Abstract
Quorum sensing, first described in marine systems five decades ago, is a well-characterized chemical communication system used to coordinate bacterial gene expression and behavior; however, the impact of quorum sensing on interkingdom interactions has been vastly understudied. In this review, we examine how these molecules mediate communication between bacteria and marine eukaryotes; influencing processes such as development, disease pathogenesis, and microbiome regulation within marine ecosystems. We describe the varied mechanisms eukaryotes have evolved to interfere with bacterial quorum sensing signaling, the crucial role these signals play in host-virus interactions, and how their exchange may be governed by outer membrane vesicles, prevalent in marine systems. Here, we present a dynamic portrayal of the impact of quorum sensing signals beyond bacterial communication, laying the groundwork for future investigations on their roles in shaping marine ecosystem structure and function.
Similar content being viewed by others
Introduction
Global biogeochemical cycling and marine ecosystem dynamics are dictated by molecular-level chemical exchanges between marine organisms1. Often, this chemical communication is orchestrated by bacteria, which secrete small diffusible quorum sensing (QS) signals2 that induce density-dependent, population-wide changes in microbial behavior and community composition3. These self-produced chemical signals accumulate locally within the environment and attain concentrations that activate the transcription of genes critical for fitness4. Since the initial observation of cell density-dependent luminescence in Vibrio fischeri over fifty years ago5, our understanding of the diversity of the quorum sensing lexicon and factors controlling the expression of these molecules has expanded significantly. At the most rudimentary level, the molecular circuitry of quorum sensing relies on one or more synthase genes, which encode the production of the diffusible signal termed an autoinducer, and one or more receptors, which dictate transcription, including the synthesis of the signal itself6,7. Various classes of QS signals have been identified in marine bacteria. These signals are characterized by their ability to diffuse from the producing cell, be recognized by a receiving cell, elicit a response in the receiver that has co-evolved alongside signal production in the producer, and confer mutual benefits to both producer and receiver cells4.
Acyl homoserine lactones (AHLs, autoinducer-1/AI-1), alkylquinolones (AQs), and α-hydroxyketones (cholera autoinducer-1/CAI-1) constitute the main classes of QS compounds in marine ecosystems8 (Fig. 1). AHLs are the most abundant QS molecules in marine systems, produced by Gram-negative bacteria from representatives of Alpha-, Beta- and Gamma-Proteobacteria. More controversial, however, is the inclusion of furanosyl borate diesters (autoinducer-2/AI-2) in this group, as not all bacteria that produce AI-2 either have the sensor or trigger a response to AI-2 consistent with canonical QS systems8. Some believe AI-2 can be at times either a signal, a cue, or metabolic byproduct used opportunistically for signaling depending on the bacterial producer or receiver4,9,10. Similarly, tropodithietic acid (TDA) might fall into this autoinducer category as well, possessing some, but not all QS signal properties11. The production of TDA is density-dependent and induces transcription of tda genes which coincide with biofilm formation12. Expanding the vocabulary of QS molecules further, one could append this list to include the signal peptide arbitrium (Fig. 1). This peptide-based communication system used by viruses that infect bacteria is analogous to QS in bacteria13. Arbitrium-based signaling depends on the cell density-dependent accumulation of phage-encoded signaling molecules, which regulate host gene expression to promote a shift from lysis to lysogeny, thereby enhancing the long-term survival and propagation of the viral population within the host community.
The acylated homoserine lactone (AHL) family of QS signals with representatives including 3-oxo-C4-HSL (N-3-oxo-butyryl-L-homoserine lactone), C6-HSL (N-hexanoyl-DL-homoserine lactone), 3-OH-C14-HSL (N-3-hydroxytetradecanoylhomoserine lactone). p-Coumaroyl-homoserine lactone (p-coumaroyl-HSL) is a representative aryl-HSL in which the acyl side chain is replaced with p-coumaric acid moiety. The cholera autoinducer-1 (CAI-1, (S)-3-hydroxytridecan-4-one) family is produced only in Vibrio species. The autoinducer-2 (AI-2) family containing furanosyl-borate diester. The alkylquinolone family of QS signals including 2-heptyl-4-quinolone (HHQ). Finally, the QS-adjacent representatives including tropodithietic acid (TDA) and the viral peptide arbitrium encoded by the phage phi3T which infects Bacillus subtilis.
QS-molecules are widespread among marine bacteria and are structurally diverse, yet together comprise a “language” allowing bacteria to behave collectively as a group. While the role of QS signaling in bacterial communication is well-established8, the exploration of QS molecules’ potential in mediating a wealth of cellular behaviors at the interkingdom level is still in the early stages14. Intricate recognition mechanisms between bacteria and eukaryotic cells may occur when host cells detect bacterial signals or when an autoinducer acts on the host cell without regulation15. Numerous studies in mammalian systems have identified targets for QS signals including nuclear receptors (aryl hydrocarbon receptor and peroxisome proliferator-activated receptor), G-protein coupled receptors, Toll-like receptors, transcription factors (NF-κB), protein kinases (p38 and p42/44 kinases), cytoskeletal proteins, and cell-surface lipid domains16. These targets broadly modulate processes like innate immune responses, lipid metabolism, inflammatory responses, cell structure, and intracellular calcium (Ca²⁺) signaling pathways (reviewed in ref. 14). In contrast, fewer studies have identified QS receptors in terrestrial plants. Notably, G-protein coupled receptors in Arabidopsis have been shown to be activated by AHLs, resulting in root elongation17,18. These studies in mammalian systems and terrestrial plants highlight the complexity of interkingdom QS between bacteria and eukaryotic host cells, and provide an excellent starting point for exploring cognate interkingdom QS receptors and associated regulatory pathways in marine eukaryotes. In this review, we examine the ability of bacterial QS signals to influence marine eukaryotic organisms, providing hosts with chemical contextual cues required for initiating developmental or physiological changes to enhance host fitness under distinct environmental conditions. Furthermore, the presence of QS signals could trigger cross-kingdom recognition, thereby allowing eukaryotic hosts to take stock of their bacterial consortia and respond by producing their own small molecules. The interdependence between hosts and their associated bacteria covers a spectrum of ecological interactions, from cooperative to competitive (Fig. 2). Here, we explore the emerging evidence that QS signals play a pivotal role in shaping co-evolutionary relationships in marine systems.
Here, we showcase the diversity of roles initiated by QS molecules and the consequences of these chemically-mediated interactions on marine invertebrates, macroalgae, phytoplankton, and viruses. For example, the quorum sensing alkylquinolone 2-heptyl-4-quinolone (HHQ) (1) arrests cell division, targets the inhibition of pyrimidine biosynthesis, and protects the coccolithophore Emiliania huxleyi from virus-induced mortality. Moreover, lumichrome (2), a derivative of the vitamin riboflavin, is a QS mimic whose release by exponentially growing E. huxleyi initiates antibiotic production in commensal bacteria, a likely strategy to thwart bacterial invaders. QS molecules can signal an impending viral threat to the community and coordinate the reduced expression of cell surface receptors critical for viral entry, and the induction of CRISPR-Cas systems to acquire immunity during viral invasion. Conversely, the viral peptide arbitrium, which is generated by bacteriophages during bacterial host infection, serves as a communication signal within the viral population, helping to determine the fate of the host cell. In the macroalgae Delisea pulchra, halogenated furanones (3) competitively inhibit acyl-homoserine lactone (AHL) (4) signalling, thereby controlling the bacterial species composition and halting AHL-induced virulence in macroalgal pathogens. The interaction between QS signalling and QS inhibition plays a crucial role in the progression of development and disease in marine invertebrates. Image credit: jenesesimre/ stock.adobe.com.
Marine ecosystems provide an opportunity to explore the multifaceted roles of QS signals and autoinducers. These molecules act as a shared molecular language understood by both bacteria and eukaryotes, conveying vital information and directing foundational processes within marine environments. In this review, we aim to provide insight into the role of QS signals as drivers of eukaryotic fitness and determinates of trophic interactions in marine systems. Indeed, the long co-evolutionary relationship between microorganisms and their hosts has enabled marine eukaryotes to colonize all ocean habitats by forming holobionts19—an emerging concept that views a eukaryotic host and its associated microbes as a single collective organism20. Given the essential role of the holobiont in host biology and the widespread presence of QS signals in marine bacteria, the influence of interkingdom signaling on host evolution merits further exploration.
Recent advances in our understanding of marine host-bacteria interactions illustrate how molecules implicated in bacterial QS contribute to disease progression, developmental trajectories, and the modification of marine eukaryotic physiology with examples from marine invertebrates21,22, macroalgae23,24, and phytoplankton25,26. Within marine ecosystems, eukaryotes both engage in and interfere with QS processes, actively participating in QS-mediated dialogs by producing compounds or proteins that disrupt bacterial QS signaling22,27. Mechanisms to disrupt QS systems are classified as either quorum quenching (QQ) molecules, which enzymatically degrade QS signals, or quorum sensing inhibitors (QSI), which interfere with QS signaling by competitively binding to QS molecule receptors on the bacterial cell surface28 or by inactivating autoinducer synthases29. The production of these molecules implies that host-driven modulation of bacterial QS signals may have evolved as a mechanism for eukaryotes to regulate the composition of their associated microbial communities22,27.
Interactions between marine viruses and microbes dictate the flow and fate of carbon in marine ecosystems. Viruses are the most abundant biological entities in the oceans, outnumbering bacteria by 10-fold, with the majority being bacteriophages—viruses that specifically target and infect bacteria30. Emerging research now reveals how QS molecules are influencing bacteriophage success. QS molecules provide critical information about bacterial population size, enabling phages to make lysis-lysogeny decisions by monitoring QS molecules. Likewise, viruses are responsible for mortality of ~20% of the oceanic photosynthetic biomass daily31. Recent work has uncovered a novel mechanistic role for a bacterial QS signal in mediating viral success within its phytoplankton host25, offering insight into a complex tri-trophic interaction governed by QS compounds.
The rapid loss of QS compounds from their source due to diffusion or fluid flow in the ocean poses a major challenge to maintaining effective concentrations. Many chemical-mediated interactions are sustained by physical contact between the microbe and host, or in specialized host anatomical structures32. Additionally, biofilms produced by bacterial consortia act as sorptive sponges, trapping and concentrating organic molecules33. Another important mechanism involves outer membrane vesicles (OMVs), which play a critical role in both stimulating and distributing QS molecules. The packaging of QS signaling molecules into OMVs by Gram-negative bacteria appears to be more widespread in marine systems than previously recognized. This delivery system not only preserves the integrity of the signal during transport but also enables targeted delivery to specific sites via receptor-mediated processes. Considering the profound influence of QS signals on aquatic ecosystems and the wide range of hosts they affect, there is a pressing need for further investigation into how these signals shape interkingdom interactions in marine environments.
QS-mediated interactions between marine invertebrates and their associated bacteria
Secondary metabolites produced by invertebrates and their associated microbes structure marine populations, communities, and ecosystems. These chemical cues dictate key processes in the life of an invertebrate, including predator-prey interactions, habitat selection, and larval settlement34. Building on the rich body of work dedicated to the chemical ecology of marine invertebrates34,35, new research has revealed that QS molecules are an understudied yet essential component of the relationship between marine invertebrates and their microbiota21,22,27.
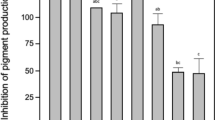
The interplay between QS signaling and QS inhibition is a key determinant of disease pathogenesis in marine invertebrates21,36,37,38,39. The ubiquitous detection of QS signals in the microbial consortia of corals afflicted with Black Band Disease40, suggests QS systems likely play a role in Black Band Disease communities41. QS signals alone can induce disease in coral by promoting growth of pathogenic bacteria that overtake the beneficial coral microbiome21,42, suggesting that QS compounds are a driving factor affecting host microbiome composition. When N-hexanoyl-DL-homoserine lactone (AHL), a quorum-sensing molecule produced by marine Vibrio pathogens43, was applied to healthy Acropora cervicornis coral fragments, all samples exhibited tissue loss and died completely within five days21. This finding suggests that quorum-sensing signals can trigger commensal bacteria to become opportunistic pathogens.
QS-induced diseases can be prevented by exogenous addition of QSI36,42 or QQ molecules that inhibit pathogenic growth and induction of virulence21,39,44. A growing body of evidence demonstrates invertebrates, including sea anemones, holothurians, and coral mucus, harbor bacteria capable of producing QSI/QQ as defensive agents against pathogen colonization45,46. However, pathogens can also use QSIs to disrupt the microbiome and induce disease by obstructing the QS signaling mechanisms of beneficial microbes41. This phenomenon is exemplified by the cyanobacterial pathogen associated with coral Black Band Disease. Although typically found in low abundance in healthy corals, once the disease takes hold, the cyanobacterial pathogen is thought to actively shape the disease community by cultivating an environment for opportunistic pathogens to attack the coral host. It does so in part by producing the secondary metabolite lyngbic acid, which inhibits QS signaling by blocking CqsS, the receptor for the hydroxyketone signal CAI-1. This blockade likely results in the modification of the microbiome composition41. A metagenomics analysis of bacterial communities associated with bleached and unbleached corals indicated pathogenicity-associated QS genes encoding for key proteins in biofilm formation, adhesion, and infection, and virulence genes were significantly increased in bleached corals29. Furthermore, researchers discovered that genes encoding molecules involved in quenching or inhibiting QS signals, annotated as QQ, decreased in bleached corals. These genes were negatively associated with the presence of virulence genes, suggesting that the interplay between QS/QQ and virulence could be a critical tipping point for the onset of disease in corals29.
These findings contribute to an ongoing paradigm shift in our understanding of disease emergence in marine holobionts, whereby disease is not caused by exposure to a single pathogen but rather an overall imbalance, or dysbiosis, in microbiome composition that can be activated by QS signaling20,47,48. Dysbiosis may result from the QS-mediated activation of virulence by commensal bacteria that are usually present in the microbiome at low abundances and become opportunistic pathogens when the host experiences physiological stress21,29. Consequently, native probiotic bacteria lose their competitiveness, leading to changes in structure and function of complex host-associated communities, opening up the door for disease to gain a foothold29,49. By identifying genes regulated by QS/QQ communication systems that drive complex interactions among the holobiont members, potential strategies for interventions can be developed to thwart disease establishment in corals. More broadly, understanding QS-mediated interactions between invertebrates and their bacterial pathogens is critical to monitoring physiological stress in marine holobionts in the context of a changing ocean.
The impact of QS and QQ signaling on marine holobionts is further exemplified by the emerging role of microbes in invertebrate development50. The moon jelly Aurelia aurita modulates its production of QQ compounds to selectively inhibit the QS signaling of bacterial colonizers at different developmental timepoints, finetuning the composition of the microbiome for each developmental stage22,51. The jelly’s enhanced QQ defense against bacteria in the larval ephyra stage may serve as a protective shield for the offspring, preserving fitness and ensuring their continued developmental progression to reproductive medusa22. Furthermore, A. aurita polyps and ephyrae express QQ enzymes at different levels in response to commensal and pathogenic bacteria, as commensal bacteria induce a decrease in QQ expression while pathogenic bacteria lead to activation of the host’s QQ repertoire to eliminate the invader. Understanding how conflict or cooperation is controlled via QQ and QSI strategies in these invertebrate-associated microbiomes can further elucidate how microbial taxa drive host development and fitness.
QS-mediated interactions between macroalgae and bacteria
The role of QS signaling in mediating interactions between macroalgae and bacteria was first established by foundational studies in the red alga Delisea pulchra, which produces halogenated furanones that competitively inhibit AHL signaling52. Located in vesicles at the algal cell surface53, halogenated furanones may act as gatekeepers that control which bacterial species can colonize the algal cell, allowing the host to determine the composition of its associated bacterial community23. Specifically, furanones inhibit signaling by covalently modifying and inactivating the AI-2 producing enzyme LuxS52,54,55. This control over microbiome composition enhances algal defense against disease by preventing host colonization by opportunistic pathogens and inhibiting AHL-induced virulence of resident bacteria23. For example, halogenated furanones significantly reduce algal susceptibility to bleaching23, a lethal disease caused by AHL-induced virulence of two bacteria native to the D. pulchra microbiome56,57. By controlling bacterial colonization through the interference with QS systems, algae can ameliorate the deleterious effects of bacterial pathogens58.
The production of QS inhibitory compounds59,60,61,62 or extracts63,64,65,66,67,68 with inhibitory activity has been documented for several other macroalgal species, suggesting that QS inhibition is a widespread mechanism used by macroalgae to control microbial community composition and evade disease by QS-sensing pathogens. The seagrass Halodule pinifolia produces 4-methoxybenzoic acid which inhibits QS-mediated virulence factor production and suppresses both QS-mediated virulence gene expression and production of extracellular polymeric substances required for biofilm formation in the pathogen Pseudomonas aeruginosa60. The brown alga Laminaria produces the microbicidal compound hypobromous acid, which rapidly and specifically reacts with acyl homoserine lactones. Deactivation of these QS signals influences biofilm development and may therefore serve as an additional strategy for controlling biofouling59. The sodium alginate oligomer OligoG CF-5/20, produced by Laminaria, acts as a QS antagonist, leading to the reduction of AHL synthesis via the interference of expression of both las and rhl QS systems, resulting in altered biofilm architecture and reduced virulence factor production61. Red algal representatives produce betonicine, floridoside, and isethionic acid believed to inhibit the production or reception of AHLs62. Both N-benzyl cinnamamide and α-resorcylic acid isolated from the red alga Gracilaria fisheri interfere with AI-2 mediated bioluminescence in Vibrio harveyi, hypothesized to bind to either the receptor or autoinducer of the AI-2 system69. Polyphenolic phlorotannins, derived from brown algae, are most notable for their function as herbivore deterrents, digestive inhibitors, and antibacterial agents in marine ecosystems70. These compounds were also found to inhibit QS activity in QS-sensing violacein reporter strains, accompanied by a decrease in virulence factor production, biofilm formation, and QS molecule production71.
Production of QS inhibitory compounds is not limited to the macroalgal host. Members of the macroalgal holobiont including fungi24,72 and epibiotic bacteria73,74 produce QS-inducing, QQ, and QSI compounds, including a recently discovered class of α-hydroxy γ-butenolides that structurally resemble host-produced halogenated furanones72. QSIs from the marine fungus Penicillium inhibit virulence factors and biofilm production via competitive binding to receptor proteins including LasR and PqsR in P. aeruginosa. This binding likely results in a conformational change causing the loss of downstream QS-mediated gene transcription75,76,77. Fungal production of these QSIs is enhanced by the presence of QS-inhibiting bacteria, hinting at layers of chemical complexity underlying the interactions between macroalgae-associated microbes. QS inhibition by epiphytic fungi and bacteria is essential for host-microbiota equilibrium and prevention of dysbiosis leading to macroalgal disease24,72. By regulating microbial community composition within the macroalgal holobiont, QS-mediated dialogs maintain the health of coastal marine habitats that depend on macroalgae for primary production and habitat formation23.
Bacterial biofilms play a significant role in the development of macroalgal communities78. The ability of representatives from green and red algae to perceive QS signals, albeit through an unknown mechanism, is more widespread than previously recognized and could be an important trait contributing to their ecological success58. Finding the ideal substrate for settlement is critical for benthic organisms and this process is often mediated by infochemicals. Zoospores of the green seaweed Ulva (= Enteromorpha) stimulate attachment only in the presence of biofilm strains that produce AHLs, compared to strains lacking quorum sensing circuits VanIR and VanMN79. The sensing of AHLs by zoospores results in a calcium influx into the cells, reducing their swimming rate and leading to their accumulation at the biofilm surface for settlement80. Paradoxically, the presence of AHLs was demonstrated to reduce Ulva zoospore germination and early growth, suggesting that AHLs are a cue to slow growth algal rate lessening metabolic burden and thereby enhancing survival in nutrient limiting conditions81. For the red epiphytic alga Acrochaetium sp., life cycle completion strongly depends on AHL-producing bacteria. Spore release was stimulated by AHLs in a dose-dependent manner and blocked when using AHL-receptor-blocking halofuranones82. The molecular mechanisms underlying the reported ecological functions of cross-domain signaling between bacteria and seaweeds requires further investigation to fully understand the algal receptors and pathways involved in QS molecule reception.
QS-mediated interactions between phytoplankton and bacteria
Interactions between phytoplankton and bacteria have been recognized as the foremost interkingdom relationship in the marine environment83,84. These interactions propel global biogeochemical cycles1, structure the foundation of marine food webs85, and contribute to the regulation of global climate86. Phytoplankton account for nearly half of global photosynthetic output87, and as much as half of the carbon they produce is eventually consumed by heterotrophic bacteria88. In exchange for the provision of carbon, bacteria provide phytoplankton with essential micronutrients and vitamins, while also acting as direct competitors for inorganic nutrients84. This dynamic relationship is mediated by chemical signals, which induce shifts between mutualism and parasitism based on the physiological state of each partner89,90. QS signals are emerging as a crucial part of chemically mediated microbial interactions that control phytoplankton population growth25,26. Given the widespread presence of phycosphere isolates from phytoplankton blooms with QS-producing capabilities11,91,92,93, further research is needed to explore how QS compounds can influence phytoplankton community dynamics, and, conversely, how algal metabolites can impact bacterial QS-mediated signaling.
Within the phycosphere, a region immediately surrounding individual phytoplankton cells where microscale chemical exchanges occur94, QS signaling drives interkingdom communication between the host and its microbial assemblage, ultimately determining the composition of the host’s colonizers19. QS signals modulate biofilm formation and bacterial motility95, both necessary for initial attachment of bacterial cells within the phycosphere. QS signals produced by phytoplankton promote a switch from a free-living mode to a surface-attached lifestyle, initiating the selective colonization of the phycosphere by QS-responsive cells and triggering the enhancement of biofilm formation and reduced motility. Two bacterial symbionts adapted to thrive off diatom metabolites, Sulfitobacter pseudonitzschiae F5 and Phaeobacter sp. F10, possess AHL synthesis luxI-like genes adjacent to the transcriptional regulator luxR gene. Both species show enhanced biofilm formation and reduced mobility in the presence of AHLs produced by diatom symbionts. An AHL-induced phenotype supporting enhanced attachment effectively retains these bacteria in the phycosphere, ensuring their success relative to other competitors96. The reliance on QS molecules of phycosphere residents to initiate a switch from free-living to phycosphere-associated is favorable in those species with dependence on phytoplankton exudates. Simultaneously, there is growing evidence that bacterial density alone is not the sole trigger of QS expression; rather, eukaryote metabolites can trigger QS processes97,98. Even among phycosphere residents that can switch phytoplankton hosts, the initiation of QS production is not universal and is likely the result of a diversity and divergency in the labile metabolites produced by different phytoplankton species99. Future studies identifying the phytoplankton metabolites orchestrating quorum sensing expression will assist in our understanding of co-evolutionary cross-talk and metabolic alliances sustaining bacteria-phytoplankton partnerships in the ocean.
There is also evidence that the diatom host Asterionellopsis glacialis can release rosmarinic acid, a QS mimic functionally similar to homoserine lactones. This molecule may serve as a signal to promote a lifestyle shift from free-living to phycosphere-enabled symbionts by suppressing motility and encouraging the attachment of bacterial symbionts100. Rosmarinic acid, which is also produced by land plants, has been shown to bind to QS regulators101. In vitro assays found rosmarinic acid was capable of binding to the QS-regulator RhlR of P. aeruginosa (PAO1), competing with the QS signal N-butanoyl-homoserine lactone and activating RhlR-mediated gene transcription. The use of QS mimics that promote attachment of beneficial bacteria while simultaneously suppressing opportunists demonstrates an innate ability of phytoplankton to curate their microbial consortia, with parallels seen in higher eukaryotes.
Quorum sensing bacteria are not restricted to a particular type of phytoplankton-bacteria interaction; rather, representatives are ubiquitous among phycosphere members11. Metagenomic sequencing across the stages of a natural phytoplankton bloom has shed light on QS-mediated dynamics of the microbial consortia in association with their phytoplankton hosts. During a bloom of the harmful algal species Gymnodinium catenatum, QQ sequences, including lactonases and acylases, were most dominant during the peak stage of the bloom, which coincided with the lowest bacterial abundance92. Further research is needed to determine whether deploying QQ at the height of the bloom provides symbiotic bacteria with a competitive advantage to maintain a foothold within the phycosphere. Additional studies using bioreactors to simulate shifts among dominant phytoplankton species have shown that phycosphere bacterial communities are dominated by QS/QQ-producing members, particularly within the attached microbial consortia102. Alongside autoinducer production genes, these consortia possess functional genes regulated by QS signals, such as those linked to antibiotic activity and iron acquisition—traits that impact both bacterial and host fitness. Although complex to interpret, further studies employing network analysis could uncover patterns of co-variation among autoinducer production, regulated functional genes, QQ enzymes, and host responses in natural populations, supporting the view that QS systems are integral to phytoplankton bloom dynamics and microbial consortia transitions.
Bacteria also regulate microalgal nutrient acquisition103,104 and population size using QS signals. These QS compounds have growth-enhancing or -inhibiting effects that can be eliminated by a change in a single functional group on the molecule25,26. Studies of Trichodesmium bacterial consortia indicate that QS signals coordinate the acquisition of phosphorus, an important nutrient in oligotrophic waters, by regulating alkaline phosphatase activity in Trichodesmium colonies104. QS molecules also modulate the growth and nitrogenase activity of the filamentous cyanobacterium Anabaena sp., which produces an acylase capable of inactivating long-chain AHLs as a possible defense strategy against bacteria communication103. QS molecules mediate their effects through various mechanisms. For example, 2-heptyl-4-quinolone (HHQ) induces cellular stasis—meaning neither growth nor mortality—in the coccolithophore Emiliania huxleyi25. Long-chained AHLs (C14-HSL) promoted growth in the benthic diatom Seminavis robusta, while N-3-hydroxytetradecanoyl homoserine lactone (OH-C14-HSL) and N-3-oxotetradecanoyl homoserine lactone (oxo-C14-HSL) inhibited growth26. The production of dimethysulfoniopropionate (DMSP) is associated with the phycosphere of phytoplankton cells, where higher nutrient concentrations exist compared to the surrounding water column. When grown in the presence of DMSP, an organosulfur osmolyte produced by phytoplankton, the bacterium Ruegeria pomeroyi produces N-(3-oxotetradecanoyl)-L-homoserine lactone concomitant with differential expression of intra- and extracellular metabolites105. These DMSP triggered QS signal-mediated changes in gene expression lead to the release of metabolically high-value biosynthetic intermediates useful in cross-feeding. Production of these intermediates is thought to switch R. pomeroyi to a cooperative lifestyle with other bacteria and phytoplankton. The episodic or strategic release of metabolites by phytoplankton can cue QS-mediated changes in bacteria metabolism to allow for scenarios favoring a mutually beneficial exchange of high-value metabolites.
Additionally, QS molecules can activate algicidal activity in commensal bacteria that retaliate against their phytoplankton hosts106,107. This is demonstrated by the phycosphere isolate Bacillus subtilis (strain JA) against the alga Alexandrium minutum108, and by the bacterium Ponticoccus sp. against its host alga Prorocentrum donghaiense as well as two other red tide microalgae, Phaeocystis and Alexandrium109. Bacterial production of algicidal compounds has previously been described as a response to host senescence that allows bacteria to quickly access the carbon produced by the dying algae before finding a new host90. Specifically, Phaeobacter gallaeciensis is able to sense the physiological state of E. huxleyi by the liberation of p-coumaric acid, a degradation product of algal lignin released by senescing cells, and induce the up-regulation of algal degrading roseobacticides90. Interestingly, p-coumaric acid serves as a precursor for p-coumaroyl-HSL (p-C-HSL) (Fig. 1), a non-fatty acyl homoserine lactone recently identified as a potential quorum sensing signaling molecule110. This discovery broadens the repertoire of QS signals and highlights a potential co-evolutionary relationship between the algal host and the p-C-HSL-producing bacterium. During E. huxleyi’s exponential phase, Phaeobacter gallaeciencis provides the alga with growth inducers like auxins and produces the potent broad-spectrum autoinducer antibiotic TDA to fight algal pathogens111,112,113 in return for the sulfur source DMSP114,115. The production of semiochemicals associated with different life stages of the phytoplankton host may convey information about phycosphere nutrient availability10. Therefore, QS signaling or the acquisition of QS-precursors may represent another strategy by which bacteria manipulate algal population dynamics to maximize access to host-derived nutrients.
On the cellular and molecular level, QS compounds fundamentally restructure phytoplankton morphology and gene expression. E. huxleyi cells exposed to nanomolar concentrations of the QS molecule HHQ exhibit increased lipid storage, nuclear envelope disintegration, and changes in chromatin structure. These morphological effects in lipid storage and DNA replication parallel the transcriptional changes induced by HHQ, which involve differential expression of genes involved in lipid metabolism, DNA repair, and cell cycle regulation25,26. In our attempts to understand how QS molecules are sensed by phytoplankton, the growth promoting molecule C14-HSL enhanced the expression of intracellular signaling transcripts in the diatom S. robusta. These transcripts include genes containing motifs found in receptor-like kinases, G-protein coupled receptors, receptor-type guanylate cyclases, and protein kinase domains—all of which initiate eukaryotic signaling networks26. Conversely, oxo-C14-HSL exposure in S. robusta led to the downregulation of cell cycle genes, induced a switch to increased fatty acid degradation, and caused photoprotection mechanisms to be induced in concert with the down-regulation of photosynthetic genes involved in light harvesting26. These studies offer the first look into the complex web of molecular interactions that QS molecules trigger in phytoplankton, as revealed through the lens of gene transcription. The recent discovery that the alkylquinolone, HHQ is a potent inhibitor of dihydroorotate dehydrogenase, an enzyme involved in pyrimidine biosynthesis in the coccolithophore Emiliania huxleyi, establishes a novel avenue through which QS molecules shape algal physiology and further suggests an intimate co-evolutionary relationship between the algal host and QS signal producing bacterium116. Building on these foundational transcriptomic and enzymatic studies, metabolomic profiling is poised to reveal whether bacteria use QS signals to induce algal production of specific metabolites, allowing bacteria to “farm” their phytoplankton hosts.
Phytoplankton are not only passive recipients of bacterial QS signals. Globally abundant microalgal species such as Chlamydomonas reinhardtii117, Phaeodactylum tricornutum118, and E. huxleyi119 are known to produce the QS mimic known as lumichrome, which was found to stimulate the LasR receptor from P. aeruginosa. Docking studies indicate that lumichrome and its precursor riboflavin are both recognized in the AHL binding pocket of LasR120. Lumichrome induces the production of antibacterial compounds by Vibrio spp., thereby protecting the phytoplankton from bacterial pathogens119. QS mimics like lumichrome and riboflavin therefore represent another mechanism by which marine eukaryotes manipulate bacterial QS signaling through LasR-like receptors to prevent host disease or modulate the phycosphere community. The production of QS mimics is a promising yet understudied area of research, and future studies may reveal a diversity of currently unknown QS-modulating compounds produced by marine phytoplankton.
Throughout their co-evolutionary history, phytoplankton and their bacterial symbionts have likely relied on mutual exchanges of infochemicals to guide their decision-making. Algal metabolites serve as metabolic signposts, allowing bacteria to assess the host’s physiological state and trigger metabolic shifts that favor mutual nutrient exchange. QS systems evolved to detect and respond to phytoplankton cues, altering bacterial gene expression to promote a transition from a free-living to a particle-associated lifestyle that benefits both partners. In turn, phytoplankton hosts likely developed structural QS mimics to encourage this lifestyle shift, gaining protection through the bacterial production of antibiotic compounds that ward off pathogens. Other algal metabolites evolved to silence QS signaling, likely fostering specific mutualistic or commensal relationships within the phycosphere community. Meanwhile, bacteria influence host fitness by deploying QS signals that activate algicidal activities, enabling them to exploit dying phytoplankton. These QS-mediated interactions highlight the intricate co-evolutionary complexity of bacteria-phytoplankton relationships. Future work aimed at identifying key bacterial and algal infochemicals, mapping their regulatory targets with host and QS pathways, and elucidating their downstream physiological effects on both bacteria and phytoplankton fitness will help decode the molecular circuitry shaping these fundamental marine interactions.
Impacts of QS signaling on host-virus dynamics
Viral infection of marine microbes drives nutrient cycling and governs microbial community composition in the oceans31. Viruses are the most numerous biological entities in the ocean and nearly 20% of primary productivity is turned over daily due to viral infection. While host-virus dynamics operate at global scales to influence carbon and nutrient cycling, recent investigations have highlighted how chemical communication strategies operating on the molecular level similar to bacterial QS systems influence viral strategy. These signaling mechanisms allow viruses to manipulate host bacterial communication pathways to optimize lysis-lysogeny strategies121,122. Chemical signaling is a pivotal aspect in the continuous evolutionary struggle between viruses and their bacterial hosts, whereby bacteria evolve new antiviral protection methods while viruses develop strategies to bypass these defenses123. In the course of a billion years of evolution, viruses that infect bacteria, termed phage, have developed the ability to monitor the number of uninfected bacteria in a population using QS signals or similar compounds used in microbial communication. This unique capacity gives phage a strategic advantage, allowing them to toggle their infection trajectory depending on the number of nearby infection attempts121.
QS compounds are a significant component of bacterial defenses against viral attack. The density-dependent nature of QS signaling provides critical information about bacterial population density that allows bacteria to weigh the costs and benefits of different antiviral defense strategies124. QS molecules can disrupt viral attachment by downregulating the expression of bacterial cell surface receptors used for viral entry124,125,126. For example, E. coli, which does not produce AHLs itself, reduces the numbers of LamB λ phage cell surface receptors in response to AHLs, resulting in a 2-fold reduction in the adsorption of phage. This reduction in turn increases the number of surviving E. coli cells. This protective cascade of events is dependent on the presence of E. coli’s AHL receptor SidA, and not a secondary side effect due to the presence of AHLs manipulating the media environment. However, downregulation of these receptors is energetically costly since the receptors are involved in key cellular functions, such as nutrient acquisition and motility124. The density-dependence of QS signaling allows bacteria to downregulate these critical receptors only in high-density populations where virions released by infected cells are most likely to infect another host124. Given that phage, once thought to have narrow host specificity, can adapt to new hosts at high rates, this strategy also benefits those bacteria that eavesdrop on QS signals rather than producing QS signals themselves127. QS signaling can therefore steer bacterial populations toward the most effective antiviral defense strategy and allow populations to adapt to dynamic infection landscapes126.
QS molecules also fortify antiviral defenses in bacteria by inducing coordinated expression of the CRISPR-Cas immune system, which allows bacterial populations to acquire immunity against a diverse range of possible invaders128,129. This diversity within the collective immune system of a population is essential for antiviral defense because viruses can develop resistance to CRISPR-Cas through a single point mutation128,130. However, CRISPR-Cas expression is costly to the host since autoimmunity can cause the CRISPR-Cas system to target the host genome itself128,131,132. Therefore, the density-dependent nature of QS signaling again functions to ensure that CRISPR-Cas is only triggered in high-density bacterial populations most at risk of viral infection128,129,133.
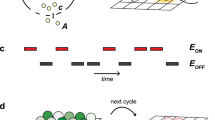
Viruses have already co-opted numerous facets of the bacterial QS system to gain an advantage in the coevolutionary arms race122. Viruses possess genes encoding bacterial QS molecules134 as well as membrane receptors that bind QS compounds135, suggesting that viruses can listen to and participate in QS-mediated bacterial communication. Detection of QS signals allows eavesdropping viruses to determine the density of a bacterial population, a key factor influencing the shift between lysis and lysogeny135,136. For example, vibriophages encode a receptor specific to the autoinducer 3,5-dimethylpyrazin-2-ol, a QS compound produced by Vibrio cholerae135. Binding of 3,5-dimethylpyrazin-2-ol to the vibriophage-encoded QS receptor and transcription factor (VqmAphage) signals a high bacterial-host cell density to the phage, triggering the lytic pathway135. Presently, the specific cue that activates vqmAphage expression is unknown. However, it is hypothesized that a low-level production of VqmAPhage is triggered in response to an environmental stimulus or unstable state. Viral recognition of QS compounds may induce either a lytic or lysogenic life cycle in different phage-bacteria systems135,136,137,138, based on the distinct advantages provided by lysis versus lysogeny to the virus135,139. As a key form of information used by viruses in making lysis-lysogeny decisions, QS molecules are a fundamental determinant of the fate of virally infected bacterial populations and, therefore, a central element shaping the structure of marine microbial communities.
Further exemplifying the dynamic coevolutionary relationship between viruses and bacteria, phage have developed their own QS-like system of small molecule communication, known as arbitrium. This system requires three phage genes to produce the signal peptide, serve as a receptor, and regulate lysogeny13. Arbitrium involves the use of small peptide signaling molecules released by phage-infected bacteria as a message for future phages infecting the same bacterial population. The concentration of these peptides indicates the number of previous viral infections in the population, and if at sufficient titer, indicates a significant number of the host cells in the population may already have been lysed. This leaves few remaining hosts for new viral progeny released through a lytic program, and shifts infection dynamics to a lysogenic model to avoid the risk of new progeny phage lacking a host to infect13. Therefore, a high concentration of the signaling peptides during later stages of infection increases the probability of lysogeny over lysis for a new phage entering the population. When modeled, phage using small-molecule signals as determinates of the switch in lytic versus lysogenic lifestyles were found to outcompete non-communicating phage, particularly when host densities become low140. Subsequent to the discovery of the ‘arbitrium’ QS system, an additional host-derived QS system in the Bacillus phage phi3T genome was discovered (Bernard et al. 2021). This system downregulates host defense mechanisms during infection when cell stress or cell abundance is high and phage titer is low, making it the first known virus with two communication repertoires141. The evolution of the arbitrium system and others demonstrates that phage have not only imitated but also improved on the bacterial QS model by innovating a molecular mechanism to facilitate transmission of information across different generations about prior infection events13.
Finally, the story of how QS molecules impact viral success gains additional complexity as we investigate tripartite interactions between a eukaryotic host, a virus, and bacterially-derived QS signals. Pollara et al. 2021 demonstrated that the coccolithophore E. huxleyi likely gains protection against viral mortality through QS signal-mediated physiological remodeling of the eukaryotic host. Phytoplankton cells exposed to nanomolar concentrations of the alkylquinolone HHQ, however, were still susceptible to viral infection by E. huxleyi virus, but displayed a significantly reduced burst size, or the total number of viral particles release per host cell, when compared to controls142. Subsequent work identified HHQ as a potent inhibitor of E. huxleyi dihydroorotate dehydrogenase, a fundamental enzyme catalyzing the fourth step in pyrimidine biosynthesis and a known antiviral drug target116. This finding established a novel avenue through which a bacterial QS molecule can shape algal physiology and revealed fundamental insights into ecologically significant tripartite interactions in the phycosphere.
Role of membrane vesicles in transport of QS molecules
Critical to understanding how QS molecules mediate interkingdom communication is describing the cellular processes of infochemical exchange between organisms. Perhaps most vital is the question of how hydrophobic QS molecules are transferred at sufficient concentrations through aqueous environments143, such as the ocean. A compelling solution to this problem is the transport of QS compounds within lipid-bound outer membrane vesicles (OMVs) that shield the hydrophobic QS compounds from the aqueous environment143. Gram-negative bacteria securely secrete QS signals and other products to their intended targets using OMVs, formed when the bacterial outer membrane buds and internalizes cargo144. In marine ecosystems, the concentration of OMVs in oligotrophic waters are approximately 105/mL145. OMVs are widespread throughout marine systems145, and the transport of QS molecules within OMVs has been reported in marine bacteria146,147, suggesting that OMVs may be a significant yet understudied mechanism of infochemical exchange in the oceans. The transport of QS signals within OMVs is dictated by complex feedback loops in which QS molecules and products of QS-regulated pathways are transported within OMVs and regulate the formation of the vesicles themselves143,148,149. Investigating the interconnections between QS signaling and OMVs is a promising area of future work that may provide key insights into the mechanisms of infochemical exchange in the oceans.
OMVs as vessels for QS delivery regulate many of the same fundamental processes controlled by QS signaling, including bacterial pathogenicity, host-virus dynamics150, and eukaryotic development151. OMVs are integral to the QS-mediated dysbiosis that causes coral disease150, as coral pathogens are known to produce OMVs containing AHLs and other virulence-related proteins147. The information-trafficking function of OMVs has also been exploited by viruses, using phage receptors and small RNAs as OMV cargo to propagate infection in bacterial and eukaryotic host populations152,153. By facilitating the transfer of phage receptors between cells in a bacterial population, OMVs can induce short-term susceptibility to viral infection in previously resistant bacterial subpopulations153. OMVs also mediate viral infection of phytoplankton cells, as infected cells release OMVs containing small RNAs that extend viral half-life in the extracellular environment and increase the rate of viral infection152,154. Viruses have therefore evolved the ability to hijack the host’s OMV machinery to accelerate viral infection of a population150. However, OMVs deliver more than just bad news to eukaryotes, as they also function as carriers of critical information required for eukaryotic development. For example, the Hawaiian bobtail squid (Euprymna scolopes) uses OMVs produced by its symbiotic bacterium Vibrio fischeri as a key checkpoint required to indicate successful bacterial colonization before the induction of irreversible light organ morphogenesis151. Considering the well-established role of QS signals in inducing bioluminescence of V. fischeri within the squid light organ155, the emerging importance of OMVs in this host-microbe symbiosis highlights the significant interconnection between OMVs and QS signals as a key area for future study.
Summary and outlook
A vast expansion is underway in our understanding of QS signaling in the oceans, revealing that quorum sensing is a crucial mechanism of interkingdom signaling through which bacteria shape eukaryotic development, physiology, and ultimately fitness (Fig. 2). In tandem, eukaryotes produce metabolites that can be recognized by bacterial QS receptors and serve as interkingdom signals. Moreover, eukaryotes have evolved the ability to eavesdrop on bacterial QS conversations, and can respond by using QQ and QSI compounds to circumvent bacterial community dynamics and modulate the composition of their associated microbiome (Table 1). These sophisticated interkingdom interactions between bacteria and eukaryotes likely have evolved to favor symbiotic exchanges between partners or mitigate deleterious interactions with eukaryotic hosts that deploy mimics or signal curtailing mechanisms as a defense strategy. With a diversity of key players in interkingdom QS signaling now identified, interrogating the molecular and biochemical mechanisms underpinning these interactions is needed to fully understand the role of QS-mediated communication in the marine environment. What specific biochemical pathways do QS molecules target to manipulate eukaryotic metabolism and physiology? What cell surface receptors or transcription factors are shared between QS-producing bacteria and QQ/QSI-producing eukaryotes that facilitate chemical communication between these organisms? What are the regulatory mechanisms that dictate how QS signals modulate viral reproduction strategies in response to host cell density and physiological state? Answering these questions will vastly expand our understanding of the microscale cellular interactions shaped by QS signaling that dictate marine community dynamics.
A major gap in our current understanding of QS signaling is how environmental conditions and host physiological state affect the production of QS compounds in natural systems. To provide a complete picture of the biotic and abiotic factors that impact QS signaling, future work must expand the study of QS signaling beyond the laboratory setting, using novel field-based methods to accurately probe and quantify the presence of QS compounds in natural systems. Laboratory studies have placed fundamental limits on our understanding of marine QS signaling, as only a small subset of marine bacteria are ultimately cultivable in the laboratory setting. Furthermore, determining ecologically relevant concentrations of QS molecules for use in laboratory research is challenging because measurements of bulk seawater do not accurately represent the concentration of QS signals in the distinct microenvironment surrounding microbial cells or in biofilms. Accurate quantification of QS molecules necessitates innovative experimental methods leveraging emerging technologies, such as live bacterial biosensors that use fluorescent reporters to detect QS compounds. These approaches can enhance our understanding of the spatial and physical heterogeneity of QS molecule gradients experienced by organisms at the microscale. Ultimately, such technologies will help determine the effective “range” of infochemicals in situ within marine ecosystems. Additionally, the use of microfluidics can allow us to precisely control flow and QS gradients to examine physiological response at the level of single cells156. Furthermore, while laboratory studies have hinted at the synergistic effects of QS-inhibiting signals produced by the co-culturing of marine microbes, it is essential to prioritize examinations of natural systems to comprehensively delineate the spectrum of interactions between QS-producing marine microorganisms and the biological entities that receive and interpret these signals157. This integrated approach to comprehending marine QS signaling will provide critical insights into the molecular interactions that structure marine ecosystems.
References
Azam, F. & Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol 5, 782–791 (2007).
Fuqua, W. C., Winans, S. C. & Greenberg, E. P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176, 269–275 (1994).
Abisado, R. G., Benomar, S., Klaus, J. R., Dandekar, A. A. & Chandler, J. R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 9, e02331–17 (2018).
Whiteley, M., Diggle, S. P. & Greenberg, E. P. Progress in and promise of bacterial quorum sensing research. Nature 551, 313–320 (2017).
Nealson, K. H., Platt, T. & Hastings, J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104, 313–322 (1970).
Dow, L. How Do Quorum-Sensing Signals Mediate Algae–Bacteria Interactions? Microorganisms 9, 1391 (2021).
Urvoy, M., Labry, C., L’Helguen, S. & Lami, R. Quorum Sensing Regulates Bacterial Processes That Play a Major Role in Marine Biogeochemical Cycles. Front. Mar. Sci. 9, 834337 (2022).
Hmelo, L. R. Quorum Sensing in Marine Microbial Environments. Ann. Rev. Mar. Sci. 9, 257–281 (2017).
Chen, X. et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549 (2002).
Lami, R. Quorum Sensing in Marine Biofilms and Environments. in Quorum Sensing 55–96 (Elsevier, 2019). https://doi.org/10.1016/B978-0-12-814905-8.00003-4.
Rolland, J. L., Stien, D., Sanchez-Ferandin, S. & Lami, R. Quorum Sensing and Quorum Quenching in the Phycosphere of Phytoplankton: a Case of Chemical Interactions in Ecology. J. Chem. Ecol. 42, 1201–1211 (2016).
Geng, H. & Belas, R. Expression of Tropodithietic Acid Biosynthesis Is Controlled by a Novel Autoinducer. J. Bacteriol. 192, 4377–4387 (2010).
Erez, Z. et al. Communication between viruses guides lysis–lysogeny decisions. Nature 541, 488–493 (2017).
Fan, Q. et al. Structure and Signal Regulation Mechanism of Interspecies and Interkingdom Quorum Sensing System Receptors. J. Agric. Food Chem. 70, 429–445 (2022).
Lowery, C. A., Dickerson, T. J. & Janda, K. D. Interspecies and interkingdom communication mediated by bacterial quorum sensing. Chem. Soc. Rev. 37, 1337 (2008).
Teplitski, M., Mathesius, U. & Rumbaugh, K. P. Perception and Degradation of N -Acyl Homoserine Lactone Quorum Sensing Signals by Mammalian and Plant Cells. Chem. Rev. 111, 100–116 (2011).
Jin, G. et al. Two G-protein-coupled-receptor candidates, Cand2 and Cand7, are involved in Arabidopsis root growth mediated by the bacterial quorum-sensing signals N-acyl-homoserine lactones. Biochemical Biophysical Res. Commun. 417, 991–995 (2012).
Liu, F., Bian, Z., Jia, Z., Zhao, Q. & Song, S. The GCR1 and GPA1 Participate in Promotion of Arabidopsis Primary Root Elongation Induced by N -Acyl-Homoserine Lactones, the Bacterial Quorum-Sensing Signals. MPMI 25, 677–683 (2012).
González-Pech, R. A. et al. The Evolution, Assembly, and Dynamics of Marine Holobionts. Annu. Rev. Mar. Sci. 16, 443–466 (2024).
Egan, S. & Gardiner, M. Microbial Dysbiosis: Rethinking Disease in Marine Ecosystems. Front. Microbiol. 7, 991 (2016).
Certner, R. H. & Vollmer, S. V. Evidence for Autoinduction and Quorum Sensing in White Band Disease-Causing Microbes on Acropora cervicornis. Sci. Rep. 5, 11134 (2015).
Weiland-Bräuer, N., Fischer, M. A., Pinnow, N. & Schmitz, R. A. Potential role of host-derived quorum quenching in modulating bacterial colonization in the moon jellyfish Aurelia aurita. Sci. Rep. 9, 34 (2019).
Longford, S. R. et al. Interactions within the microbiome alter microbial interactions with host chemical defences and affect disease in a marine holobiont. Sci. Rep. 9, 1363 (2019).
Tourneroche, A. et al. Bacterial–Fungal Interactions in the Kelp Endomicrobiota Drive Autoinducer-2 Quorum Sensing. Front. Microbiol. 10, 1693 (2019).
Pollara, S. B. et al. Bacterial Quorum-Sensing Signal Arrests Phytoplankton Cell Division and Impacts Virus-Induced Mortality. mSphere 6, https://doi.org/10.1128/msphere.00009-21 (2021).
Stock, F. et al. Distinctive Growth and Transcriptional Changes of the Diatom Seminavis robustain Response to Quorum Sensing Related Compounds. Front. Microbiol. 11, 1240 (2020).
Pietschke, C. et al. Host modification of a bacterial quorum-sensing signal induces a phenotypic switch in bacterial symbionts. Proc. Natl. Acad. Sci. USA. 114, E8488-E8497 (2017).
Borges, A. & Simões, M. Quorum Sensing Inhibition by Marine Bacteria. Mar. Drugs 17, 427 (2019).
Xu, M. et al. Interactions between quorum sensing/quorum quenching and virulence genes may affect coral health by regulating symbiotic bacterial community. Environ. Res. 238, 117221 (2023).
Breitbart, M., Bonnain, C., Malki, K. & Sawaya, N. A. Phage puppet masters of the marine microbial realm. Nat. Microbiol 3, 754–766 (2018).
Suttle, C. A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol 5, 801–812 (2007).
Martínez‐Pérez, C., Zweifel, S. T., Pioli, R. & Stocker, R. Space, the final frontier: The spatial component of phytoplankton–bacterial interactions. Mol. Microbiol. 122, 331–346 (2024).
Decho, A. W. & Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 8, 922 (2017).
Hay, M. E. Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Annu. Rev. Mar. Sci. 1, 193–212 (2009).
Puglisi, M. P., Sneed, J. M., Ritson-Williams, R. & Young, R. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 36, 410–429 (2019).
Certner, R. H. & Vollmer, S. V. Inhibiting bacterial quorum sensing arrests coral disease development and disease‐associated microbes. Environ. Microbiol. 20, 645–657 (2018).
Mohamed, A. R., Ochsenkühn, M. A., Kazlak, A. M., Moustafa, A. & Amin, S. A. The coral microbiome: towards an understanding of the molecular mechanisms of coral–microbiota interactions. FEMS Microbiol. Rev. 47, fuad005 (2023).
Peixoto, R. S. et al. Coral Probiotics: Premise, Promise, Prospects. Annu. Rev. Anim. Biosci. 9, 265–288 (2021).
Torres, M. et al. AHL-lactonase expression in three marine emerging pathogenic Vibrio spp. reduces virulence and mortality in brine shrimp (Artemia salina) and Manila clam (Venerupis philippinarum). PLoS ONE 13, e0195176 (2018).
Zimmer, B. L. et al. Quorum Sensing Signal Production and Microbial Interactions in a Polymicrobial Disease of Corals and the Coral Surface Mucopolysaccharide Layer. PLoS ONE 9, e108541 (2014).
Meyer, J. L., Gunasekera, S. P., Scott, R. M., Paul, V. J. & Teplitski, M. Microbiome shifts and the inhibition of quorum sensing by Black Band Disease cyanobacteria. ISME J. 10, 1204–1216 (2016).
Zhou, J. et al. Opportunistic bacteria use quorum sensing to disturb coral symbiotic communities and mediate the occurrence of coral bleaching. Environ. Microbiol. 22, 1944–1962 (2020).
Tait, K., Hutchison, Z., Thompson, F. L. & Munn, C. B. Quorum sensing signal production and inhibition by coral‐associated vibrios. Environ. Microbiol Rep. 2, 145–150 (2010).
Sun, X. et al. Identification of quorum sensing-regulated Vibrio fortis as potential pathogenic bacteria for coral bleaching and the effects on the microbial shift. Front. Microbiol. 14, 1116737 (2023).
Krediet, C. J., Ritchie, K. B., Paul, V. J. & Teplitski, M. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc. R. Soc. B. 280, 20122328 (2013).
Reina, J. C., Pérez, P. & Llamas, I. Quorum Quenching Strains Isolated from the Microbiota of Sea Anemones and Holothurians Attenuate Vibrio corallilyticus Virulence Factors and Reduce Mortality in Artemia salina. Microorganisms 10, 631 (2022).
Han, S. et al. AI-2 quorum sensing signal disrupts coral symbiotic homeostasis and induces host bleaching. Environ. Int. 188, 108768 (2024).
MacKnight, N. J. et al. Microbial dysbiosis reflects disease resistance in diverse coral species. Commun. Biol. 4, 679 (2021).
Tout, J. et al. Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Front. Microbiol. 6, 432 (2015).
Wilkins, L. G. E. et al. Host-associated microbiomes drive structure and function of marine ecosystems. PLoS Biol. 17, e3000533 (2019).
Weiland-Bräuer, N. et al. The Native Microbiome is Crucial for Offspring Generation and Fitness of Aurelia aurita. mBio 11, e02336-20 (2020).
Manefield, M. et al. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145, 283–291 (1999).
Dworjanyn, S. A., De Nys, R. & Steinberg, P. D. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 133, 727–736 (1999).
Givskov, M. et al. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178, 6618–6622 (1996).
Zang, T. et al. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Medicinal Chem. Lett. 19, 6200–6204 (2009).
Fernandes, N. et al. Genomes and Virulence Factors of Novel Bacterial Pathogens Causing Bleaching Disease in the Marine Red Alga Delisea pulchra. PLoS ONE 6, e27387 (2011).
Gardiner, M. et al. VarR controls colonization and virulence in the marine macroalgal pathogen Nautella italica R11. Front. Microbiol. 6, 1130 (2015).
Goecke, F., Labes, A., Wiese, J. & Imhoff, J. Chemical interactions between marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 409, 267–299 (2010).
Borchardt, S. A. et al. Reaction of Acylated Homoserine Lactone Bacterial Signaling Molecules with Oxidized Halogen Antimicrobials. Appl Environ. Microbiol 67, 3174–3179 (2001).
Danaraj, J., Mariasingarayan, Y., Ayyappan, S. & Karuppiah, V. Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb. Pathogenesis 147, 104392 (2020).
Jack, A. A. et al. Alginate Oligosaccharide-Induced Modification of the lasI-lasR and rhlI-rhlR Quorum-Sensing Systems in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 62, e02318–17 (2018).
Kim, J. S., Kim, Y. H., Seo, Y. W. & Park, S. Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnol. Bioprocess Eng. 12, 308–311 (2007).
Batista, D., Carvalho, A. P., Costa, R., Coutinho, R. & Dobretsov, S. Extracts of macroalgae from the Brazilian coast inhibit bacterial quorum sensing. Botanica Mar. 57, 441–447 (2014).
Carvalho, A. P., Batista, D., Dobretsov, S. & Coutinho, R. Extracts of seaweeds as potential inhibitors of quorum sensing and bacterial growth. J. Appl Phycol. 29, 789–797 (2017).
Jha, B., Kavita, K., Westphal, J., Hartmann, A. & Schmitt-Kopplin, P. Quorum Sensing Inhibition by Asparagopsis taxiformis, a Marine Macro Alga: Separation of the Compound that Interrupts Bacterial Communication. Mar. Drugs 11, 253–265 (2013).
Kulshreshtha, G. et al. Red Seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down Regulate Virulence Factors of Salmonella Enteritidis and Induce Immune Responses in Caenorhabditis elegans. Front. Microbiol. 7, 421 (2016).
Muthukrishnan, S., Muthar, N. I., Zakaria, M. H., Rukayadi, Y. & Natrah, I. Anti-biofilm and Anti-quorum Sensing Activities of the Red Seaweed, Gracilaria changii and its Associated Bacteria. J. Appl Phycol. 35, 2555–2566 (2023).
Skindersoe, M. E. et al. Quorum Sensing Antagonism from Marine Organisms. Mar. Biotechnol. 10, 56–63 (2008).
Karnjana, K. et al. Purification and Evaluation of N-benzyl Cinnamamide from Red Seaweed Gracilaria fisheri as an Inhibitor of Vibrio harveyi AI-2 Quorum Sensing. Mar. Drugs 18, 80 (2020).
Targett, N. M. & Arnold, T. M. Minireview - Predicting the effects of the brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 34, 195–205 (1998).
Tang, J., Wang, W. & Chu, W. Antimicrobial and Anti-Quorum Sensing Activities of Phlorotannins From Seaweed (Hizikia fusiforme). Front. Cell. Infect. Microbiol. 10, 586750 (2020).
Vallet, M. et al. Novel α-Hydroxy γ-Butenolides of Kelp Endophytes Disrupt Bacterial Cell-to-Cell Signaling. Front. Mar. Sci. 7, 601 (2020).
Blanchet, E. et al. Quorum Sensing and Quorum Quenching in the Mediterranean Seagrass Posidonia oceanica Microbiota. Front. Mar. Sci. 4, 218 (2017).
Kanagasabhapathy, M., Yamazaki, G., Ishida, A., Sasaki, H. & Nagata, S. Presence of quorum-sensing inhibitor-like compounds from bacteria isolated from the brown alga Colpomenia sinuosa. Lett. Appl. Microbiol. 49, 573–579 (2009).
Chen, J. et al. Quorum Sensing Inhibitors from Marine Microorganisms and Their Synthetic Derivatives. Mar. Drugs 17, 80 (2019).
Ji, H. et al. Citrinin Is a Potential Quorum Sensing Inhibitor against Pseudomonas aeruginosa. Mar. Drugs 21, 296 (2023).
Yu, X. et al. A Cyclic Dipeptide from Marine Fungus Penicillium chrysogenum DXY-1 Exhibits Anti-quorum Sensing Activity. ACS Omega 6, 7693–7700 (2021).
Singh, R. P. & Reddy, C. R. K. Seaweed-microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiol Ecol. 88, 213–230 (2014).
Joint, I. et al. Cell-to-Cell Communication Across the Prokaryote-Eukaryote Boundary. Science 298, 1207–1207 (2002).
Joint, I., Tait, K. & Wheeler, G. Cross-kingdom signalling: exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B 362, 1223–1233 (2007).
Twigg, M. S., Tait, K., Williams, P., Atkinson, S. & Cámara, M. Interference with the germination and growth of U lva zoospores by quorum‐sensing molecules from Ulva ‐associated epiphytic bacteria. Environ. Microbiol. 16, 445–453 (2014).
Weinberger, F. et al. Spore release in Acrochaetium sp. (Rhodophyta) is bacterially controlled. J. Phycol. 43, 235–241 (2007).
Kuhlisch, C., Shemi, A., Barak-Gavish, N., Schatz, D. & Vardi, A. Algal blooms in the ocean: hot spots for chemically mediated microbial interactions. Nat Rev Microbiol https://doi.org/10.1038/s41579-023-00975-2 (2023).
Seymour, J. R., Amin, S. A., Raina, J. B. & Stocker, R. Zooming in on the phycosphere: the ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol 2, 17065 (2017).
Cole, J. J. Interactions between Bacteria and Algae in Aquatic Ecosystems. Annu Rev. Ecol. Syst. 13, 291–314 (1982).
Yoch, D. C. Dimethylsulfoniopropionate: Its Sources, Role in the Marine Food Web, and Biological Degradation to Dimethylsulfide. Appl Environ. Microbiol 68, 5804–5815 (2002).
Falkowski, P. G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth Res 39, 235–258 (1994).
Fuhrman, J. A. & Azam, F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: Evaluation and field results. Mar. Biol. 66, 109–120 (1982).
Amin, S. A. et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 522, 98–101 (2015).
Seyedsayamdost, M. R., Case, R. J., Kolter, R. & Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 3, 331–335 (2011).
Huang, X., Cai, Z., Lin, G. & Zhou, J. Quorum sensing modulating algae–bacteria interactions. Chin. J. Appl. Environ. Biol. 22, 0708–0717 (2016).
Huang, X. et al. Profiles of quorum sensing (QS)-related sequences in phycospheric microorganisms during a marine dinoflagellate bloom, as determined by a metagenomic approach. Microbiological Res. 217, 1–13 (2018).
Zhou, J., Lyu, Y., Richlen, M. L., Anderson, D. M. & Cai, Z. Quorum Sensing Is a Language of Chemical Signals and Plays an Ecological Role in Algal-Bacterial Interactions. Crit. Rev. Plant Sci. 35, 81–105 (2016).
Platt, A. J. & Whalen, K. E. Probing the Phycosphere: Techniques to Study Bacteria-Phytoplankton Interactions. Integr. Comp. Biol. 63, 1509–1519 (2023).
Waters, C. M. & Bassler, B. L. Quorum sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 (2005).
Fei, C. et al. Quorum sensing regulates ‘swim‐or‐stick’ lifestyle in the phycosphere. Environ. Microbiol. 22, 4761–4778 (2020).
Natrah, F. M. I. et al. Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture 317, 53–57 (2011).
Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153, 3923–3938 (2007).
Landa, M., Burns, A. S., Roth, S. J. & Moran, M. A. Bacterial transcriptome remodeling during sequential co-culture with a marine dinoflagellate and diatom. ISME J. 11, 2677–2690 (2017).
Shibl, A. A. et al. Diatom modulation of select bacteria through use of two unique secondary metabolites. Proc. Natl Acad. Sci. USA 117, 27445–27455 (2020).
Corral-Lugo, A., Daddaoua, A., Ortega, A., Espinosa-Urgel, M. & Krell, T. Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci. Signal. 9, 409 (2016).
Zhu, J. et al. Dynamic patterns of quorum sensing signals in phycospheric microbes during a marine algal bloom. Environ. Res. 212, 113443 (2022).
Romero, M., Muro-Pastor, A. M. & Otero, A. Quorum sensing N-acylhomoserine lactone signals affect nitrogen fixation in the cyanobacterium Anabaena sp. PCC7120: AHLs inhibit nitrogen fixation in cyanobacteria. FEMS Microbiol. Lett. 315, 101–108 (2011).
Van Mooy, B. A. et al. Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 6, 422–429 (2012).
Johnson, W. M., Kido Soule, M. C. & Kujawinski, E. B. Evidence for quorum sensing and differential metabolite production by a marine bacterium in response to DMSP. ISME J. 10, 2304–2316 (2016).
Nakashima, T. et al. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium γ-proteobacterium. Appl Microbiol Biotechnol. 73, 684–690 (2006).
Paul, C. & Pohnert, G. Interactions of the Algicidal Bacterium Kordia algicida with Diatoms: Regulated Protease Excretion for Specific Algal Lysis. PLoS ONE 6, e21032 (2011).
Zhang, S.-J. et al. The complete genome sequence of the algicidal bacterium Bacillus subtilis strain JA and the use of quorum sensing to evaluate its antialgal ability. Biotechnol. Rep. 25, e00421 (2020).
Chi, W. et al. Quorum sensing of microalgae associated marine Ponticoccus sp. PD-2 and its algicidal function regulation. AMB Expr. 7, 59 (2017).
Schaefer, A. L. et al. A new class of homoserine lactone quorum-sensing signals. Nature 454, 595–599 (2008).
Geng, H., Bruhn, J. B., Nielsen, K. F., Gram, L. & Belas, R. Genetic Dissection of Tropodithietic Acid Biosynthesis by Marine Roseobacters. Appl Environ. Microbiol 74, 1535–1545 (2008).
Greer, E. M., Aebisher, D., Greer, A. & Bentley, R. Computational Studies of the Tropone Natural Products, Thiotropocin, Tropodithietic Acid, and Troposulfenin. Significance of Thiocarbonyl−Enol Tautomerism. J. Org. Chem. 73, 280–283 (2008).
Thiel, V. et al. Identification and biosynthesis of tropone derivatives and sulfur volatiles produced by bacteria of the marine Roseobacter clade. Org. Biomol. Chem. 8, 234–246 (2010).
González, J. M., Kiene, R. P. & Moran, M. A. Transformation of Sulfur Compounds by an Abundant Lineage of Marine Bacteria in the α-Subclass of the Class Proteobacteria. Appl Environ. Microbiol 65, 3810–3819 (1999).
Newton, R. J. et al. Genome characteristics of a generalist marine bacterial lineage. ISME J. 4, 784–798 (2010).
Garrett, O. & Whalen, K. E. A bacterial quorum sensing signal is a potent inhibitor of de novo pyrimidine biosynthesis in the globally abundant Emiliania huxleyi. Front. Microbiol. 14, 1266972 (2023).
Teplitski, M. et al. Chlamydomonas reinhardtii Secretes Compounds That Mimic Bacterial Signals and Interfere with Quorum Sensing Regulation in Bacteria. Plant Physiol. 134, 137–146 (2004).
Brisson, V. et al. Identification of Effector Metabolites Using Exometabolite Profiling of Diverse Microalgae. mSystems 6, e00835-21 (2021).
Mausz, M. A. & Pohnert, G. Phenotypic diversity of diploid and haploid Emiliania huxleyi cells and of cells in different growth phases revealed by comparative metabolomics. J. Plant Physiol. 172, 137–148 (2015).
Rajamani, S. et al. The Vitamin Riboflavin and Its Derivative Lumichrome Activate the LasR Bacterial Quorum-Sensing Receptor. MPMI 21, 1184–1192 (2008).
Dahan, Y., Wingreen, N. S. & Meir, Y. The value of information gathering in phage–bacteria warfare. PNAS Nexus 3, pgad431 (2023).
Duddy, O. P. & Bassler, B. L. Quorum sensing across bacterial and viral domains. PLoS Pathog. 17, e1009074 (2021).
Golais, F., Hollý, J. & Vítkovská, J. Coevolution of bacteria and their viruses. Folia Microbiol 58, 177–186 (2013).
Høyland-Kroghsbo, N. M., Mærkedahl, R. B. & Svenningsen, S. L. A Quorum-Sensing-Induced Bacteriophage Defense Mechanism. mBio 4, e00362–12 (2013).
Hoque, M. M. et al. Quorum Regulated Resistance of Vibrio cholerae against Environmental Bacteriophages. Sci. Rep. 6, 37956 (2016).
Tan, D., Svenningsen, S. L. & Middelboe, M. Quorum Sensing Determines the Choice of Antiphage Defense Strategy in Vibrio anguillarum. mBio 6, e00627–15 (2015).
Letarov, A. & Kulikov, E. The bacteriophages in human- and animal body-associated microbial communities. J. Appl. Microbiol. 107, 1–13 (2009).
Høyland-Kroghsbo, N. M. et al. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc. Natl Acad. Sci. Usa. 114, 131–135 (2017).
Patterson, A. G. et al. Quorum Sensing Controls Adaptive Immunity through the Regulation of Multiple CRISPR-Cas Systems. Mol. Cell 64, 1102–1108 (2016).
Van Houte, S. et al. The diversity-generating benefits of a prokaryotic adaptive immune system. Nature 532, 385–388 (2016).
Stern, A., Keren, L., Wurtzel, O., Amitai, G. & Sorek, R. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends Genet. 26, 335–340 (2010).
Vercoe, R. B. et al. Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands. PLoS Genet 9, e1003454 (2013).
Mion, S., Plener, L., Rémy, B., Daudé, D. & Chabrière, É. Lactonase SsoPox modulates CRISPR-Cas expression in gram-negative proteobacteria using AHL-based quorum sensing systems. Res. Microbiol. 170, 296–299 (2019).
Hargreaves, K. R., Kropinski, A. M. & Clokie, M. R. J. What Does the Talking?: Quorum Sensing Signalling Genes Discovered in a Bacteriophage Genome. PLoS ONE 9, e85131 (2014).
Silpe, J. E. & Bassler, B. L. A Host-Produced Quorum-Sensing Autoinducer Controls a Phage Lysis-Lysogeny Decision. Cell 176, 268–280.e13 (2019).
Tan, D. et al. High cell densities favor lysogeny: induction of an H20 prophage is repressed by quorum sensing and enhances biofilm formation in Vibrio anguillarum. ISME J. 14, 1731–1742 (2020).
Laganenka, L. et al. Quorum Sensing and Metabolic State of the Host Control Lysogeny-Lysis Switch of Bacteriophage T1. mBio 10, e01884-19 (2019).
Liang, X., Wagner, R. E., Li, B., Zhang, N. & Radosevich, M. Quorum Sensing Signals Alter in vitro Soil Virus Abundance and Bacterial Community Composition. Front. Microbiol. 11, 1287 (2020).
Knowles, B. et al. Lytic to temperate switching of viral communities. Nature 531, 466–470 (2016).
Doekes, H. M., Mulder, G. A. & Hermsen, R. Repeated outbreaks drive the evolution of bacteriophage communication. eLife 10, e58410 (2021).
Bernard, C., Li, Y., Lopez, P. & Bapteste, E. Beyond arbitrium: identification of a second communication system in Bacillus phage phi3T that may regulate host defense mechanisms. ISME J. 15, 545–549 (2021).
Harvey, E. et al. Quorum sensing signal disrupts viral infection dynamics in the coccolithophore Emiliania huxleyi. Aquat. Microb. Ecol. 89, 75–86 (2023).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol 13, 605–619 (2015).
Biller, S. J. et al. Bacterial Vesicles in Marine Ecosystems. Science 343, 183–186 (2014).
Brameyer, S. et al. Outer Membrane Vesicles Facilitate Trafficking of the Hydrophobic Signaling Molecule CAI-1 between Vibrio harveyi Cells. J Bacteriol 200, https://doi.org/10.1128/jb.00740-17 (2018).
Li, J., Azam, F. & Zhang, S. Outer membrane vesicles containing signalling molecules and active hydrolytic enzymes released by a coral pathogen Vibrio shilonii AK1. Environ. Microbiol. 18, 3850–3866 (2016).
Batista, J. H. et al. Interplay between two quorum sensing‐regulated pathways, violacein biosynthesis and VacJ /Yrb, dictates outer membrane vesicle biogenesis in Chromobacterium violaceum. Environ. Microbiol. 22, 2432–2442 (2020).
Choi, S. Y. et al. Chromobacterium violaceum delivers violacein, a hydrophobic antibiotic, to other microbes in membrane vesicles. Environ. Microbiol. 22, 705–713 (2020).
Schatz, D. & Vardi, A. Extracellular vesicles — new players in cell–cell communication in aquatic environments. Curr. Opin. Microbiol. 43, 148–154 (2018).
Aschtgen, M.-S., Wetzel, K., Goldman, W., McFall-Ngai, M. & Ruby, E. Vibrio fischeri -derived outer membrane vesicles trigger host development: OMV deliver signals in the squid/vibrio symbiosis. Cell. Microbiol. 18, 488–499 (2016).
Schatz, D. et al. Ecological significance of extracellular vesicles in modulating host-virus interactions during algal blooms. ISME J. 15, 3714–3721 (2021).
Tzipilevich, E., Habusha, M. & Ben-Yehuda, S. Acquisition of Phage Sensitivity by Bacteria through Exchange of Phage Receptors. Cell 168, 186–199.e12 (2017).
Schatz, D. et al. Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga. Nat. Microbiol 2, 1485–1492 (2017).
Verma, S. & Miyashiro, T. Quorum Sensing in the Squid-Vibrio Symbiosis. IJMS 14, 16386–16401 (2013).
Cui, Y. et al. SlipO2 Chip – single-cell respiration under tuneable environments. Lab Chip 24, 4786–4797 (2024).
O’Toole, G. A. We have a community problem. J Bacteriol e00073-24 https://doi.org/10.1128/jb.00073-24 (2024).
Tait, K. et al. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ. Microbiol. 7, 229–240 (2005).
Wheeler, G. L., Tait, K., Taylor, A., Brownlee, C. & Joint, I. Acyl‐homoserine lactones modulate the settlement rate of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism. Plant Cell Environ. 29, 608–618 (2006).
Huang, Y.-L., Dobretsov, S., Ki, J.-S., Yang, L.-H. & Qian, P.-Y. Presence of Acyl-Homoserine Lactone in Subtidal Biofilm and the Implication in Larval Behavioral Response in the Polychaete Hydroides elegans. Micro. Ecol. 54, 384–392 (2007).
Tait, K. & Havenhand, J. Investigating a possible role for the bacterial signal molecules N‐acylhomoserine lactones in Balanus improvisus cyprid settlement. Mol. Ecol. 22, 2588–2602 (2013).
Romero, M., Diggle, S. P., Heeb, S., Cámara, M. & Otero, A. Quorum quenching activity in Anabaena sp. PCC 7120: identification of AiiC, a novel AHL-acylase. FEMS Microbiol. Lett. 280, 73–80 (2008).
Syrpas, M. et al. Haloperoxidase Mediated Quorum Quenching by Nitzschia cf pellucida: Study of the Metabolization of N-Acyl Homoserine Lactones by a Benthic Diatom. Mar. Drugs 12, 352–367 (2014).
Stock, F. et al. N -Acyl Homoserine Lactone Derived Tetramic Acids Impair Photosynthesis in Phaeodactylum tricornutum. ACS Chem. Biol. 14, 198–203 (2019).
Dow, L. et al. The Multifaceted Inhibitory Effects of an Alkylquinolone on the Diatom Phaeodactylum tricornutum. ChemBioChem 21, 1206–1216 (2020).
Wang, R., Gallant, É. & Seyedsayamdost, M. R. Investigation of the Genetics and Biochemistry of Roseobacticide Production in the Roseobacter Clade Bacterium Phaeobacter inhibens. mBio 7, e02118–15 (2016).
Acknowledgements
Preparation of this manuscript was funded by NSF IOS 2041748 and by the Charles E. Kaufman Foundation Integrated Research Education Grant (KA2021-121932) to K.E.W. In addition, M.C. was supported by the Arnold and Mable Beckman Fellowship. We would like to acknowledge Elizabeth Harvey and Jiahe Wu for their helpful comments on drafts of the manuscript.
Author information
Authors and Affiliations
Contributions
M.C. developed the outline and first draft of the manuscript. K.E.W. edited the first draft. Both authors edited the revised manuscript, which K.E.W. finalized.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Jin Zhou, Irene Wagner-Döbler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Linn Hoffmann and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Coolahan, M., Whalen, K.E. A review of quorum-sensing and its role in mediating interkingdom interactions in the ocean. Commun Biol 8, 179 (2025). https://doi.org/10.1038/s42003-025-07608-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07608-9
This article is cited by
-
Mechanistic insights of smart biofilms in environmental bio-monitoring: from growth to detection
Microbial Cell Factories (2026)
-
Extracellular vesicles as structured vectors of quorum sensing signals influence aquatic microbial communities
npj Biofilms and Microbiomes (2026)
-
Exploring the interaction between symbiotic bacteria from seagrass-associated sponges and biofilm-forming bacteria
International Microbiology (2026)
-
Valorizing agro-food waste for microbial B vitamin biosynthesis: impacts on gut microbiota dynamics and microbial communication
Reviews in Environmental Science and Bio/Technology (2026)
-
Effects of a novel Paraburkholderia phage IPK on the phenanthrene degradation efficiency of the PAH-degrading strain Paraburkholderia caledonica Bk
Biodegradation (2025)