Abstract
Pregnancy is a period of profound biological transformation. However, we know remarkably little about pregnancy-related brain changes. To address this gap, we chart longitudinal changes in brain structure during pregnancy and explore potential mechanisms driving these changes. Ten participants (Mean age = 28.97 years) are assessed 1–6 times (median = 3) during their pregnancy. Each visit includes anatomical and diffusion-weighted MRI, and assessments of waking salivary hormones, hair hormones, and inflammatory cytokines. Here we observe a reduction in gray matter volume and an increase in neurite density index (NDI), a proxy of axon density, in white matter tracts across pregnancy. Progesterone levels are associated with reductions in brain volumetric measurements, and both progesterone and estradiol levels are linked to increases in NDI in white matter tracts. This study highlights the profound neurobiological changes experienced by pregnant individuals and provides insights into neuroplasticity in adulthood.
Similar content being viewed by others
Introduction
Pregnancy is a period characterized by profound changes, including hormonal surges, increased blood volume, heightened oxygen consumption, as well as morphological adjustments such as uterine expansion and relocation of organs1,2,3. These adaptations support fetal development and preparation for labor and delivery. The body’s response to pregnancy may also change the pregnant person’s brain, potentially as adaptive, nonadaptive, or merely evolutionary byproducts4,5. However, we know remarkably little about pregnancy-related brain changes. This knowledge gap regarding how, why, and to what extent the brain changes during pregnancy is, at least in part, attributable to historical reluctance of medical studies to include pregnant individuals due to concerns over potential risks and ethical complexities6,7,8,9. However, magnetic resonance imaging (MRI) has been used clinically among pregnant patients for the last four decades10, and numerous studies find no evidence that MRI is associated with increased risk for adverse effects on pregnant individuals or their offspring (e.g., congenital anomalies, neoplasm, or vision or hearing loss)11,12,13. Given that approximately 85% of US women give birth in their lifetimes14 and the existence of safe imaging procedures, it is critical to identify how a pregnant person’s brain changes across pregnancy.
Non-human animal studies of pregnancy report diminished hippocampal cell proliferation15,16 and reduced microglial cell density17, both of which may manifest as detectable reductions in gray matter volume. Research on structural brain changes associated with pregnancy in humans remains limited. While several studies suggest potential reductions in brain volume during pregnancy18,19,20,21,22, these findings, however, come with limitations. For example, only four participants were assessed repeatedly (3–7 times) during pregnancy in Oatridge et al.21, and anatomical scans were collected via a 1.0-T MRI system, which has relatively low signal-to-noise ratio (SNR) and spatial resolution. Further, neither Hoekzema et al.18,19, which compared women’s brains before conception to 2-3 months postpartum, nor Paternina-Die et al.20, which examined differences between pregnant individuals and nulliparous controls at late pregnancy and early postpartum, addressed changes occurring within pregnancy. More recently, Pritschet et al.22 leveraged precision imaging to map neuroanatomical changes in a single individual from preconception through two years postpartum, suggesting decreases in gray matter volume and cortical thickness across the brain during pregnancy. While this work offers intriguing evidence of remarkable neuroplasticity during pregnancy, the findings are based on a single subject, which limits the generalizability and scope of broader inferences. As such, the current literature regarding brain changes during pregnancy remains inconclusive. Within-subject longitudinal studies are essential for mapping brain dynamics throughout gestation, offering insights into pregnancy-related neuroplasticity.
In terms of potential pregnancy-related changes in white matter microstructure, non-human animal research has observed an increase in myelinated axons during pregnancy, potentially resulting from augmented proliferation of oligodendrocytes—a type of brain cell vital for axonal myelination23,24,25. In particular, Chan et al.26 reported a general increase in diffusivity across the whole brain during pregnancy, as well as regional increases in mean diffusivity (MD) and fractional anisotropy (FA) that were derived from Diffusion Tensor Imaging (DTI). The current human studies, however, have generated inconsistent findings. Hoekzema et al.19 found no significant changes in white matter diffusion metrics derived from DTI or in white matter volume when comparing pregnant women to nulliparous controls. In contrast, Pritschet et al.22 observed nonlinear increases in white matter quantitative anisotropy across the brain—indicating greater tract integrity—as gestational weeks progressed in a single subject.
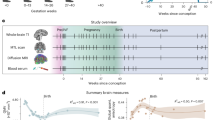
In vivo longitudinal studies are crucial for detecting and understanding the dynamics of brain changes during pregnancy. Moreover, there are increasing calls for a more inclusive approach to biomedical research that recognizes and investigates the distinctive biological experiences of females27,28,29. Responding to this need, we initiated a pilot longitudinal study of pregnant people (n = 10, assessed 1–6 times [median = 3], spanning 12–39 gestational weeks). We used anatomical MRI to assess brain morphometry (e.g., gray matter volume and cortical thickness). White matter microstructure was evaluated with multi-shell diffusion-weighted MRI and advanced neurite orientation dispersion and density (NODDI)30 modeling, which provided metrics such as the neurite density index (NDI) and orientation dispersion index (ODI). The primary goals of the current study were twofold. The first objective was to chart the brain structural changes occurring during pregnancy. The second goal was to explore potential mechanisms driving these changes, particularly focusing on pregnancy hormones, inflammatory cytokines, and the intracranial cerebrospinal fluid (CSF) volume.
Results
Brain changes during pregnancy
Brain morphometry changes
Covarying for total intracranial volume (TIV) and age, total brain volume decreased across pregnancy (β = −0.105, 95% CI [−0.169, −0.040], p = 0.004). This translates to a 2.6% decline over the observed gestational range in this study (i.e., 12–39 weeks). We further conducted leave-one-out analyses, which resulted in estimated β values that ranged from −0.184 to −0.090. Similarly, total gray matter volume exhibited decreases across pregnancy (β = −0.192, 95% CI [−0.301, −0.082], p = 0.002). This translates to a 3.9% decline over the observed gestational range in this study (see Fig. 1). With leave-one-out analyses, β values ranged from −0.328 to −0.179. There were decreases in total cortical volume (β = −0.238, 95% CI [−0.374, −0.102], p = 0.002) and in subcortical volume (β = −0.094, 95% CI [−0.196, 0.008], p = 0.068), although the latter was not statistically significant. Additionally, there was a large decrease in cortical thickness (β = −0.516, 95% CI [−0.915, −0.116], p = 0.015), yet cortical surface area remained unchanged (β = −0.018, 95% CI [−0.052, 0.017], p = 0.297). These results suggest that the observed reduction in total gray matter volume may primarily result from decreases in cortical volume, particularly cortical thickness (see Fig. S2 for detailed statistics). Lastly, total white matter volume did not exhibit a statistically significant change across pregnancy (β = −0.031, 95% CI [−0.120, 0.057], p = 0.460). All findings were consistent using leave-one-out analyses, as detailed in Table S2.
The plots are based on the linear mixed-effects model shown in Eq. (1). Specifically, brain metrics were the dependent variables, and gestational week was the predictor, covarying for age and total intracranial volume. GM: gray matter, WM: white matter. The shaded area represents the 95% confidence interval (95% CI) for the fitted line. Note. **p < 0.01.
Changes in cortical regions
To further characterize the reduction in total cortical volume, we fitted linear mixed-effects (LME) models for all Desikan–Killiany atlas regions, using gestational week as the fixed effect and hemisphere, TIV, and age as covariates. A surface map with the standardized regression coefficients for all cortex regions is shown in Fig. 2a. Overall, we observed a consistent trend of decreasing cortical volume in most cortical regions, except for three regions in the occipital lobe (pericalcarine, lingual, and cuneus) and two regions in the medial temporal lobe (entorhinal and temporal pole). Statistically significant reductions in cortical volume were observed in prefrontal cortex regions, including the medial orbitofrontal, superior frontal, and lateral orbitofrontal cortex, as well as two regions surrounding the central sulcus: the precentral and postcentral cortex (Fig. 2b). Detailed statistical reports, including leave-one-out analyses consistent with the full sample results, are provided in Table S3.
a The figure is color-coded to reflect the standardized regression coefficients derived from linear mixed-effects models, which were fitted for each cortical region volume against gestational week while covarying for age and total intracranial volume (Eq. 2). b The figure selectively presents the cortical regions that exhibited a significant volume decrease throughout pregnancy. No multiple comparison correction was implemented.
White matter microstructure changes
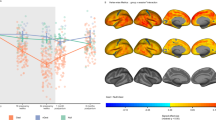
In our investigation of changes in white matter microstructure, we implemented LME models for each of the NODDI metrics (NDI and ODI) across each of the 50 tracts generated by TractSeg with gestational week as the fixed effect and hemisphere (where applicable), age, and relative motion as covariates. To facilitate the understanding of the global pattern of metric changes, a heat map was generated, representing the standardized regression coefficients for all segmented tracts (see Fig. S3). Throughout the gestation period, we observed a predominant increase in NDI across most tracts, whereas ODI metrics remained relatively stable. Notably, the most pronounced increases in NDI were observed in projection tracts such as the corticospinal tract (CST), fronto-pontine tract (FPT), parieto-occipital pontine (POPT), in addition to the striato-premotor tract (ST_PREM) and arcuate fascicle (AF) (see Fig. 3). In line with these NDI findings, our analysis of DTI metrics revealed a predominant trend of increasing FA. Tracts that exhibited the most significant FA increases largely overlapped with those displaying notable NDI increases. Comprehensive statistical results for NDI and ODI metrics are available in Tables S6 and S7, and analyses of DTI metrics can also be found in the supplementary materials results section. Lastly, an examination of average measures across all segmented tracts revealed no significant global changes in NDI and ODI metrics during pregnancy, consistent with our secondary method for assessing global diffusion metrics (see supplementary materials results section).
The figure is color-coded to represent the standardized regression coefficients from linear mixed-effects models fitted for NDI against gestational week while covarying for age and relative motion (Eq. 2). CST: Corticospinal Tract, FPT: Fronto-Pontine Tract, POPT: Parieto‐Occipital Pontine, SCP: Superior Cerebellar Peduncle, STR: Superior Thalamic Radiation, T_PREM: Thalamo-Premotor, ST_PREM: Striato-Premotor.
CSF changes
We observed a statistically significant increase in total intracranial CSF (β = 0.187, 95% CI [0.021, 0.353], p = 0.030) after covarying for age and TIV (see Fig. 4). Using a leave-one-out cross-validation approach, β values ranged from 0.145 to 0.267 (see Table S4). This pattern was consistent when varying the threshold of CSF volume fraction between 60% and 95% (see Table S5).
The plot is based on the linear mixed-effects model shown in Eq. (1). Specifically, CSF was the dependent variable and gestational week was the predictor, covarying for age and total intracranial volume. The shaded area represents the 95% confidence interval (95% CI) for the fitted line. Note. *p < .05.
Associations between brain dynamics and salivary hormones in pregnancy
Salivary hormones and inflammatory cytokine changes during pregnancy
Covarying for age, we observed statistically significant increases in waking salivary levels of progesterone (β = 0.717, 95% CI [0.391, 1.042], p < 0.001), estradiol (β = 0.792, 95% CI [0.533, 1.050], p < 0.001), and testosterone (β = 0.393, 95% CI [0.061, 0.724], p = 0.024) across pregnancy (see Fig. 5a). However, the increase in cortisol levels was not statistically significant (β = 0.198, 95% CI [−0.014, 0.410], p = 0.065). Additionally, we did not observe statistically significant changes in C-reactive protein (CRP) levels throughout the course of pregnancy (β = −0.067, 95% CI [−0.248, 0.114], p = 0.441).
a The observed changes in hormone levels (progesterone, estradiol, testosterone, and cortisol) during pregnancy. Linear mixed-effects models were applied with hormone levels as the dependent variable and gestational week as the predictor, covarying for age. b Associations between progesterone levels and brain structural metrics, including total brain volume, total gray matter volume, total cortical volume, and cortical thickness. The plot is based on Eq. 3. Specifically, brain metrics were the dependent variable and progesterone was the predictor, covarying for age and total intracranial volume. The shaded area represents the 95% confidence interval (95% CI) for the fitted line. Note. **p < .01. ***p < .001.
Associations between salivary hormones and brain structure
After covarying for TIV and age, we found statistically significant negative associations between total brain volume and salivary levels of progesterone (β = −0.103, 95% CI [−0.170, −0.036], p = 0.006) and cortisol (β = −0.213, 95% CI [−0.393, −0.032], p = 0.025), indicating that increasing salivary levels of progesterone and cortisol are associated with decreasing total brain volume. Associations between total brain volume and either salivary estradiol or testosterone levels were not statistically significant (ps > 0.144). Additionally, total gray matter volume was negatively associated with salivary progesterone (β = −0.189, 95% CI [−0.305, −0.073], p = 0.003), but not with salivary estradiol, testosterone, or cortisol (all ps > 0.134). Total cortical volume (β = −0.254, 95% CI [−0.390, −0.118], p = 0.002) and mean cortical thickness (β = −0.691, 95% CI [−1.049, −0.333], p = 0.001) were also negatively associated with salivary progesterone (see Fig. 5b), but not with salivary estradiol, testosterone, or cortisol levels (all ps > 0.137).
Associations between salivary hormones and diffusion metrics
Fig. S8 shows the standardized regression coefficients of hormone levels across all segmented tracts, illustrating the associations between NDI and salivary hormone levels. Most notably, our results indicated that NDI across nearly all tracts was positively associated with salivary estradiol levels and moderately with salivary progesterone (see Fig. 6). Moreover, NDI demonstrated a negative link with salivary cortisol levels, while no significant association was found with salivary testosterone. Detailed statistical outcomes of these analyses are available in Tables S12–S15.
The color-coding reflects the standardized regression coefficients obtained from linear mixed effects models, which were used to analyze the relation between NDI and each of the three hormones while covarying for age and relative motion (Eq. 4). Note. *p < .05. **p < .01. ***p < .001. An asterisk in the color bar indicates the level of significance for standardized regression coefficients.
Associations between salivary hormones and CSF
Covarying for TIV and age, no statistically significant associations were detected between CSF volume changes and salivary hormonal levels, including progesterone (β = 0.130, 95% CI [−0.060, 0.319], p = 0.160), estradiol (β = 0.151, 95% CI [−0.060, 0.361], p = 0.143), testosterone (β = 0.153, 95% CI [−0.119, 0.425], p = 0.242), or cortisol (β = 0.187, 95% CI [−0.284, 0.668], p = 0.401; see Table S16–S19).
Associations between CSF and brain structure
There were statistically significant negative associations between total intracranial CSF and total brain volume (β = −0.277, 95% CI [−0.452, −0.102], p = 0.004), total gray matter volume (β = −0.388, 95% CI [−0.678, −0.097], p = 0.013), and total cortical volume (β = −0.391, 95% CI [−0.754, −0.028], p = 0.037), suggesting that reductions in brain volumetrics may be associated with increases in total intracranial CSF volume. However, the link between total intracranial CSF and mean cortical thickness was not statistically significant (β = −0.515, 95% CI [−1.069, 0.039], p = 0.066).
Associations between CSF and diffusion metrics
Across all segmented tracts, there were no statistically significant associations between total intracranial CSF and NDI. Detailed statistical outcomes of these analyses are available in Table S20.
Discussion
In a sample of 10 pregnant participants who underwent multimodal MRI scans, hormone assessments, and inflammatory cytokine assessments between 1 and 6 times during pregnancy (median = 3 times), we explored whether, and to what degree, neurobiological changes occur during pregnancy. There were four main findings. First, there were pronounced reductions in overall brain and gray matter volume, while an increase was found in the total intracranial CSF volume. Second, using NODDI metrics to assess white matter microstructure, we found an increase in NDI across the majority of segmented tracts, with the most pronounced increase observed in tracts associated with sensorimotor processing. Third, there were significant correlations between pregnancy-related salivary hormones and observed brain changes (i.e., volumetric reduction in overall brain and gray matter, and increased NDI in segmented tracts). Fourth, the increase in the total intracranial CSF volume was associated with the decrease in brain volumetrics, but not with NDI changes.
The decreases in estimates of total brain volume in the present study were consistent with earlier evidence involving both humans18,19,20,21,22 and non-human animal models15,16,17. Comparing mothers before and after their first pregnancy, Hoekzema et al.18,19 reported a reduction in gray matter volume across multiple brain regions, notably within the anterior and posterior midline structures. Another study found that during late pregnancy, mothers exhibited smaller global cortical volume and thickness compared to nulliparous controls, with the most pronounced differences in the somatomotor, attentional, and default mode networks20. A recent study by Pritschet et al.22 focused on neuroanatomical changes from preconception to two years postpartum in a single individual, documenting significant reductions in gray matter volume and cortical thickness. Non-human animal studies suggest that the observed brain volume reduction may be linked to decreased microglial proliferation, as evidenced by the reduced microglial cell density in pregnant rats during late gestation and the early postpartum period compared to virgin rats17. Expanding upon previous pre- and post-pregnancy comparisons and cross-sectional analyses, our findings demonstrate a significant rate of reduction in gray matter volume during pregnancy. Additionally, we observed a significant correlation between CSF volume and overall brain and gray matter volumes. This association suggests that intracranial CSF volume increases during pregnancy, consistent with the prior report of ventricular volume expansion21, may exert pressure on the brain and compress the cortex, possibly contributing to the observed decreases in brain volume31,32.
Additionally, we observed an increase in NDI across tracts, especially those linked with sensorimotor processing, whereas ODI remained largely unchanged. NDI quantifies the tissue volume fraction occupied by neurites, such as axons and dendrites, and is considered a proxy for axonal density in white matter33,34. Meanwhile, ODI reflects the variance in neurite orientation. Our findings suggest that, across pregnancy, there may be an increase in axonal density, potentially stemming from increased myelination and/or axonal diameter, without significant shifts in white matter geometric complexity. This aligns with prior non-human animal studies that have documented an increase in myelinated axons during pregnancy, potentially as a result of amplified proliferation of oligodendrocytes24,25. However, there is a discrepancy between our results and those reported by Hoekzema et al.19 who did not observe significant changes in DTI metrics during pregnancy. The discrepancy may arise from differences in imaging sequence parameters. For instance, Hoekzema et al.19 used a single shell protocol (b = 1000) in contrast to the multi-shell protocol (b = 500, 1500, 2500) in the present study, which has been shown to affect the estimated scalar diffusion metrics35. Another consideration is the methodological divergence between the two studies. Our study adopted a longitudinal design, capturing dynamic changes over the course of pregnancy, while Hoekzema et al.19 employed a pre-post pregnancy comparison approach. Their post-pregnancy data acquisition took place, on average, 100 days after childbirth with a wide variability (SD = 70 days). This extended interval could potentially obscure white matter alterations occurring during pregnancy. Indeed, the recent study by Pritschet et al.22 demonstrated an increase in global quantitative anisotropy (QA), an index of white matter microstructural integrity, during gestation, which was concomitant with rises in estradiol and progesterone. However, extended analyses into the postpartum period revealed non-linear patterns of global QA, with increases during the first and second trimesters, returning to baseline levels postpartum. Thus, it is possible that white matter quickly reverts to its pre-pregnancy state, thereby remaining undetected in the delayed post-pregnancy assessment. Alternatively, the postpartum period itself, characterized by hormonal fluctuations, physiological adaptations, and new caregiving demands, might induce its own set of white matter microstructural adaptations, which might counteract those taking place during pregnancy. Additionally, the absence of global changes in white matter microstructural metrics, specifically NDI, may be due to regional variability. While we observed increases in NDI across most tracts, other tracts exhibited small to subtle increases. As such, the effects may not be detectable at the global level. Although it is also possible that our study is underpowered to detect subtle global changes that may indeed be present, this observation, if generalized, highlights the importance of understanding focal tract changes in NDI that occur during pregnancy in future studies.
The current study also explored candidate mechanisms underlying the observed neurobiological changes during pregnancy. First, our data revealed a correlation between pregnancy-associated hormones and brain alterations in both gray matter and white matter. Estradiol and progesterone are pivotal neuroprotective agents, modulating processes like neurogenesis36,37, dendritic spine formation and branching38,39, myelination40,41, axonal growth, and synaptogenesis42. Our findings of positive correlations between white matter NDI and progesterone and estradiol levels are consistent with results from animal studies. However, the observed inverse associations between pregnancy-related hormones and gray matter volumes are somewhat counterintuitive, especially when considering that non-human animal research suggests estradiol and progesterone promote neurogenesis43,44. It might be that increased dendritic length, branching, and spine density in gray matter due to pregnancy-related hormones38,39,42,45 and/or myelination lead to misclassification of voxels at tissue interfaces, thereby appearing as reductions in gray matter volume46,47, either in addition to or instead of fluid-related compression. It is also possible that hormones influence other physiological functions that are linked to a decrease in gray matter volume. For example, suppressed microglial proliferation might underlie the connections between hormonal shifts and grey matter reduction during pregnancy47. Furthermore, the observed inverse associations raise the possibility of a non-linear, U-shaped relationship, where progesterone’s links to brain structure may not be straightforward and could vary depending on concentration levels48. Indeed, inverted U-shaped relationships have also been observed for other hormones such as estradiol49 and cortisol50 in humans.
Additionally, it is interesting that we observed a link between brain morphometry and progesterone, but not estradiol. However, we should acknowledge that salivary hormone assays can be affected by numerous factors, including collection time and sample storage51,52. If these effects generalize, it might suggest that progesterone and estradiol have distinct links to brain morphometry. This distinction has been evidenced by their different effects on receptors53 and functional network organization54, as well as their complex interactions in synaptic plasticity and neuroprotection55. It has been reported that intrinsic fluctuations in progesterone, but not estradiol, were associated with medial temporal lobe morphology across the human menstrual cycle56. In mice, during pregnancy, both estradiol and progesterone affect the galanin (Gal)-expressing neurons in the mouse medial preoptic area (MPOA), but in different ways—estradiol silences MPOAGal neurons and increases neuronal excitability, while progesterone promotes dendritic spine formation. Those effects on MPOAGal neurons are linked to increased selectivity for pup stimuli57. However, whether these previously mentioned findings from animal studies translate to humans remains unclear, given the challenges inherent in comparing animal models to human experiences58. It is important to note that our findings reflect associations and cannot be interpreted as evidence of causality. The observed relations between hormone levels and brain structure could be due to a variety of factors, such as underlying physiological changes that occur during pregnancy and/or the influence of other biological processes not measured in the current study.
Moreover, the influence of pregnancy-related hormones likely represents only one of the factors contributing to observed brain changes. Other pregnancy-associated alterations, such as increased water content59,60 and blood flow variations61,62 during pregnancy, may also play significant roles. For example, studies have documented a significant and progressive rise in total body water, as well as extracellular and intracellular water values throughout pregnancy59,60, which might help explain the observed elevation in NDI. Yet, it is important to note that in vivo MRI methods offer insights at a macroscopic level and inherently lack the resolution to elucidate the cellular and molecular mechanisms at play during pregnancy. Future research across multiple levels of analysis is needed to supplement this work and enhance our understanding of the neurobiological changes observed during pregnancy.
Although cortisol levels in our study did not reach statistical significance at the .05 level, they demonstrated a small to moderate increasing trend over the course of pregnancy. This observation is consistent with previous literature indicating that salivary cortisol levels progressively rise during pregnancy63,64. Additionally, we observed negative associations between cortisol levels and total brain volume, as well as between cortisol levels and white matter NDI. These findings align with existing research that implicates cortisol in neurodevelopmental processes, including neurogenesis65,66, synaptic plasticity67,68, and myelination69. Higher cortisol levels have been linked to reduced brain volume and alterations in white matter microstructures70. Therefore, despite the lack of statistical significance in the observed cortisol changes, the upward trend might still hold biological relevance in influencing brain structure in pregnant individuals. However, it is important to acknowledge the limitations of the waking salivary cortisol assay in our study, as it was assessed only once upon waking71,72, and hormone assays can be affected by various factors, as mentioned above. As such, interpretations should be made with caution. Future research may benefit from more frequent cortisol measurements to better capture dynamic changes during pregnancy and explore their implications for brain structure.
Additionally, we did not observe significant changes in CRP levels during the observed period (12 to 39 gestational weeks). This finding aligns with previous literature, which demonstrates that CRP levels can vary widely among individuals during pregnancy—some studies report an increase73,74,75, some report consistency73,76,77, and others report a decrease73,74 in CRP levels across gestation. Therefore, while CRP is an important marker of inflammation78, it has not been consistently shown to track with gestation and is likely influenced by various factors.
While not the focus of the current study, there are multiple hypotheses regarding the potential implications of pregnancy-related brain changes. While some contend that changes to the pregnant person’s brain are most likely byproducts of promoting fetal survival and childbirth4, others point to the possibility that these changes are in the service of promoting the parent–child relationship19,79,80. There is substantial evidence that interactions with children post-birth shape the brain. Indeed, evidence from non-birthing parents, which allows for distinctions between pregnancy-specific vs. caregiving-related brain changes, shows brain alterations following childbirth81,82,83,84 and child rearing85,86. Additionally, research has shown that brain changes can persist up to six years postpartum87, and persistent changes may relate to the cognitive and emotional challenges of caring for a child88,89. Future work would benefit from careful consideration of the potential functions of these changes, with designs that help to rule out plausible rival hypotheses.
Several limitations merit consideration. First, our study was constrained by a small sample size. This limited our power to detect subtle effects as well as our ability to adjust for potential confounds and examine the potential effect of moderators. For example, prior research suggests that the number of live births is related to brain age88. It is possible that the number of live births might moderate the neurobiological changes during pregnancy, such that individuals with more births experience less (or more) neurobiological changes (see supplementary materials results section for our exploration of these associations within our sample). Given that the current literature has focused nearly exclusively on first-time mothers, it is especially important for future research to consider the number of prior births on pregnancy-related brain changes. Second, our study is limited by the number of data points available (27 unique scan sessions from 10 individuals), with some participants attending only a single session. For example, while our calculation of percentage declines in brain morphometry using estimated LME models offers a useful estimate of average changes during pregnancy, it is important to interpret these data with caution. The focus on the observed range of gestational weeks (i.e., 12–39 weeks) is intentional to accommodate the limited coverage of gestational weeks and small sample size, which could nevertheless affect the precision of this estimate. Third, our study does not have benchmarking data. Including such comparisons in future research would provide additional context for interpreting the uniqueness of pregnancy-related neurobiological changes. For example, benchmarking against non-birthing parents could help isolate changes unique to pregnancy from those due to shared environmental or experiential factors, offering a more nuanced understanding of the impacts of pregnancy. Fourth, our sample did not cover the entire duration of pregnancy. Without preconception data, we have an incomplete picture of when during pregnancy such alterations become evident. Fifth, given the preliminary nature of our investigation, we opted not to implement corrections for multiple comparisons. Although this decision increases sensitivity to potential effects, it simultaneously raises the risk of type I errors. However, the consistency in patterns and lack of randomness suggest that the results may not be artifacts but are likely indicative of genuine underlying phenomena. It is important for subsequent, larger-scale studies to incorporate appropriate corrections to further validate and replicate our observations. Sixth, our exploration of the underlying mechanisms of neural dynamics primarily focused on hormonal fluctuations during pregnancy. Although these hormonal shifts are important, they might represent just one aspect of a more multifaceted interplay. Other potential factors, such as metabolic changes, vascular dynamics, and alterations in neurotransmitter levels, may also significantly contribute. Future research adopting an interdisciplinary approach, integrating insights from these domains would shed light on the multifaceted nature of brain changes during pregnancy. Lastly, careful monitoring of pregnancy complications (e.g., preeclampsia and eclampsia) which may significantly affect brain structure21, is crucial to ensure accurate interpretation of the findings in the future.
The present study elucidates neurobiological changes occurring across pregnancy. Specifically, we observed a decline in total brain and gray matter volume, along with increases in total intracranial CSF volume and NDI in white matter tracts across pregnancy. Our data also suggest that increases in CSF and pregnancy-related hormones may, at least partially, contribute to these observed neural alterations. These findings highlight the neuroplasticity associated with pregnancy, establishing a foundation for further investigations in this essential yet understudied area of research.
Methods
Participants
A total of 10 pregnant women aged between 20.00 and 39.25 years (M = 28.97, SD = 6.50) years were recruited for the current longitudinal study. In terms of race, 7 identified as White, 2 as Black or African American, and 1 selected “Other.” In terms of ethnicity, 1 participant identified as Hispanic/Latine. Participants were recruited through online advertisements and referral/word of mouth and 1 participant was the senior author. Participant eligibility was initially screened via telephone. Inclusion criteria required participants to be pregnant, at least 18 years old, English-proficient, and have no immediate plans to relocate. Exclusion criteria encompassed a medical history of neurological injury or impairment, a history of psychosis, non-removable metal or electronic implants, having undergone in vitro fertilization (IVF), and use of medication(s) that could potentially influence hormonal or neurological changes during pregnancy (e.g., progesterone). The mean number of prior pregnancies carried to term was 0.50 (SD = 0.71, range = 0–2). The mean weeks gestation at data collection was 23.90 (SD = 7.70, range = 12.29–39.14). The number of data collection sessions for each participant ranged from 1 and 6 times (mean number of repeated measures = 2.70 times, SD = 1.49), contingent on individual circumstances and pregnancy progression. Figure 7 provides a detailed illustration of the gestational timing range captured for each participant. Each session included an MRI scan and collection of hair samples and dried blood spots. Additionally, participants were asked to collect saliva samples at their homes on the morning prior to and the morning of each session. All participants provided written informed consent prior to participation. Study procedures were approved by the Vanderbilt University Institutional Review Board. All ethical regulations relevant to human research participants were followed.
Hormone assessments
Hormonal assessments were performed by measuring levels of progesterone, estradiol, testosterone, and cortisol in saliva samples obtained from participants, in accordance with Salimetrics collection and assaying procedures. Two saliva samples were collected for each participant: one on the morning prior to the MRI scan and another on the day of the scan. The obtained values were then averaged for analysis. Additionally, we obtained estimates of cortisol, cortisone, and progesterone from hair samples. Further details on the saliva and hair sample collection and assay procedures can be found in the supplementary materials sections titled Salivary hormone assessments and Hair hormone assessments. We primarily reported on salivary hormones, and results from hair hormone analysis were included in the supplementary materials results section.
Inflammatory cytokines assessments
Inflammatory cytokine assessments were performed by measuring levels of Interleukin-6 (IL-6), Interleukin-10 (IL-10), Tumor Necrosis Factor alpha (TNFɑ), and CRP in dried blood spots (DBS) obtained from participants. At each visit, up to 5 drops of capillary whole blood were collected on filter paper (Whatman 903) following a finger stick with a sterile microlancet. Blood was then dried at room temperature and stored at −20 degrees Celsius in accordance with protocols established by Northwestern University’s Laboratory for Human Biology Research90,91, which performed assays on DBS samples. Further details on the DBS collection and assay procedures can be found in the supplementary materials section titled Inflammatory cytokines assessments. The usability of the samples for IL-6, IL-10, and TNFɑ was constrained by the high volume requirements, resulting in a low yield of successfully assessed data for these cytokines (less than 50%). Thus, only CRP was utilized in the analyses. Detailed descriptive statistics are provided in Supplementary Table S1.
MRI acquisition
MRI data acquisition was conducted on a 3T Philips Achieva scanner using a 32-channel head coil at Vanderbilt University Institute of Imaging Science. High-resolution T1- and T2-weighted images were obtained for each participant through 3D-quantification using an interleaved Look–Locker acquisition sequence with a T2 preparation pulse (3D-QALAS) sequence92 with the following parameters: repetition time (TR) of 5.0 ms, echo time (TE) of 2.3 ms, a flip angle of 4 degrees, a field of view (FOV) of 230 ×193 × 150 mm, a matrix size of 224 × 224 × 146, and a voxel size of 1 × 1 × 1 mm. The acquisition parameters of diffusion-weighted imaging (DWI) were as follows: TR of 4300 ms, TE of 91.5 ms, flip angle of 90 degrees, FOV of 240 ×240 x 135 mm, matrix size of 112 × 112 × 63, yielding a voxel size of 2 × 2 × 2 mm. The diffusion weighting was distributed across 23 directions with a b-value of 500 s/mm², 47 directions with a b-value of 1500 s/mm², and 70 directions with a b-value of 2500 s/mm². The scanner used in this study underwent regular calibrations and quality control assessments at least weekly, conducted by the Vanderbilt University Institute of Imaging Science. Additionally, vendor engineers performed quarterly preventative maintenance and periodic image quality tests to assess system performance across various specifications, including SNR, geometry, scale, and uniformity. Furthermore, the scanner was remotely monitored by Philips, with daily performance reports covering parameters such as cryogenic system stability, noise levels, coil functionality, hardware integrity, cooling efficiency, and environmental factors like temperature and humidity. Throughout the study, all reports and test results indicated no significant deviations in scanner performance. Further details can be found in the supplementary materials section titled MRI acquisitions.
MRI Processing
Anatomical MRI
Synthetic T1-weighted and T2-weighted images were found from 3D-QALAS images using SyntheticMR software (version 0.45.35; Linkoping, Sweden). Both T1-weighted and T2-weighted anatomical images were then used to extract structural MRI data through FreeSurfer v7.1.0’s longitudinal pipeline93. Scans at every time point were independently processed for each participant (including skull stripping, Talairach transformation, subcortical structure labeling, surface extraction, spherical registration, and cortical parcellation), followed by the creation of an unbiased within-subject template space using all the subject’s time points94. Note that the FreeSurfer longitudinal pipeline assumes a fixed head size over time, meaning that TIV is considered constant across time in the final longitudinal output. By accounting for the expected stability, the longitudinal pipeline is particularly sensitive to detecting small, gradual changes over time. To ensure that TIV remained relatively stable and to better support the inferences made from our subsequent morphological analyses, we used the cross-sectional estimates, treating each dataset as a separate scan before feeding it into the longitudinal pipeline. The results showed that total TIV did not exhibit a meaningful or statistically significant change over the course of pregnancy (β = −0.014, 95% CI [−0.060, 0.033], p = 0.539; see Fig. S1). Following the automated processing steps, all the cortical reconstructions were visually inspected for potential inaccuracies. We observed no processing failures and no manual edits were required. Please refer to the supplementary materials for the Euler number report and example anatomical images in the section titled Anatomical MRI. Subsequently, we obtained measurements of volume for 34 cortical regions in both the left and right hemispheres (68 regions in total) using FreeSurfer’s cortical parcellation based on the Desikan-Killiany atlas95, which were used for cortical morphometric analyses.
CSF
To quantify the total CSF volume in the entire brain, we used the aggregated volume fraction of the CSF image generated by SyntheticMR software from the 3D-QALAS images. Specifically, we first applied a threshold to retain voxels in which the CSF volume faction exceeded this threshold. Then, we multiplied the CSF volume fraction by the voxel volume across the brain. For our primary analyses, we set a threshold at 80%, meaning that a voxel was classified as containing CSF if it has 80% or more CSF. To test if this threshold influenced findings, we repeated analyses using different thresholds across a range of 60% to 95% (reported in Table S4). We observed consistent results across these different probability thresholds.
Diffusion-weighted MRI
The raw diffusion data were processed using MRtrix396 and FMRIB’s Software Library (FSL)97. Preprocessing steps involved noise estimation and removal via the dwidenoise function from MRtrix398,99. The eddy and susceptibility distortion corrections were performed using the dwifslpreproc function in MRtrix397,100,101,102. Subsequent to preprocessing, we computed the NDI and ODI using the NODDI Matlab Toolbox30. All preprocessing steps and NODDI metrics were carried out in native space. For comparison, we also fitted the standard DTI model103. In order to analyze changes across tracts, we first estimated fiber orientation distributions (FOD) using MRtrix3’s dwi2fod msmt_csd and then normalized them using MRtrix3’s mtnormalise104,105. The normalized FODs were then fed to TractSeg to automatically segment fiber tracts, resulting in NODDI and DTI metrics for each tract across 50 established tracts, which were subsequently used in our statistical analyses106,107. Lastly, we characterized the global changes for each NODDI metric and investigated if observed tract-level changes reflected a general alteration across all segmented tracts using two different approaches. For a more detailed description of diffusion data processing and metric quantification, please refer to the supplementary materials section titled Diffusion-weighted MRI.
Statistics and reproducibility
Given the longitudinal nature of our data, analyses were conducted using LME models, utilizing the “lme4” package in R108. One of the main advantages of using LME models with longitudinal data is their flexibility and robustness in handling missing data points and designs with variable numbers and intervals between measurements109. This approach can weigh the data points based on the number of observations per subject, thus subjects with different numbers of observations can be included without biasing the overall estimates, enabling us to use all available data points110. Note that we included all available time points, including those from subjects with only a single time point, to maximize data usage. While individuals with data at only a single-time point do not contribute to the estimation of within-subject changes, they provide information about between-subject variability, and inclusion results in improved intercept estimation and overall model accuracy111.
We organized analyses into two primary sections. First, we examined alterations in brain metrics throughout the course of pregnancy, where brain metrics (BrainMetricij) were the dependent variable and gestational week (GesWeekij) was the predictor. We also included age (Agej) and total intracranial volume (TIVj) as covariates for volumetric analyses (see Eq. 1), and included Agej and relative motion (Motionij) as covariates for diffusion metric analyses112; see Eq. (2).
where \({\gamma }_{10}\) is the fixed effect of gestational weeks (i.e., slope), and \({\gamma }_{00}\) and \({u}_{0j}\) are the fixed and random effects of the model intercept which allow each subject to have their own baseline level. To calculate the average percentage change in brain morphometry, we used the estimated LME models to predict the average change in brain morphometry over the range of observed gestational weeks (i.e., 12–39 weeks). Specifically, we used the estimated model to predict the brain metric at 12 and 39 weeks, respectively, and then computed the percentage change as
The second set of analyses focused on the potential mechanisms underlying the observed brain alterations by examining the associations between brain metrics and potential influencing factors, including hormone levels, CRP, and CSF volume. We first applied LME models to examine variations in hormone levels (progesterone, estradiol, testosterone, and cortisol) and CRP throughout pregnancy, with hormone levels and CRP as the dependent variable and gestational week as the fixed effect. CRP was log-transformed using a base 10 logarithm to normalize their distribution113. For brain metrics demonstrating significant alterations during pregnancy, we examined potential links with hormone levels and the total intracranial CSF volume using LME models in which BrainMetricij was the dependent variable and hormone levels (Hormoneij) or CSF volume was the predictor. Given the absence of significant changes in CRP, associational analyses between CRP levels and observed changes in brain metrics were not implemented. Moreover, we included Agej and total TIVj as covariates for volumetric analyses (see Eq. 3); we included Agej and Motionij as covariates for diffusion metric analyses (see Eq. 4).
where \({\gamma }_{10}\) is the fixed effect of gestational weeks (i.e., slope), and \({\gamma }_{00}\) and \({u}_{0j}\) are the fixed and random effects of the model intercept. We reported standard beta values and 95% confidence intervals (95% CI), with p-values tested based on a two-sided significance test.
Given the small sample size and the exploratory nature of our study, we did not correct for multiple comparisons114. Results with p-values < 0.05 were considered statistically significant. This decision was made to prioritize sensitivity over specificity, as it is particularly important in exploratory research to identify potential effects for further investigation in larger studies. Our results should therefore be interpreted with caution. We mitigated potential concerns by emphasizing our interpretation of the direction and magnitude of effect sizes. Additionally, recognizing the possibility that individual outliers could disproportionately influence the outcome in our small sample, we implemented leave-one-out analyses for our main findings, in which each participant was systematically removed and the analyses recomputed. Specifically, a custom R script was programmed to loop through the subject IDs, sequentially removing each participant by instructing the script to filter out the subject ID for each iteration. In each iteration, one subject was automatically excluded from the dataset and the LME models were estimated using the remaining subjects. This approach helps to validate that the observed effects are consistent and not artifacts of unique data points, thus enhancing the reliability of our findings115. Additionally, we included all available data, including those with single time points, in our LME models to maximize the use of the data and increase the reliability of the findings110. To further validate our approach, we have also included results excluding the three participants with only a single time point in the supplementary materials results section. These results are consistent with those presented in the main text. We also considered the patterns of results across related dependent measures, as opposed to individual tests in isolation. The results of our study thus serve as a preliminary exploration into the complex domain of neurobiological dynamics during pregnancy.
Data availability
Data supporting the findings and figures in this study are available at https://github.com/vanderbiltsealab/NCAP. All original data in this study are also available from the corresponding author upon reasonable request.
Code availability
The code utilized in this study is publicly available at https://github.com/vanderbiltsealab/NCAP.
References
Kazma, J. M., van den Anker, J., Allegaert, K., Dallmann, A. & Ahmadzia, H. K. Anatomical and physiological alterations of pregnancy. J. Pharmacokinet. Pharmacodyn. 47, 271–285 (2020).
Tan, E. K. & Tan, E. L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 27, 791–802 (2013).
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H. & Mebazaa, A. Physiological changes in pregnancy: review articles. Cardiovasc. J. Afr. 27, 89–94 (2016).
Cárdenas, E. F., Kujawa, A. & Humphreys, K. L. Neurobiological changes during the peripartum period: implications for health and behavior. Soc. Cogn. Affect. Neurosci. 15, 1097–1110 (2020).
Martínez-García, M., Cardenas, S. I., Pawluski, J., Carmona, S. & Saxbe, D. E. Recent neuroscience advances in human parenting. Patterns Parent. Behav. Anim. Sci. Comp. Ethol. Neurosci 239, 267 (2022).
Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies (National Academies Press (US), 1999).
Weld, E. D., Bailey, T. C. & Waitt, C. Ethical issues in therapeutic use and research in pregnant and breastfeeding women. Br. J. Clin. Pharmacol. 88, 7–21 (2022).
Merkatz, R. B. Inclusion of women in clinical trials: a historical overview of scientific ethical and legal issues. J. Obstet. Gynecol. Neonatal Nurs. 27, 78–84 (1998).
Chartier, A. L. et al. The safety of maternal and fetal MRI at 3 T. Am. J. Roentgenol. 213, 1170–1173 (2019).
Lowe, T. W., Weinreb, J., Santos-Ramos, R. & Cunningham, F. G. Magnetic resonance imaging in human pregnancy. Obstet. Gynecol. 66, 629–633 (1985).
Choi, J. S. et al. A case series of 15 women inadvertently exposed to magnetic resonance imaging in the first trimester of pregnancy. J. Obstet. Gynaecol. 35, 871–872 (2015).
Tee, L. M., Kan, E. Y., Cheung, J. C. & Leung, W. Magnetic resonance imaging of the fetal brain. Hong Kong Med. J. 22, 270 (2016).
Ray, J. G., Vermeulen, M. J., Bharatha, A., Montanera, W. J. & Park, A. L. Association between MRI exposure during pregnancy and fetal and childhood outcomes. Jama 316, 952–961 (2016).
U. S. Census Bureau. Fertility of women in the United States. Current Population Reports: US Census Bureau https://www.census.gov/data/tables/2018/demo/fertility/women-fertility.html (2018).
Eid, R. S. et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol. Aging 78, 1–17 (2019).
Pawluski, J. L. et al. Effect of sertraline on central serotonin and hippocampal plasticity in pregnant and non-pregnant rats. Neuropharmacology 166, 107950 (2020).
Haim, A. et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain. Behav. Immun. 59, 67–78 (2017).
Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 (2017).
Hoekzema, E. et al. Mapping the effects of pregnancy on resting state brain activity, white matter microstructure, neural metabolite concentrations and grey matter architecture. Nat. Commun. 13, 6931 (2022).
Paternina-Die, M. et al. Women’s neuroplasticity during gestation, childbirth and postpartum. Nat. Neurosci. 27, 319–327 (2024).
Oatridge, A. et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am. J. Neuroradiol. 23, 19–26 (2002).
Pritschet, L. et al. Neuroanatomical changes observed over the course of a human pregnancy. Nat. Neurosci. 1–8 https://doi.org/10.1038/s41593-024-01741-0 (2024).
Gregg, C. Pregnancy, prolactin and white matter regeneration. J. Neurol. Sci. 285, 22–27 (2009).
Kalakh, S. & Mouihate, A. Enhanced remyelination during late pregnancy: involvement of the GABAergic system. Sci. Rep. 9, 7728 (2019).
Gregg, C. et al. White matter plasticity and enhanced remyelination in the maternal CNS. J. Neurosci. 27, 1812–1823 (2007).
Chan, R. W. et al. Structural and functional brain remodeling during pregnancy with diffusion tensor MRI and resting-state functional MRI. PLoS ONE 10, e0144328 (2015).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Clayton, J. A. & Collins, F. S. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 (2014).
Ferretti, M. T. Improving pharmacological treatment in brain and mental health disorders: the need for gender and sex analyses. Front. Neuroendocrinol. 50, 1–2 (2018).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016 (2012).
Sakka, L., Coll, G. & Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 309–316 (2011).
Kang, K., Kwak, K., Yoon, U. & Lee, J.-M. Lateral ventricle enlargement and cortical thinning in idiopathic normal-pressure hydrocephalus patients. Sci. Rep. 8, 13306 (2018).
Jespersen, S. N. et al. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy. NeuroImage 49, 205–216 (2010).
Sepehrband, F. et al. Brain tissue compartment density estimated using diffusion‐weighted MRI yields tissue parameters consistent with histology. Hum. Brain Mapp. 36, 3687–3702 (2015).
Correia, M. M., Carpenter, T. A. & Williams, G. B. Looking for the optimal DTI acquisition scheme given a maximum scan time: are more b-values a waste of time? Magn. Reson. Imaging 27, 163–175 (2009).
Azcoitia, I., Barreto, G. E. & Garcia-Segura, L. M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 55, 100787 (2019).
Montes, P. et al. Progesterone treatment in rats after severe global cerebral ischemia promotes hippocampal dentate gyrus neurogenesis and functional recovery. Neurol. Res. 41, 429–436 (2019).
Cooke, B. M. & Woolley, C. S. Gonadal hormone modulation of dendrites in the mammalian CNS. J. Neurobiol. 64, 34–46 (2005).
Sanchez, A. M., Flamini, M. I., Genazzani, A. R. & Simoncini, T. Effects of progesterone and medroxyprogesterone on actin remodeling and neuronal spine formation. Mol. Endocrinol. 27, 693–702 (2013).
Ibanez, C. et al. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol. Appl. Neurobiol. 30, 80–89 (2004).
Ibanez, C. et al. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog. Neurobiol. 71, 49–56 (2003).
Haraguchi, S. et al. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. The Cerebellum 11, 416–417 (2012).
Arevalo, M. A. et al. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: Recent advances. J. Steroid Biochem. Mol. Biol. 131, 52–56 (2012).
Chen, S., Kumar, N., Mao, Z., Sitruk-Ware, R. & Brinton, R. D. Therapeutic progestin segesterone acetate promotes neurogenesis: implications for sustaining regeneration in female brain. Menopause 25, 1138 (2018).
Keyser-Marcus, L. et al. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res. Bull. 55, 737–745 (2001).
Natu, V. S. et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc. Natl. Acad. Sci. 116, 20750–20759 (2019).
Servin-Barthet, C. et al. The transition to motherhood: linking hormones, brain and behaviour. Nat. Rev. Neurosci. 24, 605–619 (2023).
Stein, P. et al. Relation of progesterone and DHEAS serum levels to 5-HT1A receptor binding potential in pre- and postmenopausal women. Psychoneuroendocrinology 46, 52–63 (2014).
Bayer, J., Gläscher, J., Finsterbusch, J., Schulte, L. H. & Sommer, T. Linear and inverted U-shaped dose-response functions describe estrogen effects on hippocampal activity in young women. Nat. Commun. 9, 1220 (2018).
Shi, X., Wu, J. & Smyth, N. An inverted U-shaped relationship between cortisol awakening response and same-day error monitoring function in healthy males. Biol. Psychol. 160, 108052 (2021).
Kudielka, B. M., Hellhammer, D. H. & Wüst, S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 34, 2–18 (2009).
Lewis, J. G. Steroid analysis in saliva: an overview. Clin. Biochem. Rev. 27, 139 (2006).
Cyr, Ghribi & Paolo, D. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J. Neuroendocrinol. 12, 445–452 (2000).
Pritschet, L. et al. Functional reorganization of brain networks across the human menstrual cycle. NeuroImage 220, 117091 (2020).
Baudry, M., Bi, X. & Aguirre, C. Progesterone–estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience 239, 280–294 (2013).
Taylor, C. M. et al. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. NeuroImage 220, 117125 (2020).
Ammari, R. et al. Hormone-mediated neural remodeling orchestrates parenting onset during pregnancy. Science https://doi.org/10.1126/science.adi0576 (2023).
Brett, Z. H., Humphreys, K. L., Fleming, A. S., Kraemer, G. W. & Drury, S. S. Using cross-species comparisons and a neurobiological framework to understand early social deprivation effects on behavioral development. Dev. Psychopathol. 27, 347–367 (2015).
Valensise, H. et al. Multifrequency bioelectrical impedance analysis in women with a normal and hypertensive pregnancy12. Am. J. Clin. Nutr. 72, 780–783 (2000).
Van Loan, M. D., Kopp, L. E., King, J. C., Wong, W. W. & Mayclin, P. L. Fluid changes during pregnancy: use of bioimpedance spectroscopy. J. Appl. Physiol. 78, 1037–1042 (1995).
Nevo, O., Soustiel, J. F. & Thaler, I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am. J. Obstet. Gynecol. 203, 475.e1–.e6 (2010).
Osol, G., Ko, N. L. & Mandalà, M. Plasticity of the maternal vasculature during pregnancy. Annu. Rev. Physiol. 81, 89–111 (2019).
Allolio, B. et al. Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clin. Endocrinol. (Oxf.) 33, 279–289 (1990).
Obel, C. et al. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology 30, 647–656 (2005).
Egeland, M., Zunszain, P. A. & Pariante, C. M. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 16, 189–200 (2015).
Gould, E. & Tanapat, P. Stress and hippocampal neurogenesis. Biol. Psychiatry 46, 1472–1479 (1999).
Krugers, H. J., Hoogenraad, C. C. & Groc, L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat. Rev. Neurosci. 11, 675–681 (2010).
Mikasova, L. et al. Stress hormone rapidly tunes synaptic NMDA receptor through membrane dynamics and mineralocorticoid signalling. Sci. Rep. 7, 8053 (2017).
Antontseva, E., Bondar, N., Reshetnikov, V. & Merkulova, T. The effects of chronic stress on brain myelination in humans and in various rodent models. Neuroscience 441, 226–238 (2020).
Echouffo-Tcheugui, J. B. et al. Circulating cortisol and cognitive and structural brain measures. Neurology 91, e1961–e1970 (2018).
Hellhammer, J. et al. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology 32, 80–86 (2007).
Stalder, T. et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432 (2016).
Belo, L. et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 123, 46–51 (2005).
De Oliveira, L. C., Franco-Sena, A. B., Farias, D. R., Rebelo, F. & Kac, G. Maternal C-reactive protein concentrations during pregnancy and birth weight in a prospective cohort in Rio de Janeiro, Brazil. J. Matern. Fetal Neonatal Med. 30, 2346–2353 (2017).
De Oliveira, L. C. et al. Factors associated with maternal serum C-reactive protein throughout pregnancy: a longitudinal study in women of Rio de Janeiro, Brazil. Nutrition 31, 1103–1108 (2015).
Nielsen, F. R., Bek, K. M., Rasmussen, P. E., Qvist, I. & Tobiassen, M. C-reactive protein during normal pregnancy. Eur. J. Obstet. Gynecol. 35, 23–27 (1990).
Watts, D. H., Krohn, M. A., Wener, M. H. & Eschenbach, D. A. C-Reactive protein in normal pregnancy. Obstet. Gynecol. 77, 176 (1991).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Barba-Müller, E., Craddock, S., Carmona, S. & Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens Ment. Health 22, 289–299 (2019).
Drew, L. How pregnancy transforms the brain to prepare it for parenthood. Nature 632, 22–24 (2024).
Aviv, E. C. et al. Prenatal prolactin predicts postnatal parenting attitudes and brain structure remodeling in first-time fathers. Psychoneuroendocrinology 156, 106332 (2023).
Cárdenas, S. I. et al. White matter microstructure organization across the transition to fatherhood. Dev. Cogn. Neurosci. 67, 101374 (2024).
Kim, P. et al. Neural plasticity in fathers of human infants. Soc. Neurosci. 9, 522–535 (2014).
Saxbe, D. & Martínez-García, M. Cortical volume reductions in men transitioning to first-time fatherhood reflect both parenting engagement and mental health risk. Cereb. Cortex 34, bhae126 (2024).
Abraham, E. et al. Father’s brain is sensitive to childcare experiences. Proc. Natl. Acad. Sci. 111, 9792–9797 (2014).
Orchard, E. R. et al. Protective role of parenthood on age-related brain function in mid- to late-life. https://doi.org/10.1101/2024.05.03.592382 (2024).
Martínez-García, M. et al. Do pregnancy-induced brain changes reverse? The brain of a mother six years after parturition. Brain Sci 11, 168 (2021).
De Lange, A. M. G. et al. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proc. Natl. Acad. Sci. 116, 22341–22346 (2019).
Orchard, E. R., Rutherford, H. J. V., Holmes, A. J. & Jamadar, S. D. Matrescence: lifetime impact of motherhood on cognition and the brain. Trends Cogn. Sci. 27, 302–316 (2023).
McDade, T. W. Development and validation of assay protocols for use with dried blood spot samples. Am. J. Hum. Biol. 26, 1–9 (2014).
Miller, E. M. & Mcdade, T. W. A highly sensitive immunoassay for interleukin-6 in dried blood spots. Am. J. Hum. Biol. 24, 863–865 (2012).
Fujita, S. et al. 3D quantitative synthetic MRI-derived cortical thickness and subcortical brain volumes: Scan–rescan repeatability and comparison with conventional T1-weighted images. J. Magn. Reson. Imaging 50, 1834–1842 (2019).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61, 1402–1418 (2012).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980 (2006).
Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Veraart, J., Fieremans, E. & Novikov, D. S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 76, 1582–1593 (2016).
Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. NeuroImage 142, 394–406 (2016).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 141, 556–572 (2016).
Bastiani, M. et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. NeuroImage 184, 801–812 (2019).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).
Jeurissen, B., Tournier, J.-D., Dhollander, T., Connelly, A. & Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage 103, 411–426 (2014).
Tournier, J.-D., Calamante, F., Gadian, D. G. & Connelly, A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage 23, 1176–1185 (2004).
Wasserthal, J. et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacology 45, 1750–1757 (2020).
Wasserthal, J., Neher, P. & Maier-Hein, K. H. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage 183, 239–253 (2018).
Bates, D., Machler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. Journal of Statistical Software (2015).
Fitzmaurice, G. M., Laird, N. M. & Ware, J. H. Applied Longitudinal Analysis (John Wiley & Sons, 2012).
Snijders, T. A. & Bosker, R. Multilevel analysis: An introduction to basic and advanced multilevel modeling. (2011).
Bernal-Rusiel, J. L., Greve, D. N., Reuter, M., Fischl, B. & Sabuncu, M. R. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. NeuroImage 66, 249–260 (2013).
Yendiki, A., Koldewyn, K., Kakunoori, S., Kanwisher, N. & Fischl, B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage 88, 79–90 (2014).
Ragsdale, H. B., Kuzawa, C. W., Borja, J. B., Avila, J. L. & McDade, T. W. Regulation of inflammation during gestation and birth outcomes: Inflammatory cytokine balance predicts birth weight and length. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 31, e23245 (2019).
Althouse, A. D. Adjust for multiple comparisons? It’s not that simple. Ann. Thorac. Surg. 101, 1644–1645 (2016).
Hastie, T., Tibshirani, R., Friedman, J. H. & Friedman, J. H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Vol. 2 (Springer, 2009).
Acknowledgements
The authors extend our sincere gratitude to all participants for their contributions to this study. This research was supported by the Peabody Small Grant, Vanderbilt Brain Institute Trans-Institutional Program (TIPs) Grant, Vanderbilt Institute for Clinical and Translational Research Grant (VR61621) to KLH, and the Vanderbilt University Institute of Imaging Science Center for Human Imaging (1 S10OD021771 01). Author contributions were supported in part by T32MH18921 (HAP; LGB).
Author information
Authors and Affiliations
Contributions
K.L.H., conceived and designed the study; H.A.P., S.R., W.B. collected data; Y.N., B.N.C., M.C.C., M.K.M. cleaned and processed the data; Y.N., D.A.C., L.G.B., M.C.C. performed data analysis; Y.N., M.C.C., K.L.H., B.N.C. interpreted results; Y.N. wrote the first draft of the paper; all authors (Y.N., B.N.C., M.C.C., S.R., H.A.P., L.G.B., W.B., M.K.M., D.A.C., E.W.C., S.S.O., S.A.S., A.K., K.L.H.) contributed to the manuscript final writing, editing, and reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niu, Y., Conrad, B.N., Camacho, M.C. et al. Longitudinal investigation of neurobiological changes across pregnancy. Commun Biol 8, 82 (2025). https://doi.org/10.1038/s42003-024-07414-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-07414-9