Abstract
Using chromosomal barcoding, we observed that >97% of the Streptococcus pneumoniae (Spn) population turns over in the lung within 2 days post-inoculation in a murine model. This marked collapse of diversity and bacterial turnover was associated with acute inflammation (severe pneumococcal pneumonia), high bacterial numbers in the lungs, bacteremia, and mortality. Intra-strain competition mediated by the blp locus, which expresses bacteriocins in a quorum-sensing-dependent manner, was required for each of these effects. Bacterial turnover from the activity of Blp-bacteriocins increased the release of the pneumococcal toxin, pneumolysin (Ply), which was sufficient to account for the lung pathology. The ability of Ply to evade complement, rather than its pore-forming activity, prevented opsonophagocytic clearance of Spn enabling its multiplication in the lung, facilitating the inflammatory response and subsequent invasion into the bloodstream. Thus, our study demonstrates how an appreciation for bacterial population dynamics during infection provides new insight into pathogenesis.
Similar content being viewed by others
Introduction
Competition is a hallmark feature of bacterial ecology that shapes microbial communities and influences their behavior. Bacteria have evolved numerous tactics to outwit their competitors and emerge victorious. One way they can achieve this is indirectly through resource competition, wherein the acquisition of a limiting resource by a bacterial strain restricts its availability for the competitor. Alternatively, bacteria can produce an arsenal of weapons that directly damage their competitors. These include elaborate machineries such as the Type VI secretion system that can deliver anti-bacterial toxins to target cells1,2,3, or small diffusible bacteriocin molecules that eliminate competitors4,5. Bacteria utilize these weapons not only for inter-species and inter-strain competitive behaviors, but also to engage in intra-strain rivalry6,7. Intra-strain competition is an intriguing phenomenon whereby genetically identical bacterial kin cells within a population battle each other for survival. Bacteria employ collective behaviors such as quorum sensing to carefully coordinate these interactions and regulate weapon production. For example, a sub-population reaching its quorum produces bacteriocins and consequently, lyses the remaining sub-populations that were yet to activate their cognate immunity proteins8,9.
Streptococcus pneumoniae (Spn) encodes for a vast array of bacteriocin molecules that can mediate inter- and intra-strain competition9,10,11,12. Like other bacteria, Spn also employs quorum sensing pathways that allow it to carefully regulate bacteriocin production, and emerge successful during competition. Our recent work has demonstrated an example of this behavior, wherein Spn activates BlpC-induced quorum sensing pathway to regulate production of Blp bacteriocins and mediate intra-strain competition during colonization of the host nasopharynx9. Using a molecularly barcoded library, we showed that the induction of the Blp bactericidal program causes a rapid loss of clonal diversity with over 70% of the clonal population turning over within the first day of colonization. While these findings demonstrated the existence of tight population bottlenecks during host colonization, the consequences of these phenomena on the pathogenesis of Spn remain unclear.
Here, we investigate how bacteriocin-mediated turnover impacts pneumococcal pathogenesis in a lung infection model. Spn is a major human pathogen and the leading cause of pneumonia morbidity and mortality globally13,14,15,16,17. Spn’s ability to induce inflammation is a key feature characteristic of its pathogenesis during pneumococcal pneumonia18,19,20. We hypothesized that bacteriocin-mediated Spn lysis and turnover results in the release of inflammatory mediators, including its sole toxin, pneumolysin (Ply). Ply is a member of the cholesterol-dependent cytolysin (CDC) family of pore-forming toxins21. Unlike other CDCs, Ply does not possess an N-terminal secretion signal or any other known secretion mechanism making it unclear how it is released from Spn22. Using both in vitro and in vivo studies, we demonstrated that Blp bacteriocin-mediated intra-strain competition and Spn turnover facilitates Ply release from the cell. Our results reveal that bacteriocin-mediated turnover and the subsequent Ply release is responsible for the inflammatory pathology that characterizes pneumococcal pneumonia.
Results
Blp bacteriocin-mediated intra-strain competition drives pneumococcal pneumonia
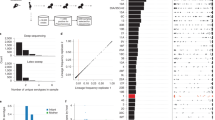
To investigate the impact of Blp bacteriocins (Supplementary Fig. 1) on Spn turnover during lung infection, we first established a pneumonia model with a virulent type 4 Spn strain (TIGR4). Following the establishment of infection at 1 day post-inoculation (dpi), WT Spn rapidly multiplied in the lung resulting in >50-fold increased bacterial load by 2 dpi (Fig. 1A). We constructed a diverse molecularly barcoded library of the TIGR4 strain to examine the population dynamics of Spn during this duration within the lung. We analyzed the population structure of barcoded Spn at 2 dpi relative to the structure observed immediately following inoculation within the lung (0 h), since the overall bacterial burden at these time points is comparable (Fig. 1A, Supplementary Fig. 2). By 2 dpi, there was a collapse of diversity with only 2.4% of the successfully inoculated clones being detected in the lungs (Fig. 1B, Supplementary Fig. 3). The remaining >97% of the clones could not be detected at this time point (Supplementary Fig. 3). In contrast to 0 h, the Spn population at 2 dpi was dominated by a single clonal lineage (Fig. 1C), reminiscent of the extensive intra-strain competition observed during colonization of the upper respiratory tract (URT)9. In addition, barcoding had no impact on the virulence of the parental TIGR4 strain.
A Burden of Spn strains per gram of lung tissue at 1 & 2 dpi. Whiskers on the plot show min to max values. B–E Spn undergoes extensive turnover in the lungs. B, C Graphs depict B Hill’s N1 (clonal diversity) and C proportion of the most abundant clone of WT Spn detected in the lung at 0 h & 2 dpi. D, E Graphs depict D fold change in Hill’s N1 (clonal diversity), and E proportion of most abundant clone for WT and Δblp strains at 1 dpi. F–H Burden of Spn strains per gram of (F, H) lung, and G spleen (Δblp & Δblp::blp: P = 0.0022 by Mann–Whitney test) at 2 dpi. Animals were infected with either A–G TIGR4, or H type 6A Spn strains. P values were calculated by (A) two-way ANOVA followed by Tukey’s multiple comparisons, (B–E) two-tailed t test, and (F–H) Kruskal–Wallis test followed by Dunn’s multiple comparisons. Each dot represents an individual animal (Geometric mean ± 95% CI). I Table represents survival of mice inoculated with TIGR4 and Type 6A Spn strains until humane end point at 2 dpi. P values denote comparison with WT by Mantel–Cox test. ‘ns’ denotes statistically not significant.
Our recent work had shown that quorum sensing-mediated induction of bacteriocins encoded by the blp locus promote intra-strain competition during pneumococcal colonization of the URT9. Thus, we investigated whether the blp locus also impacts Spn population structure and pathogenesis of disease in the pneumonia model. For this, we validated that the Δblp strain did not show any fitness defect in vivo during nasopharyngeal colonization in the infant mouse model (Supplementary Fig. 2). Thereafter, we tested this strain in the pneumonia model. The Δblp strain showed equivalent numbers to WT Spn in the lung immediately following inoculation and at 1 dpi (Fig. 1A and Supplementary Fig. 2). In contrast to the WT, there was >2000-fold reduction in the lung bacterial burden of mice at 2 dpi when inoculated with Δblp strain (Fig. 1A, F). Owing to this vast difference in overall bacterial loads at 2 dpi, we compared the population structure of WT and Δblp strains at 1 dpi when there is no difference in the lung bacterial burden. At 1 dpi, while WT Spn had lost over 78% of its clonal diversity, there was a less than 10% reduction in diversity of the Δblp strain (Fig. 1D). Further, the most abundant WT clone was present at a frequency of 14.6%, while the corresponding frequency for the Δblp clone was 2.7% at 1 dpi (Fig. 1E). These results suggested that Blp bacteriocins-mediated intra-strain competition was responsible for the bacterial turnover and collapse in Spn diversity observed within the lung.
In addition, following infection of the lungs, WT Spn is able to invade into the bloodstream as evidenced by high bacterial load in the spleen by 2 dpi (Fig. 1G). At this humane endpoint, 44.4% of the mice had succumbed to the infection (Fig. 1I). In contrast, there was no mortality by 2 dpi with Δblp strain, which correlated with an over a 5000-fold reduction in the splenic bacterial load (Fig. 1G, I). In the Δblp::blp corrected strain, WT levels of mortality and bacterial burdens in the lung and spleen were restored (Fig. 1F, G, I). Together, these results suggest that Blp bacteriocin-mediated bacterial turnover promotes the development of pneumococcal pneumonia and bacteremia.
We also validated whether the loss of blp locus impacted pneumococcal burden in the lungs and spleen using another virulent pneumococcal strain of type 6A (Spn 6A). As with TIGR4, infection with 6A Δblp also resulted in a dramatic reduction of the Spn burden both in the lung and spleen, relative to 6A WT strain (Fig. 1H and Supplementary Fig. 4). Further, infection with 6A Δblp strain also resulted in no mortality by 2 dpi, compared to 100% mortality observed with 6A WT (Fig. 1I). These results demonstrate that the blp locus drives the development of pneumococcal disease.
Blp bacteriocin-mediated Spn turnover promotes host inflammation
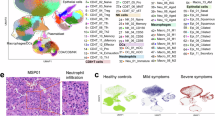
To examine the pulmonary pathology of infected animals, we performed hematoxylin and eosin (H&E)-staining of lung tissues at 2 dpi. Compared to mock-infected controls, lungs from animals infected with TIGR4 WT Spn showed dramatic pathological alterations, characteristic of acute inflammation, including marked lung edema, and infiltration of inflammatory cells, typical of pneumococcal pneumonia (Fig. 2A). However, lungs from animals infected with the Δblp strain showed minimal inflammation (Fig. 2A). Immunofluorescent staining of Spn, and Ly6G+ cells, a marker for neutrophils, from WT Spn-infected lung tissue demonstrated the presence of high bacterial loads and infiltration of neutrophils (Fig. 2B). Inoculation with Δblp strain also led to the recruitment of neutrophils, consistent with their role in clearing infection (Fig. 2B). These lungs showed fewer pneumococci consistent with a lower lung CFU burden compared to WT-infected lungs. Further, the bacterial staining was distinct in the case of infection with WT versus Δblp strains (Fig. 2B, insets). While intact diplococci predominated following inoculation with WT Spn, the staining in the case of Δblp strain showed a pattern consistent with uptake and lytic killing by professional phagocytes23. These results showed that infection with WT Spn, but not the Δblp strain, led to severe pneumonia characterized by high numbers of viable bacteria and suggested this was due to reduced bacterial clearance.
Images denote lung sections, 2 days following intranasal inoculation of mice with either PBS or Spn (WT, Δblp, and Δply). A H&E-stained lung sections. Scale: 100 µm. B Immunofluorescence images. Scale: 50 µm. White arrows denote phagocytosed and lysed Spn. These are also depicted at ×5 magnification in insets, and in comparison with intact WT diplococci.
Blp bacteriocin-mediated Spn turnover facilitates Ply release
We hypothesized that Blp bacteriocins-mediated Spn turnover results in the release of inflammatory mediators, such as Ply, that trigger host inflammation and result in severe pneumonia. To test this hypothesis and validate that Blp bactericidal activity results in Spn turnover, we utilized in vitro agar overlay inhibition assays24. As previously reported, in vitro inhibition assays are not sensitive enough to demonstrate bactericidal activity using certain genomic lineages such as TIGR424,25. As such, we validated this bactericidal activity by employing strains belonging to Spn 6A lineage. Spn 6A WT and Blp bacteriocin effector-deficient (ΔblpMNO) Spn were tested for their ability to inhibit the growth of a bacteriocin-deficient overlay strain. As expected, WT Spn showed inhibitory activity against the overlay strain as evidenced by the appearance of zones of inhibition (Fig. 3A, B). However, this inhibitory activity was not observed with the ΔblpMNO strain, confirming that the bacteriocins of the blp locus mediate bacterial competition and result in Spn lysis and turnover (Fig. 3A, B).
A, B Blp bacteriocins mediate Spn turnover in vitro. Overlay inhibition assay depicting lysis of ΔblpMNO overlay strain by producer strains (WT or ΔblpMNO). A Images depicting zones of inhibition in the overlay strain. B Sizes of zones of inhibition. Each dot represents an independent measurement (Arithmetic mean ± standard deviation). P values were calculated using two-tailed t test. C, D Spn turnover by Blp bacteriocins facilitates Ply release. Equal numbers of the two strains were mixed, followed by incubation for 2 h. Equal volumes of the cell-free supernatants from in vitro competition between the strains was loaded and used to detect Ply using (C) anti-Ply immunoblot (rPly was used as a positive control), and D hemolysis of horse erythrocytes (two representative replicates shown, n = 3).
We then investigated whether bacteriocin-mediated Spn turnover facilitates Ply release from the cell. Ply-deficient (Δply) “attacker” strain was competed with blp-deficient (Δblp) “target” strain in vitro for 2 h followed by testing for Ply levels in the cell-free supernatant. Any Ply detected in the supernatant would have been released solely from the target cells, since the attacker strain was Ply-deficient. Western blot showed that the Δblp strain released high levels of Ply in competition with Blp bacteriocin-expressing (Δply) attacker strain (Fig. 3C). In contrast, Δblp strain released 3.4-fold lower levels of Ply in competition with a Blp bacteriocin-deficient (ΔblpΔply) attacker strain (Fig. 3C). The residual Ply released in this case is not due to the activity of Blp bacteriocins, but possibly due to basal turnover of target cells. To further test our hypothesis, we utilized the hemolyzing ability of Ply to test for its release in the supernatant from target Spn cells26,27,28. Similar to Western blots, the Δblp strain released ~four-fold more Ply in competition with Δply strain, compared to when competed with ΔblpΔply strain (Fig. 3D). No hemolysis was observed when both attacker and target strains were Ply-deficient indicating that the observed hemolysis was specific to Ply activity (Fig. 3D). Further, the loss of Blp bacteriocins did not affect basal Ply levels in the cell (Supplementary Fig. 5). These results establish that bacterial competition facilitated by Blp bacteriocins leads to Spn turnover and subsequent Ply release from the cell.
Blp bacteriocin-mediated Ply release triggers lung inflammation and results in pneumococcal pneumonia
Then, we tested whether Blp-dependent release of Ply promotes pneumococcal pneumonia in our model. Similar to Δblp, inoculation with Δply strain also resulted in marked reduction in the bacterial burden both in the lung and spleen at 2 dpi relative to WT strain and no mortality by this time point in both TIGR4 and 6A backgrounds (Fig. 1A, G–I and Supplementary Fig. 4). Histopathology showed that the lung sections from mice inoculated with Δply strain resembled those from the Δblp infection (Fig. 2A). Lungs infected with Δply strain also displayed a bacterial staining pattern consistent with Spn uptake and lysis (Fig. 2B). These results indicated that loss of either Blp bacteriocins or Ply protects the host from development of severe pneumonia.
We investigated whether Ply release facilitated by activity of the Blp system promotes pneumonia. The addition of purified recombinant Ply (rPly) to the Δblp strain inoculum led to increased bacterial loads both in the lung and spleen at 2 dpi (Fig. 4A and Supplementary Fig. 6). This suggested that extracellular Ply in the absence of Blp bacteriocin-mediated Spn turnover is sufficient in leading to multiplication of Spn in the lung and promoting pneumonia. Further, we investigated whether trans-complementation with Ply achieved through Blp bacteriocins-mediated bacterial competition can drive pneumonia. For this, we inoculated mice with a 1:1 mix of Δply with either Δblp or ΔblpΔply strains. Each of these strains is individually attenuated in their virulence. We hypothesized that Blp bacteriocins from the Δply strain will lyse the Δblp strain, thereby facilitating Ply release from the latter and subsequent bacterial multiplication in the lung. In accordance, we observed that the mixture of Δply with Δblp strain, but not with Δblp Δply strain, resulted in increased bacterial loads in the lung and spleen at 2 dpi (Fig. 4B and Supplementary Fig. 6). Of the two competing strains that result in increased bacterial load, over 75% of Spn in the lungs at 2 dpi was the Δply strain (Supplementary Fig. 7). The higher prevalence of the Δply strain was consistent with this strain outcompeting and killing the Δblp strain via the activity of Blp bacteriocins. Together, these results confirmed that the activity of Blp bacteriocins in mediating bacterial competition and turnover facilitate Ply release, resulting in pneumococcal pneumonia.
Bacterial burden in lungs at 2 dpi following Spn infection. Ply, but not its pore-forming activity, is critical for increased bacterial burden in the lungs. A Complementation of Δblp strain with 250 ng of recombinant (rPly) or non-pore forming, toxoid version of pneumolysin (rPdB) increases Spn levels in the lungs. B Mice were inoculated with a 1:1 mix of Δply with either Δblp or ΔblpΔply strains. C W433F point mutation ablates the pore-forming activity of Ply. Each dot represents an individual animal (Geometric mean ± 95% CI). P values were calculated using A, C Kruskal–Wallis test followed by Dunn’s multiple comparisons, or B Mann–Whitney test.
Role of Ply in evading complement leads to higher bacterial load in lungs
Ply is a multifunctional toxin that promotes host inflammation and Spn pathogenesis during pneumonia in a number of ways29,30,31. To test whether its pore-forming cytolytic activity is responsible for augmenting bacterial growth in our pneumonia model, we utilized a mutant strain that contains a single amino acid change in Ply (W433F) that renders the toxin deficient in pore-formation (Δply::PlyW433F)23,32. Unlike Δply, the Δply::PlyW433F strain did not result in reduced bacterial load in the lungs or the spleen at 2 dpi (Fig. 4C and Supplementary Fig. 6). Addition of the purified recombinant toxoid containing the W433F mutation (PdB) to the Δblp strain also resulted in increased bacterial loads in both the organs at this time point (Fig. 4A and Supplementary Fig. 6). These data suggested that the pore-forming activity of Ply is not required for Spn multiplication in the lungs and subsequent invasion into the bloodstream.
It is well established that Ply also promotes Spn pathogenesis by serving as a molecular decoy that activates and locally depletes complement, thereby preventing complement deposition on the bacterial surface29,30,33,34,35,36,37. We then investigated whether this role of Ply was critical in leading to increased bacterial load in our pneumonia model. To this end, we treated mice either with cobra venom factor (CoVF) to deplete complement systemically or PBS vehicle control prior to intranasal infection38,39,40,41. In case of inoculation with the Δply strain, CoVF treatment was sufficient to increase the lung bacterial load relative to PBS control-treated mice at 2 dpi (Fig. 5A). This suggested that the activity of Ply in inhibiting complement deposition on Spn surface is important for augmenting bacterial growth in the lungs. If Blp bacteriocin-mediated Ply release was responsible for reduced Spn load in the lung, then prior complement depletion should also result in increased bacterial load following inoculation with Δblp strain. In agreement, CoVF treatment was also sufficient to increase the load of the Δblp strain both in the lung and spleen at 2 dpi (Fig. 5B and Supplementary Fig. 8). Complement depletion prevents opsonization and subsequent phagocytosis of Spn cells by professional phagocytes42. To test if neutrophil depletion also led to increased bacterial loads following infection, mice were treated either with anti-Gr-1 (αGr-1) monoclonal antibody to deplete neutrophils (and other Gr-1+ professional phagocytes) or isotype (IgG) control prior to intranasal bacterial inoculation. αGr-1 treatment was sufficient in depleting the numbers of circulating neutrophils, while not significantly affecting monocyte counts (Supplementary Fig. 9). αGr-1 treatment resulted in increased Spn loads in the lung following inoculation of both Δply (Fig. 5C) or Δblp strains (Fig. 5D) relative to their isotype controls. Depletion of Gr-1+ cells also resulted in increased splenic loads of the Δblp strain at 2 dpi (Supplementary Fig. 8). Thus, these data showed that the role of Ply in activating and locally depleting complement is important in inhibiting phagocyte-mediated clearance in the lungs, and the subsequent development of bacteremia. Further, our results demonstrate that the activity of Blp bacteriocins in facilitating bacterial turnover and Ply release promotes pneumococcal pneumonia via Ply’s role in evading the activity of complement.
Bacterial burden in lungs at 2 dpi following Spn infection. A, B Cobra venom factor (CoVF) treatment to deplete complement results in an increased burden of A Δply, and B Δblp strains in the lungs relative to PBS controls. C, D Neutrophil depletion using αGr-1 results in increased levels of C Δply, and D Δblp strains in the lungs relative to isotype controls. Each dot represents an individual animal (Geometric mean ± 95% CI). P values were calculated using the Mann–Whitney test.
Discussion
To date, there has been minimal investigation regarding processes that shape population structures of bacterial pathogens throughout their entire infection lifecycle. In this study, we demonstrate that Spn exhibits a collapse of clonal diversity during lung infection. Within 2 days following inoculation, the structure of infecting Spn in the lung changes dramatically with a single clonal lineage dominating the population. This intra-strain bacterial competition mediated by Blp bacteriocins leads to bacterial lysis and turnover that is responsible for the inflammation that characterizes pneumococcal pneumonia. Our results suggest that this constant bacterial turnover within a population is necessary to promote bacterial multiplication and establish the dominance of a single clonal lineage in the lung.
Spn population is known to experience tight population bottlenecks that limit clonal success both during nasopharyngeal colonization and host-to-host transmission9. In this study, we show that the same is true for lung infection, wherein sustained bacterial turnover and loss of diversity following inoculation impose a tight population bottleneck within the lung. This is consistent with previous work that demonstrated existence of a tight population bottleneck in a pneumonia model using CRISPRi-Seq43. Here, we demonstrate that Blp bacteriocin-mediated intra-strain competition is responsible for tightening of this population bottleneck, resulting in the success of a single clonal lineage. We have recently shown that in an example of quorum-sensing-induced phenotypic heterogeneity, the sub-population that reaches quorum and activates Blp bacteriocins first is able to eliminate others that have yet to activate their cognate immunity proteins9. This competitive process, moreover, is observed even in the case of isogenic bacteria as shown in this report.
Interestingly, our results highlight that Blp system-mediated bacterial turnover is necessary to promote expansion of Spn population in the lung. WT and Δblp strains showed no difference in overall bacterial burdens at 1 dpi despite stark differences in their population structures. By 2 dpi, there was a >58-fold increase in WT Spn burden within the lung. In contrast, there was a sustained reduction in the overall load of Δblp strain. These data highlight that Blp bacteriocin-mediated change in WT strain’s population structure is necessary to promote expansion of Spn in the lung. This increase in bacterial load is not observed in the absence of bacterial turnover and subsequent Ply release.
Our study of Spn population dynamics reveal novel insights into facets of bacterial infection biology that have long remained puzzling. For example, the classic 1964 study by Austrian and Gold reported the unexpected finding that penicillin treatment had no effect on the outcome of infection in pneumococcal pneumonia patients during the first five days of illness44,45. Penicillin is a bactericidal antibiotic that induces a lytic response in Spn, and triggers host inflammation46,47,48. Our study shows that Spn undergoes extensive turnover within the lung in a model of pneumococcal pneumonia in the absence of antibiotics. This Blp bacteriocin-mediated turnover may explain Austrian and Gold’s clinical observation, wherein bacteriocin-dependent killing results in a similar inflammatory effect as penicillin-induced lysis.
Our study reveals how bacteriocins that mediate bacterial competition are also able to promote bacterial pathogenesis by facilitating the release of inflammatory mediators. The toxin, Ply, is just one example of such inflammatory mediators; Spn turnover will also be expected to result in the release of other PAMPs. However, these other PAMPs do not appear to significantly contribute to Spn multiplication in the lung since, trans-complementation in the absence of Ply had no effect on lung Spn load. Bacteriocin-mediated turnover among other pathogenic bacteria could promote the release of a range of toxins and PAMPs that can be detected by the host innate immune system. Thus, such turnover triggered host inflammation may be an underappreciated aspect of host-pathogen interactions conserved across numerous bacterial pathogens.
An open question in the field has been the mechanism leading to Ply export from the bacterial cell to exert its cytotoxic effects. One proposed mechanism for its release is through Spn autolysis49,50,51,52. Alternatively, it has been postulated that Ply is actively exported outside the cell via an autolysis-independent but yet unknown mechanism53,54,55. Our studies reveal that in vivo Ply is predominantly released from the cell through bacteriocin-mediated bacterial competition and Spn turnover. The release of Ply due to Blp bacteriocin-mediated turnover results in increased bacterial load in the lung, leading to the development of severe pneumonia and subsequent bacteremia. Our results show that by 2 dpi, there was over a 50-fold increase in WT Spn levels in the lung. In contrast, both the Δblp and Δply strains continued to be cleared from the tissue. The bacterial staining in the lung tissues from animals inoculated with these mutant strains was also consistent with their uptake and lytic killing by professional phagocytes such as neutrophils. Further, depleting complement or inhibiting Gr-1+ cell infiltration was sufficient to result in increased Δblp and Δply levels in the lung. Together, these results indicate that bacteriocin-mediated turnover and subsequent Ply release, result in the inhibition of complement deposition on Spn surface, thereby leading to reduced opsonophagocytic bacterial clearance from the lung. In addition, previous work has also demonstrated that Ply promotes an anti-inflammatory response in mouse alveolar macrophages resulting in enhanced pneumococcal burden in the airways56,57. This alternative mechanism is also consistent with our finding that increased Ply release facilitates the development of pneumococcal pneumonia. While we cannot rule out the possibility that Ply activity also contributes to the release of nutrients that, in turn, facilitate increased Spn replication, this is unlikely since the pore-forming ability of Ply is not required for an increase in the lung bacterial load.
An interesting aspect of the blp locus is its hierarchical regulatory interplay with the competence quorum-sensing system11,58,59. In addition to being activated by the BlpC quorum-sensing pheromone, the blp locus has also been shown to be induced upon competence stimulating peptide (CSP) stimulation58,59. The competence system machinery also contributes to the export of peptides of the blp system11,60. How this complex regulatory interplay with the competence system influences the dynamics of bacterial turnover during lung infection will be the subject of future investigations.
The blp locus, which is under negative frequency-dependent selection, is one of the most heterogenous regions in the Spn genome61,62. The locus exhibits exceptional diversity at all levels of genomic organization—the number of putative bacteriocin and immunity genes encoded, allelic variation, and presence or absence of specific genes61. The two strains (TIGR4 and type 6A) under investigation in this study also show substantial variation in the number and types of bacteriocin peptides encoded within their respective blp loci. Our results show that despite this variation, the blp locus from both strains contributes to a similar increase in bacterial load in the lung. The blp mutants used in these infection studies encompassed deletion of the entire blp locus. As such, we cannot fully exclude the possibility that other hypothetical or putative immunity proteins encoded by the locus also contribute to the observed phenotypes.
The blp locus is an example of a selfish genetic element that promotes the survival of a clonal linage that is first to activate its quorum sensing program, contributing to collapse of diversity and clonal success in the URT9. Spn colonization episodes are not as inflammatory in comparison to lower respiratory tract (LRT) infections. As such, in contrast to the lungs, Blp bacteriocins do not significantly impact the overall bacterial loads during colonization9. The low level of complement found at the mucosal surface relative to serum likely minimizes the impact of Blp bacteriocin-mediated Ply release in mediating evasion of phagocyte clearance as seen in the lungs. Thus, changes in population structure can have distinct effects at different sites of infection. Additionally, RNA-seq has shown that genes of blp locus are upregulated in the lungs relative to the nasopharynx63. This suggests that Blp bacteriocins may have a more dramatic impact on the population dynamics of Spn in the pneumonia model. However, since infection of the lung is likely an ecological dead end for the bacteria, the Blp-mediated clonal success in the LRT will not be advantageous to Spn in facilitating its transmission to a new host. In contrast, in the URT, the mild inflammation induced by Ply stimulates nasal secretions that increase host-to-host Spn transmission promoting its spread within the host population64.
Thus, our findings demonstrate the importance of studying population dynamics during infection and how they can enable mechanistic investigations into bacterial pathogenesis. A unifying feature during infection by opportunistic pathogens is the release of bacterial toxins and PAMPs that trigger the host inflammatory cascade. Many of these toxins are regulated through quorum-sensing pathways. For instance, activation of the Agr quorum sensing system in Staphylococcus aureus induces transcription of hemolysins, including the α-hemolysin65. As such, our work that explores the role of competitive interactions and quorum sensing-induced mediators of competition in shaping host-pathogen interactions has broad relevance for the field of bacterial pathogenesis. Future investigations that explore the role of quorum sensing pathways in impacting population dynamics of other bacterial species in vivo will provide greater insight into their mechanisms of disease.
Methods
Ethics statement
All animal experiments were performed according to the guidelines laid by the National Science Foundation Animal Welfare Act (AWA) and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. We have complied with all relevant ethical regulations for animal use. NYU’s Grossman School of Medicine’s Institutional Animal Care and Use Committee (IACUC) oversees the welfare, well-being, proper care, and use of all animals. They have approved the protocols used in this study: IA16-00538.
Bacterial strains and growth conditions
Spn TIGR4 strain, P2406, was used for animal studies in this work. P2406 is a streptomycin-resistant derivative of the lab TIGR4 strain which has previously been used for in vivo studies9,66,67. All bacterial strains used in this experimental work are listed in Supplementary Table 1. Colonies were grown from frozen stocks by streaking on TSA-II agar plates supplemented with 5% sheep blood (BD BBL, NJ, USA). Unless otherwise stated, starter cultures were prepared by inoculating streaked colonies in tryptic soy (TS) broth statically at 37 °C until they reached an optical density at 620 nm (OD620) of 1.0. The cells were then pelleted, washed, and resuspended in sterile phosphate-buffered saline (PBS) for mouse inoculations. Bacterial numbers were enumerated by plating serial dilutions on TSA plates supplemented with 100 µl of catalase (38,000 U/ml; Worthington Biochemical Corporation, NJ) and the desired antibiotic (250 µg/ml kanamycin, 200 µg/ml streptomycin, or 200 µg/ml spectinomycin) and incubated overnight at 37 °C with 5% CO2.
Construction of mutants
Spn mutants were constructed as previously described9,67,68,69,70. Briefly, colonies were picked and inoculated in acidic Columbia broth (pH 6.6) and grown until an OD595 of 0.05 followed by the addition of 5 µg/ml of 1:1 mix of CSP1 and CSP2 along with 500 ng of transforming DNA. Cultures were incubated statically at 37 °C with 5% CO2 followed by plating on TSA plates supplemented with 100 µl of catalase (38,000 U/ml; Worthington Biochemical Corporation, NJ) and the desired antibiotic (250 µg/ml kanamycin or 200 µg/ml streptomycin). The blp locus contained a number of hypothetical peptides in addition to bacteriocins, immunity proteins, regulatory genes and ABC transporters (Supplementary Fig. 1). The entire blp locus spanning from blpT to sp_0547 was deleted in the Δblp locus mutants. Site-directed homologous recombination was used to create the clean deletion strains, Δblp locus (P2838), Δply (P2851), 6A Δply (P2436) and the corrected mutant Δblp::blp (P2862). These mutants were created in a two-step process. In the first step, the blp locus (or ply) were replaced with a Janus cassette (containing ~1 kb in flanking regions both upstream and downstream of the region of interest) in P2406 to obtain blp::Janus (P2702) and ply::Janus (P2423)64 constructs. 6A mutants were created by replacing the blp locus and ply with a Janus cassette in 6A WT Spn to obtain 6A Δblp locus (P2720) and ply::Janus (P2435)71 constructs. These strains were kanamycin-resistant. Thereafter, unmarked, clean deletions were made by replacement of the Janus cassette. P2702, P2423, and P2435 were transformed with PCR fragments containing ~1 kb each upstream and downstream of the regions of interest joined together to obtain P2838, P2851 and P2436, respectively. P2862 was obtained by transforming P2702 with a PCR fragment containing ~1 kb each upstream and downstream of the blp locus. The desired mutants were streptomycin-resistant but kanamycin sensitive. The double mutant ΔblpΔply (P2861) was prepared by transforming P2838 with ply::Janus PCR fragment from P2423. P2861 was kanamycin-resistant but streptomycin sensitive. Mutants were confirmed by PCR following each step. All the primers used in this work are listed in Supplementary Table 2.
Construction of barcoded library
The barcoded library was designed as previously described9. Briefly, 7-nt barcodes of the sequence NNMCAATGNNMCAAN with intervening fixed sequences were designed to avoid the presence of start and stop codons. The pooled plasmid library previously obtained from Escherichia coli was transformed into P2406 as described above to obtain molecularly barcoded TIGR4 strain. The barcoded Spn transformants were selected on TS plates supplemented with 100 µl of catalase (38,000 U/ml; Worthington Biochemical Corporation, NJ) and 200 µg/ml spectinomycin. The resulting barcoded Spn library was grown, sequenced and stocked at −80 °C.
Library sequencing
Genomic DNA from the samples was isolated using MasterPure Complete DNA & RNA Purification Kit (Lucigen, Middleton, WI) as per the manufacturer’s instructions. Barcodes were amplified from genomic DNA using Nested PCR; wherein, the first step consisted of amplifying the iga region (5 cycles) followed by amplification of the barcodes (35 cycles). Primers used for amplification of the barcodes contained the adapters to be used for sequencing library preparation. These amplicons were then purified using QIAquick PCR purification kit (Qiagen, Germantown, MD) as per the manufacturer’s instructions. Purified samples were then shipped to Azenta Life Sciences (South Plainfield, NJ) for sequencing using their Next-Gen Amplicon-EZ service.
Analysis of sequencing data
The data was analyzed as previously described9,72. Reads were aligned to a reference sequence using Python. First, trimmomatic was used for quality control to trim adaptor sequences and low-quality bases from the reads (sliding window size: 3, sliding window quality: 20, leading and trailing quality: 15, minimum length: 75)73. The reads were then aligned to a reference sequence by BWA (Matching Score: 10, Mismatch Penalty: 2) and outputted in a .sam file74. The remainder of the analysis was done using R. The barcode sequence was extracted from the aligned reads by concatenating bases at known variable positions while filtering out incomplete or ambiguous barcodes. A table detailing each barcode detected and the number of times it was found was compiled. To account for variability in the number of total reads, we standardized samples by computing rarefaction and extrapolation of clonal diversity using iNEXT75,76. The clonal diversity was expressed using Hill numbers with q = 0 (‘clonal richness’ or number of unique clones present) and q = 1 (Hill’s N1)76. Shannon diversity index (H) was calculated as H = −⅀pi.ln(pi) where pi denotes the proportion of the population made up of the clone i.
Animal studies
Wild-type C57BL/6J (strain 00664) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The mouse colony was bred and maintained in a conventional mouse facility. Infant pups were housed with the dam until weaning at 3 weeks of age. Adult mice were fed ad lib the PicoLab Rodent Diet 20, a 20% protein diet formulation, and were given water for consumption. All the animals were kept on a light cycle of 12 h on, 12 h off with a temperature in the animal facility of 70 °F ( ± 2 °F).
Pneumonia model
Adult male mice (6–9 weeks) were used for the pneumonia model. Mice were anesthetized with 4% isoflurane and infected intranasally with 8 × 107 CFU of TIGR4 or 2 × 107 CFU of 6A Spn in 50 µl of sterile PBS with a pipette tip. Mice were monitored for clinical signs of disease and euthanized if they appeared sick, at pre-determined, and humane end points. Mice were euthanized by CO2 asphyxiation followed by cardiac puncture. Thereafter, animal tissues were harvested for bacterial enumeration (lungs and spleen), or for immunohistochemistry (lungs). For bacterial enumeration, mouse tissues were weighed, and homogenized with a Mini-BeadBeater 16 Disruptor (BioSpec Products, Bartlesville, OK) using 2.7-mm Glass Beads (BioSpec Products, Bartlesville, OK) followed by viable plating. For assessing bacterial population dynamics, mice were infected with a ten-fold lower dose of Spn. At the indicated time points, the entirely homogenized lung was plated on TSA-catalase plates supplemented with 200 µg/ml spectinomycin and incubated overnight at 37 °C with 5% CO2. The following day, all the colonies were grown in TS broth supplemented with 200 µg/ml spectinomycin until an OD620 of 1.0 for genomic DNA isolation. For experiments utilizing recombinant protein, 250 µg of purified Ply (10 hemolytic units) or PdB32 in PBS was mixed with the inoculum.
Depletion of complement and neutrophils
To systemically deplete complement, mice were treated with 25 µg of cobra venom factor (Quidel Corporation, San Diego, CA, cat. A600) diluted in 100 µl dPBS, or dPBS control, intraperitoneally twice: 1 day prior, and 1 day post-intranasal Spn inoculation. For neutrophil depletion experiments, mice were intraperitoneally treated with 200 µg of either rat anti-mouse αGr-1 monoclonal antibody (BioXcell; RB6-8C5; cat. #BE0075) or rat IgG2b isotype control (BioXcell; IgG2b; cat. #BE0090) diluted in 100 µl of dPBS 4–5 h prior to intranasal inoculation of Spn. To quantify neutrophil and monocyte numbers, blood was harvested at the time of euthanasia through a cardiac puncture in an EDTA-coated microtainer tube (Becton Dickinson, Franklin Lakes, NJ). Samples were run on the Element HT5 Veterinary Hematology Analyzer (Heska, Loveland, CO) for data acquisition.
Infant colonization model
Three to four-day-old infant pups were inoculated intranasally with 105 CFU of Spn in 3 µl of sterile PBS with a pipette tip, without anesthesia. The pups were returned to their dam for the duration of the experiment. At the end of the experiments, mice were euthanized at the indicated time point by CO2 asphyxiation followed by cardiac puncture. The Spn colonization density of the upper respiratory tract was measured, as previously described77. Briefly, the trachea was lavaged using a 30-gauge needle for infants with 300 µl of sterile PBS collected from the nares. In total, 40 µl of this retrotracheal lavage was used to enumerate bacteria by viable plating serial dilutions on TSA-catalase plates supplemented with the appropriate antibiotic (200 µg/ml streptomycin) and incubated overnight at 37 °C with 5% CO2.
Immunohistochemistry and immunofluorescence
Following euthanasia, the lungs were harvested and fixed for 24 h in 4% paraformaldehyde at 4 °C. The lungs were then washed with PBS and placed in 70% ethanol. Lungs were then processed through graded ethanol solutions to xylene and infiltrated with paraffin in a Leica Peloris automated tissue processor. In all, 5-µm paraffin sections were stained with hematoxylin and eosin on a Leica ST5020 automated stainer, or immuno-stained with Akoya Biosciences® Opal™ multiplex immunofluorescence reagents on a Leica BondRx autostainer, according to the manufacturer’s instructions. In brief, sections were incubated with Type 4-specific antisera (1:4000 dilution, Statens Serum Institut; cat. #16747) for 60 min followed by an anti-rabbit secondary polymer (Akoya ARH1001EA) and HRP-mediated tyramide signal amplification with the 520 Opal® fluorophore (Akoya FP1487001KT). Primary and secondary antibodies were subsequently removed with a heat retrieval step (ER1, Leica AR9961) for 60 min, leaving the Opal fluorophore covalently linked to the antigen. Prior to the second immunostaining cycle, endogenous mouse IgG was blocked with Rodent Block M (Biocare, RBM961 L) and subsequently incubated with anti-mouse Ly6g (1:300 dilution, 60 min, BD Biosciences, Cat #551459), anti-rat-1step-HRP polymer (Biocare, BRR4016) and 690 Opal fluorophore (Akoya FP1497001KT). Sections were counterstained with spectral DAPI (Akoya Biosciences, FP1490), and mounted with ProLong Gold Antifade (ThermoFisher Scientific, P36935). Semi-automated image acquisition was performed on a Akoya PhenoImagerHT (formerly Vectra® Polaris) multispectral imaging system. After whole slide scanning at 20× in the MoTIF mode, regions of interest were selected for spectral unmixing and image processing using InForm® version 2.4.10 software from Akoya Biosciences.
Overlay inhibition assays
The in vitro overlay inhibition assays were performed, as previously described24,78. The producer test strains were grown in TS broth until an OD620 of 0.7, pelleted, and resuspended in PBS. These strains were then stabbed into TS plates containing 0.5% agar, and supplemented with catalase followed by overnight incubation at 37 °C with 5% CO2. The next day, overlay strains were grown from frozen stocks in TS broth until an OD620 of 0.3. Plated stabbed with Spn were then overlaid with 300 µl of overlay strain, and 8 ml of TS containing 0.5% agar which was pre-warmed at 37 °C. These plates were returned to 37 °C for incubation overnight. Test strains that led to the appearance of clear zones of inhibition of the overlay strain were scored as being bacteriocin-producers. The bacteriocin activity was compared across strains by measuring the size of the zone of inhibition. Briefly, the boundary of the clear zone of inhibition was outlined using ImageJ. In the event that no zone of inhibition was visible, the boundary of the producer test strain was outlined. The area of these outlined zones was measured using ImageJ. The graph depicts the normalized size, defined as the fold change in the area of the zone of inhibition relative to ΔblpMNO (which was normalized to 1). Because of the reduced sensitivity of the TIGR4 strain to in vitro overlay inhibition assays, the assay was performed in a serotype 6A strain (P376)24,25.
In vitro competition experiment and Ply release
All bacterial strains were grown to an OD620 of 0.1 in TS broth. An equal volume (6 ml) of attacker (e.g., Δply, P2408) and target (e.g., Δblp, P2706) strains were mixed and incubated at 37 °C with 5% CO2 for 2 h. The attacker cells were defined by their genotype and were always pneumolysin-deficient (Δply). This ensured that any Ply present in the supernatant was being released from the target cells. Thereafter, the bacterial cells were pelleted at 4300 rpm for 10 min. The supernatant was then filtered using 0.22-µm filters. The resulting cell-free supernatant was then concentrated 20× using Amicon Ultra spin columns (Millipore Sigma, Burlington, MA) and used for analysis further.
Western blot
The concentrated cell-free supernatants from in vitro assays as well as purified rPly were mixed with NuPAGE LDS sample buffer and NuPAGE reducing agent (Invitrogen, Waltham, MA), and prepared by treatment at 100 °C for 5 min. Samples were separated using 10% Bolt Bis-Tris Plus gels (ThermoFisher Scientific, Waltham, MA) and transferred onto PVDF membranes via iBlot3, a dry blotting system (Invitrogen, Waltham, MA). The membranes were blocked with 5% nonfat dry milk in TBST (TBS/0.1% Tween-20) for 1 h at room temperature and washed three times with TBST. The membrane was probed with primary monoclonal antibody specific to Ply (1F11, ThermoFisher Scientific, Waltham, MA) at a dilution of 1:1000 in TBST. Following three washes, the membrane was incubated with an HRP-conjugated goat anti-mouse IgG (Cat. No. 31430, ThermoFisher Scientific, Waltham, MA) at a dilution of 1:10,000 in TBST for 1 h at room temperature. Membranes were washed thrice and developed with SuperSignal Maximum Sensitivity Substrate kit (Cat. No. 34095, ThermoFisher Scientific).
Hemolysis assays
Hemolysis assay was performed as previously described79. The assay was performed either using cell lysates or concentrated cell-free supernatants obtained from the in vitro competition assay. For obtaining cell lysates, WT or Δblp strains were grown to an OD620 of 0.4 in TS broth, pelleted and resuspended in lysate buffer (0.01% SDS, 0.1% sodium deoxycholate, and 0.015 M sodium citrate in distilled water) and incubated at 37 °C for 30 min. Thereafter, 160 µl sample buffer (0.1% bovine serum albumin, 10 mM dithiothreitol, and PBS) was added to a 96-well V-bottom plate. Thereafter, 80 µl of the concentrated cell-free supernatants or cell lysates (and their respective 2-fold serial dilutions) were added to the wells. This was followed by the addition of 80 µl pf PBS-washed horse erythrocytes (2%) to the plate. The plate was incubated at 37 °C for 30 min and centrifuged at 2000 rpm for 10 min. The plates were scored for complete lysis of horse erythrocytes as indicated by the absence of red blood cell pellets.
Statistics and reproducibility
For comparisons between two groups, either nonparametric (Mann–Whitney test) or parametric Student’s t test was used. For comparisons between more than two groups, either nonparametric (Kruskal–Wallis test) or parametric ANOVA followed by correction for multiple comparisons was used. For each of these comparisons, the parametric test was chosen when the data followed a normal distribution. In all other cases, nonparametric tests were performed. The details of the statistical analyses are included in the figure legends. The statistical analyses were performed using GraphPad Prism v10.1.1 (GraphPad Software Inc., San Diego, CA) unless stated otherwise. Data from at least three independent biological replicates is included in the figures. For figures, each data point (or “n”) corresponds to an individual animal.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source values for the figures have been made available as Supplementary Data.
Code availability
The code for analyzing the sequencing data is available at https://github.com/sda26/pneumo_diversity. It can also be accessed on Zenodo at https://doi.org/10.5281/zenodo.13983312.
References
Sana, T.G. et al. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl Acad. Sci. USA. 113, E5044–E5051 (2016).
MacIntyre, D.L., Miyata, S.T., Kitaoka, M. & Pukatzki, S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl Acad. Sci. USA. 107, 19520–19524 (2010).
Fu, Y., Waldor, M.K. & Mekalanos, J.J. Tn-seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host and Microbe: Elsevier Inc.; 14, 652–663 (2013).
Hibbing, M. E., Fuqua, C., Parsek, M. R. & Peterson, S. B. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Micro 8, 15–25 (2010).
Riley, M. A. & Gordon, D. M. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 7, 129–133 (1999).
Hols, P., Ledesma-García, L., Gabant, P. & Mignolet, J. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trend Microbiol. 27, 690–702 (2019).
Claverys, J.-P., Martin, B. & Håvarstein, L.S. Competence-induced fratricide in streptococci. Mol. Microbiol. 64, 1423–1433 (2007).
Trappetti, C. & Paton, J.C. A selfish bacteriocin dictates pneumococcal domination. Cell Host Microbe 31, 7–8 (2023).
Aggarwal, S.D. et al. BlpC-mediated selfish program leads to rapid loss of Streptococcus pneumoniae clonal diversity during infection. Cell Host Microbe 31, 124–134.e125 (2023).
Javan, R.R., van Tonder, A.J., King, J.P., Harrold, C.L. & Brueggemann, A.B. Genome sequencing reveals a large and diverse repertoire of antimicrobial peptides. Front. Microbiol. 9, 2012 (2018).
Aggarwal, S.D., Yesilkaya, H., Dawid, S. & Hiller, N.L. The pneumococcal social network. PLoS Pathogens 16, e1008931 (2020).
Guiral, S., Mitchell, T.J., Martin, B. & Claverys, J.-P.P. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: Genetic requirements. Proc. Natl Acad. Sci. USA. 102, 8710–8715 (2005).
Collaborators, G.A.R. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248 (2022).
Lancet. Acute respiratory infections in under-fives: 15 million deaths a year. Lancet 326, 699–701 (1985).
O’Brien, K.L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902 (2009).
CDC. Fast Facts About Pneumococcal Disease (Centers for Disease Control and Prevention, 2015).
Torres, A. et al. Pneumonia. Nat. Rev. Dis. Primers. 7, 25 (2021).
Weiser, J. N., Ferreira, D. M. & Paton, J. C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 16, 355–367 (2018).
Tuomanen, E.I., Austrian, R. & Masure, H.R. Pathogenesis of pneumococcal infection. N. Eng. J. Med. 332, 1280–1284 (1995).
Loughran, A.J., Orihuela, C.J. & Tuomanen, E.I. Streptococcus pneumoniae: Invasion and inflammation. Microbiol. Spectrum. 7, https://doi.org/10.1128/microbiolspec.gpp1123-0004-2018 (2019).
Tweten, R.K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73, 6199–6209 (2005).
Walker, J.A., Allen, R.L., Falmagne, P., Johnson, M.K. & Boulnois, G.J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 55, 1184–1189 (1987).
Matthias, K.A., Roche, A.M., Standish, A.J., Shchepetov, M. & Weiser, J.N. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J. Immunol. 180, 6246–6254 (2008).
Dawid, S., Roche, A.M. & Weiser, J.N. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect. Immun. 75, 443–451 (2007).
Son, M.R. et al. Conserved mutations in the pneumococcal bacteriocin transporter gene, blpA, result in a complex population consisting of producers and cheaters. mBio 2, e00179–00111 (2011).
Sanders, M. et al. A comparison of pneumolysin activity and concentration in vitro and in vivo in a rabbit endophthalmitis model. Clin. Ophthalmol. 2, 793–800 (2008).
Bokori-Brown, M. et al. Red blood cell susceptibility to pneumolysin: Correlation with membrane biochemical and physical properties. J. Biol. Chem. 291, 10210–10227 (2016).
Cohen, B., Halbert, S.P. & Perkins, M.E. Pneumococcal hemolysin: the preparation of concentrates, and their action on red cells. J. Bacteriol. 43, 607–627 (1942).
Nishimoto, A.T., Rosch, J.W. & Tuomanen, E.I. Pneumolysin: pathogenesis and therapeutic target. Front. Microbiol. 11, 1543 (2020).
Pereira, J.M., Xu, S., Leong, J.M. & Sousa, S. The yin and yang of Pneumolysin during pneumococcal infection. Front. Immunol. 13, 878244 (2022).
Gilbert, R.J.C. Inactivation and activity of cholesterol-dependent cytolysins: What structural studies tell us. Structure 13, 1097–1106 (2005).
Kuipers, K. et al. Age-related differences in IL-1 signaling and capsule serotype affect persistence of Streptococcus pneumoniae colonization. PLoS Pathogens 14, e1007396 (2018).
Paton, J.C., Rowan-Kelly, B. & Ferrante, A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43, 1085–1087 (1984).
Mitchell, T.J., Andrew, P.W., Saunders, F.K., Smith, A.N. & Boulnois, G.J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute‐phase protein. Mol. Microbiol. 5, 1883–1888 (1991).
Rubins, J.B. et al. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95, 142–150 (1995).
Yuste, J., Botto, M., Paton, J.C., Holden, D.W. & Brown, J.S. Additive inhibition of complement deposition by Pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 175, 1813–1819 (2005).
Ali, Y.M. et al. Human L-ficolin, a recognition molecule of the lectin activation pathway of complement, activates complement by binding to pneumolysin, the major toxin of Streptococcus pneumoniae. PLoS ONE 8, e82583 (2013).
Cochrane, C.G., Müller-Eberhard, H.J. & Aikin, B.S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 105, 55–69 (1970).
Müller-Eberhard, H.J. & Fjellström, K.-E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J. Immunol. 107, 1666–1672 (1971).
Morgan, B.P. & Harris, C.L. Complement therapeutics; history and current progress. Mol. Immunol. 40, 159–170 (2003).
Kock, M.A., Hew, B.E., Bammert, H., Fritzinger, D.C. & Vogel, C.W. Structure and function of recombinant cobra venom factor. J. Biol. Chem. 279, 30836–30843 (2004).
Merle, N.S., Noe, R., Halbwachs-Mecarelli, L., Fremeaux-Bacchi, V. & Roumenina, L.T. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
Liu, X. et al. Exploration of bacterial bottlenecks and Streptococcus pneumoniae pathogenesis by CRISPRi-seq. Cell Host Microbe 29, 107–120.e106 (2021).
Austrian, R. & Gold, J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med.; 60, 759–776 (1964).
Burgos, J. et al. The problem of early mortality in pneumococcal pneumonia: A study of risk factors. Eur. Respir. J. 46, 561–564 (2015).
Tomasz, A. & Waks, S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc. Natl Acad. Sci. USA. 72, 4162–4166 (1975).
English, B.K. Limitations of beta-lactam therapy for infections caused by susceptible Gram-positive bacteria. J. Infect. 69, S5–S9 (2014).
Wolf, A.J., Liu, G.Y. & Underhill, D.M. Inflammatory properties of antibiotic-treated bacteria. J. Leukocyte Biol. 101, 127–134 (2017).
Canvin, J.R. et al. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a Type 2 pneumococcus. J. Infect. Dis. 172, 119–123 (1995).
Berry, A.M. & Paton, J.C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68, 133–140 (2000).
Paton, J.C., Andrew, P.W., Boulnois, G.J. & Mitchell, T.J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: The role of pneumococcal proteins. Annu. Rev. Microbiol. 47, 89–115 (1993)
Jacques, L.C. et al. Increased pathogenicity of pneumococcal serotype 1 is driven by rapid autolysis and release of pneumolysin. Nat. Commun. 11, 1–13 (2020).
Price, K.E. & Camilli, A. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J. Bacteriol. 191, 2163–2168 (2009).
Price, K.E., Greene, N.G. & Camilli, A. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 194, 3651–3660 (2012).
Balachandran, P., Hollingshead, S.K., Paton, J.C. & Briles, D.E. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183, 3108–3116 (2001).
Subramanian, K. et al. Pneumolysin binds to the Mannose Receptor C type 1 (MRC-1) leading to anti-inflammatory responses and enhanced pneumococcal survival. Nat. Microbiol. 4, 62–70 (2019).
Subramanian, K. et al. Mannose receptor‐derived peptides neutralize pore‐forming toxins and reduce inflammation and development of pneumococcal disease. EMBO Mol. Med. 12, 1–17 (2020).
Kjos, M. et al. Expression of Streptococcus pneumoniae bacteriocins Is induced by antibiotics via regulatory interplay with the competence system. PLOS Pathogens 12, e1005422 (2016).
Wholey, W.-Y., Kochan, T.J., Storck, D.N. & Dawid, S. Coordinated bacteriocin expression and competence in Streptococcus pneumoniae contributes to genetic adaptation through neighbor predation. PLOS Pathogens 12, e1005413 (2016).
Wang, C.Y., Patel, N., Wholey, W.-Y. & Dawid, S. ABC transporter content diversity in Streptococcus pneumoniae impacts competence regulation and bacteriocin production. Proc. Natl Acad. Sci. 115, E5776–E5785 (2018).
Miller, E.L., Abrudan, M.I., Roberts, I.S. & Rozen, D.E. Diverse ecological strategies are encoded by Streptococcus pneumoniae bacteriocin-like peptides. Genome Biol. Evol. 8, 1072–1090 (2016).
Bogaardt, C., van Tonder, A.J. & Brueggemann, A.B. Genomic analyses of pneumococci reveal a wide diversity of bacteriocins - including pneumocyclicin, a novel circular bacteriocin. BMC Genom. 16, 554 (2015).
D’Mello, A. et al. An in vivo atlas of host–pathogen transcriptomes during Streptococcus pneumoniae colonization and disease. Proc. Natl Acad. Sci. USA 117, 33507–33518 (200).
Zafar, M.A., Wang, Y., Hamaguchi, S. & Weiser, J.N. Host-to-host transmission of Streptococcus pneumoniae Is driven by its inflammatory toxin, pneumolysin. Cell Host Microbe 21, 73–83 (2017).
Novick, R.P. et al. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12, 3967–3975 (1993).
Zafar, M.A., Kono, M., Wang, Y., Zangari, T. & Weiser, J.N. Infant mouse model for the study of shedding and transmission during Streptococcus pneumoniae monoinfection. Infect. Immun. 84, 2714–2722 (2016).
Abruzzo, A.R., Aggarwal, S.D., Sharp, M.E., Bee, G.C.W. & Weiser, J.N. Serotype-dependent effects on the dynamics of pneumococcal colonization and implications for transmission. mBio. 13, e00158–00122 (2022).
Aggarwal, S.D. et al. Function of BriC peptide in the pneumococcal competence and virulence portfolio. PLoS Pathogens 14, e1007328 (2018).
Aggarwal, S.D. et al. A molecular link between cell wall biosynthesis, translation fidelity, and stringent response in Streptococcus pneumoniae. Proc. Natl Acad. Sci. 118, e2018089118 (2021).
Aggarwal, S.D. et al. Competence-associated peptide BriC alters fatty acid biosynthesis in Streptococcus pneumoniae. mSphere 6, e00145–00121 (2021).
Lokken-Toyli, K. L. et al. Impaired upper respiratory tract barrier function during postnatal development predisposes to invasive pneumococcal disease. PLoS Pathog. 20, e1012111 (2024).
Aggarwal, S.D. Code for analyzing clonal diversity (v1.0.0) Zenodo. https://doi.org/10.5281/zenodo.13983312 (2024).
Bolger, A.M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv; 2013. p. 1303.3997v1302.
Chao, A. et al. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monograp. 84, 45–67 (2014).
Hsieh, T.C., Ma, K.H. & Chao, A. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456 (2016).
Richard, A.L., Siegel, S.J., Erikson, J. & Weiser, J.N. TLR2 signaling decreases transmission of Streptococcus pneumoniae by limiting bacterial shedding in an infant mouse Influenza A co-infection model. PLoS Pathogens 10, e1004339 (2014).
Maricic, N. & Dawid, S. Using the overlay assay to qualitatively measure bacterial production of and sensitivity to pneumococcal bacteriocins. J. Vis. Exp. 91, e51876 (2014).
Ratner, A.J. et al. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem. 281, 12994–12998 (2006).
Acknowledgements
We thank Drs. Ifrah Shahi and Adam Ratner for providing purified recombinant pneumolysin. The tissue sectioning, staining and imaging was performed by Dr. Cynthia Loomis and members of NYU Langone’s Experimental Pathology Research Laboratory (RRID:SCR_017928). We thank Dr. Adam Ratner for his feedback on the manuscript. This work was funded by NIH grants R01 AI50893, and R01 AI038446 to JNW. The Experimental Pathology Research Laboratory is partially supported by the Cancer Center Support Grant P30CA016087 at NYU Langone’s Laura and Isaac Perlmutter Cancer Center.
Author information
Authors and Affiliations
Contributions
Conceptualization, SDA, and JNW; methodology, SDA; investigation, SDA, KLL-T; resources, SDA; software, SDA; formal analysis, SDA and KLL-T; writing—original draft, SDA; writing—review and editing, SDA, KLL-T, and JNW; funding acquisition, JNW.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aggarwal, S.D., Lokken-Toyli, K.L. & Weiser, J.N. Pneumococcal pneumonia is driven by increased bacterial turnover due to bacteriocin-mediated intra-strain competition. Commun Biol 7, 1628 (2024). https://doi.org/10.1038/s42003-024-07176-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07176-4