Abstract
Mapping the spatial organization of tissues is critical to understanding organ biology in health and disease. Developments in multiplexed antibody-based, fluorescence labelling methods have provided unique insights into tissue microenvironments. However, many current methods have a variety of limitations that reduce their practical utilization including cost, time and technical complexity. To address these drawbacks, we developed Spatial Photo-inactivation Enhanced Cyclic Target REsolved multiPlexing (SPECTRE-Plex). SPECTRE-Plex is a relatively low cost, end-to-end technique based on a series of methods that significantly improve speed, automation, and resolution of cyclic multiplex immunofluorescence imaging. We describe a representative example of the application of the method by investigating spatial cellular and neighborhood changes in the proximal small intestine between healthy tissue and active celiac disease.
Similar content being viewed by others
Introduction
Describing the spatial context of how proteins and cells organize, interact, and change is critical to understanding tissue and organ biology in health and disease. Several different high dimensionality spatial biology approaches for tissues have emerged that detect and measure either mRNA transcripts (e.g., MERFISH, Slide-seq)1,2 or protein (e.g., CODEX, cyCIF)3,4,5,6,7.
Detection and localization of proteins within tissues allows assessment of cellular function, local communication, and accurate mapping of tissue state. Imaging-based protein methods center around cognate antibody binding to targets of interest, with either fluorescence or mass-spectroscopy-based detection. To detect multiple targets in the same tissue, most multiplexed protein techniques are based on cyclical, iterative antibody binding8. Discrimination of individual targets between cycles can be achieved in a variety of ways, such as fluorescence dye inactivation (t-cyCIF, IBEX), barcoding (CODEX), or antibody elution3,5,9,10. Each of the major methods has relative advantages and disadvantages varying by application, ease of use, and resource investment. For example, while CODEX enables highly amplified and specific signal identification, the need for specific oligonucleotide conjugation substantially increases cost (for commercially available targets/panels) and/or time (for custom antibody conjugation and validation). Antibody elution/stripping methods have advantages in linear signal amplification but can be hampered by incomplete antibody removal and loss of tissue morphology after a finite number of cycles8. Dye inactivation methods based on directly conjugated fluorescent antibodies are attractive because of the large array of cheap, validated, commercially available antibodies, and relatively simple procedures and reagents. However, current implementations of dye inactivation methods have limitations related to cost, speed, resolution, and fluidics.
We therefore developed an end-to-end system, based on a series of novel or optimized methods to allow rapid, high-resolution, and relatively low-cost multiplex immunofluorescence. Spatial Photo-inactivation Enhanced Cyclic Target REsolved multiPlexing (SPECTRE-Plex) broadly encompasses three modules. Firstly, an optimized upright water dipping objective-based imaging and fluidic system, including custom solutions for repeatable, iterative image capture. Secondly, a novel microfluidic imaging chamber system incorporating a refractive index matching optical window that enables imaging of tissues at a variety of resolutions, including the use of high NA (>1.0) objectives in conjunction with repeated solution changes. Thirdly, a new fast, non-gas producing dye inactivation method that reduces cycle times. We show application of this system for a 22-plex, 7-round panel in healthy and diseased human duodenal tissue, including downstream analysis. In addition, we highlight additional applications of the method, including direct tissue-based measurements of antibody binding kinetics for quantitative validation and assessment of staining conditions.
Results and Discussion
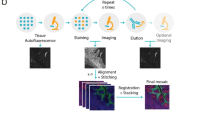
Figure 1a, Supplementary Fig. 1, and Supplementary Note 1 outline the overall system design, including custom optics with a water dipping objective, small volume fluidics system, and python-based imaging and device control. The system is designed so that slide-mounted samples undergo staining, washing, imaging, and dye inactivation in situ allowing efficient and rapid repetition of cycles (Fig. 1b). Overall, the method significantly reduces experimental time. As an example, a 7-cycle run can be completed in 8 h as compared to 14–36 h for most manual or semi-automated cyclical methods (Fig. 1b, Supplementary Fig. 2 and Supplementary Table 1). Tiled imaging in place is enabled by a highly repeatable motorized stage. Integrated control of imaging, positioning, and fluidics is achieved by custom python and μ-manager software (Supplementary Notes 2–4, Supplementary Fig. 3–5).
a Schematic of SPECTRE-plex system. b Overview of the cyclical steps and experimental times for SPECTRE-plex tissue staining. c Kinetics of dye inactivation of Alexa 488, 532, 555, and 647 showing time constant (τ, s/mM) for m-CPBA, LiBH4, and H2O2 (left) and time course of m-CPBA dye-inactivation for susceptible fluorophores Alexa 555 and 647 and non-susceptible Alexa 594 (right) mean ± SD, n = 5 replicates). Falcon tubes containing 10 mM mCPBA, 4.5% H2O2, and 1 mg/ml LiBH4 showing gas production over 20 min (inset) (d) Schematic for imaging chamber system including microfluidic (‘invisi-slip’) device. e High magnification image of human duodenum using 60x, 1.2NA objective. f Antibody binding association of anti-MUC2 IgG on duodenal tissue with alteration of solution pH (top), and temperature (bottom), with representative images of antibody fluorescence (right). Schematics created using Biorender Thiagarajah, J. (2025) https://BioRender.com/shedphj.
To further reduce cycle times, we examined the chemistry underlying inactivation of cyanine and rhodamine-based fluorophores (Supplementary Fig. 6). This led to the discovery that meta-chloroperoxybenzoic acid (m-CPBA) potentially functions more efficiently than previously used dye-inactivation agents. m-CBPA is orders of magnitude faster against a set of commonly used conjugated fluorophores, than lithium borohydride (LiBH4) or hydrogen peroxide (H2O2) (Fig. 1c, and Supplementary Fig. 7). In addition, m-CPBA does not generate oxygen in solution overcoming a major issue with LiBH4 and H2O2 for imaging and the application of microfluidics (Supplementary Note 5). We found that m-CPBA does not cause appreciable deterioration of tissue quality (tested up to 40 cycles, Supplementary Fig. 6), and the lack of bubble formation allows easy implementation of small volume fluidic flow control.
Imaging resolution and magnification of current automated cyclical fluorophore staining methods is limited by the use of air objectives (usually NA ≤ 0.8) and/or glass chamber windows, which introduce refractive index mismatching (Supplementary Note 4). To solve this issue, we developed a PDMS-based microfluidic device (“invisi-slip”) with an embedded fluorinated ethylene propylene (FEP) window enabling aberration free imaging as the FEP polymer has the same refractive index as water and can be coupled to water-based objectives (Supplementary Fig. 8). This device is incorporated into an integrated imaging chamber system which includes ports and tubing for fluids and temperature control (Fig. 1d). For whole tissue imaging and to reduce the number of imaging tiles we use a large field of view (FOV) 16 × 0.8 NA water dipping objective. To illustrate the wide range of objectives that can be used, we also carried out SPECTRE-plex runs using a 60 × 1.2NA water dipping objective, which allowed imaging of target proteins at sub-cellular resolution (Fig. 1e).
The in-situ nature of the SPECTRE-plex design allows for simultaneous imaging and conjugated antibody labeling on tissues. This enables experiments assessing the kinetics of antibody-target association directly on tissues. To illustrate this, we tested the binding kinetics of a specific antibody (anti-Muc2 IgG) and the effect of altering solution pH or temperature. As shown in Fig. 1f, at 22 °C, anti-Muc2 exhibited a one-phase association with a mean half-time of 50 min, which was not altered by solution pH but was significantly reduced (t1/2 = 13 min) by increasing solution temperature to 37 °C. We observe that increased binding kinetics with increasing temperature occurred for many but not all antibodies (Supplementary Fig. 9, Supplementary Table 2), and further studies are ongoing to provide a more detailed assessment of the kinetic behavior of antibody binding. This capability of the SPECTRE-plex system is relevant to a variety of applications, including tissue-specific validation of antibodies and rapid assessment of therapeutic antibody or small molecule binding.
To demonstrate the use of our system, a 22-plex image data set (Supplementary Table 3) was generated using duodenal tissues from children with active celiac disease and age-matched healthy controls. Figure 2a shows representative registered and stitched whole tissue SPECTRE-plex images including markers of specific cell types such as epithelial cells (Na+K+-ATPase), T-cells (CD3d), enteroendocrine cells (ChromograninA), and myofibroblasts (smooth muscle actin), among others. A custom image analysis pipeline involving adaptive segmentation and cell coordinate mapping (Fig. 2b, Supplementary Note 7–8, and Supplementary Fig. 10–12) enables several downstream analyses. Cell compositional analysis (Fig. 2b–d) revealed differences between healthy and celiac disease tissue, including increases in intestinal enteroendocrine cells, immune cell expansion, and increased spatial cellular heterogeneity. Celiac disease tissues showed relative expansion of proliferating (PCNA+) epithelial cells with focal high-density areas and increases in cellular density within non-epithelial compartments (Fig. 2e–g). Analysis of tuft cells, a lineage of epithelial cells involved in luminal nutrient and pathogen sensing11 showed differences in both distribution and clustering between healthy and celiac disease tissues (Fig. 2h, i). Neighborhood cell-cell analysis for pEGFR+ tuft cells indicated increases in spatial association with both proliferating and differentiated epithelial cells in celiac disease compared to healthy controls (Fig. 2j, Supplementary Fig. 13). As a proof of generalizability, we have included two more multiplex immunofluorescence runs, one on colon tissue and one on spleen tissue (Supplementary Fig. 14).
a Example tiled, stitched, processed whole-section tissue images from healthy control duodenum and active celiac disease. Main image showing lactase, mucin-2 (MUC2), smooth muscle actin (SMactin), sodium-potassium ATPase (Na+K+-ATPase), and nuclei (DAPI), with inset target antigens as indicated. b Cell-type spatial map of healthy and celiac disease duodenum. c Uniform Manifold Approximation and Projection (UMAP) for spatial clustering of cell-types. d Mean cell numbers and cell percentages for healthy versus celiac disease tissue (mean ± SEM, n = 3). e Mean epithelial sub-type cell percentages for healthy versus celiac disease tissue. f Example cellular density analysis for non-epithelial cells. g Example spatial cluster density analysis for PCNA+ cycling epithelial cells. h Example spatial cluster size analysis for pEGFR+ tuft cells. i Mean cluster size frequency for pEGFR+ tuft cells (mean ± SEM, n = 3) (j) Cell-cell neighborhood association analysis for pEGFR+ tuft cells. The p values for the different comparisons are total cells (0.9287), PCNA(0.0989), ChgA(0.0010), pEGFR(0.6480), and CD3D(0.5317).
Future improvements to our method include the addition of a multi-slide configuration to enable high-throughput experiments and screening, and addition of confocal optics to enable three-dimensional sample imaging. Although SPECTRE-Plex was primarily designed for dye-inactivation multiplex methods, the imaging and microfluidic modules can be readily applied to other tissue-based imaging applications, including RNA profiling, barcoding, and other use cases such as quantitative tissue binding studies.
Methods
Human tissue sections
De-identified formalin-fixed paraffin-embedded duodenal, spleen, and colon tissue sections for multiplex staining were obtained under Boston Children’s Hospital IRB protocol # P00026726. Celiac disease (active disease, serologically positive) and age-matched healthy control sections were obtained from <12yo subjects. All ethical regulations and informed consent relevant to secondary use of human biological specimens and data were followed and obtained.
Imaging system
The custom optical train, chamber system, and fluidics are described in detail in Supplementary Note 1.
Invisi-slip system
Details of the Invisi-slip system can be found in Supplementary Note 6.
Slide preparation
Slides are deparaffinized at 60 °C for 15 min and then incubated twice with 100% Histoclear first for 5 min and then for 10 min at RT. They are then rehydrated by repeated incubation for 5 min with decreasing concentrations of Ethanol (100%, 95%, 75%, 50%, and 0%) at RT. The slides are then incubated in Antigen Retrieval Buffer and heated to 100 °C for 20 min. After cooling to RT for 20–40 min, the slides are washed twice with PBS and incubated in blocking buffer for 1 h. The slides were then stored in PBS at 4 °C.
Standard SPECTRE-Plex protocol
Full protocol methods are outlined in detail in Supplementary Note 9. Cost of running a typical panel is given in Supplementary Table 4.
Multiplex imaging
Prior to the multiplex run, the slides are stained with 1:5k Hoechst dilution for 5 min and then rinsed. The antibody dilutions are prepared as necessary. Image acquisition is set up using the Micro-Magellan plug-in for μ-manager along with custom Python code. The first step in the process is to define the surface of the slide to be imaged. An initial acquisition is performed to capture autofluorescence with default exposure times. The DAPI images are segmented as a binary image using Stardist12 and run through a size exclusion filter. The focus map is updated with this information for each tile to image only the tiles where tissue exists. Next, in the cycle process, antibody solutions are pumped into the chamber and incubated for 45 min and washed with PBS for 2.5 min.
The next step in the cycle is the imaging, which has three parts (a) an autofocus step (b) an auto-exposure step that updates the focus map, and (c) an acquisition step to acquire the images. The autofocus part of the program is detailed in Supplementary Note 3, while the autoexposure code is elaborated in Supplementary Note 4. Very briefly, this is implemented as follows. When the acquisition phase of the cycle starts, the auto focus program calculates the ∆Z between the current focus maxima with previous cycle’s maxima to center the stack around the current maxima and append that value to the focus map. Following this, the autoexposure code is run to determine the optimal exposure times, and the images are acquired.
Following this is the dye inactivation step in which the imaging chamber is incubated with the dye inactivation solution for 3 min, followed by 2 min of washing. The fluorophore-inactivated images were acquired using the exact same settings as the stained images. All code is available in GitHub at the following link: https://github.com/mdanderson03/SpectrePlex.git.
Comparison of different dye-inactivation solutions
Dye-inactivation solutions were prepared at the concentrations described and based on previous studies5,9. All experiments were conducted as triplicates in a 96-well format using a Tecan Spark multi-well plate imaging system in inverted fluorescence mode with the monochromator positions chosen to have 25 nm bandwidths centered on excited and emission peaks for each fluorophore. Each fluorophore was diluted at 1:1000 in PBS and with the appropriate concentration of the different bleach solutions with the bleach solution without any fluorophores as their respective controls. The average fluorescence intensity normalized to the control was measured every 15 s for 10 min. The resulting kinetic data were fit in Graphpad Prism using a double exponential decay model for 5 technical replicates. The proposed mechanism of the bleach, along with the comparison, is elaborated in Supplementary Fig. 6.
Antibody staining kinetics
The kinetics experiment was conducted on the SPECTRE-Plex system and modified from the standard SPECTRE-Plex imaging protocol. A slide stained with DAPI was loaded onto the microscope’s stage and washed with PBS. For these experiments, a fixed exposure time and illumination intensity (50% power and 50 ms exposure time for both Alexa-488 and Alexa-647) were used. An initial image was taken as the background autofluorescence image, and then the fluidic system was used to dispense the stain solution. Next, every 2 min, the system would execute the Stardist12 recursive autofocus algorithm and each channel imaged. A final image was taken 90 min after a PBS wash. Temperature and pH were modulated for these kinetic experiments. For the pH experiments, Phosphate-citrate buffer was used due to its large buffering range. Temperature was modulated using an embedded heater in the imaging chamber. For these experiments, the heater was then turned on for 1.5 h prior to achieving a stable chamber fluid temperature as measured by an infrared thermometer prior to antibody staining.
Images were processed by subtracting the background, zero-time frame and the average pixel intensity of the tissue region was calculated for each frame. A binary tissue mask was generated by thresholding the final background-subtracted image via Otsu’s method. An exception was made for the pH 5.0 for MUC2 data, as the signal was too low for Otsu’s method to threshold accurately. In this case, the mask was set by manually tracing out 3 small regions of goblet cells in the image. The temporal change in average pixel intensity data was fit to a single exponential function in GraphPad Prism.
Assessment of fluidic bubble generation
H2O2, LiBH4, and m-CPBA were mixed at normal working concentrations (4.5%, 50 mM, 10 mM, respectively) in 15 mL tubes, vortexed, and left to sit for 20 min for observing the propensity of each solution to form bubbles. The advantages of mCPBA along with our observations are detailed in Supplementary Note 5.
Initial Image pre-processing
A modified Brenner score (see Supplementary Note 3) with a skip parameter of 17 is used to identify the z slice that is in focus. The equivalent image in the dye-inactivated (“bleach”) stack is obtained by using the same slice index as used for the stained z-stack. Next, both the stained and the bleach images that contain tissue regions are used to train a BaSiC13 flat field correction surface. Pystackreg14 is used to register the bleached image to the stained image, and the registered bleached image is subtracted from the stained image. The images are then converted as TIFF stacks and populated with metadata and inputted into McMicro15 for stitching using the Ashlar module. The non-tissue regions are then removed using a tissue mask to output a finalized dataset. Details of the pre-processing steps can be found in Supplementary Note 7.
Image processing
As the first step in image analysis, a tissue mask was created. All individual channels are downsampled by twofold, normalized, and finally merged to generate the composite stain image. This normalized image is then thresholded by Otsu’s method to create a binary image, which is then dilated with filled holes, and the largest contoured structure is defined as the tissue mask. All subsequent image processing was conducted on images with the applied tissue mask.
Four channels (DAPI, Sodium Potassium ATPase, EpCAM, and Pan Cytokeratin) were normalized using Min Max Scaler in the sklearn Python package. Sodium Potassium ATPase, EpCAM, and Pan Cytokeratin were then averaged together to create the final image for segmentation. CellPose (Ver 3.0.7)16 was then used for segmentation with the normalized cell boundary image and the normalized DAPI image being the “chan to segment” and “chan2” inputs, respectively. The model used was “cyto3” with the default settings except for the “flow threshold” which was changed to 0.0. The mask was used to determine the characteristic intensity of each marker, along with the centroid coordinates for each cell in the image. Otsu’s thresholding was used to identify and label the stained cells from cells that have only background fluorescence for different markers, and this was used to assign the various cell types.
All further analysis was performed using a custom-written R program utilizing several CRAN packages (listed below).
The spatial centroid position and the identity were used to generate a point-map position of each run to visually identify potential changes in tissue architecture. These visual observations were used as a starting point to quantify spatial changes between normal and celiac tissue (Supplementary Fig. 15–16). UMAPs were generated using normalized intensity data using the CRAN-UMAP package (Supplementary Fig. 17–18). Cluster size estimation was performed using HBDscan clustering algorithm17 with 5 points as the minimum clustering parameter. Busyness of tissue was estimated using point density function of DBSCAN with a bandwidth of 300. Neighborhood analysis was performed using the KNN function of DBSCAN.
The following packages from CRAN were used for analysis: readr, ggplot2, reshape2, tidyverse, hrbrthemes, viridis, tidyverse, readxl, janitor, dplyr, corrplot, dbplyr, dbscan, umap, plotly, spatialcluster, spdep, geosphere, usedist, dbscan, nabor, stringr, and lessR.
Statistics and reproducibility
Unpaired two-tailed t-test was used to compare healthy versus celiac groups.
Reporting summary
Further information on research design is available in Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data underlying graphs can be obtained from Supplementary data.
Code availability
All code is available in GitHub at the following link: https://github.com/mdanderson03/SpectrePlex.git.
References
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981.e15 (2018).
Gerdes, M. J. et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc. Natl. Acad. Sci. USA 110, 11982–11987 (2013).
Lin, J.-R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. Elife 7, e31657 (2018).
Saka, S. K. et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat. Biotechnol. 37, 1080–1090 (2019).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40, 1794–1806 (2022).
Chen, Y. & Guo, J. Multiplexed single-cell in situ protein profiling. ACS Meas. Sci. Au 2, 296–303 (2022).
Radtke, A. J. et al. IBEX: a versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc. Natl. Acad. Sci. USA 117, 33455–33465 (2020).
Zrazhevskiy, P. & Gao, X. Quantum dot imaging platform for single-cell molecular profiling. Nat. Commun. 4, 1619 (2013).
Billipp, T. E. et al. Tuft cell-derived acetylcholine promotes epithelial chloride secretion and intestinal helminth clearance. Immunity S1074761324001444 https://doi.org/10.1016/j.immuni.2024.03.023 (2024).
Weigert, M. & Schmidt, U. Nuclei instance segmentation and classification in histopathology images with Stardist. In Proc. 2022 IEEE International Symposium on Biomedical Imaging Challenges (ISBIC) 1–4 https://doi.org/10.1109/ISBIC56247.2022.9854534 (2022).
Peng, T. et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 8, 14836 (2017).
Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process 7, 27–41 (1998).
Schapiro, D. et al. MCMICRO: a scalable, modular image-processing pipeline for multiplexed tissue imaging. Nat. Methods 19, 311–315 (2022).
Stringer, C. & Pachitariu, M. Cellpose3: one-click image restoration for improved cellular segmentation. Nat. Methods 22, 592–599 (2025).
Density-Based Clustering Based on Hierarchical Density Estimates|SpringerLink. https://link.springer.com/chapter/10.1007/978-3-642-37456-2_14.
Acknowledgements
Analysis supported the Cell Function and Imaging Core/Harvard Digestive Disease Center (NIH grant P30DK034854). Plasma etching and fluidic device manufacturing were performed at The Microfluidics Core Facility at Harvard Medical School with the assistance of Calixto Saenz and Belinda Jean-Calixte. Schematics created using Biorender.
Author information
Authors and Affiliations
Contributions
M.D.A.: Conceptualization, methodology, investigation, writing—original draft, writing— review & editing, A.P.: methodology, investigation, writing—review & editing J.L.: methodology, investigation, writing—review & editing, M.W.: investigation, writing—review & editing, K.R.: Conceptualization, methodology, writing—original draft. writing—review & editing, J.A.S.: Conceptualization, writing—review & editing, supervision, JRT: Conceptualization, methodology, writing—original draft, writing—review & editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors (J.R.T. and M.D.A.) are named inventors on a patent application (PCT/US24/31703) related to the methods and designs described in this manuscript. All other authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr Shan E Ahmed Raza and Dr Ophelia Bu. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Anderson, M.D., Plone, A., La, J. et al. SPECTREPlex: an automated, fast, high-resolution enabled approach for multiplexed cyclic imaging and tissue spatial analysis. Commun Biol 8, 636 (2025). https://doi.org/10.1038/s42003-025-08052-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08052-5