Abstract
Lipid-containing vacuoles in microglia were discovered more than one hundred years ago in the brain of patients showing neurodegenerative processes. Recently, molecular-biological studies demonstrated specific changes in lipid-metabolism related to neurodegeneration. Despite that already Alzheimer described a distinct glia phenotype having large, lipid-containing vacuoles (Gitterzellen), little is known about how microglia convert lipid metabolites into a vacuolated phenotype. We studied the impact of liver-derived, insoluble, lipid-enriched nanoparticles (Lef-NP) ( ~ 20 nm) and of ceramide-coated Percoll-nanoparticles (Cer-NP) ( ~ 20 nm) on vacuolization in microglia. Lipidomic analysis of Lef-NP revealed numerous distinct lipids, including pro-inflammatory ceramides, which are enriched in the brain of Alzheimer patients. Video microscopy revealed that hepatocyte-derived Lef-NP and Cer-NP enhanced macropinocytosis, followed by macropinosome swelling and formation of the Gitterzellen phenotype. Neither ceramide nor Percoll-nanoparticles induced Gitterzellen-formation. Electron-tomography visualized membrane contact-sites between nanoparticle-loaded endosomes, endoplasmic reticulum cisternae and mitochondria. Suppression of lipid-nanoparticle-induced Gitterzellen-formation by amiloride, which supresses macropinocytosis, and bafilomycin A, an endosomal acidification inhibitor, further confirmed a pinocytotic pathway in Gitterzellen-formation. Bafilomycin A also reversed Gitterzellen to a ramified microglia phenotype. Our experimental findings suggest that lipid-nanoparticles but not emulsified lipids provoke vacuolization in microglia, and provide a simple in-vitro model for a pathogenic process taking years in the human brain.
Similar content being viewed by others
Introduction
Microglia, representatives of the innate immune system in the central nervous system, prevents accumulation of soluble and, consequently, deposition of extracellular insoluble β-amyloid (Aß)1,2 by receptor-mediated endocytosis of fibrillar Aß1, and by nonselective macropinocytosis3. Following uptake, microglia and macrophages degrade soluble Aß in the endolytic pathway3,4. Accordingly, failure of Aß clearance by microglia in transgenic mouse models of Alzheimer disease (AD) accelerates cerebral deposition of Aß and spatial memory decline1,5,6,7,8,9. Research in the past three decades has favoured that the neuron-derived proteins, Aß and tau, as the cause of AD. However, recent genome-wide association studies revealed that about half of the 30 risk alleles for AD are associated with the innate immune system and microglia function8. The two most prominent proteins encoded by AD risk alleles are APOE, a lipid-binding protein, and TREM-2, a lipid receptor in the plasma membrane. Both proteins play an important role in lipid homeostasis8. Further studies indicate that lipid-containing particles may enter the brain across an impaired blood-brain-barrier, as summarized in the Lipid-Invasion-Model (for review, see Rudge, 202310). Interestingly, in human brains afflicted by degenerative or traumatic processes, Alois Alzheimer and his contemporaries identified lipid-enriched vacuolated cells (for historical review see Rezaie and Hanisch, 201411). Alois Alzheimer called this microglia phenotype “Gitterzellen” because of its grid-like appearance.
Cell culture studies reveal that ceramide promotes cell death in neurons12. This finding in combination with the observations that ceramides are increased in the brain of AD patients13, as well as following brain injury12 led to the assumption that the metabolism of sphingolipids is central in neurodegenerative processes. While in the healthy brain, ceramide is metabolized to sphingomyelin and sphingosine 1-phosphate (S1P), ceramide accumulates during neurodegenerative processes and brain injury12,14. In general, the phosphorylated sphingolipids are neuroprotective metabolites and non-phosphorylated forms of ceramide promote cell death in neurons12. When sphingomyelinase, an enzyme that metabolizes sphingomyelin to ceramide, is pharmacologically blocked, the neurotoxic effect of ceramide could be inhibited12 .
Experiments on microglia show that different sphingolipids affect cytokine release and phagocytosis. S1P activates microglia and triggers the release of pro-inflammatory cytokines15,16. Other experiments show that S1P impairs inflammation, decreases autophagy17 and activates phagocytosis in microglia18. While short-chain ceramides promote neuroprotective effects of microglia19,20, long-chain ceramides trigger pro-inflammatory processes21. Blockade of de-novo ceramide synthesis suppresses palmitate-induced release of the pro-inflammatory cytokine, IL-1ß22. Again, sphingomyelinase inhibition suppressed tau activation of microglia12. These findings on sphingosine metabolism combined with current genomic studies showing the lipid-binding proteins apolipoprotein APOE-ε4 allele, CLU (APOJ; encodes clusterin, a brain apolipoprotein), and TREM allele among AD risk alleles5,8,23 indicate that distinct lipids may affect clearance efficacy of microglia.
While these results clearly indicate the neurotoxic and pro-inflammatory properties of ceramides, their impact on vacuolization in microglia is unknown. Given the prominent position of vacuolated, lipid-containing microglia in neurodegenerative and neurotraumatic processes, there is a need for cell culture models to evaluate the discrete nature of microglial vacuolization; yet, the vacuolated, lipid-containing Gitterzellen according to Alzheimer could not be reproduced in cell-culture experiments. Here we show that lipid-rich nanoparticles promote the transformation of primary mouse microglial cells to Gitterzellen in vitro; findings that demonstrate that lipid—bound nanoparticles but not the dissolved lipid alone induce a vacuolated microglial phenotype.
Results
Lipid-enriched nanoparticles (Lef-NP)
Dissociation of organs into single cells is a substantial injury leading to the release of injury signals by the isolated cells. Because the liver is essential to lipid metabolism, and parenchymal hepatocytes can be readily isolated and cultured under serum-free conditions24,25, we harvested conditioned media from freshly isolated primary hepatocytes cultured for three hours and isolated a precipitable lipid-enriched fraction (Lef-NP). (see Supplementary methods).
Table 1 lists the most prominent lipid components identified in Lef-NP by metabolomic analysis. As evidenced by transmission electron microscopy (TEM) / electron energy loss spectroscopy (EELS), the Lef-NP consists of oxygen-enriched nanoparticles with a diameter of about 20 nm (Fig. 4a-c).
Lef-NP induces a Gitterzellen phenotype
Bridging the historic findings of lipid-containing vacuoles in microglia26,27 with the recent findings of changes in lipid metabolism in AD28,29,30, we exposed primary microglial cells to Lef-NP, acting as mimetic for tissue injury, and monitored phenotypical changes over up to 100 hours by LCI.
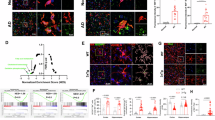
Following Lef-NP exposure, cells initially stopped to migrate, but dynamically protruded and retracted lamellipodium-like extensions, and subsequently started to develop vacuoles. Finally, Lef-NP-treated microglial cells transformed in a Gitterzellen phenotype (Fig. 1b, d-k; Suppl.Video 1) that resembles a phenocopy of the Gitterzellen documented by Alzheimer’s camera lucida drawings (Fig. 1a). No comparable morphological changes were observed in control cultures.
a Gitterzellen shown in Alzheimer’s camera lucida drawings in post mortem tissue (from Kettenmann et al. 27; with permission of the American Physiological Society). b,c Gitterzellen seen by live cell imaging in mouse primary microglial cells exposed to Lef-NP [1:1000] (b) or ceramide-coated nanoparticles (Cer-NP) (c). d–k Characteristic vacuolated phenotypes seen in Lef-NP-treated microglial cells (phase contrast, live cell imaging) (d-g) and scanning electron microscopy (h–k): foam phenotype (d, h), early (multivacuolar) and advanced (oligovacuolar) Gitterzellen (e,f and i,j) and ballooning (monovacuolar) Gitterzellen (g,k). Frequently, the progression of Gitterzellen towards advanced stages is marked by the formation of one or two enlarged vacuoles surrounded by numerous smaller ones, a phenotype that is also documented by Alzheimer’s camera lucida drawings (blue arrows in Fig. a–c). Notably, the cell nucleus is condensing during Gitterzellen progression, but remains detectable until terminal stages (red arrows in d–g). Scale bars are: 20 µm (b,c), 10 µm (d–k).
Lef-NP-treated microglial cell transformation was driven by pinosome expansion and fusion events as documented by LCI and Dextran 3000 uptake experiments (Suppl. Fig. 2). Initially, the pinosomes were centripetally transported and aggregated in the perinuclear area. Then, the vesicles expanded and fused with each other (Suppl. Video 2). Because we observed distinct cellular variations in the amount as well as in the size of vesicles, we distinguished three phenotypes of vesicle-containing cells in Lef-NP-treated cultures: (i) cells marked by a perinuclear array of small to medium-sized vesicles revealing a foam phenotype (Fig. 1d,h; Suppl.Fig. 2a), (ii) Gitterzellen characterized by enlarged vesicles (vacuoles), which increased in size but decreased in number with time (Fig. 1e,f,i,j; Suppl.Fig. 2e-g, h-k) and (iii) terminal oligo- or monovacuolar, ballooning Gitterzellen (Fig. 1g,k; Suppl. Fig. 2f,l). At this terminal stage, the balloons occupied almost the entire cell and could reach enormous dimensions, exerting a lens-like, magnifying property (Suppl.Video 1-3). Gitterzellen development was accompanied by fusion events between neighbouring vacuoles contributing to the transition from a multi- to oligo- to monovacuolar phenotype (Suppl.Video 2). In addition, while cells developing a foam phenotype still showed a continuous transport of newly formed pinosomes from the periphery to the perinuclear region, Gitterzellen did not reveal marked centripetal vesicle movement. Further, vacuoles in the Gitterzellen were expanding from the perinuclear area throughout the whole cell body, also extending to the cell periphery (Fig. 1e,f and i,j). In a complementary approach to substantiate the causative role of macropinocytosis in Lef-NP-mediated Gitterzellen formation, macropinocytosis was monitored by employing LCI in combination with a micropinocytosis-tracking dye (Texas-red labelled Dextran 3000). This allowed following vesicle / vacuole growth readily by vesicular dye accumulation (Suppl. Fig. 2a-f). Surprisingly, however, the tracking-dye did not accumulate in enlarged, giant vacuoles as well as balloons seen in the Gitterzellen (Suppl. Fig. 2d-f). This suggests the effect of other than solely endocytic (macropinocytosis) mechanisms fuelling vacuole growth during advanced Gitterzellen stages.
Onset and time-course of Lef-NP-induced changes were asynchronous (Fig. 2a, Suppl. Fig. 1). Occasionally, the foam phenotype, as well as few Gitterzellen were detectable earliest after 5 -10 h following Lef-NP addition (Suppl.Video 1). Typically, the changes became significant within 24 hours of exposure to Lef-NP and could be followed subsequently in about 50% of the cells (Fig. 2a, Suppl. Fig. 1, Suppl.Video 1,2). Beginning of Gitterzellen ballooning was most variable and started between 20 and 60 hours after Lef-NP addition. Interestingly, early ballooning onset was frequently associated with a rapid, terminal burst of the balloon cell (Suppl.Video 1,3), while Gitterzellen with late ballooning onset generally survived till the end of Lef-NP exposure (Suppl. Video 2). Surprisingly, Gitterzellen having a ballooning phenotype even reverted spontaneously to an amoeboid or ramified, dystrophic phenotype (Suppl. Video 3). Switching back to Lef-NP-free culture medium promoted revision of vacuolated cells to amoeboid or dystrophic phenotypes.
a Time-course of cellular vacuolisation in Lef-NP-treated cells. Box-Whisker Plots represent the frequency of cells revealing a vacuolated phenotype (i.e. foam, gitter and balloon cells) as described in Fig. 1. Individual experimental data are highlighted by coloured circles. ++ p < 0.005 for the differences between the time points as indicated (Friedman test); ** p < 0.005 compared to 0 h (Bonferroni post-hoc test). N ≥ 3 independent experiments. Insert: comparison between initial treatment for 5 h and 45 h (i.e. the „plateau-phase”); bars represent the mean±2 SEM. * p < 0.05 compared to 5 h (two-tailed independent samples t-test). b Effect of solvent control (Control), ceramide (Cer), Percoll-nanoparticles (NP) and ceramide-coated Percoll (Cer-NP) on vacuolisation. Bars represent the mean±2 SEM of the percentage of vacuolated cells seen after 45 hours (plateau phase) of incubation. p < 0.005 for the observed differences (ANOVA). ** p < 0.005 compared to the control or as indicated (Tukey post-hoc test). The number of independent experiments is given in parentheses. Addition of MBCD had no effect on the vacuolization. c Comparison between the different vacuolated phenotypes in cells treated with NP or Cer-NP. Bars represent the mean±2 SEM of the percentage of vacuolated cells seen after 45 hours (plateau phase) of incubation. * p < 0.05; ** p < 0.005 compared to the corresponding NP group (two-tailed independent samples t-test); + p < 0.05; ++ p < 0.005 compared as indicated (paired samples t-test). The number of independent experiments is given in parentheses.
Ceramide-coated Percoll
Considering the lipid character of Lef-NP (also containing ceramide), the transformation of microglial cells towards a Gitterzellen phenotype could be due to dissolved or emulsified lipids or to lipid-containing nanoparticles. To address these questions, we treated primary microglial cells with either (1) ceramide [10 µM], (2) solvent control [0.181% ethanol + 0.045% DMSO], (3) Percoll [diluted 1:400; i.e. 0.25%] or (4) solvent control with methylbetacyclodextrin (MBCD) [1 µM MBCD, 10 µM ceramide] or (5) ceramide-coated Percoll (Cer-NP) [equivalent to a content of 10 µM ceramide].
Treatment with ceramide, the solvent control alone or the solvent containing MBCD had no vacuolizing effect. However, Percoll and even stronger Cer-NP increased the number of vacuolated cells (p < 0.005) (Fig. 2b; Suppl. Fig. 3). Critically, Percoll and Cer-NP stimulated the foam phenotype, but only Cer-NP promoted the further transformation into Gitterzellen (p < 0.005) (Fig. 2c). Therefore, Gitterzellen transition mechanistically depended on a combination of a nanoparticle coated with distinct lipids, like ceramide.
Macropinocytosis, pH regulation, and bafilomycin sensitivity
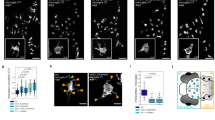
Macropinosomes contain a V-ATPase proton pump and, accordingly, have a low pH (for review see Ritter et al. 31). Employing the acridine orange (AO) uptake assay revealed a marked heterogeneity of vacuolar acidification in Lef-NP-treated microglial cells independent of vacuolar size (Fig. 3i-l). This heterogeneity was most prominent in advanced Gitterzellen (Fig. 3k). A subpopulation of this phenotype showed acidified vacuoles as visualized by AO retention. This indicated a sufficient ATP supply to fuel the proton pump even in ballooning Gitterzellen. To further investigate pH regulation and macropinocytosis on nanoparticle / Lef-NP-stimulated Gitterzellen formation, we used (i) the V-ATPase antagonist bafilomycin A (BafA), a well-described inhibitor of endosomal acidification and (ii) amiloride, an inhibitor of macropinocytosis acting via Na+/H+ exchange inhibition32.
a, b Effect of amiloride. The cells were treated for 48 hours either with (a) Lef-NP [1:1000] alone (arrows point at Gitterzellen) or (b) a combination of Lef-NP and amiloride [100 µM] (Live cell imaging). c,d Effect of bafilomycin A (BafA). Gitterzellen (c; arrowheads) formed within 24-hours pre-incubation with Lef-NP [1:1000] (c; 0 h – addition of BafA) revert upon further incubation in the presence of 25 nM BafA (d; 12 hours), yielding a dystrophic phenotype (d, arrowheads). (Live cell imaging). e–h Scanning electron microscopy of characteristic stages of BafA-mediated Gitterzellen reversion (e–h). i–l Acridine orange (AO)-retention assay for vacuolar acidification. The AO accumulation (red colour) in Lef-NP-treated cells (24 hours) indicates the presence of acidified (j,l; red arrowheads) and non-acidified (i,k; yellow arrowheads) enlarged vacuoles. Green: nuclear AO stain. Scale bars: 20 µm (a–d), 10 µm (e–bl).
Addition of BafA [25 nM] to cultures pre-exposed to Lef-NP for 24 hours (Fig. 3c) led to a rapid reversion of the already established Gitterzellen phenotype (Fig. 3d). Generally, Lef-NP-induced Gitterzellen reverted to an amoeboid or ramified, dystrophic phenotype (Fig. 3 e-h) within 6-12 hours after BafA addition (Suppl.Video 4). In some advanced Gitterzellen, giant vacuoles disappeared within a few minutes. Extended exposure to 25 nM BafA ( > 15 hours) markedly increased in a subpopulation of reverted cells cell death by a necrotic cell burst (Suppl.Video 4). The BafA-induced reversion of Gitterzellen started with fenestrations of the cell soma forming a “Swiss-cheese-like” pattern (Fig. 3f). Fenestration radiated towards the cell periphery. Finally, a dystrophic phenotype with a small cell soma and few, long extending processes was established (Fig. 3g,h). Noteworthy, primary microglial cell exposed to 25 nM BafA alone promoted a ramified phenotype as seen in Lef-NP+BafA treated cells (Fig. 3e-h).
Microglial cells exposed to a combination of Lef-NP and 100 µM amiloride did not transform to Gitterzellen (Fig. 3a,b; Suppl. Video 5). In these cultures, vacuole formation or Gitterzellen formation was rarely observed.
Finally, experiments using the widely used macroautophagy inhibitor 3-methyladenine (3MA) were carried out to address the possible involvement of macroautophagy in Lef-NP-induced vacuolisation. In microglia cultures exposed to Lef-NP in the presence of 5 mM 3MA, we still observed prominent Gitterzellen formation, also developing advanced ballooning states (Suppl. Fig. 2g-l). Hence, it is unlikely that macroautophagy plays a causative role in Lef-NP-induced Gitterzellen formation.
Taken together, these observations suggest a causal, mechanistic role of macropinocytosis, H+-ATPase activity and endosomal acidification in nanoparticle / Lef-NP-induced Gitterzellen transformation of microglial cells.
Ultrastructural aspects
Transmission electron microscopy of Lef-NP treated microglial cells revealed in endosomes an aggregation of electron-dense nanoparticles resembling in their spherical structure, size ( ~ 20 nm), and electron density Lef-NPs (Fig. 4d,e). While early endosomes were filled with Lef-NPs, NPs were less abundant in endosomes having intraluminal vesicles indicating late endosomes (Fig. 4d). This may indicate a gradual disassembly of the Lef-NP. Interestingly, Lef-NPs containing endosomes showed membrane contact sites with dilated ER or dilated mitochondria (Fig. 4e). Electron microscopic serial tomography revealed large-scaled interdigitated physical interactions between the organelles (Fig. 4f), indicating maximal surface contact. Thus, our 3D reconstructions suggest that membrane contact sites stabilize lipid exchange sites between interacting organelles and, accordingly, drive lipid metabolism in mitochondria and ER.
a, b TEM analysis of Lef-NP reveals the presence of electron-dense nanoparticles of ~20 nm size at different degrees of aggregation. c EELS measurements indicates a high oxygen content of the electron-dense Lef-NP. d Endosomal accumulation of electron-dense nanoparticles (ed-NP) in Lef-NP-treated microglia. With respect to the presence of intraluminal vesicles and degree of ed-NP accumulation, early (EE; ed-NP-rich) and late endosomes (LE; ed-NP poor) can be distinguished. e The endosomes of Lef-NP treated cells often form close contact zones with mitochondria (M; note the dilated morphology) and the ER (e; arrows in d). f 3D tomography modelling of the endosome (yellow)—ER (blue)—mitochondria (purple) contact zones.
Discussion
Neurodegeneration-related changes in lipid metabolism may induce neuronal cell death and activation of the innate immune system. Here, we developed and analysed a cell-culture-based approach to evaluate the impact of inflammation-related lipids as potential drivers for Gitterzellen-formation. We demonstrate that Lef-NPs or ceramide-coated nanoparticles promote the transformation of microglial cells to a “Gitterzellen” phenotype. Notably, emulsified ceramide itself does not trigger Gitterzellen formation. Most Gitterzellen survive exposure to lipid-containing nanoparticles and some even revert spontaneously to a dystrophic microglia phenotype. We also obtained evidence that Lef-NP-containing macropinosomes form extensive membrane contact areas with the ER and mitochondria suggesting a lipid-metabolism-associated mechanism. Figure 5 proposes a model for lipid-nanoparticle-induced Gitterzellen formation based on our findings.
Macropinocytosis and perinuclear vesicle aggregation is stimulated by Lef-/Cer-NP as well as NP(Percoll) yielding a foam-phenotype. Further transition to the Gitterzellen phenotype is promoted specifically by the endosomal accumulation of lipid-nanoparticles (Lef-NP/Cer-NP). Endosome/vacuole fusion events - potentially involving lysosomes (LYS) - and pH-dependent vacuole swelling drive the expansion of the endo-lysosomal compartment (ELC). This is accompanied by the formation of contact zones between Lef-NP-rich endosomes with the ER and mitochondria, hypothetically serving lipid processing. Progressive vacuole fusion and swelling, also involving the non-pinocytotic uptake of extracellular fluid will terminally establish ballooning Gitterzellen which either may survive, revert spontaneously (promoted by BafA) or undergo necrotic cell death upon balloon collapse.
The factors that initiate neurodegenerative processes are still poorly understood. Our observation that lipid-nanoparticles elicit vacuolization in microglia together with (i) the equivocal description of lipid-containing vacuoles in glia (Gitterzellen) localized in plaques by Alzheimer himself (1911)26 and (ii) the outcome of genome-wide association studies in AD patients showing disease-related changes in lipid-metabolism33 and demonstrating that about half of the 30 risk alleles for AD are connected with the innate immune system and microglia function8 suggest that lipid metabolism, as well as microglia activation are drivers in neurodegenerative processes. Previous data demonstrate that peripheral nano-sized particles can enter the brain10,34. The Lipid Invasion Model assumes that lipid-containing vesicles, like lipoprotein particles (around 20 nm in diameter), enter the brain when the BBB is damaged. The greater the BBB damage, the greater the LDL-entrance into the brain, and hence the higher the concentration of cholesterol favoring amyloid-beta generation10. The finding that ApoE-coated nanoparticles enter the brain via transcytosis across capillaries34 indicates a brain-entering pathway of nano-particles even before BBB-damage. Interestingly, alcohol-induced liver damage reveals lipoprotein particles - having similar size and electron-density as our Lef-NPs - in the Golgi-apparatus of hepatocytes35,36. Consequently, if cells under stress show massive lipid nanoparticles exocytosis, which are decorated by ApoE, then capillary transcytosis will promote an accumulation of Lef-NP in the brain and, finally, vacuolization of microglia. Moreover, sphingolipids too participate in endocytosis, endosomal sorting, and exocytosis37. C6-ceramide increases via cellular C16-ceramide production phagocytosis in Kupffer cells, resident liver macrophages38, but decreases pinocytosis of horseradish peroxidase in fibroblasts39, and ceramide increases exocytosis of glutamate from neuronal synapses40. Endogenous ceramides also promote oxLDL transcytosis across isolated umbilical venous wall endothelial cells41. As Lef-NP are rich in ceramides, a similar ceramide-dependent process across the BBB could cause an accumulation of lipid-nanoparticles in the brain. Hence, our observation that ceramide-coated Percoll induces vacuolization is intriguing given recent observations of ingestion or inhalation of plastic nanoparticles in humans42,43,44. If plastic nano-particles coated with exogenous or endogenous inflammation-promoting factors, like ceramides from peripheral inflammatory areas (e.g., hepatitis), enter the brain via transcytosis, this pathway is a testable link between environmental factors and neurodegeneration.
Although the identity and concentration of lipids extracted from nano-particles within late endosomes, the precise direction of lipid transfer across the organelles, and their metabolism remains to be determined, we assume that membrane contact sites between late endosomes, mitochondria and endoplasmic reticulum are the key structures in Gitterzellen-formation. Membrane contact sites are characterized by tethering of membranes of juxta-positioned organelles to ensure maintenance of contact, by a distance of 10 – 80 nm between the membranes for a rapid diffusion of molecules between the organelles, and by a specific function, like accurate, unidirectional, non-vesicular lipid transfer via lipid-transfer proteins45,46. Hanada suggests that the unidirectional lipid transfer from one organelle to the next is driven by co-transport of distinct lipids, like ceramide-transport-protein (CERT)-mediated ceramide/diacylglycerol exchange between the ER and Golgi apparatus, and their subsequent enzyme-mediated metabolism to maintain the required substrate gradient45. This model is also a good candidate for the mechanism of Gitterzellen-formation, as it predicts a failure of lipid transfer when the enzymes are saturated due to an overload of substrates and, accordingly, an accumulation of lipids in endosomes. Accumulation of lipid metabolites within endosomes drives an influx of water and, therefore, swelling of the endosome. Notably, the lipid-storage disorder, Niemann-Pick type C disease, also shows vacuolization in neuronal and non-neuronal cells47. Again, a saturation of NPC2 (Niemann-Pick C intracellular cholesterol transporter 2), which translocate intra-endosomal cholesterol to a lipid transfer protein for export47,48, may cause an accumulation of intra-endosomal cholesterol. Thus, given the significant changes in lipid metabolism in AD and neural injuries, we suggest that vacuolization of microglia is a consequence of an unbalanced distribution of lipids across cell organelles and, consequently, a failure in their metabolism.
Our observation of a transition from an initial increase in endocytic activity (vacuolated foam phenotype, early Gitterzellen stages) to a later non-endocytic phase (giant vacuole containing or ballooning late-stage Gitterzellen) may also occur in-vivo. In transgenic AD mice, microglia phagocyte amyloid-containing neurites in pre-plaque animals49, phagocyte Aβ plaques in a later stage50, but declines in phagocytic activity as Aβ deposition increases51,52. In AD patients, activated microglia is observed in early amyloid formation53, whereas extensive Aβ deposition depositions are associated with non-responsive microglia, and extensive tau pathology is associated with microglial cytorrhexis, characterized by fragmentation of their processes54. Interestingly, Streit and co-workers argue “that these cells were exhausted by their attempts to remove the aggregated, insoluble Aβ”53. Thus, in transgenic AD mice as well as AD patients, microglia show an early endocytic phase and a later non-endocytic phase.
BafA, a specific inhibitor of V-ATPase, caused a rapid reversion of ballooning Gitterzellen to a ramified, dystrophic microglia phenotype. This phenotype resembles microglia upon contact with amyloid plaques46. Although V-ATPase-dependent acidification of lysosomes and endocytic uptake of environmental substances at the plasma membrane are spatially segregated processes, they are both sensitive to BafA155. In a previous in-vitro study on viral infection, BafA1 exposed cells contain fewer endocytic vesicles and, according to the authors, BafA1 strongly inhibits the internalization of viral proteins55. Our observations on Lef_NP internalization also show that preincubation of cells with BafA1 inhibits macropinosome formation by an unknown mechanism. However, our study further demonstrates that the application of BafA1 to Gitterzellen reversed the Gitterzellen phenotype to a ramified dystrophic microglia phenotype. Whether both processes rely on the acidification of lysosomes, acting as a sink for degrading substances, or are related to an unknown molecular target of BafA1 is critical for the mechanistic understanding of Gitterzellen-formation and formation of a dystrophic microglia phenotype.
Interestingly, only about 50-70% of the microglia cells exposed to lipid-nanoparticles show vacuolization to different degrees and only few of these cells proceed to a necrotic cell death. This corresponds well with the heterogeneity of microglial cells as demonstrated by cytological criteria56 as well as transcriptomic analyses at the single cell level57,58. These studies identify microglia subsets for housekeeping tasks, but also disease associated microglia (DAM)58. DAM shows an upregulation of lipid metabolism-associated genes encoding for ApoE, Cst7 and Lpl59. Interestingly, an antigen-presenting microglia subset with a reduced expression of DAM-specific genes is found in AD patients58. Whereas DAM is a single subset in murine microglia, in human microglia the DAM-associated expression profile is distributed among few different microglia subsets58. Hence, the heterogeneous response we have observed in Gitterzellen formation may be due to different sensitivities of microglia subsets for the metabolic handling of lipid nanoparticles. It is tempting to speculate, that the Lef-NP-sensitive phenotype resembles a microglia phenotype that in AD, upon contact with plaques declines.
Summarizing, our study suggests the lipid nanoparticles, but not emulsified lipids, promote vacuolization in microglia, replicating the Gitterzellen-phenotype described by Alzheimer himself in plaques. Given that membrane contact areas between endosomes, ER, and mitochondria are important in lipid transport and metabolism45, the intense membrane contact areas between lipid-nanoparticle-containing macropinosomes, ER, and mitochondria described here may indicate a link between molecular biological observations and cellular responses to changes in lipid metabolism. Thus, our model and experimental approach may be useful in testing the impact of lipid metabolites in microglia in the context of neurodegenerative or traumatic processes.
Material and methods
Animals
Experiments were conducted in compliance with ethical regulation as defined by national (Austrian national animal experimentation law 2012) and European guidelines (Directive 2010/63//EU of the European Parliament on the protection of animals used for scientific purposes). According to Directive 2010/63/EU “killing of animals solely for the use of their organs and tissues” is not considered as ‘procedure’ or ‘animal experiment’. As in our study, animals were killed solely for the purpose of organ use / cell isolation (i.e. for establishing primary cell cultures) no permissions were required. Animals were obtained from Charles River (Germany) and kept at the certified central facility husbandry of the University of Salzburg. Animals were kept in a temperature (20–24 °C)—and humidity (55 ± 10%)—controlled room with a 12 h light–dark cycle. Food and water were provided ad libitum.
Preparation of liver-derived lipid nanoparticles [Lef-NP]
The Lef-NP was prepared from serum-free culture supernatants (conditioned media; CM) collected three hours after establishing primary cultures of parenchymal rat hepatocytes isolated from 8 – 12 weeks old female Fischer 344 rats by the two-step in-situ perfusion described by Michalopoulos and co-workers24. The CM was concentrated by ultrafiltration (molecular weight cutoff:100 kDa) and precipitated with 30% saturated (NH4)2SO4 solution (SAS). The SAS-precipitate was reconstituted in 50 mM sodium acetate buffer, pH 5.0 (NaAc) and precipitated again with 6% SAS. The resulting, non-dissolvable precipitate was washed twice in NaAc and finally reconstituted in 8 M Urea by applying several urea-washing steps. The final, nonurea soluble pellet (Lef-NP) was washed several times in Ca2+ / Mg2+ - free PBS and recovered as Lef-NP suspension in a small volume of PBS.
Metabolomics / lipidomics of Lef-NP
Analysis of the Lef-NP lipid content was carried out by use of a targeted metabolomic assay employing the MxP® Quant500 Kit (Biocrates Life Sciences AG).
Establishing primary cultures of mouse microglia
Primary microglial cells were isolated and cultured from newborn Black-6 mice of both sexes according to Lian et al. 60.
Live cell imaging (LCI) / in-vitro testing
Lef-NP and Cer-NP effects were monitored in primary mouse microglial cells under serum-free conditions using a NIKON Biostation IQ over a total incubation interval of maximum 96 hours (acquired at a time resolution of 1 frame per minute). For in-vitro testing of Lef-NP, the cells were treated with Lef-NP (at a dilution of 1:1000) alone or with combinations of (i) Lef-NP + 100 µM amiloride (macropinocytosis inhibition), (ii) Lef-NP + 5 mM 3-methyladenine (macroautophagy inhibition), (iii) Lef-NP + 25 nM bafilomycin A (V-ATPase inhibition) after 24 h Lef-NP pre-incubation or (iv) Texas-red labelled Dextran 3000 [10 mg/mL] for monitoring macropinocytosis. For addressing in-vitro effects of Cer-NP, the cells were treated either with 10 µM ceramide [C18 Ceramide from porcine brain] or Percoll (diluted 1:400 yielding a final Percoll content of 0.25%) or Cer-NP (Percoll : Ceramide [4.43 mM] mixed 1:1] adjusted to a final ceramide (free/NP-bound ceramide) concentration of 10 µM (yielding a final Percoll content of 0.23%).
Acridine orange (AO) relocation assay
Primary cultured microglia treated with Lef-NP [1:1000] for 24–48 hours was incubated prior to analysis for 5 minutes with AO [5 µg/mL], washed twice in pre-warmed PBS and the living cells analysed by fluorescence microscopy (Excitation 480 nm) as described by Krenn et al.61. The accumulation of AO in acidic organelles gives a bright red fluorescence.
Electron microscopy
For scanning electron microscopy, glutaraldehyde-fixed cells were dehydrated, critical point dried, sputter coated with gold and visualized using a CAMBRIDGE Stereoscan 250 scanning electron microscope. For Transmission electron microscopy, high pressure freeze fixation was employed, followed by cryosubstitution (2% osmium tetroxide, 0.05% uranyl acetate). The specimens were embedded in epoxy resin and processed for TEM analyses.
Statistics and reproducibility
A minimum of 200 cells was analysed per treatment group and experiment by scoring individual LCI video frames. At least three independent LCI experiments (biological replicates; i.e. employing independent primary cultures) were performed. Areas selected for scoring and quantification were selected randomly. Statistical evaluation followed standard procedures using SPSS statistical software package version 29 as outlined in the supplemental methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Source data and statistical outputs for Fig. 2 and Suppl.Fig. 1 are provided in Supplementary Data (Fig. 2 and Suppl.Fig. 1); a complete list of lipids identified in Lef-NP is provided in Supplementary Table 1. A diagram compiling Lef-NP preparation is given in Suppl.Fig. 4. All other materials are available on reasonable request from the corresponding author: Dr. Hubert Kerschbaum, University of Salzburg, Faculty of Natural and Life Sciences, Department of Biosciences and Medical Biology, Hellbrunnerstrasse 34, 5020 Salzburg, Austria. e-mail: Hubert.kerschbaum@plus.ac.at.
References
Lee, C. Y. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. neural Transm. (Vienna, Austria : 1996) 117, 949–960 (2010).
Pluta, R. et al. Ischemic rats as a model in the study of the neurobiological role of human beta-amyloid peptide. Time-dependent disappearing diffuse amyloid plaques in brain. Neuroreport 10, 3615–3619 (1999).
Mandrekar, S. et al. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J. Neurosci. 29, 4252–4262 (2009).
Zhao, L. et al. Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J. Neurosci. 29, 3603–3612 (2009).
Huang, K. L. et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci. 20, 1052–1061 (2017).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med 13, 432–438 (2007).
Heneka, M. T., McManus, R. M. & Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 19, 610–621 (2018).
Shi, Y. & Holtzman, D. M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18, 759–772 (2018).
Tenner, A. J. Complement-Mediated Events in Alzheimer’s Disease: Mechanisms and Potential Therapeutic Targets. J. Immunol. 204, 306–315 (2020).
Rudge, J. D. The Lipid Invasion Model: Growing Evidence for This New Explanation of Alzheimer’s Disease. J. Alzheimers Dis. 94, 457–470 (2023).
Rezaie, P. & Hanisch, U.-K. Historical context. Microglia in Health and Disease, 7-46 (2014).
Ouro, A. et al. Involvement of Ceramide Metabolism in Cerebral Ischemia. Front Mol. Biosci. 9, 864618 (2022).
Panchal, M. et al. Ceramides and sphingomyelinases in senile plaques. Neurobiol. Dis. 65, 193–201 (2014).
Ayub, M., Jin, H. K. & Bae, J. S. Novelty of Sphingolipids in the Central Nervous System Physiology and Disease: Focusing on the Sphingolipid Hypothesis of Neuroinflammation and Neurodegeneration. Int J Mol Sci 22 https://doi.org/10.3390/ijms22147353 (2021).
Nayak, D. et al. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience 166, 132–144 (2010).
Zhong, L. et al. Lipid transporter Spns2 promotes microglia pro-inflammatory activation in response to amyloid-beta peptide. Glia 67, 498–511 (2019).
Karunakaran, I. et al. Neural sphingosine 1-phosphate accumulation activates microglia and links impaired autophagy and inflammation. Glia 67, 1859–1872 (2019).
Kihara, Y. Systematic Understanding of Bioactive Lipids in Neuro-Immune Interactions: Lessons from an Animal Model of Multiple Sclerosis. Adv. Exp. Med Biol. 1161, 133–148 (2019).
Nakajima, K., Tohyama, Y., Kohsaka, S. & Kurihara, T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J. Neurochem 80, 697–705 (2002).
Jung, J. S. et al. Anti-inflammatory mechanism of exogenous C2 ceramide in lipopolysaccharide-stimulated microglia. Biochim Biophys. Acta 1831, 1016–1026 (2013).
Crivelli, S. M. et al. CERT(L) reduces C16 ceramide, amyloid-β levels, and inflammation in a model of Alzheimer’s disease. Alzheimers Res Ther. 13, 45 (2021).
Scheiblich, H. et al. Activation of the NLRP3 inflammasome in microglia: the role of ceramide. J. Neurochem 143, 534–550 (2017).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet 41, 1088–1093 (2009).
Michalopoulos, G. et al. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res 42, 4673–4682 (1982).
Eckl, P. M., Whitcomb, W. R., Michalopoulos, G. & Jirtle, R. L. Effects of EGF and calcium on adult parenchymal hepatocyte proliferation. J. Cell Physiol. 132, 363–366 (1987).
Alzheimer, A. Uber eigenartige Krankheitsfalle des spateren Alters. Psychiatr. Nervenkr Z. Gesamt. Neurol. Psychiatr. 4, 356–385 (1911).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Su, H. et al. Exploring the significance of lipids in Alzheimer’s disease and the potential of extracellular vesicles. PROTEOMICS, e2300063 https://doi.org/10.1002/pmic.202300063 (2023).
de Wit, N. M., Mol, K., Rodríguez-Lorenzo, S., de Vries, H. E. & Kooij, G. The Role of Sphingolipids and Specialized Pro-Resolving Mediators in Alzheimer’s Disease. Front. Immunol. 11, 620348 (2020).
Yang, L. G., March, Z. M., Stephenson, R. A. & Narayan, P. S. Apolipoprotein E in lipid metabolism and neurodegenerative disease. Trends Endocrinol. Metab. 34, 430–445 (2023).
Ritter, M., Bresgen, N. & Kerschbaum, H. H. From Pinocytosis to Methuosis-Fluid Consumption as a Risk Factor for Cell Death. Front. cell developmental Biol. 9, 651982 (2021).
Koivusalo, M. et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563 (2010).
Baker, E. et al. What does heritability of Alzheimer’s disease represent? PLoS One 18, e0281440 (2023).
Zensi, A. et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control Release 137, 78–86 (2009).
Ehrenreich, J. H., Bergeron, J. J., Siekevitz, P. & Palade, G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J. Cell Biol. 59, 45–72 (1973).
Schulze, R. J., Schott, M. B., Casey, C. A., Tuma, P. L. & McNiven, M. A. The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. 218, 2096–2112 (2019).
Breiden, B. & Sandhoff, K. Lysosomal Glycosphingolipid Storage Diseases. Annu Rev. Biochem 88, 461–485 (2019).
Choi, J. M. et al. C(6)-ceramide enhances phagocytic activity of Kupffer cells through the production of endogenous ceramides. Molecules cells 32, 325–331 (2011).
Chen, C. S., Rosenwald, A. G. & Pagano, R. E. Ceramide as a modulator of endocytosis. J. Biol. Chem. 270, 13291–13297 (1995).
Bian, F., Xiong, B., Yang, X. & Jin, S. Lipid rafts, ceramide and molecular transcytosis. Front Biosci. (Landmark Ed.) 21, 806–838 (2016).
Li, W. et al. Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med Cell Longev. 2014, 823071 (2014).
Kopatz, V. et al. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials (Basel) 13 https://doi.org/10.3390/nano13081404 (2023).
Qian, N. et al. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl Acad. Sci. USA 121, e2300582121 (2024).
Marfella, R. et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med 390, 900–910 (2024).
Hanada, K. Lipid transfer proteins rectify inter-organelle flux and accurately deliver lipids at membrane contact sites. J. Lipid Res 59, 1341–1366 (2018).
Scorrano, L. et al. Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019).
Pacheco, C. D. & Lieberman, A. P. The pathogenesis of Niemann-Pick type C disease: a role for autophagy? Expert Rev. Mol. Med 10, e26 (2008).
Pfrieger, F. W. The Niemann-Pick type diseases - A synopsis of inborn errors in sphingolipid and cholesterol metabolism. Prog. Lipid Res 90, 101225 (2023).
von Saucken, V. E., Jay, T. R. & Landreth, G. E. The effect of amyloid on microglia-neuron interactions before plaque onset occurs independently of TREM2 in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 145, 105072 (2020).
Grubman, A. et al. Transcriptional signature in microglia associated with Aβ plaque phagocytosis. Nat. Commun. 12, 3015 (2021).
Sebastian Monasor, L. et al. Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. Elife 9 https://doi.org/10.7554/eLife.54083 (2020).
Krabbe, G. et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One 8, e60921 (2013).
Streit, W. J. et al. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 66, 2550–2562 (2018).
Streit, W. J. & Xue, Q. S. Life and death of microglia. J. Neuroimmune Pharm. 4, 371–379 (2009).
Miao, L. et al. SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells. Cell Chem. Biol. 30, 248–260.e244 (2023).
Hanisch, U. K. Functional diversity of microglia - how heterogeneous are they to begin with? Front Cell Neurosci. 7, 65 (2013).
Hanisch, U. K. Proteins in microglial activation-inputs and outputs by subsets. Curr. Protein Pept. Sci. 14, 3–15 (2013).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Keren-Shaul, H. et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e1217 (2017).
Lian, H., Roy, E. & Zheng, H. Protocol for Primary Microglial Culture Preparation. Bio Protoc 6 https://doi.org/10.21769/BioProtoc.1989 (2016).
Krenn, M. A. et al. Ferritin stimulated lipid peroxidation, lysosomal leak and macroautophagy promote lysosomal “metastability” in primary hepatocytes determining in-vitro cell survival. Free Radic. Biol. Med. 80, 48–58 (2014).
Acknowledgements
Ancuela Andosch is highly acknowledged for assistance in transmission electron microscopy and EELS measurements. This open access publication was supported by the Paris Lodron University of Salzburg Publication Fund.
Author information
Authors and Affiliations
Contributions
Kerschbaum, H.H., conceptualization, manuscript preparation, transmission electron microscopy. Gerner, C., metabolomic analysis, manuscript preparation. Oberascher, K., in-vitro testing and live cell imaging, scoring for phenotype distributions, scanning electron microscopy. Steiner, P., transmission electron microscopy, TEM analysis (3D tomography modelling). Schürz, M., in-vitro testing and live cell imaging, acridine orange assay. Bresgen, N, conceptualization, manuscript preparation, experimental work (Lef-NP preparation), LCI media editing, statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr Huan Bao and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kerschbaum, H.H., Gerner, C., Oberascher, K. et al. Lipid-nanoparticle-induced vacuolization in microglia. Commun Biol 7, 1558 (2024). https://doi.org/10.1038/s42003-024-07271-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07271-6