therapeutic strategies for individual patients or patient cohorts with the most similar characteristics.
Methods
Human hepatocytes and non-parenchymal cells (NPCs)
Cryopreserved human primary hepatocytes (post-thaw plateability with >90% viability) were purchased from Thermo Fisher (HU1960). Hepatocyte donor information is available in supplementary Table 1. Liver sinusoidal endothelial cells (LSECs) were purchased from LifeNet Health (cat. no. NPC-AD-LEC-P1). To preserve a consistent LSECs stock, cells were thawed and expanded in EGM-2 endothelial cell growth medium (Lonza, cat. no. CC-3124) for two passages (P-2) and re-frozen at 1×106 cells ml−1. Human hepatic stellate cell line LX-2 were acquired from Sigma (cat. no. SCC064) and were cultured in DMEM-2 (ThermoFisher, cat. no. 10564011) supplemented with 2% FBS (Fisher Scientific, NC9525043) and 10 mg/ml Pen-Strep (Hyclone, cat. no. SV30010). Human monoblast cell line THP-1 were purchased from ATCC (cat. no. TIB-202). THP-1 cells were used as Kupffer-like cells, as they can be differentiated into activated macrophages83. The cells were expanded in RPMI 1640 (ThermoFisher, cat. no. 11875093) supplemented with 10% FBS, 10 mg ml−1 Pen-Strep, and 2 mM L-Glutamine (Hyclone, cat. no. SH30034.01). Forty-eight hours prior to harvesting for seeding into the vLAMPS, cells were treated with 200 ng ml−1 phorbol myristate acetate (PMA) (Calbio, cat. no. 524400) in RPMI84. Prior to MPS assembly, all NPCs were maintained at maximum of 80% confluency with their respective cell culture media and incubated at 37 °C in 5% CO2. LX-2 and THP-1 cells were kept under 20 passages. Hepatocytes were maintained in liquid nitrogen until thawed to be seeded in the vLAMPS.
Pancreatic Islets
Human cadaveric pancreatic islets were procured from Prodo Labs (San Francisco, CA). Donor details can be found in supplementary Table 2. The islets were maintained in a petri dish (Fisher Scientific, cat. no. FB0875711) suspended in 10 mL Prodo islet media for recovery (PIM(R)) (Prodo Labs, cat. no. PIMCR001GMP), for a minimum of 4 days. Fifty percent medium changes were carried out every other day under static suspension and incubated at 37 °C in 5% CO2.
Normal fasting and early metabolic syndrome media
The base formulation for normal fasting (NF) and early metabolic syndrome (EMS) media have been previously described57. Shortly, EMS medium consisted of NF medium with the modification of 20 mM glucose (Sigma, cat. no. G8644ML), 30 pM glucagon (Sigma, cat. no. G2044), 10 nM insulin (Gibco, cat. no. 12585-014), and addition of 200 µM oleic acid-BSA conjugated (Cayman, cat. no. 29557) and 100 µM sodium palmitate-BSA conjugated (Cayman, cat. no. 29558) (Supplementary Table S1).
Microfluidic glass device design
The vLAMPS and PANIS were constructed using the Micronit Organ-on-Chip glass microfluidic platform36. The 2-chamber flow device consists of three borosilicate glass layers (Micronit, cat. no. OOC 00739). The middle layer contains an oval orifice the bottom of which is covered by porous Polyethylene terephthalate (PET) membrane, creating an oval-well on the top, partitioning the top and the bottom chambers. The vLAMPS has an oval area of 77 mm2 with 0.4 µm pores (Micronit, cat. no. 03237), while the PANIS uses the 100mm2 oval with 3 µm pores (Micronit, cat. no. 02861). The layers are held together within a 4-device aluminum clamp (Micronit, cat. no. 4515) which has the openings for the corresponding inlets and outlets.
The vLAMPS fluid flow configuration was achieved by parallel perfusion of the 2 chambers denoted hepatic and vascular chambers (Supplementary Fig. S1A), with two inlets and two outlets. The PANIS fluid flow configuration consisted of a single inlet/outlet achieved by a looped path through the chip by interconnecting the two chambers (top and bottom chambers) (Supplementary Fig. S1B).
Computational Fluid Dynamics (CFD) and vLAMPS Oxygen Tension
A perfusion flow rate was estimated based on computational fluidic dynamics (CFD) to establish a physiological oxygen zonation gradient in vLAMPS. The CFD modeling of the oxygen zonation using a transport of diluted species model and a flow model has been previously described36. In the geometry of the current middle layer (oval area and PET membrane) the number of hepatocytes was decreased from published study36, but the cell’s oxygen consumption rate was maintained at 0.45 nmol s−1 106cells ml−1 85,86. The PET membrane’s porosity, thickness, and oxygen diffusion coefficient were updated accordingly87 in the CFD model (Supplementary Table 3). The estimated flow rate of 35 µl h−1 was determined to generate an oxygen tension gradient at the hepatocytes level, that ranges from 1.7 × 10−1 to 6 × 10−2 mol m−3 with ~85,000 hepatocytes on a parallel perfusion in a glass device (Supplementary Fig. S2A–C).
The flow rate and oxygen tension were empirically validated with the previously described ratiometric imaging of oxygen sensitive beads (ex/em 405/706 nm) to reference beads (ex/em 405/455 nm)36. Beads were initially anchored in the apical side of the PET membrane by ethanol evaporation at 1:2000 density in a 1:1 ratio of each bead type. Beads were then covered by seeding ~85,000 hepatocytes on top of them inside the oval of the middle layer. Additionally, ~85,000 LSEC were seeded in the basolateral side of the membrane. The oxygen sensitive beads were manufactured as previously reported36, loading of palladium(II)-5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin (PdTFPP, Sigma Aldrich cat. no. 673587) to 4.6 µm diameter polystyrene beads (Bangs Laboratories cat. no. FCGB008). Auto fluorescence polystyrene 5 µm diameter beads (Bangs Laboratories cat. no. PS05003) were utilized as reference beads. Following the cell seeding, the middle layers was assembled into the aluminum clamp and perfusion with NF medium was initiated at 35 µl h−1 inside an incubator at 37 °C and 5% CO2. On day 4, the vLAMPS was imaged by loading the clamp into the IN Cell Analyzer 6000 microscope (General Electric) and kept at 37 °C and maintained under perfusion. In the workstation software (v4.5) we defined inside the vLAMPS oval area the regions of interest (ROIs). These were arrangements of 12 columns equidistantly distributed across the long axis of the oval. Each column is made up of 4 fields (20× magnification) evenly distributed perpendicular to each side of the center of the oval’s long axis, a total of 48 fields across 12 ROIs. 2D images of the ROI were acquired at the beads’ specific spectrum every hour for three hours. The fluorescence intensity of the three image sets was measured using Fiji (ImageJ version 1.53c), and the corresponding mean value of each field associated to a ROIs was averaged for both their O2 sensitive and reference beads (Supplementary Fig. S2D). Syringes were swapped for freshly prepared ambient oxygen medium (18%) and perfused, with the ROIs imaged every hour for two hours. Lastly syringes were again swapped for freshly prepared medium with 0.14 U ml−1 of glucose oxidase (Sigma-Aldrich cat. no. G2133-10KU) to generate oxygen-depleted medium (0%). After one hour of stabilization, two sets of images are taken one hour apart. The mean of the fluorescence intensity of the 18% and 0% conditions are used to generate the calibration curve (Supplementary Fig. S2F). Using these calibration curve, we extrapolated the oxygen percentage of each ROIs from the fluorescence ratio value between O2 sensitive and reference beads (Supplementary Fig. S2E-G).
vLAMPS fabrication
The fabrication of the vLAMPS was as previously described36 with the following modifications. The vLAMPS middle layer was treated with a solution of 100 μg ml−1 fibronectin (Sigma, cat. no. F1141) and 150 μg ml−1 collagen (Corning, cat. no. 354249) in 1× phosphate buffered saline (PBS) (Gibco, cat. no. 21600-069) overnight in a 4 °C cold room. Based on the proportions of liver cell types in vivo seeding numbers were determined by allometric scaling to be 40: 14: 6, Hepatocytes: Kupffer: Stellate37,88. LSEC seeding ratio is 1:1 to hepatocytes to cover the extended surface area of the PET membrane as previously described36. The cell densities used here were: P-2 LSECs 8.5×105 cells ml−1, LX-2 at 1.3 × 104 cells ml−1, PMA-treated THP-1 at 2.4 × 104 cells ml−1, and hepatocytes at 2.12 × 106 cells ml−1. The decellularized porcine liver extracellular matrix (LECM) was provided by Dr. Badylak89. To promote hepatocyte polarization, an overlay of rat tail collagen type 1 solution (2.5 mg ml−1) in NF medium (or EMS for disease conditions) was deposited on top of the hepatocytes inside the borders of the oval-well. Lastly, the middle layer was fully submerged in NF or EMS medium and kept at 37°C and 5% CO2 until assembly of vLAMPS.
For the standalone vLAMPS assembly, the three glass layers were secured in the aluminum clamp. The bottom side of the membrane forms the vascular chamber, and the top side forms the hepatic chamber (Supplementary Fig. S1A). The parallel perfusion of the 2 chambers was set up by two 10 ml syringes in via a syringe pump (Harvard Apparatus, cat. no. PHD ULTRA) and connected via PEEK tubing (IDEX, cat. no. 1569XL) into the vLAMPS inlets. Efflux collection of ~1.7 ml was carried out in channel-independent (hepatic and vascular chamber) 4 ml glass vials.
PANIS fabrication
Prior to encapsulation into the PANIS, islets were washed twice in NF medium and equilibrated overnight at 37°C and 5% CO2. With the aid of a dissection microscope, ~100 individual islets (per PANIS) were transferred using a 200 µL pipette (Fisher Scientific, cat. no. 02-707-430) into a suspension of 1.1% alginate solution. In 2 µL of the islet-alginate solution ~10-20 islets were dispensed into the oval-well of the middle layer in an alternating array of ~10 droplets. The alginate was polymerized by adding the solution of 10 mM BaCl2 (Sigma, cat. no. 1.01716) through the bottom side of the membrane for 20 min at 37 °C and 5% CO2, encapsulating the islets into each alginate bead. Lastly, the middle layer was fully submerged in NF (or EMS for disease studies) medium and placed at 37 °C and 5% CO2 until assembly of PANIS.
Similar to the vLAMPS, the standalone PANIS assembly consisted of clamping the three glass layers in the aluminum clamp to form the top and bottom chambers (Supplementary Fig. S1B). The PANIS was placed under flow (35 µl hr−1) of medium from a single 10 mL syringe connected by PEEK tubing into the single PANIS inlet top chamber, and a loop was created by connecting the top chamber outlet to the bottom chamber inlet using C-flex tubing (Fisher Scientific, cat. no. NC0142679). The ~1.7 mL efflux was collected from the bottom chamber outlet.
Physical coupling
The 4-device aluminum clamp was used to form a duplicate coupling of a vLAMPS and a PANIS. Assembly of the vLAMPS and PANIS follows the same steps as described above, with coordination between vLAMPS cell seeding and PANIS islet incubation (Supplementary Fig. S1D). Two vLAMPS modules in parallel to each other were assembled first, followed by the assembly of the two PANIS modules. Following hepato-pancreatic axis and to ensure that the medium influx had the highest oxygen tension possible, the vLAMPS was connected to the PANIS via C-flex tubing from the hepatic chamber (vLAMPS outlet number 8) into the PANIS top chamber (inlet number 5) (Supplementary Fig. S1C). With the system sealed, media perfusion was initiated in the inlets of the vLAMPS (inlet number 2 and 5) by connecting two 10 ml syringes to the device with PEEK tubing. Perfusion passed through the vLAMPS to the PANIS and followed the looped path as described above. Finally, the bottom chamber of the PANIS (outlet number 2) was fed into a collection tube.
Efflux collection
Efflux medium from the vLAMPS, PANIS, and coupled vLAMPS-PANIS was collected in glass vials which were changed every other day for 8 days after initiating flow. Samples for the standalone vLAMPS were collected from the hepatic chamber (Fig. S1A), standalone PANIS samples were collected from the bottom chamber (Fig. S1B), and lastly medium collected for the coupled system was from the shared flow between the vLAMPS’s hepatic chamber, into PANIS top chamber, and looped to the bottom chamber (Fig. 1C). The collected samples were maintained at 4 °C until the completion of the experiment. Efflux samples were then aliquoted in 500 µl samples and stored at -20 °C until analysis.
Live and Immunofluorescence Islet Imaging
Islet viability and morphology were evaluated after 8 days under perfusion. The islet-alginates beads were divided into four groups, one for live imaging, one for oxidative stress, one for immunohistochemistry, and one for glucose stimulated insulin secretion (GSIS) evaluation. The islets were decapsulated from the alginate by adding 100 mM EDTA and incubating for 2 min at room temperature. The viability of the islets was determined as previously described46. Briefly, the islets were incubated with 1:4000 Calcein AM (live), 1:1000 Ethidium homodimer-1 (EthD-1) (dead) (Invitrogen, cat. no. L3224) and 1:2000 Hoechst (nuclear staining) (ThermoFisher, cat. no. 62249) in DMEM-2. Imaging was done at 20× magnification using Olympus widefield fluorescence microscope with 494/517 nm, 528/617, and 340/488 (excitation/emission) filters, respectively. Quantification was carried out using Fiji (ImageJ version 1.53c). Image segmentation was carried out using the “Yen” threshold function option. The selected objects were tabulated using the -Analyze Particles- function to quantify the number of EthD-1 and DAPI positive objects to identify the number dead cells and total number of cells (nuclei) within the islets. The percentage of live cells was determined by calculating \(1-\left(\frac{{dead\; cells}}{{total\; cells}}\right)\).
The second subset of islets was evaluated for oxidative stress by 2′,7′-Dichlorodihydrofluorescein diacetate (DCFDA) (Abcam, cat. no. ab113851) staining of reactive oxygen species (ROS). Freshly decapsulated islets were washed with 1× buffer supplied in the kit and later transferred to a 20 μM DCFDA solution and incubated at room temperature for 1 h before a final wash with 1× buffer. To capture the total dimensions of each islet, we counterstained the islets with 1 μM MitotrackerTM Red CMX ROS (Invitrogen, cat. no. ab112143). Imaging was carried out on an Olympus Fluoview laser scanning confocal microscope (Olympus, Japan) with 490/520 nm and 485/535 nm (excitation/emission) for Mitotracker and DCFDA, respectively (20x magnification). For the ROS positive control, islets were preincubated with 50 μM of Tert-Butyl hydroperoxide (TBHP) (Sigma, cat. no. 416665) for 1 h before staining with DCFDA and Mitotracker. Image processing and quantification was carried out using Fiji. ROS in the islets was measured by quantifying the intensity of DCFDA signal in islets normalized to the area90. To account for islet size variability and the associated inconsistent signal to noise ratio of the islet’s core, the fluorescence intensity measurement was limited to the periphery of each islet. The periphery area was delineated as the distal one-third of the radius of each islet. The mean intensity in the selected region was normalized to the established periphery area of each islet. Normalized ROS intensity for islet groups was represented as a fraction of the corresponding value for the islets treated with TBHP (positive control). Area and intensity were measured using Fiji.
The third subset of released islets was fixed with 4% Paraformaldehyde (PFA) (Thermo Scientific, cat. no. J19943.K2) for 1 hour at room temperature. Following the previously descried methodology46, islets were immunofluorescence labeled using primary C-peptide (R&D Systems, cat. no. MAB80561) and Glucagon (Abcam, cat. no. ab92517) antibodies with a 1:100 dilution. The primary antibodies were incubated at 4 °C overnight. The islets were subsequently incubated at 4 °C overnight with anti-mouse Alexa Fluor 488 (Invitrogen, cat. no. A28175) and anti-rabbit Alexa Fluor 555 (Invitrogen, cat. no. A27039) at a dilution of 1:500, and nuclei were stained using DAPI (1:2000) (Thermo Fisher, cat. no. D1306). Before imaging, islets were whole mounted on depression glass slides using Gold Antifade Mountant (Invitrogen, cat. no. P36930) and imaged using the Fluoview 1000 laser scanning confocal microscope with 40× objective with 1.32 NA. The images were acquired with an exposure time of 10 μs/pixel, and a line Kalman filter was applied to remove background noise. The images acquired for C-peptide, glucagon were quantified using a basic machine learning algorithm executed in Qupath (version 0.5.0) to detect the number of cells expressing C-peptide and glucagon.
Glucose-stimulated insulin secretion
The fourth set of islet-alginate capsules was not decapsulated. Glucose stimulated insulin secretion (GSIS) was conducted under static conditions using 8 μm-membrane transwells (Corning, cat. no. 3464). Islet-alginate capsules were washed five times with G55 medium (500 mL HAMS F10 (Cytiva, cat. no. SH30025.01), 500 mL DMEM without glucose (Gibco, cat. no. A1443001), 600 mg NaHCO3, 110 mg CaCl2, and balanced to pH 7.4) prior to incubating overnight at 37 °C and 5% CO2. Following the incubation, the islets were washed five more times and exposed to the low (3 mM) and high glucose (16 mM) G55 solutions for 1-hour spans. Insulin was measured from supernatant using an ELISA kit (ALPCO, cat. no. 80-INSHU-E01.1) as per the manufacturer’s instructions. Insulin stimulation index is calculated by dividing the levels of insulin measured under high glucose conditions by the levels of insulin measured under low glucose conditions.
Secretome measurements
Albumin, urea, LDH, COL1A1, and TIMP1 were measured as previously described36,91. Albumin assays were performed in a 1:100 efflux dilutions by enzyme linked immunosorbent assay (ELISA) using commercial antibodies (Bethyl Laboratories, cat. no. A80-129A and A80-129P) and an ELISA accessory kit (Bethyl Laboratories, E101) with a human albumin standard prepared in house (MilliporeSigma, cat. no. 126658). COL1A1 was measured using the Human pro-collagen 1A1 ELISA kit (R&D Systems, cat. no. DY6220-05) in a 1:50 efflux dilution, and TIMP1 was measured using the TIMP1 ELISA kit (R&D Systems, cat. no. DTM100) with a efflux dilution of 1:20. Urea was measured using the Stanbio BUN liquid reagent for diagnostic set (Stanbio Laboratory, cat. no. SB-0580-250). Lactate dehydrogenase was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, cat. no. G1780). The protocols for urea and LDH assay were modified to a 384 well microplate format with no efflux dilution. Controls were run in duplicate, and only sample values inside the range of the standard curve were used for analysis.
Multiplex immunoassay
Amylin, C-peptide, Pancreatic Polypeptide, GLP-1, and Glucagon were measured using a customized MILLIPLEX human metabolic Hormone Panel V3 (Millipore Sigma, cat. no. HMH3-34K). CCL2, CCL3, CXCL1, Fetuin A, G-CSF, GM-CSF, HGF, IGFBP-7, IL-1β, IL-6, IL-8, PDGF-AA, RBP4, TNF-α, and VEGF-A were measured using a custom Luminex Human Discovery LXSAHM-15 panel (R&D Systems). The measurements were carried out according to the manufacturer’s instructions at the University of Pittsburgh Cancer Proteomics Facility Luminex® Core Laboratory using the xMAP platform. All samples and controls were run in triplicate and only values inside the range of the standard curve were used for analysis.
vLAMPS Immunofluorescence Imaging
On day 8 the vLAMPS cells were fixed with 2% PFA at 4 °C for 30 min. Following the previously described methodology36, hepatocytes were immunofluorescence labeled for either cytokeratin 8 (CK-8) (1:200) (Invitrogen, cat. no. MA1-06318), or insulin receptor substrate 2 (IRS-2) (1:250) (Abcam, cat. no. ab134101). Stellate cells were labeled for alpha-smooth muscle actin (α-SMA) (1:100) (Sigma, cat. no. A2547). The primary antibodies were incubated at 4°C overnight. Secondary antibodies anti-mouse Alexa Fluor 488 (Invitrogen, cat. no. A-11001) and anti-rabbit Alexa Fluor 594 (Invitrogen, cat. no. A-11012) were incubated at room temperature for 2 h (1:250). Lipid droplets in the hepatocytes were stained with LipidTOX (1:500) (Invitrogen, cat. no. H34476) fluorescent staining (595/615 nm). The F-actin of LSECs were fluorescence stained with 568-Phalloidin (1:500) (Invitrogen, cat. no. A12380). Cell nuclei were stained using Hoechst (1:2000) (Thermo Fisher, cat. no. 62249).
To capture the appropriate metabolic zonation across the vLAMPS, images were taken at the specific fields in the ROI denoted Zone 1, Zone 2, and Zone 3 across the vLMAPS oval area (Fig. S2E). Image stacks were taken in a grid of 2 by 4 fields (length × height for a total of 8 fields per ROI). Images were acquired with a Nikon 20x (0.45 NA) objective using the Operetta CLS High Content Scanning system (Perkin Elmer), to accommodate the distance of the vLAMPS middle layer inside the aluminum clamp to the objective’s working distance. Applying confocal mode and maximum projection acquisition mode, all images were taken with at 3.3 µm stack size on a 160 µm height, using the 490/525 nm, 561/572, and 650/671 nm excitation/emission settings. Fluorescence intensity analysis was performed using Fiji. To exclude background fluorescence for IRS-2 and α-SMA the images were segmented with an intensity threshold based on a secondary antibody only control. To measure the total fluorescence intensity of the lipid droplets, particle quantification was applied by excluding small objects ( < 10 μm2). The average fluorescence intensity of all fields (regardless of the metabolic zone) was used for statistical normalization and analysis of LipidTOX, IRS-2, and α-SMA.
Reproducibility analysis
Data from all studies were uploaded to the Numa Biosciences EveAnalytics™ platform. Inter-study reproducibility analysis followed the Pittsburgh Reproducibility Protocol (PReP)55 and used the Graphing/Reproducibility feature in the EveAnalyticsTM platform for statistical comparisons. Reproducibility status based on one-way ANOVA ranged from Excellent (p-value ≥ 0.1) to Poor (p-value < 0.01)53,54. All metrics with a p-value ≥ 0.01 were used for the multivariate correlation analysis.
Multivariate correlation analysis
The correlation between the levels of the various secreted factors in the vLAMPS-PANIS coupled model was assessed using the Multivariate method analysis in JMP (version 17.1). The input data were the levels of the secreted analytes measured in the effluent from the bottom chamber of vLAMPS-PANIS coupled model. The correlation of each target analyte to each of the other target analytes measured under the same media conditions (NF or EMS) was estimated by the Pairwise method to calculate the Pearson correlation coefficient and the correlation probability p-value. The p-value is the probability that the true correlation between two analytes is zero. Correlations with p-values < 0.05 were considered to be statistically different from zero, i.e., a true correlation.
To verify for vLAMPS-derived and islet-derived factors, the levels of detection of the corresponding analyte had to be at least of 2.5:1 to the same factor under their corresponding medium between standalone vLAMPS (Supplementary Fig. S4) and standalone PANIS (Supplementary Fig. S6).
For the selection criteria of the key analytes’ correlation presented in Table 2 the following criteria were used: paired analytes with reproducible p-values (>0.01) in EMS and NF medium, for one or both of the correlation coefficient p-values to be significant ( ≥ 0.05), and the magnitude of the significant correlation coefficients to be at least moderately correlated (r > 0.6).
Statistics and reproducibility
Statistical comparisons between NF and EMS media groups as well as standalone and coupled systems were made using PRISM (version 10.1.2). All data are presented as mean ± standard error (SEM) for n = 2–7 devices per study. Normality was first determined with all data by Shapiro-Wilk test. When at least one of the groups or studies did not follow Gaussian distribution, a nonparametric test was applied. For media comparison studies, EMS values were normalized to the average NF values of their corresponding study. For parametric measurements a One-sample t test was applied, and a Wilcoxon signed-rank test was applied as nonparametric alternative. The two-way ANOVA with Sidak’s test was applied to multiple day efflux comparison between NF and EMS. An unpaired two-tail t test with Welch’s correction was applied to islet data collected outside of the PANIS, as no equal distribution and sample size could be secure due to the heterogeneric nature of islets derived from 3 different donors (Supplementary Table 1). Alternatively, Mann-Whitney test was implemented as the nonparametric test for one-to-one comparison of two unpaired groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All of the source data and metadata pertaining to the findings presented in the main paper and supplementary information were uploaded in EveAnalytics (previously BioSystics-Analytics Platform and MPS-Database53). Standalone and coupling studies ID numbers are: 1124, 1121, 1120, 1119, 1118, 1060, and 1058.
References
Control, C. f. D. & Prevention. National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services (2017).
American Diabetes, A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 36, 1033–1046 (2013).
Defronzo, R. A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
Hartwig, S. et al. Secretome profiling of primary human skeletal muscle cells. Biochim Biophys. Acta 1844, 1011–1017 (2014).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481 (2011).
Paula, F. M. et al. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 29, 1805–1816 (2015).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463 (2012).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014).
Giudice, J. & Taylor, J. M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharm. 34, 49–55 (2017).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425 (1994).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Halberg, N., Wernstedt-Asterholm, I. & Scherer, P. E. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. 37, 753–768 (2008).
Lehr, S., Hartwig, S. & Sell, H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl 6, 91–101 (2012).
Lehr, S. et al. Identification and validation of novel adipokines released from primary human adipocytes. Mol. Cell Proteom. 11, M111 010504 (2012).
Li, Q. et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J. Clin. Endocrinol. Metab. 101, 4886–4894 (2016).
Yang, S. et al. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 96, E1325–E1329 (2011).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635 (2005).
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 22, 1078–1089 (2015).
Meex, R. C. R. & Watt, M. J. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 13, 509–520 (2017).
Calle, M. C. & Fernandez, M. L. Inflammation and type 2 diabetes. Diabetes Metab. 38, 183–191 (2012).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Ciardullo, S., Vergani, M. & Perseghin, G. Nonalcoholic fatty liver disease in patients with type 2 diabetes: screening, diagnosis, and treatment. J. Clin. Med. 12, https://doi.org/10.3390/jcm12175597 (2023).
Morrison, A. E., Zaccardi, F., Khunti, K. & Davies, M. J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 39, 557–567 (2019).
Targher, G., Corey, K. E., Byrne, C. D. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 18, 599–612 (2021).
Harrison, S. A. et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 390, 497–509 (2024).
Tilg, H., Moschen, A. R. & Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42 (2017).
El Ouaamari, A. et al. SerpinB1 promotes pancreatic β cell proliferation. Cell Metab. 23, 194–205 (2016).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Scoditti, E., Sabatini, S., Carli, F. & Gastaldelli, A. Hepatic glucose metabolism in the steatotic liver. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-023-00888-8 (2024).
Lopez-Bermudo, L. et al. Contribution of liver and pancreatic islet crosstalk to beta-cell function/dysfunction in the presence of fatty liver. Front. Endocrinol. (Lausanne) 13, 892672 (2022).
Flessa, C. M. et al. Genetic and diet-induced animal models for non-alcoholic fatty liver disease (NAFLD) Research. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms232415791 (2022).
Hebbard, L. & George, J. Animal models of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 8, 35–44 (2011).
Kleinert, M. et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162 (2018).
Neff, E. P. Farewell, FATZO: a NASH mouse update. Lab Anim. (NY) 48, 151 (2019).
Brennan, P. N. et al. Antifibrotic therapy in nonalcoholic steatohepatitis: time for a human-centric approach. Nat. Rev. Gastroenterol. Hepatol. 20, 679–688 (2023).
Li, X., George, S. M., Vernetti, L., Gough, A. H. & Taylor, D. L. A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip 18, 2614–2631 (2018).
Vernetti, L. A. et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. (Maywood) 241, 101–114 (2016).
Shi, Q. et al. Co-culture of human primary hepatocytes and nonparenchymal liver cells in the emulate(R) liver-chip for the study of drug-induced liver injury. Curr. Protoc. 2, e478 (2022).
Rajan, S. A. P. et al. A novel milli-fluidic liver tissue chip with continuous recirculation for predictive pharmacokinetics applications. AAPS J. 25, 102 (2023).
Ouchi, R. et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 30, 374–384 e376 (2019).
Kostrzewski, T. et al. Modelling human liver fibrosis in the context of non-alcoholic steatohepatitis using a microphysiological system. Commun. Biol. 4, 1080 (2021).
Kang, S. Y. et al. A pillar and perfusion plate platform for robust human organoid culture and analysis. Adv. Healthc. Mater, e2302502, https://doi.org/10.1002/adhm.202302502 (2023).
Schlunder, K. et al. Microphysiological pancreas-on-chip platform with integrated sensors to model endocrine function and metabolism. Lab Chip 24, 2080–2093 (2024).
Patel, S. N. et al. Organoid microphysiological system preserves pancreatic islet function within 3D matrix. Sci. Adv. 7, https://doi.org/10.1126/sciadv.aba5515 (2021).
Jun, Y. et al. In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci. Adv. 5, eaax4520 (2019).
Wiegand, C. R. Pancreatic Islet Microphysiology System for Disease Modeling of Type 2 Diabetes, University of Pittsburgh (2023).
Bauer, S. et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 7, 14620 (2017).
Aizenshtadt, A. et al. Pump-Less, Recirculating Organ-on-Chip (rOoC) Platform to Model the Metabolic Crosstalk between Islets and Liver. Adv. Healthc. Mater. e2303785, https://doi.org/10.1002/adhm.202303785 (2024).
Kim, J. J. et al. Pathophysiological reconstruction of a tissue‐specific multiple‐organ on‐a‐chip for Type 2 diabetes emulation using 3D cell printing. Adv. Funct. Mater. 33, 2213649 (2023).
Tao, T. et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv. Sci. (Weinh.) 9, e2103495 (2022).
Gough, A. et al. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat. Rev. Gastroenterol. Hepatol. 18, 252–268 (2021).
El Ouaamari, A. et al. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Rep. 3, 401–410 (2013).
Schurdak, M. et al. Applications of the microphysiology systems database for experimental ADME-Tox and disease models. Lab Chip 20, 1472–1492 (2020).
Sakolish, C. et al. Analysis of reproducibility and robustness of a human microfluidic four-cell liver acinus microphysiology system (LAMPS). Toxicology 448, 152651 (2021).
Miedel, M. T. et al. Reproducibility Analytics and Metadata are Required to Qualify Microphysiology Systems for Interpreting Biologically/Clinically Relevant Heterogeneity for Precision Medicine. bioRxiv (2024).
Xia, M. et al. Comparison of Wild-Type and High-risk PNPLA3 variants in a Human Biomimetic Liver Microphysiology System for Metabolic Dysfunction-associated Steatotic Liver Disease Precision Therapy. Front. Cell Dev. Biol. 12, https://doi.org/10.3389/fcell.2024.1423936 (2024).
Saydmohammed, M. et al. Quantifying the progression of non-alcoholic fatty liver disease in human biomimetic liver microphysiology systems with fluorescent protein biosensors. Exp. Biol. Med. (Maywood) 246, 2420–2441 (2021).
Lee-Montiel, F. T. et al. Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp. Biol. Med. (Maywood) 242, 1617–1632 (2017).
Ben-Moshe, S. & Itzkovitz, S. Spatial heterogeneity in the mammalian liver. Nat. Rev. Gastroenterol. Hepatol. 16, 395–410 (2019).
Hijmans, B. S., Grefhorst, A., Oosterveer, M. H. & Groen, A. K. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie 96, 121–129 (2014).
Honma, M. et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J. Obes. (Lond.) 42, 1544–1555 (2018).
Chainy, G. B. N. & Sahoo, D. K. Hormones and oxidative stress: an overview. Free Radic. Res. 54, 1–26 (2020).
Leloup, C. et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58, 673–681 (2009).
Sanches, J. M., Zhao, L. N., Salehi, A., Wollheim, C. B. & Kaldis, P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. FEBS J. 290, 620–648 (2023).
Nguyen-Tu, M. S. et al. Opposing effects on regulated insulin secretion of acute vs chronic stimulation of AMP-activated protein kinase. Diabetologia 65, 997–1011 (2022).
Yamasandhi, P. G., Dharmalingam, M. & Balekuduru, A. Fetuin-A in newly detected type 2 diabetes mellitus as a marker of non-alcoholic fatty liver disease. Indian J. Gastroenterol. 40, 556–562 (2021).
Dogru, T. et al. The evolving role of fetuin-a in nonalcoholic fatty liver disease: an overview from liver to the heart. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22126627 (2021).
Stefan, N. et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 57, 2762–2767 (2008).
Mathews, S. T. et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol. Cell Endocrinol. 164, 87–98 (2000).
Mathews, S. T. et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 51, 2450–2458 (2002).
Stefan, N. et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29, 853–857 (2006).
Goustin, A. S., Derar, N. & Abou-Samra, A. B. Ahsg-fetuin blocks the metabolic arm of insulin action through its interaction with the 95-kD beta-subunit of the insulin receptor. Cell Signal. 25, 981–988 (2013).
Mukhuty, A., Fouzder, C. & Kundu, R. Blocking TLR4-NF-kappaB pathway protects mouse islets from the combinatorial impact of high fat and fetuin-A mediated dysfunction and restores ability for insulin secretion. Mol. Cell Endocrinol. 532, 111314 (2021).
Nag, S., Mandal, S., Majumdar, T., Mukhopadhyay, S. & Kundu, R. FFA-Fetuin-A regulates DPP-IV expression in pancreatic beta cells through TLR4-NFkB pathway. Biochem Biophys. Res Commun. 647, 55–61 (2023).
Holter, M. M., Saikia, M. & Cummings, B. P. Alpha-cell paracrine signaling in the regulation of beta-cell insulin secretion. Front Endocrinol. (Lausanne) 13, 934775 (2022).
Gerst, F. et al. Metabolic crosstalk between fatty pancreas and fatty liver: effects on local inflammation and insulin secretion. Diabetologia 60, 2240–2251 (2017).
Zhang, Z. H. et al. Serum retinol-binding protein levels are associated with nonalcoholic fatty liver disease in Chinese patients with type 2 diabetes mellitus: a real-world study. Diabetes Metab. J. 46, 129–139 (2022).
Flores-Cortez, Y. A. et al. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 26, https://doi.org/10.3892/mmr.2022.12760 (2022).
Huang, R., Bai, X., Li, X., Wang, X. & Zhao, L. Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic beta-Cell Dysfunction in Type 2 Diabetes. Diabetes 70, 449–463 (2021).
Campbell, S. A., Johnson, J. & Light, P. E. Evidence for the existence and potential roles of intra-islet glucagon-like peptide-1. Islets 13, 32–50 (2021).
Hwang, J. J., Chan, J. L., Ntali, G., Malkova, D. & Mantzoros, C. S. Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide: interventional studies in humans. Diabetes Care 31, 945–951 (2008).
Fiocca, R. et al. Pancreatic polypeptide (PP) cells in the PP-rich lobe of the human pancreas are identified ultrastructurally and immunocytochemically as F cells. Histochemistry 77, 511–523 (1983).
Chanput, W., Mes, J. J. & Wichers, H. J. THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharmacol. 23, 37–45 (2014).
Baxter, E. W. et al. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNgamma+LPS), M(IL-4) and M(IL-10) phenotypes. J. Immunol. Methods 478, 112721 (2020).
Balis, U. J. et al. Oxygen consumption characteristics of porcine hepatocytes. Metab. Eng. 1, 49–62 (1999).
Stephenne, X. et al. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transpl. 16, 409–419 (2007).
Ochs, C. J., Kasuya, J., Pavesi, A. & Kamm, R. D. Oxygen levels in thermoplastic microfluidic devices during cell culture. Lab Chip 14, 459–462 (2014).
Vekemans, K. & Braet, F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J. Gastroenterol. 11, 5095–5102 (2005).
Loneker, A. E., Faulk, D. M., Hussey, G. S., D’Amore, A. & Badylak, S. F. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. J. Biomed. Mater. Res. A 104, 1846–1847 (2016).
Oparka, M. et al. Quantifying ROS levels using CM-H(2)DCFDA and HyPer. Methods 109, 3–11 (2016).
Lefever, D. E. et al. A Quantitative Systems Pharmacology Platform Reveals NAFLD Pathophysiological States and Targeting Strategies. Metabolites 12, https://doi.org/10.3390/metabo12060528 (2022).
Acknowledgements
This work was supported by the following grants from the National Institute of Health (NIH) and National Science Foundation (NSF): UH3TR003289-NIH/NCATS (J. Behari, A. Soto-Gutierrez, and D.L. Taylor), U24TR002632-NIH/NCATS (M.E. Schurdak, A. Gough, and D.L. Taylor), UH3DK119973-NIH/NIDDK (A. Soto-Gutierrez, I. Banerjee, and D.L. Taylor), U24TR002632-NIH/NCATS (M.E. Schurdak, A. Gough, and D.L. Taylor), S10OD028450-NIH/OD (D.L. Taylor), R01DK117881-NIH/NIDDK (D.L. Taylor), 1706674-NSF/CBET (I. Banerjee), 2229156-NSF/CBET (I. Banerjee), and R01DK135606-NIH/NIDDK (M.T. Miedel and A. Soto-Gutierrez). The authors would like to acknowledge valuable discussions with members of the Drug Discovery Institute and the Pittsburgh Liver Research Center (PLRC). We would like to thank the Biostatistical Consulting Laboratory at the University of Pittsburgh School of Public Health for its assistance with correlation analysis; however, any errors of fact or interpretation remain the sole responsibility of the authors. The cytokine data presented here were obtained using the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory.
Author information
Authors and Affiliations
Contributions
J.A., R.K., C.W., and L.V. conceptualized and designed the experiments under the supervision of A.G., V.K.Y., M.T.M., A.M.S., I.B., and D.L.T. J.A., R.K., and C.W. participated in MPS experiments. J.A., R.K., C.W., D.G., C.R., R.D., and G.L. participated in the efflux and imaging quantification. J.A., M.E.S., and Y.D.A. participated in reproducibility and correlation analysis. A.S.G., J.B., and V.K.Y. lead the clinical input. J.A., R.K., C.W., M.E.S., M.T.M., and A.M.S. wrote the manuscript under the supervision of V.K.Y., I.B., and D.L.T. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.L.T., A.G., and M.E.S. have equity in Nortis, a company supplying MPS chips/some automation and EveAnalytics (accessing, analyzing, and computationally modeling data on patient-derived microphysiology systems).
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Simona Chera and Ophelia Bu. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aleman, J., K, R., Wiegand, C. et al. A metabolic dysfunction-associated steatotic liver acinus biomimetic induces pancreatic islet dysfunction in a coupled microphysiology system. Commun Biol 7, 1317 (2024). https://doi.org/10.1038/s42003-024-07006-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07006-7