Abstract
The prefrontal cortex (PFC) is important for learning and performing working memory tasks. However, its precise role for spatial and non-spatial working memory, and the role of different cell types in the circuits that maintain working memory remain poorly understood. To investigate this issue, we analyzed single-unit recordings from the PFC of monkeys during the passive viewing phase before they learned the task rules and after learning, during the execution of active working memory tasks (spatial and feature). Through cluster analysis of extracellular spike waveform features, we identified two classes of narrow-spiking neurons (putative inhibitory cells) and two classes of broad-spiking neurons (putative pyramidal cells). These putative cell classes exhibited distinct physiological characteristics, including baseline firing rates, baseline neural firing variability, and visual stimulus-evoked responses. Neuronal response modulation varied heterogeneously across these cell classes after training and performing active tasks. Training and execution of spatial working memory resulted in higher activity in all class types, highlighting the involvement of diverse prefrontal circuits in spatial information processing. In contrast, feature working memory training and execution affected activity of broad-spiking cell classes alone, suggesting less involvement of a prefrontal circuit in the representation of feature information. We also revealed hitherto unknown, differential effects of training and task execution on different broad-spiking cell types. One broad-spiking neuron subtype exhibited significant response modulation, with increased baseline firing rate, stimulus-evoked responses, and working memory-related firing rates. Another broad-spiking subtype showed decreased baseline firing rate and variability, which may optimize neural coding efficiency. This study advances our understanding of the functional heterogeneity within the PFC and the specialized contributions of different neuronal subtypes to cognitive processes.
Similar content being viewed by others
Introduction
Working memory (WM), an essential cognitive skill involved in encoding, retaining, and manipulating information in the conscious mind for a limited period, plays a pivotal role in higher cognitive functions such as decision-making, control of attention, planning, reasoning, and learning abilities1,2,3,4. It is widely known that the prefrontal cortex (PFC) has a significant role in WM tasks across humans and non-human primates5,6,7,8, however its precise role and the neural circuits that mediate working memory have been the matter of considerable debate9,10. There is broad agreement for a role of prefrontal cortex in spatial working memory, and the bump attractor model provides mechanistic insights on how working-memory related activity can be maintained, by virtue of recurrent excitation between neurons tuned for similar spatial locations and similarly spatially-targeted inhibition11,12. Less agreement exists for a role of prefrontal cortex in the maintenance of object-related working memory and the circuits that could maintain such information.
We address the question of the neural circuits maintaining spatial and object information, capitalizing on two developments. First, it has been observed that learning and performing WM tasks induces changes in the PFC activity of monkeys13. Specifically, a higher number of neurons respond to identical stimuli, and their activity levels are elevated after training14,15. This provides us the opportunity to examine changes in different cell types after training and execution of spatial and object working memory tasks, as cortical neurons have significant functional distinctions16,17. We reasoned that critical functions of the prefrontal cortex can be revealed by determining what types of tasks induce changes in its activity, and in which circuit elements.
Secondly, cell classes have been identified through clustering extracellular spike waveforms in cortical circuits. Early in vitro studies have leveraged the features of action potential (AP) waveforms to categorize cell types16,18,19. These investigations have distinguished two cell classes based on waveform duration: narrow-spiking, characterized by shorter waveform durations, and broad-spiking, characterized by longer waveform durations. The findings demonstrate that narrow-spiking units predominantly correspond to GABAergic inhibitory interneurons, whereas broad-spiking units to pyramidal cells16,18,19. The intracellular spike characteristics can also be extended to extracellular spike features20, thereby enabling the identification of putative cell classes in extracellular recordings. Indeed, numerous extracellular studies have investigated cell classes in various neocortical areas, such as the parietal cortex21, motor cortex22,23,24, prefrontal cortex25,26,27,28,29,30, frontal eye field31,32,33, auditory cortex34, visual cortex35,36,37,38,39,40, and hippocampus41. More recently, studies have begun to identify cell classes beyond the narrow- and broad-spiking types in primates and demonstrated specific functions for these classes42,43,44. For instance, a study employed two features, trough-to-peak duration and repolarization time of extracellular AP shapes, for spike waveform clustering, resulting in the extraction of four cell-class-specific functional categories45. Moreover, in the macaque premotor cortex, applying non-linear dimensionality reduction techniques on extracellular waveforms has led to the discovery of various cell classes46. Therefore, diverse features associated with extracellular spike waveforms can enable the identification of further putative cell types, promoting a more comprehensive understanding of brain functionality.
Our study relied on single-unit recordings collected from monkeys before and after learning to perform working memory tasks. We analyzed how training and active performance of spatial and feature working memory tasks modulated the activity of distinct cell classes, relative to their responses during passive viewing of stimuli. The results emphasize the varying degrees of neural changes elicited across different cell types. Consequently, this investigation provides new pathways for understanding the circuits of WM.
Results
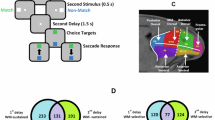
In this study, spiking activity was recorded from the lateral prefrontal cortex of three rhesus monkeys with no previous training in laboratory tasks. Data collection was performed in two phases: passive viewing (pre-training; Fig. 1A, B, first five frames), before the animals learned the task rules, and active working memory tasks involving decision-making (post-training; Fig. 1A, B), after the animals learned the task rules. Two distinct stimulus sets were used: a spatial set, which varied the location of a white square, and a feature set, which included different geometric shapes (Fig. 1C). During the pre-training phase, the animals were required only to maintain fixation while two visual stimuli were sequentially presented with a 1.5 s delay interval (Fig. 1A, B; first five frames). A total of 1282 neurons were isolated during the passive viewing task. After training to perform the WM tasks, the post-training phase commenced, during which the subjects were tasked with remembering the locations or features of the stimuli (Fig. 1A, B). The stimuli, timing, and sequence of presentation remained identical to the pre-training phase, except that two choice targets appeared after the second delay period. The animals indicated whether the two stimuli matched by making a saccadic eye movement to one of the choice targets. In total, 1178 neurons were isolated during the spatial and feature WM tasks.
A Spatial task. The sequence of events in the spatial task included fixation, stimulus presentation, and delay periods. During the pre-training phase, monkeys maintained fixation while stimuli appeared passively in different spatial locations. In the post-training phase, after the second delay period, choice targets appeared, requiring the monkey to saccade to a target based on the spatial match of the stimuli. B Feature task. The sequence of events in the feature task included fixation, stimulus presentation, and delay periods. During pre-training, monkeys maintained fixation while different shapes were presented passively. In the post-training phase, after the delay period, choice targets appeared, requiring the monkey to saccade to a target based on the feature match of the stimuli. C Stimulus sets. The spatial set consisted of nine possible locations arranged on a 3 × 3 grid. The feature set included eight distinct shapes: square, triangle, circle, diamond, plus sign, H, number sign, and inverted Y.
Distinction of cell types based on extracellular spike waveforms
Initially, we analyzed the spike waveforms obtained during the passive task to identify cell types. To distinguish numerous distinct cell classes, we selected three features derived from the AP waveform for each single unit: the trough-to-peak duration, first peak-to-trough ratio, and peak ratio (Fig. 2A). We determined diverse cell classes by applying unsupervised Gaussian mixture model (GMM) clustering to these waveform features. Additionally, we employed the Bayesian Information Criterion (BIC) to determine the optimal number of clusters in the GMM. The BIC analysis indicated that a model with four clusters had the lowest value, leading to the choice of four cell classes (Fig. 2B; minimum BIC shown by the purple arrow). Consequently, we identified four cell classes using GMM clustering in three-dimensional feature space (Fig. 2C): narrow-spiking 1 (NS1), narrow-spiking 2 (NS2), broad-spiking 1 (BS1), and broad-spiking 2 (BS2), comprising 6.55%, 21.29%, 41.81%, and 30.34% of the neurons, respectively (Fig. 2D, E; showing the spike waveforms of each class). The assignment into narrow- or broad-spiking categories was determined based on the trough-to-peak duration of each class. The mean trough-to-peak durations for NS1, NS2, BS1, and BS2 were 239.29, 420.79, 523.13, and 623.52 ms, respectively.
A Extracted waveform features for clustering: trough-to-peak duration, first peak-to-trough ratio (A1/A3), and peak ratio (A2/A1). B Bayesian information criterion (BIC) analysis for determining the optimal number of clusters. Four clusters were chosen as the optimal number based on the minimum BIC, as shown by the purple arrow. C Three-dimensional representation of spike waveform features and clustering using Gaussian mixture modeling (GMM). The clusters were identified as narrow-spiking 1 (NS1, blue, n = 84), narrow-spiking 2 (NS2, red, n = 273), broad-spiking 1 (BS1, yellow, n = 536), and broad-spiking 2 (BS2, purple, n = 389). D Spike waveforms of different cell classes. The average spike waveform for each class is displayed in black. E Comparison of average waveforms between clusters. F Confusion matrix for validation of cluster separation. Using the fitted GMM distributions, synthetic data points were generated, and their true labels were evaluated against the GMM-predicted assignments. The main diagonal represents the accuracy rate of classifying each class (cluster separation), while the elements outside the main diagonal indicate the percentage of misclassification (cluster overlap). The overall average accuracy was 95.45%.
The separation between clusters was assessed by generating 10,000 random data points from the fitted GMM distributions and comparing the true labels with the predicted cluster assignments45. Classification accuracy was quantified using a confusion matrix (Fig. 2F). The average accuracy across the four identified cell classes was 95.45%, indicating that the clusters are well-separated with minimal overlap.
Cell classes in post-training neurons
At this stage, our goal was to determine the cell types in the post-training units. Similar to the pre-training data, three waveform-based features (trough-to-peak duration, first peak-to-trough ratio, and peak ratio) were derived for every unit (Fig. 2A). We then employed the GMM, previously trained on pre-training data, to cluster the waveforms of post-training data. The outcome of this clustering analysis is depicted in Fig. 3A, which shows four distinct cell classes: NS1 (5.52%), NS2 (24.7%), BS1 (48.73%), and BS2 (21.05%) (Fig. 3B, C; showing the spike waveforms of each class). The waveforms of these identified cell classes closely matched those observed in the pre-training phase. Additionally, the percentage distribution of each cell class in the post-training phase was nearly identical to that of the pre-training phase. These results underscore the stability and robustness of cell classes determined from extracellular waveforms.
A Three-dimensional representation of spike waveform features in post-training recordings and clustering using the GMM trained on pre-training units. NS1 (n = 65) and NS2 (n = 291) clusters are blue and red, respectively, while BS1 (n = 574) and BS2 (n = 248) are yellow and purple. B Spike waveforms of different cell classes. The average spike waveform for each class is displayed in black. C Comparison of average waveforms between clusters.
Physiological characteristics of cell classes
We recognized neurons as responsive units that demonstrated a significant increase in firing rate during either stimulus presentation or the delay period relative to the fixation period (p < 0.05, paired t-test), consistent with previous investigations14,15,47. The proportion of responsive units (responsive to stimulus or delay) was computed for each cell type in both the pre- and post-training tasks (Fig. 4). In the pre-training phase, 27.4% (23/84) of NS1 neurons, 30.8% (84/273) of NS2 neurons, 23.1% (124/536) of BS1 neurons, and 25.7% (100/389) of BS2 neurons were responsive during the stimulus presentation period (Fig. 4A, light colors). Additionally, 25.0% (21/84) of NS1 neurons, 20.2% (55/273) of NS2 neurons, 16.2% (87/536) of BS1 neurons, and 17.5% (68/389) of BS2 neurons were responsive (Fig. 4B, light colors). In the post-training phase, 46.2% (30/65) of NS1 neurons, 39.2% (114/291) of NS2 neurons, 36.4% (209/574) of BS1 neurons, and 24.6% (61/248) of BS2 neurons were responsive during the stimulus presentation period (Fig. 4A, dark colors). Similarly, 38.5% (25/65) of NS1 neurons, 35.7% (104/291) of NS2 neurons, 35.4% (203/574) of BS1 neurons, and 27.4% (68/248) of BS2 neurons were responsive during the delay period (Fig. 4B, dark colors). Furthermore, the total proportion of responsive neurons during either the stimulus presentation or delay period (or both) was 41.7% (35/84), 37.0% (101/273), 31.0% (166/536), and 31.6% (123/389) of NS1, NS2, BS1, and BS2 neurons, respectively, in the pre-training phase (Fig. 4C, light colors). These proportions increased in the post-training phase to 55.4% (36/65), 50.2% (146/291), 49.0% (281/574), and 39.1% (97/248) of NS1, NS2, BS1, and BS2 neurons, respectively (Fig. 4C, dark colors). Narrow-spiking cells exhibited a higher percentage of responsiveness compared to broad-spiking cells. Additionally, as the mean trough-to-peak duration of spike waveforms decreased in cell classes, the percentage of responsive neurons elevated. It is essential to underscore that all analyses in this study were performed on responsive neurons.
A Percentage of responsive neurons to visual stimuli in each class. These neurons exhibit a significant increase in firing rate during the stimulus presentation period compared to baseline. Pre-training recordings for each class are depicted in light colors, while post-training units are depicted in dark colors. In the pre-training phase, 27.4% (n = 23/84), 30.8% (n = 84/273), 23.1% (n = 124/536), and 25.7% (n = 100/389) of NS1, NS2, BS1, and BS2 neurons were responsive, respectively. In the post-training phase, the percentages were 46.2% (n = 30/65), 39.2% (n = 114/291), 36.4% (n = 209/574), and 24.6% (n = 61/248) for NS1, NS2, BS1, and BS2 neurons, respectively. B Percentage of responsive neurons during the delay period in each class. These neurons exhibit a significant increase in firing rate during the delay period compared to baseline. In the pre-training phase, 25.0% (n = 21/84), 20.2% (n = 55/273), 16.2% (n = 87/536), and 17.5% (n = 68/389) of NS1, NS2, BS1, and BS2 neurons were responsive, respectively. In the post-training phase, the percentages were 38.5% (n = 25/65), 35.7% (n = 104/291), 35.4% (n = 203/574), and 27.4% (n = 68/248), respectively. C Total percentage of responsive neurons during either the stimulus presentation or delay period (or both) in each class. These neurons exhibit a significant increase in firing rate during either or both periods compared to baseline. In the pre-training phase, 41.7% (n = 35/84), 37.0% (n = 101/273), 31.0% (n = 166/536), and 31.6% (n = 123/389) of NS1, NS2, BS1, and BS2 neurons were responsive, respectively. In the post-training phase, the percentages were 55.4% (n = 36/65), 50.2% (n = 146/291), 49.0% (n = 281/574), and 39.1% (n = 97/248), respectively. D Changes in the percentage of responsive units during the stimulus presentation period after training and execution of the WM tasks for each class. E Changes in the percentage of responsive units during the delay period after training and execution of the WM tasks for each class. F Changes in the total percentage of responsive units during either the stimulus presentation or delay period (or both) after training and execution of the WM tasks for each class.
Various physiological cell types show unique physiological characteristics18,19,48. Putative clusters should exhibit distinct physiological properties if they correspond to known physiological cell types. To explore the physiological differences among the putatively identified cell classes, we computed four firing measures during the fixation period: mean firing rate (FR), Fano factor (FF), coefficient of variation of the interspike interval distribution (CV), and local variation (LV). Both FF (variance-to-mean ratio of spike counts across trials) and CV (standard deviation relative to the mean of interspike intervals) provide insights into firing variability or regularity. LV (cross-correlation of consecutive interspike intervals) serves as an indicator of spike train variability or regularity (see Methods for details). Comparing these physiological properties among putative cell classes helps determine their correlation with known physiological cell types.
In this analysis, since the fixation period is similar in spatial and feature WM tasks, we integrated the spatial and feature tasks in both the passive and active phases. All measures showed a significant difference among cell classes in both the pre- and post-training tasks (p < 0.05, Kruskal-Wallis test; Fig. 5). NS1 class demonstrated the greatest baseline activity and firing variability both before and after training (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 5A–C, E–G). Additionally, NS1 showed less local spike train variability in comparison to the other classes (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 5D, H). NS2 displayed intermediate activity levels in both pre- and post-training phases (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 5A, E). Furthermore, the broad-spiking classes (BS1 and BS2) exhibited low baseline activity and high local variation in both tasks (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 5A, D, E, H). These results are consistent with previous studies indicating that narrow-spiking cells exhibit higher activity levels and broad-spiking cells demonstrate lower activity levels in the primate prefrontal cortex25,27,28,29,30,42. Additionally, we observed that as the mean trough-to-peak durations of spike waveforms increased across the four classes, the baseline activity of each class decreased, which aligns with findings from another study conducted during the fixation period45. Therefore, our findings suggest that the physiological characteristics of the putatively identified cell classes are distinct during the fixation period.
A Baseline firing rate (FR) of each cell class for pre-training units. B Baseline Fano factor (FF) of each cell class for pre-training units. C Baseline coefficient of variation of the interspike interval distribution (CV) of each cell class for pre-training units. D Baseline local variation (LV) of each cell class for pre-training units. The number of pre-training units was n = 35, 101, 166, and 123 for NS1, NS2, BS1, and BS2 classes, respectively. E Baseline FR of each cell class for post-training units. F Baseline FF of each cell class for post-training units. G Baseline CV of each cell class for post-training units. H Baseline LV of each cell class for post-training units. All measures were calculated during a 1000 ms baseline fixation period. The number of pre-training units was n = 36, 146, 281, and 97 for NS1, NS2, BS1, and BS2 classes, respectively. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. Statistical significance was assessed using the Kruskal-Wallis test for multiple comparisons (p < 0.05) and the Mann-Whitney U test with FDR adjustment for pairwise comparisons. Dotted, dashed, and solid lines indicate p < 0.05, p < 0.01, and p < 0.005 (Mann-Whitney U test, FDR adjusted), respectively.
Visual stimulus-evoked responses across distinct cell types
We assessed whether there are variations in neuronal response to visual stimuli depending on specific cell classes. The average spike density function (SDF) for each cell class across all trials in both pre- and post-training phases is illustrated in Fig. 6 (left panels). The SDF was computed within the interval of −0.5 to 4 s following the onset of the first stimulus presentation. We analyzed spatial and feature WM tasks separately. The first and second stimulus-evoked responses in each neuron were calculated by subtracting the mean SDF during the fixation period from the maximum peak of the SDF during visual stimulus presentation.
A, B Firing dynamics in the pre-training phase for the spatial and feature tasks, respectively. C, D Firing dynamics in the post-training phase for the spatial and feature tasks, respectively. The left panels display the average spike density function (SDF) of each cell class during the interval from −0.5 s to 4 s relative to the onset of the first stimulus. Gray bars indicate stimulus presentation intervals (0–0.5 s and 2–2.5 s). NS1, NS2, BS1, and BS2 classes are represented in blue, red, yellow, and purple, respectively. The right panels represent the first and second stimulus-evoked responses for each cell class. Stimulus-evoked response refers to the increase in firing rate during stimulus presentation. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. Statistical significance was assessed using the Kruskal-Wallis test for multiple comparisons (n.s. indicates no significant difference) and the Mann-Whitney U test with FDR adjustment for pairwise comparisons. Dotted, dashed, and solid lines indicate p < 0.05, p < 0.01, and p < 0.005, respectively (Mann-Whitney U test, FDR adjusted).
To investigate differences in firing dynamics among various cell types in response to visual stimuli, we conducted comparative analyses of the stimulus-evoked responses of cell classes (Fig. 6, right panels). The statistical analysis revealed significant differences between cell classes in the pre-training phase, specifically in the first stimulus-evoked response during spatial WM, as well as the first and second stimulus-evoked responses during feature WM (p < 0.05, Kruskal-Wallis test; Fig. 6A, B, right panels). In the post-training phase, the Kruskal-Wallis test indicated significant differences in the visual stimulus-evoked response among the four distinct classes across all conditions (p < 0.05; Fig. 6C, D, right panels). Furthermore, pairwise comparisons of responses between class pairs were performed using the Mann-Whitney U test (Fig. 6, right panels). During the pre-training phase, for instance, in the first stimulus-evoked response of the spatial WM task, the BS1 class exhibited a significant difference compared to the NS2 and BS2 classes (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 6A, right panel, 1st stimulus-evoked). In the post-training phase, the number of significant class pairs increased compared to the pre-training task. Specifically, in the first stimulus-evoked response of the spatial WM task, all pairwise class comparisons yielded significant differences (p < 0.05, Mann-Whitney U test, FDR adjusted; Fig. 6C, right panel, 1st stimulus-evoked). Similar to the observed pattern in fixation activity, NS1 generally demonstrated the most transient responses to visual stimuli, NS2 exhibited intermediate transient responses, and BS1 and BS2 displayed less transient responses compared to the narrow classes. It is noteworthy that as the mean trough-to-peak durations of spike waveforms increased from NS1 to BS2 classes, the mean stimulus-evoked response of each class decreased, particularly in the post-training phase. These results are consistent with previous literature indicating that narrow-spiking cells exhibit more modulation in response to stimuli than broad-spiking cells29,40,45,49.
Cell-Class-Specific Changes in Baseline Activity
Changes in baseline firing properties within each putative prefrontal cortex cell class were explored during the execution of WM tasks after training compared to the passive viewing of stimuli phase. Prior studies have reported a slight increase in neuronal activity preceding stimulus presentation in the PFC of monkeys after learning to perform WM tasks14,50. The baseline firing rate was compared between pre- and post-training phases for each cell class (Fig. 7A). Interestingly, the baseline FR of the NS1 class remained unchanged after training compared to the passive viewing phase. In contrast, both the NS2 and BS1 classes exhibited a significantly higher baseline FR after training (p < 0.01, Mann-Whitney U test). Unexpectedly, a significant decrease in baseline FR was observed in the BS2 class after training compared to before (p < 0.005, Mann-Whitney U test).
A Comparison of baseline firing rates between pre-training passive (light color) and post-training active (dark color) phases for each cell class. B Comparison of baseline Fano factor between pre-training and post-training phases for each cell class. C Comparison of baseline coefficient of variation between pre-training and post-training phases for each cell class. D Comparison of baseline local variation between pre-training and post-training phases for each cell class. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.005, respectively (Mann-Whitney U test).
Beyond firing rate changes, the variability of neural activity is also altered during the execution of WM tasks relative to prior to training51. Thus, we analyzed the changes of baseline firing variability in each cell type between pre- and post-training using the Fano factor and coefficient of variation (Fig. 7B, C). While a slight decrease of FF was observed in the NS1 class after training, no significant difference was found (p = 0.13, Mann-Whitney U test; Fig. 7B). Additionally, there was no significant difference in the CV of the NS1 class between pre- and post-training conditions (Fig. 7C). Furthermore, no significant difference was observed in the baseline FR variability of the NS2 and BS1 classes between before and after training (Fig. 7B, C). In contrast to the other classes, the BS2 class exhibited a notable reduction in both FF and CV after training (p < 0.05 for FF, p < 0.005 for CV, Mann-Whitney U test; Fig. 7B, C). The decrease of trial-to-trial variability has been observed during the baseline fixation period after training in PFC51, which we also observed in the BS2 class. Moreover, local variation decreased across all classes during the baseline fixation period after training, but only the BS2 class showed a significant decrease (p < 0.005, Mann-Whitney U test; Fig. 7D). In other words, spike train variability during the baseline period was notably lower after training and performance of the task, particularly in the BS2 class.
These findings underscore distinct patterns of changes in baseline neural activity among cell classes of PFC after training and performance of WM tasks. While NS1 exhibited no notable change following training, NS2 and BS1 showed elevated levels of baseline activity. In contrast, BS2 demonstrated a decrease in both firing rate and firing variability after training.
Cell-class-specific changes in visual response dynamics
Next, our study delved into how the neural response of each cell class to stimulus presentation changed during the execution of WM tasks after training, compared to the passive viewing phase, in both spatial and feature tasks. Prior studies have observed a significant elevation in the percentage of visually responsive neurons within the PFC after learning to perform WM tasks14,15,47. Consistent with these findings, our analysis revealed a higher percentage of NS1, NS2, and BS1 neurons responding to visual stimuli in the post-training phase compared to pre-training, with increases of 18.8%, 8.4%, and 13.3%, respectively (Fig. 4D). However, the BS2 class did not show an elevation in responsive neurons post-training. This observation suggests that specific cell classes of PFC exhibited distinct changes in responsiveness to visual stimuli after training compared to before.
Furthermore, we analyzed the differentiation in visual stimulus-evoked responses of each cell class between the pre- and post-training phases for both spatial and feature tasks (Fig. 8). In the spatial task, the NS1 class exhibited an increase in transient responses to the first and second visual stimuli after training (p < 0.05 for the first stimulus, p < 0.01 for the second stimulus, Mann-Whitney U test; Fig. 8A, B). Moreover, the first and second stimulus-evoked responses were significantly elevated across the NS2 and BS1 classes after training compared to the pre-training phase (p < 0.005, Mann-Whitney U test; Fig. 8A, B). Conversely, BS2 neurons exhibited no significant alterations in the response modulation to the first and second stimuli (Fig. 8A, B). In the feature task, the BS1 class displayed significantly stronger responses to both the first and second stimuli in the post-training phase compared to the pre-training phase (p < 0.05 for the first stimulus, p < 0.005 for the second stimulus, Mann-Whitney U test; Fig. 8C, D). In contrast, the BS2 class exhibited a significant decrease in the response to the first stimulus after training (p < 0.005, Mann-Whitney U test; Fig. 8C), although no significant difference was observed for the second stimulus presentation between pre- and post-training phases (Fig. 8D). Despite the changes observed in the broad-spiking classes, particularly the BS1 class, no discernible differences were noted in the responses of the narrow-spiking classes to stimulus presentation after training and the execution of the feature task (Fig. 8C, D). Another notable observation is the increased distinctiveness of stimulus-evoked responses between the broad-spiking cell classes in the post-training phase compared to the pre-training phase (Fig. 6, right panels). This suggests that patterns of change in the modulation of response dynamics to visual stimuli vary across different cell types after training and execution of WM tasks.
A Comparison of first stimulus-evoked responses between pre-training passive (light color) and post-training active (dark color) phases for each cell class in the spatial task. B Comparison of second stimulus-evoked responses between pre-training and post-training phases for each cell class in the spatial task. C Comparison of first stimulus-evoked responses between pre-training and post-training phases for each cell class in the feature task. D Comparison of second stimulus-evoked responses between pre-training and post-training phases for each cell class in the feature task. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.005, respectively (Mann-Whitney U test).
Cell-class-specific changes in memory dynamics
According to prior studies, it has been established that prefrontal neurons exhibit heightened firing rate and reduced firing variability during the delay period of working memory tasks after training14,15,47,51. These alterations indicate an improved ability to retain information after training that requires active memory maintenance. Our next aim was to investigate the WM-related changes in each cell class after training and performance of the active tasks.
After training, the percentage of activated neurons during the delay period increased in four cell classes, albeit at different rates (Fig. 4D). Specifically, the BS1 class showed the highest increase in responsive neurons during the delay period, with a 19.1% rise. NS1 and NS2 exhibited increases of 13.5% and 15.6% in the percentage of responsive neurons, respectively. BS2 exhibited the lowest elevation in delay period activity among the cell classes, with a 9.9% increase in the percentage of responsive neurons. An elevation in the percentage of responsive neurons during the delay epoch in the monkey PFC after learning to perform WM tasks has been observed in previous studies14,47. In this study, we found that the increase in the percentage of neurons with delay period activity varies across distinct cell types. Notably, the overall increase in the proportion of responsive neurons during either the stimulus presentation or delay period (or both) also differed across cell classes (Fig. 4F), following the order: BS1 (18.0%), NS1 (13.7%), NS2 (13.2%), and BS2 (7.6%).
The firing properties of each cell class during the first delay epoch were compared before and after training for spatial and feature tasks (Fig. 9). Notably, in our analysis of WM-related changes in firing rate, we normalized the firing rate of each neuron during the delay period. Specifically, we subtracted the firing rate during the fixation period from that during the delay period for each neuron. This measure reflects increased activity during the delay epoch over baseline activity, which indicates active memory maintenance during the execution of the WM tasks.
A Comparison of firing rate during the first delay period between pre-training passive (light color) and post-training active (dark color) phases for each cell class in the spatial task. B Comparison of Fano factor during the delay period between pre-training and post-training phases for each cell class in the spatial task. C Comparison of coefficient of variation during the delay period between pre-training and post-training phases for each cell class in the spatial task. D Comparison of local variation during the delay period between pre-training and post-training phases for each cell class in the spatial task. E Comparison of firing rate during the delay period between pre-training and post-training phases for each cell class in the feature task. F Comparison of Fano factor during the delay period between pre-training and post-training phases for each cell class in the feature task. G Comparison of coefficient of variation during the delay period between pre-training and post-training phases for each cell class in the feature task. H Comparison of local variation during the delay period between pre-training and post-training phases for each cell class in the feature task. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.005, respectively (Mann-Whitney U test).
In the spatial task, a significant increase in firing rate during the delay period was observed in the BS1 class after training compared to the passive viewing phase (p < 0.005, Mann-Whitney U test; Fig. 9A). The BS2 class showed a significant reduction in the coefficient of variation after training compared to before training (p < 0.01, Mann-Whitney U test; Fig. 9C), but there were no significant changes in FR, FF, and LV (Fig. 9A, B, D). Moreover, there was a significant increase in the firing rate of the NS2 class during the delay epoch after training (p < 0.01, Mann-Whitney U test; Fig. 9A). Also, the NS2 class exhibited a reduction in local spike train variability after training (p < 0.05, Mann-Whitney U test; Fig. 9D), implying an increase in the regularity of spike trains during the memory maintenance period of the active task. In contrast, firing measures during the delay period remained insignificantly different for the NS1 class in the post-training compared to the pre-training phase (Fig. 9A–D).
In the feature task, the firing rate of the BS1 class significantly increased during the delay epoch, akin to the findings observed in the spatial task after training, although the level of significance was lower compared to the spatial task (p < 0.05, Mann-Whitney U test; Fig. 9E). The CV and LV of the BS2 class significantly declined during the delay period, indicating an increase in spike train regularity, similar to the fixation period (p < 0.005 for both CV and LV, Mann-Whitney U test; Fig. 9G, H). The narrow-spiking cell classes had no statistically significant variations in firing metrics after training and the performance of the feature task (Fig. 9E–H).
We found that cell-class-specific changes in firing properties during the delay period of the active phase after training, relative to the passive phase, vary depending on whether the task involves maintaining stimulus location or shape information, emphasizing distinct patterns across cell classes. Specifically, the BS1 class demonstrated an increase in delay activity, and the BS2 class exhibited a decrease in firing variability during the delay epoch across both feature and spatial tasks. The NS2 class showed increased delay activity and spike train regularity after training and execution of the spatial task, but no significant changes were observed in the feature task.
Cell-class-specific neural response and behavioral performance
We analyzed neural activity under varying performance conditions during active WM tasks. It has been previously demonstrated that neuronal firing rates increase as behavioral performance improves in this paradigm14. Specifically, we examined whether the mean firing rate of the second delay period in each cell class varied between sessions with high and low choice accuracy.
In the spatial WM task, we compared the mean firing rates of each cell class between high and low choice accuracy sessions (Fig. 10A). NS2 neurons exhibited significantly higher firing rates in high choice accuracy conditions than in low choice accuracy conditions (p < 0.05, Mann-Whitney U test). Similarly, BS1 neurons demonstrated a strong elevation in firing rate during high choice accuracy conditions (p < 0.005, Mann-Whitney U test). However, no significant differences in firing rates were observed for NS1 and BS2 neurons between high and low choice accuracy conditions in the spatial task. In the feature WM task, we also compared the mean firing rates of each cell class between high and low choice accuracy sessions (Fig. 10B). Only BS1 neurons exhibited significantly greater firing rates in high choice accuracy conditions (p < 0.05, Mann-Whitney U test). In contrast, NS1, NS2, and BS2 neurons showed no significant differences in firing rates between high and low choice accuracy conditions.
A Comparison of firing rates during the second delay period between low choice accuracy (light color) and high choice accuracy (dark color) sessions for each cell class in the spatial WM task. The number of units with low and high choice accuracy was n = 16 and 19 for NS1, n = 69 and 77 for NS2, n = 147 and 134 for BS1, and n = 48 and 49 for BS2, respectively. B Comparison of firing rates during the second delay period between low choice accuracy (light color) and high choice accuracy (dark color) sessions for each cell class in the feature WM task. The number of units with low and high choice accuracy was n = 18 and 17 for NS1, n = 52 and 94 for NS2, n = 122 and 159 for BS1, and n = 42 and 55 for BS2, respectively. Each box plot shows the median (horizontal line), interquartile range (box), and whiskers extending to 1.5 times the interquartile range. Gray dots represent individual data points for each unit, and larger black dots indicate group means. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.005, respectively (Mann-Whitney U test).
These findings reveal that different cell classes exhibit distinct neural modulation based on behavioral performance. Specifically, different levels of choice accuracy in sessions were associated with changes in the neural responses of BS1 and NS2 neurons during the judgment of whether the spatial location of stimuli matched. Additionally, BS1 neurons exhibited significant changes in neural responses related to choice accuracy during the judgment of whether the shape of stimuli matched.
Discussion
It has recently come to light that learning and executing working memory tasks can lead to changes in prefrontal cortex activity13,47,52. We explored how response modulation of extracellular spike waveform-based cell classes within the monkey prefrontal cortex differs between active task execution after working memory training and the passive viewing phase. Through unsupervised clustering analysis based on specific characteristics of the spike waveform, four distinct cell classes—NS1, NS2, BS1, and BS2—were dissociated. Robust differences were found in both pre- and post-training phases among four putative identified cell types, encompassing distinct percentages of responsive units and baseline physiological properties, as well as response dynamics to visual stimuli. Hence, it is probable that the identified cell classes correspond to distinct physiological cell types. Our main finding reveals heterogeneous levels of neural changes across PFC cell classes after training and task execution. The observed alterations in baseline firing properties, visual responses, and memory maintenance dynamics underscore the diverse functional roles of putative cell classes in processing and executing working memory tasks.
Physiological insights into identified cell classes
Electrophysiological studies have demonstrated that broad-spiking neurons are associated with excitatory pyramidal cells, while narrow-spiking neurons are linked to inhibitory interneurons53,54,55. According to our findings, the prefrontal cortex contains 28.98% narrow-spiking neurons (putatively inhibitory) and 71.02% broad-spiking neurons (putatively excitatory). These proportions are consistent with anatomical investigations suggesting that approximately 70–80% of prefrontal neurons are excitatory, with inhibitory comprising the remaining 20–30%56. A pivotal aspect of this study involves the identification of two specific subtypes of narrow-spiking cells (NS1 and NS2), distinguished not only by distinct waveform features but also by differing physiological characteristics. NS1, featuring the thinnest action potential waveform, demonstrates elevated neural activity, whereas NS2, with an intermediate waveform duration overlapping with broad spiking, exhibits activity levels lower than NS1 but higher than broad-spiking neurons. Furthermore, these narrow-spiking subtypes exhibit differential neural responses during working memory execution after training, suggesting distinct functional roles within the monkey PFC. Thus, the evidence supports the hypothesis that NS1 and NS2 classes likely contain varying concentrations of distinct GABAergic interneuron subtypes. Previous extracellular studies, in alignment with our outcome, have reported various subtypes of narrow-spiking cells based on spike waveform in cortical regions including the ventral striatum57, frontal eye field58, and premotor cortex46. Major subtypes of GABAergic inhibitory interneurons in the cortex include parvalbumin-expressing (PV+) and somatostatin-expressing (SST+) neurons59. PV+ cells are typically characterized as fast-spiking neurons with narrow waveform action potentials and high firing rates. In contrast, SST+ neurons, another subtype of GABAergic cells, are identified as non-fast-spiking. Compared to excitatory pyramidal cells, the action potential duration of SST+ neurons is narrower but wider than that observed in fast-spiking cells60,61,62, although the spike waveform features of SST+ neurons are generally broader and show overlap with the spike waveform characteristics of other neuron classes63. Furthermore, SST+ neurons reveal lower levels of spontaneous and evoked firing activity relative to PV+ cells64. The SST+ cell class encompasses various subtypes, including regular-spiking non-pyramidal cells, low-threshold spiking cells, and bursting cells60,65,66. The NS1 class, characterized by a high baseline firing rate, robust stimulus-evoked response, and minimal bursty activity (low LV), shares some similarities with GABAergic PV+ fast-spiking interneurons. Most NS2 neurons, displaying an intermediate baseline firing rate, regular firing patterns (low FF and CV), and bursty activity (high LV), as well as lower stimulus-evoked responses than NS1 neurons, show some similarities to GABAergic SST+ inhibitory interneurons. Based on this evidence, it is probable that NS1 and NS2 classes contain a higher concentration of PV+ and SST+ cells, respectively. The findings suggest that specific characteristics of spike waveforms contribute to the detection of distinct subtypes of inhibitory interneurons. However, recent extracellular studies in the mouse cortex, utilizing optogenetic labeling of neuron subtypes, have shown that while waveform-based unsupervised clustering methods offer valuable information on cell classes, they may not fully capture the boundaries between PV+ and SST+ interneurons63,67,68.
Furthermore, two distinct subtypes of broad-spiking neurons with relatively different physiological characteristics were identified in our analysis. It is crucial to emphasize the functional differences between the two subtypes of broad-spiking neurons, as evidenced by their differential mechanisms after training and performance of WM tasks. Both subtypes show lower neural activity levels compared to narrow-spiking neurons and manifest highly regular firing patterns (low FF and CV). Consequently, it can be inferred that both BS1 and BS2 likely correspond to excitatory pyramidal neurons, which constitute the majority of neurons in the cortex. Recent studies employing high-density extracellular recording probes have confirmed the existence of two broad-spiking cell classes based on spike waveform features69,70. Notably, in the BS2 class, there is an initial positive spike before the trough, a characteristic commonly observed in neurons with triphasic waveforms46,71,72. While triphasic neurons have been identified as corticospinal excitatory pyramidal cells73, it remains uncertain whether the BS2 class corresponds definitively to this cell type. This uncertainty arises from previous studies indicating that the majority of corticospinal excitatory pyramidal cells typically exhibit a narrow waveform74.
Despite these interpretations, assigning narrow-spiking neurons to inhibitory interneurons and broad-spiking neurons to excitatory pyramidal cells may be associated with some inaccuracies. For example, it has been reported that vasoactive intestinal peptide-expressing interneurons (VIP) display low-firing activity and broad-spiking waveforms75,76. This observation was supported by an extracellular study in the macaque prefrontal cortex, which identified a subtype of broad-spiking neurons exhibiting similar properties to VIP neurons42. Additionally, a subtype of pyramidal cells in the monkey motor and premotor cortex exhibited narrow-spiking waveforms, according to recent studies74,77. An extracellular study has substantiated the presence of a narrow-spiking class displaying physiological characteristics consistent with pyramidal neurons in the macaque premotor cortex46. Some in vivo studies in mice have proposed approaches that combine multiple electrophysiological features (spike waveform, interspike interval distribution, and peri-stimulus time histogram) to reliably identify cell types. These methods outperform approaches relying solely on spike waveforms and are validated using ground truth data from optogenetic tagging63,67. Therefore, while waveform features offer valuable insights into neuronal function, caution is warranted when generalizing these findings to categorize neuronal subtypes.
Cell-class-specific changes in neural response after training: passive vs. active phases
Learning and performing working memory tasks affect the neuronal circuit and response modulation of the prefrontal cortex47. These effects in response modulation can manifest in various forms, such as the activation of a greater number of neurons, increased firing rate during the delay period, and shifts in baseline firing rate, firing variability, and overall neural dynamics13. Several neurophysiological studies have investigated differences in PFC neural dynamics between naive monkeys, which only viewed stimuli passively without knowledge of the task rules, and the same monkeys after being trained in the rules and execution of working memory tasks14,15,47. These studies have demonstrated that a greater percentage of PFC neurons exhibit activation during both stimulus presentation and delay periods after training. An increase in the firing rate of responsive neurons has been observed across different task periods, particularly during the delay period. Furthermore, the variability of firing rates decreases during various periods of spatial WM tasks compared to pre-training passive conditions51.
We investigated the impact of training and performing the WM tasks on two subtypes of narrow-spiking (NS1 and NS2) and two subtypes of broad-spiking (BS1 and BS2) neurons, identified based on their extracellular spike waveforms. Our findings revealed varying levels of cell-class-specific changes in PFC response modulation during the execution of WM tasks (spatial and feature) compared to the passive condition with identical stimuli before learning task rules. The BS1 subtype of broad-spiking neurons had the most significant changes. These changes encompassed the greatest rise in the percentage of neurons activated, an elevated baseline firing rate, stronger stimulus-evoked responses, and increased WM-related firing rates in both spatial and feature tasks. Contrary to expectations, there was no rise in the proportion of BS2 neurons responding to the stimulus after training. Additionally, the baseline firing rate decreased, contrary to a previous study that reported increases in firing rate during baseline fixation and delay periods for PFC neurons with broad waveforms (putative pyramidal cells)14. However, BS2 neurons showed a decrease in spike train variability during both baseline and delay periods of active tasks compared to passive, consistent with a prior study51. It has been found that decreased variability enhances the encoding of information in neuronal firing, thereby optimizing the efficiency of neural coding78. These results confirm that the two subtypes of broad-spiking neurons, both putatively pyramidal cells, exhibit different behaviors after training and performing the WM task.
The NS1 class, characterized by fast spiking, exhibited the highest increase in the percentage of neurons responsive to stimulus presentation but showed no changes in firing properties during baseline and delay periods after training. However, stimulus-evoked responses rose relative to passive viewing, specifically in the spatial WM task. This result aligns with a neurophysiological study indicating that the firing rate of neurons with narrow waveforms (putative inhibitory interneurons) is elevated during stimulus presentation in the spatial WM task14. A different pattern was observed in the NS2 class with increased baseline firing rates after training. Additionally, the NS2 neurons displayed elevated WM-related firing rates, stimulus-evoked responses, and spike train regularity in the spatial task. These findings indicate that, unlike a previous study which reported no changes in the firing rates of putative inhibitory interneurons during baseline and delay periods, the NS2 subtype shows increased firing rates during these periods14. Therefore, the evidence underscores distinct cell-class-specific modulation in the prefrontal cortex after learning and execution of working memory tasks.
Beyond changes in firing rates and variability, the selectivity of prefrontal neurons to spatial and feature information represents a critical aspect of working memory that can undergo significant changes after WM training15. A prior study demonstrated that broad-spiking neurons (putative pyramidal cells) in PFC exhibited a significant reduction in spatial selectivity after WM training in PFC while narrow-spiking neurons (putative interneurons) exhibited no changes14. This finding highlights the possibility that different cell classes contribute uniquely to spatial tuning, a hypothesis that could be further explored in future work. Moreover, future studies could explore how selectivity to spatial and feature information varies across distinct cell classes (beyond narrow- and broad-spiking classes) before and after WM training.
Effects of learning and task execution
The differences in neural responses between the pre-training passive viewing phase and the post-training active task execution phase can arise from the cognitive demands of task execution and the potential long-term effects of training. The behavioral state after training differs fundamentally from the pre-training phase, not only due to the acquisition of task rules but also due to the necessity for active engagement in stimulus processing, WM maintenance, and decision-making. Prior studies have demonstrated that task execution itself modulates PFC neuronal activity in response to task demands79,80. For instance, during the stimulus presentation period in the active phase, the monkey must actively attend to the stimulus to encode task-relevant information, whereas this requirement is absent during passive viewing. The observed increase in visual stimulus-evoked responses of certain cell classes (particularly BS1 neurons in both spatial and feature tasks) may be linked to these cognitive demands. This aligns with previous findings demonstrating that PFC activity is enhanced in response to increased attentional demands81. Additionally, some cell classes (particularly broad-spiking neurons) exhibited changed activity during the delay period after training, which may indicate their enhanced role in maintaining task-relevant information. This aligns with evidence that persistent activity in PFC neurons underlies WM retention5,10,82. However, while these changes could be attributed to the demands of active task performance, the possibility of training-induced changes in neural dynamics cannot be ruled out. Chronic recordings in a prior study have shown that training in a WM task can induce long-lasting changes in neuronal firing patterns in the monkey PFC47. The researchers have reported increased neuronal responsiveness and elevated firing rates as training progressed. Thus, these findings suggest that training and task execution lead to changes in the prefrontal cortical circuitry.
It is important to acknowledge that behavioral factors such as eye movements, motor preparation, and arousal could influence neuronal activity. However, these factors alone are unlikely to fully account for the neural changes observed across different cell classes14. Future studies could more directly investigate learning-related changes by comparing neural activity during early versus late training phases rather than contrasting passive viewing with active WM task execution. Additionally, analyzing activity during a passive viewing condition after training, alongside WM task execution, could help isolate the impact of task performance. Such approaches would provide deeper insights into how learning and task execution independently modulate neural circuits in the PFC.
Functional roles of prefrontal putative pyramidal neurons in working memory
The involvement of PFC broad-spiking neurons, putative pyramidal cells, in the spatial WM task has been previously recognized28,83. Research has indicated that putative pyramidal cells exhibit a working memory-related increase in neural activity and a decrease in firing rate variability after monkeys were trained to perform spatial WM tasks14,51. The role of dopamine in supporting WM is partly mediated by its preferential excitatory effect on broad-spiking neurons26,84,85. Experimental evidence from optogenetic studies in mice has further demonstrated that prefrontal pyramidal neurons play a crucial role in spatial WM, not only in the maintenance phase but also in encoding and decision-making processes86. These findings suggest a fundamental role for prefrontal pyramidal neurons across all phases of spatial WM processing. In agreement with these findings, our results reveal that both BS1 and BS2 subtypes contribute to spatial WM processing, albeit through distinct but complementary mechanisms. BS1 neurons showed increased neural activity across all phases of the spatial WM task, particularly during the delay period, an effect that may indicate a key role in maintaining spatial information in WM5,10. However, BS2 neurons exhibited reduced firing variability during the delay period compared to the passive phase, a phenomenon that has been proposed to potentially optimize neural coding efficiency and enhance spatial information representation in cortical circuits51,87. Moreover, we found that the firing rate of BS1 neurons during the second delay period was positively correlated with behavioral performance, indicating their likely role in the retrieval and decision-making of task-relevant spatial information. This supports prior evidence that pyramidal neurons are crucial not only for maintaining WM representations but also for retrieving stored information to guide behavior86. Beyond spatial WM, we observed a similar functional role of broad-spiking neurons in feature WM tasks. The involvement of these neurons in both spatial and feature tasks suggests a generalized role for putative pyramidal neurons in processing and retaining task-relevant information. These findings support the hypothesis that pyramidal neurons contribute to all phases of WM (encoding, maintenance, and retrieval) and highlight their essential function in PFC circuits for both spatial and non-spatial information processing.
Task-dependent differences of narrow-spiking neurons: spatial vs. feature tasks
The prefrontal cortex is implicated in advanced cognitive processes47,88. There is some evidence that the PFC elicits distinct patterns of neural activity in response to various tasks, suggesting task-dependent differences in processing and functioning79,89. Recent research has revealed task-dependent differences in the processing patterns of the monkey PFC for feature and spatial working memory during a matching-rule decision-making task90. Furthermore, an electrophysiological study has reported that narrow-spiking neurons in the PFC show task-dependent activity differences in prosaccade and antisaccade tasks30. Our study extends these findings by demonstrating that narrow-spiking neurons, putative inhibitory interneurons, within the monkey PFC exhibit task-specific modulation in response to the cognitive demands of processing spatial and feature-based WM tasks. Specifically, narrow-spiking neurons exhibited raised stimulus-evoked responses in the spatial WM task after training, but no significant modulation was observed in the feature task. The observed changes in neural responses of narrow-spiking neurons during stimulus presentation in the spatial task may reflect their involvement in spatial attention and encoding processes, which are crucial for maintaining stimulus location in working memory. This interpretation supports prior research suggesting that some inhibitory interneurons play a critical role in spatial working memory encoding during the sample phase91. However, our results indicate that similar processing is not required for the feature WM task.
There is further evidence that task-dependent modulation in the NS2 class includes changes beyond those observed in stimulus-evoked responses. A rise in the firing rate and spike train regularity of NS2 neurons was observed during the delay period of the spatial WM task compared with the spatial passive task, but this effect was not replicated in the feature task. These differences in delay responses during the spatial task likely reflect their role in maintaining location-specific information and enhancing neural coding10,51. Additionally, NS2 neurons exhibited elevated firing rates during sessions associated with high behavioral performance in the spatial WM task, further supporting the notion that their functional mechanism is tuned to processing location-specific information. Consistent with our findings, narrow-spiking neurons have been previously shown to play a crucial role in spatial working memory by exhibiting sustained activity during the delay period, with their firing rates predicting behavioral performance83. However, these effects were specific to NS2 neurons, while NS1 neurons played a lesser role in maintaining spatial information and influencing behavior. This is in line with an optogenetic study reporting that a subtype of inhibitory interneurons (SST+) is essential for WM maintenance, with their activity correlating with task performance, whereas PV+ interneurons have a more limited role in this process92. Furthermore, the functional roles of these neurons are dependent on the specific task and brain region91. Our results extend this understanding by demonstrating that, unlike in the spatial task, narrow-spiking neurons did not exhibit similar modulation during the feature WM task. This suggests that increased demands for shape-based encoding and maintenance do not necessarily engage NS2 neurons in the same manner as spatial WM.
In conclusion, our study elucidates the task-dependent neural response of narrow-spiking neurons within the prefrontal cortex of monkeys. The observed changes in neural activity patterns, particularly in response to spatial versus feature tasks, underscore the critical role of narrow-spiking neurons in maintaining and retrieving spatial information. These insights contribute to our understanding of the functional heterogeneity of inhibitory interneurons and their specialized roles in cognitive processes.
Methods
Neural signals were recorded from the PFC of three male rhesus monkeys (Macaca mulatta) before and after training to perform working memory tasks. Specifically, recordings were obtained from areas 8, 9, 12, 45, and 46 of the lateral PFC. These monkeys were between 5 and 9 years old, weighed 5–12 kg, and had no prior experience in experimental settings. We have complied with all relevant ethical regulations for animal use. Animals were either held single-housed or pair-housed in communal rooms, where they had sensory interactions with other monkeys. Animal experimental procedures were carried out according to the guidelines set forth by the National Institutes of Health and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee. The experimental paradigm, including surgery and neurophysiology procedures, experimental setup, anatomical localization, and task details, has been discussed previously14,15. We presented a brief overview of the essential information for this study.
Neurophysiology
A 20 mm recording cylinder was surgically implanted over the PFC of the monkeys. Anatomical magnetic resonance imaging (MRI) was utilized to assess the position of the cylinders. Extracellular neural activity was recorded utilizing arrays of multiple epoxylite- or glass-coated tungsten microelectrodes (up to 8), spaced 0.2–1.5 mm apart. A microdrive system (EPS drive, Alpha-Omega Engineering, Nazareth, Israel) advanced arrays into the PFC through the dura. The recorded neural signals were amplified and passed through a band-pass filter with a frequency range of 0.5–8 kHz and recorded using the APM modular data acquisition system (FHC, Bowdoin, ME). Spike waveforms were detected using a threshold method, sampled at a 25 μs resolution, and digitized. The KlustaKwik algorithm was then utilized to sort the spike waveforms via an automated cluster analysis technique93. The algorithm applies principal component analysis to waveform features to generate clusters of units with similar waveforms. All clusters were manually inspected by the investigators before being accepted as single units to ensure high-quality spike sorting. To further confirm the exclusion of multiunit recordings, we examined the interspike interval distribution for each neuron and verified the presence of a well-defined refractory period, as used by Mitchell et al.40.
Behavioral tasks
Data recordings were conducted in two phases: pre-training (passive viewing; Fig. 1A, B, first five frames) and post-training (active task execution; Fig. 1A, B). Both phases involved two distinct tasks based on the type of stimulus: spatial and feature tasks. In the spatial task, the stimuli consisted of a white 2° square, randomly displayed at one of nine possible locations arranged in a 3 × 3 grid (Fig. 1C). In the feature task, the stimuli were one of eight distinct white shapes—square, triangle, circle, diamond, plus sign, H, number sign (#), or inverted Y (Fig. 1C). During the pre-training passive phase, before learning the WM tasks, the monkeys were only required to fixate their gaze on the center of the screen while visual stimuli were passively presented (Fig. 1A, B; first five frames). Each trial commenced with a 1 s baseline fixation period, where only the fixation point was shown. The first stimulus was then presented for 0.5 s, followed by a 1.5 s delay period (first delay) with the fixation point displayed. Subsequently, the second stimulus was presented for 0.5 s, followed by an additional 1.5 s delay period (second delay). In the spatial task, the second stimulus was presented either at the same location as the first stimulus or at a different location, typically diametric (Fig. 1A, first five frames). In the feature task, the second stimulus appeared at the same location but was either identical to or a non-matching shape, with each shape paired with a specific non-matching shape (Fig. 1B, first five frames). If the monkeys maintained their gaze on the fixation target until the trial ended, they were rewarded with fruit juice. Importantly, this phase involved only passive viewing, meaning that the monkeys were not required to make judgments or responses and thus were not actively engaged in working memory or decision-making processes.
After completing this phase, the same monkeys underwent training to perform working memory tasks that required them to retain information about spatial locations or shape features and make decisions based on whether the stimuli matched or not. Once they had fully learned the task rules, the post-training phase commenced, during which they actively executed the WM tasks. The trial structure, including stimulus timing and sequence, remained identical to the passive phase, with the addition of a choice target stage following the second delay period. At this point, two choice targets (green and blue squares) appeared on the screen (Fig. 1A, B), and the monkeys were required to make a saccadic eye movement to indicate their decision. The positions of the choice targets were randomized across trials to ensure that motor planning did not interfere with the mnemonic component15. In the spatial WM task, if the spatial location of the first and second stimuli matched, the monkeys needed to saccade to the green choice target; if they did not match, the monkeys had to saccade to the blue choice target (Fig. 1A). In the feature WM task, if the shapes of the first and second stimuli matched, the monkeys needed to saccade to the green choice target; if they did not match, the monkeys had to saccade to the blue choice target (Fig. 1B). The animals received a reward for selecting the correct choice. This phase required monkeys to actively compare stimuli, maintain information in working memory, and execute a decision, engaging cognitive processes absent in the passive phase.
Data analysis
Waveform features extraction
For each single unit, we first normalized all aligned waveforms using min-max normalization, followed by centering. Next, we smoothed and computed the average of these processed waveforms for every neuron. Then, we extracted three specific features from the average waveform of each cell: trough-to-peak duration, first peak-to-trough ratio, and peak ratio (Fig. 2A). Trough-to-peak duration represents the temporal interval between the global minimum (trough) and the subsequent local maximum. First peak-to-trough ratio denotes the ratio between the amplitude of the first peak (local maximum preceding the trough) and the amplitude of the trough. Peak ratio indicates the ratio between the amplitude of the second peak (local maximum after the trough) and the amplitude of the first peak. Analyzing spike waveform characteristics beyond the trough-to-peak duration allowed us to identify more subtypes of narrow- and broad-spiking neurons.
Waveform clustering
To determine cell classes within pre-training units, we performed an unsupervised clustering analysis. Since feature scaling in machine learning guarantees that each feature contributes equally to the estimate of the model parameters, we scaled the extracted features from pre-training units via z-score normalization. The three-dimensional normalized features of spike waveforms were then clustered using a Gaussian mixture model (GMM) (Fig. 2C). The GMM is a robust unsupervised clustering technique that uses a probabilistic approach to comprehend data structure. It supposes that the data is a sum of multiple Gaussian distributions, where each Gaussian component depicts a separate cluster, as follows:
where \(P\left(X\right)\) represents the probability density function, \({\pi }_{k}\) represents the mixing coefficients of the k-th Gaussian component, and \({{\mathcal{N}}}\left(X|{\mu }_{k},\,{\Sigma }_{k}\right)\) represents the k-th Gaussian component with mean \({\mu }_{k}\) and covariance matrix \({\Sigma }_{k}\). The expectation-maximization (EM) algorithm iteratively estimates the parameters of the GMM by maximizing the likelihood of observed data. In the expectation step (E-step), the algorithm calculates the probability of each data point belonging to every cluster based on the current parameters. In the maximization step (M-step), the model parameters are updated to maximize the likelihood of the data, utilizing the probabilities computed in the previous step. These two steps are iterated until the parameters converge. We utilized the “fitgmdist” function in MATLAB with the diagonal covariance matrix. After fitting the model, each neuron from the pre-training phase was assigned to the cluster with the highest probability.
Next, to determine the cluster for each post-training unit, we first normalized the extracted features using the mean and standard deviation of the features from pre-training units. We then applied the fitted model from the pre-training units to cluster the post-training data (Fig. 3A). This approach allowed us to verify whether the same cell classes could be obtained with this clustering model. If the waveform of the cell classes is consistent across both phases, it can be concluded that the model is robust across different datasets.
Optimal number of cell classes
We used the Bayesian information criterion (BIC) to select the optimal number of cell classes (Fig. 2B), defined as follows:
where \(P\left(X|\theta \right)\) is the maximum likelihood, K is the number of parameters, and n is the number of data points. In the BIC cost function, the first term \(-2\,{{\mathrm{ln}}}\,P\left(X|\theta \right)\) indicates how well the model fits the data. The second term \(K{\mathrm{ln}}\left(n\right)\) penalizes models with many numbers of parameters, preventing overfitting. A lower BIC value indicates a better trade-off between model fit and complexity. Therefore, we computed the BIC for different cluster numbers (ranging from 2 to 10 clusters) and selected the cluster number with the minimum BIC value.
Validation of cluster separation
To validate the separation between clusters, we employed a Monte Carlo-based approach similar to that used by Trainito et al.45. First, 10,000 synthetic data points were generated from the fitted GMM distributions. Each data point was then assigned to a cluster according to the GMM’s probabilistic assignment. A confusion matrix was constructed to compare the assigned cluster labels with the true cluster labels (Fig. 2F). The degree of separation and overlap between clusters (GMM components) was quantified using the diagonal and off-diagonal elements of the confusion matrix, respectively.
Analysis of cell-class-specific spike train statistics
To characterize cell-class-specific neural profiles, we analyzed spike train statistics, including mean firing rate (FR), Fano factor (FF), coefficient of variation (CV), and local variation (LV). The mean firing rate reflects neural activity and is computed by averaging the number of spikes per second across all trials for each single neuron. The Fano factor is obtained by dividing the variance of spike counts by the mean of spike counts across trials. The coefficient of variation of the interspike interval distribution is the standard deviation relative to the mean of the interspike interval. The local variation is defined as the proportionality of the squared difference between consecutive interspike intervals to their sum94. FF and CV are metrics that provide in-depth insights into various aspects of firing variability, where low FF and CV values indicate higher regularity and high values denote increased variability (irregularity). CV reflects the global variability of the entire sequence of interspike intervals and is particularly sensitive to firing rate fluctuations94. In contrast, LV provides a measure of the local regularity of spike trains independent of the overall firing rate94,95. Low LV values indicate regular spike trains, whereas high LV values suggest high spike train variability. It has been demonstrated that elevated LV values are associated with bursty activity in neuronal firing patterns96.
First, we computed the firing rate for each neuron across all task intervals. Responsive neurons were identified as those that exhibited a significant increase in firing rate during the stimulus (location or shape) or delay period compared to the 1 s fixation period (paired t-test, p < 0.05)14,15. We analyzed the percentage of responsive neurons for each cell class in both the pre- and post-training phases. Subsequent analyses were conducted exclusively on these responsive neurons.
The four firing measures were examined during the baseline fixation period to understand the physiological traits of cell classes. Given the similarity of the fixation interval in both spatial and feature tasks, we combined the baseline firing metrics from both tasks. The firing metrics were compared among the different cell classes in both pre- and post-training phases. Since there was no normal distribution for all measures (evaluated with a Kolmogorov-Smirnov test), we employed the Kruskal-Wallis test for comparisons among the four cell types for each firing measure (p < 0.05). Furthermore, pairwise comparisons of cell class differences were conducted using the Mann-Whitney U test. To correct for multiple comparisons, all statistical analyses were adjusted using the false discovery rate (FDR) correction method.
Analysis of cell-class-specific neuronal response dynamic
For a more comprehensive analysis of the neuronal response dynamics to visual stimuli in each class, we initially examined the spike density function (SDF) within a time window of −0.5 to 4 s for spatial and feature tasks. The SDF for each responsive neuron was computed by convolving the spike train with a Gaussian kernel (σ = 20) and averaging the results across all trials. To quantify the neural response to visual stimuli (spatial or feature), we computed the stimulus-evoked response for each unit. The evoked responses to the first and second stimuli were calculated by subtracting the mean fixation SDF from the peak SDF during the presentation periods of the first and second stimuli, respectively. The differences in stimulus-evoked responses among the cell classes were statistically evaluated using the Kruskal-Wallis test (p < 0.05). The Mann-Whitney U test, with FDR correction, was utilized for pairwise comparisons between cell classes.
Comparison analysis between pre-training and post-training phases
An essential aspect of this study involved exploring how neuronal activity changes across different cell classes after training and performance of WM tasks. To achieve this, we compared various measures, including baseline firing rates and stimulus-evoked responses, between the pre-training and post-training phases for each cell class during both spatial and feature tasks. These comparisons were conducted using the Mann-Whitney U test to assess statistical significance. Additionally, we computed four firing metrics during the first delay period to assess changes in WM-related responses following learning and task execution. Given that an increase in firing rate during the delay period relative to the baseline firing rate is often associated with active memory maintenance97, we calculated the difference between the delay firing rate and the baseline firing rate.
Analysis of neural responses in relation to behavioral performance
To investigate the relationship between neuronal firing rates and behavioral performance across different cell classes, we compared neural activity between sessions with high and low choice accuracy. Choice accuracy was defined as the proportion of correct trials within a session. Sessions with above-mean accuracy were classified as high choice accuracy, while those below the mean were classified as low choice accuracy. Each neuron was categorized according to the choice accuracy classification of its recording session. Importantly, this analysis focused on the second delay period of the active task, a critical phase for WM maintenance and the judgment of whether the stimuli constituted a match or non-match. For each cell class, mean firing rates were computed separately for neurons recorded in high and low choice accuracy sessions. Statistical comparisons of firing rates between these conditions were performed using the Mann-Whitney U test. This analysis was conducted separately for the spatial and feature WM tasks to determine whether neural modulation differed as a function of behavioral performance.
Statistics and reproducibility
In this study, all statistical analyses were carried out in MATLAB R2022a (MathWorks, Natick, MA). The normality of each measurement was first assessed using the Kolmogorov–Smirnov test. For comparisons across multiple groups, we applied the non-parametric Kruskal–Wallis test, followed by pairwise Mann–Whitney U tests with FDR correction for multiple comparisons. The Mann–Whitney U test was also used for independent comparisons between two conditions (e.g., pre- vs. post-training). Data are visualized using box plots that display the median (horizontal line), interquartile range (box), whiskers extending to 1.5 times the interquartile range, individual data points (gray dots), and group means (large black dots). In pairwise comparisons among cell classes, significance is denoted by dotted (p < 0.05), dashed (p < 0.01), and solid (p < 0.005) lines (Mann–Whitney U test, FDR-adjusted). In comparisons between two conditions, significance is denoted by * (p < 0.05), ** (p < 0.01), and *** (p < 0.005) (Mann–Whitney U test).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are not publicly available but can be shared by the lead contact upon request. The source data underlying all graphs presented in the main figures have been provided in Supplementary Data 1.
References
Baddeley, A. Working memory. Science 255, 556–559 (1992).
Gathercole, S. E., Brown, L. & Pickering, S. J. Working memory assessments at school entry as longitudinal predictors of National Curriculum attainment levels. Educ. Child Psychol. 20, 109–122 (2003).
Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 63, 1–29 (2012).
Jaffe, R. J. & Constantinidis, C. Working memory: from neural activity to the sentient mind. Compr. Physiol. 11, 1–41 (2021).
Riley, M. R. & Constantinidis, C. Role of prefrontal persistent activity in working memory. Front. Syst. Neurosci. 9, 181 (2016).
Wilson, F. A., Scalaidhe, S. P. & Goldman-Rakic, P. S. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260, 1955–1958 (1993).
Fuster, J. M. & Alexander, G. E. Neuron activity related to short-term memory. Science 173, 652–654 (1971).
Szczepanski, S. M. & Knight, R. T. Insights into human behavior from lesions to the prefrontal cortex. Neuron 83, 1002–1018 (2014).
Lundqvist, M., Herman, P. & Miller, E. K. Working memory: delay activity, yes! Persistent activity? Maybe not. J. Neurosci. 38, 7013–7019 (2018).
Constantinidis, C. et al. Persistent spiking activity underlies working memory. J. Neurosci. 38, 7020–7028 (2018).
Compte, A., Brunel, N., Goldman-Rakic, P. S. & Wang, X.-J. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb. cortex 10, 910–923 (2000).
Wimmer, K., Nykamp, D. Q., Constantinidis, C. & Compte, A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat. Neurosci. 17, 431–439 (2014).
Li, S., Zhou, X., Constantinidis, C. & Qi, X.-L. Plasticity of persistent activity and its constraints. Front. neural circuits 14, 15 (2020).
Qi, X.-L., Meyer, T., Stanford, T. R. & Constantinidis, C. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb. cortex 21, 2722–2732 (2011).
Meyer, T., Qi, X.-L., Stanford, T. R. & Constantinidis, C. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J. Neurosci. 31, 6266–6276 (2011).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546 (2017).
Connors, B. W. & Gutnick, M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 13, 99–104 (1990).
McCormick, D. A., Connors, B. W., Lighthall, J. W. & Prince, D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 54, 782–806 (1985).
Henze, D. A. et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 84, 390–400 (2000).
Yokoi, I. & Komatsu, H. Putative pyramidal neurons and interneurons in the monkey parietal cortex make different contributions to the performance of a visual grouping task. J. Neurophysiol. 104, 1603–1611 (2010).
Kaufman, M. T. et al. Roles of monkey premotor neuron classes in movement preparation and execution. J. Neurophysiol. 104, 799–810 (2010).
Kaufman, M. T., Churchland, M. M. & Shenoy, K. V. The roles of monkey M1 neuron classes in movement preparation and execution. J. Neurophysiol. 110, 817–825 (2013).
Giordano, N. et al. Fast-spiking interneurons of the premotor cortex contribute to initiation and execution of spontaneous actions. J. Neurosci. 43, 4234–4250 (2023).
Hussar, C. R. & Pasternak, T. Flexibility of sensory representations in prefrontal cortex depends on cell type. Neuron 64, 730–743 (2009).
Jacob, S. N., Stalter, M. & Nieder, A. Cell-type-specific modulation of targets and distractors by dopamine D1 receptors in primate prefrontal cortex. Nat. Commun. 7, 13218 (2016).
Hussar, C. R. & Pasternak, T. Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons. J. Neurosci. 32, 2747–2761 (2012).
Constantinidis, C. & Goldman-Rakic, P. S. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J. Neurophysiol. 88, 3487–3497 (2002).
Diester, I. & Nieder, A. Complementary contributions of prefrontal neuron classes in abstract numerical categorization. J. Neurosci. 28, 7737–7747 (2008).
Johnston, K., DeSouza, J. F. & Everling, S. Monkey prefrontal cortical pyramidal and putative interneurons exhibit differential patterns of activity between prosaccade and antisaccade tasks. J. Neurosci. 29, 5516–5524 (2009).
Ding, L. & Gold, J. I. Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb. cortex 22, 1052–1067 (2012).
Thiele, A. et al. Attention induced gain stabilization in broad and narrow-spiking cells in the frontal eye-field of macaque monkeys. J. Neurosci. 36, 7601–7612 (2016).
Cohen, J. Y., Pouget, P., Heitz, R. P., Woodman, G. F. & Schall, J. D. Biophysical support for functionally distinct cell types in the frontal eye field. J. Neurophysiol. 101, 912–916 (2009).
Tsunada, J., Lee, J. H. & Cohen, Y. E. Differential representation of auditory categories between cell classes in primate auditory cortex. J. Physiol. 590, 3129–3139 (2012).
Zhang, Y., Li, S., Jiang, D. & Chen, A. Response properties of interneurons and pyramidal neurons in macaque MSTD and VPS areas during self-motion. Front. Neural Circuits 12, 105 (2018).
Mruczek, R. E. & Sheinberg, D. L. Stimulus selectivity and response latency in putative inhibitory and excitatory neurons of the primate inferior temporal cortex. J. Neurophysiol. 108, 2725–2736 (2012).
Woloszyn, L. & Sheinberg, D. L. Effects of long-term visual experience on responses of distinct classes of single units in inferior temporal cortex. Neuron 74, 193–205 (2012).
Chen, Y. et al. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat. Neurosci. 11, 974–982 (2008).
Vinck, M., Womelsdorf, T., Buffalo, E. A., Desimone, R. & Fries, P. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron 80, 1077–1089 (2013).
Mitchell, J. F., Sundberg, K. A. & Reynolds, J. H. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55, 131–141 (2007).
Csicsvari, J., Hirase, H., Czurkó, A., Mamiya, A. & Buzsáki, G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci. 19, 274–287 (1999).
Ardid, S. et al. Mapping of functionally characterized cell classes onto canonical circuit operations in primate prefrontal cortex. J. Neurosci. 35, 2975–2991 (2015).
Onorato, I. et al. A distinct class of bursting neurons with strong gamma synchronization and stimulus selectivity in monkey V1. Neuron 105, 180–197.e5 (2020).
Banaie Boroujeni, K., Tiesinga, P. & Womelsdorf, T. Interneuron-specific gamma synchronization indexes cue uncertainty and prediction errors in lateral prefrontal and anterior cingulate cortex. Elife 10, e69111 (2021).
Trainito, C., von Nicolai, C., Miller, E. K. & Siegel, M. Extracellular spike waveform dissociates four functionally distinct cell classes in primate cortex. Curr. Biol. 29, 2973–2982.e5 (2019).
Lee, E. K. et al. Non-linear dimensionality reduction on extracellular waveforms reveals cell type diversity in premotor cortex. Elife 10, e67490 (2021).
Tang, H. et al. Prefrontal cortical plasticity during learning of cognitive tasks. Nat. Commun. 13, 90 (2022).
Contreras, D. Electrophysiological classes of neocortical neurons. Neural Netw. 17, 633–646 (2004).
Ditz, H. M., Fechner, J. & Nieder, A. Cell-type specific pallial circuits shape categorical tuning responses in the crow telencephalon. Commun. Biol. 5, 269 (2022).
Qi, X. et al. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front. Syst. Neurosci. 4, 1261 (2010).
Qi, X.-L. & Constantinidis, C. Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS One 7, e41053 (2012).
Singh, B., Wang, Z., Qi, X.-L. & Constantinidis, C. Plasticity after cognitive training reflected in prefrontal local field potentials. Iscience. 25, 104929 (2022).
Mountcastle, V. B., Talbot, W. H., Sakata, H. & Hyvärinen, J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J. Neurophysiol. 32, 452–484 (1969).
Azouz, R., Gray, C. M., Nowak, L. G. & McCormick, D. A. Physiological properties of inhibitory interneurons in cat striate cortex. Cerebral cortex (New York, NY: 1991)7, 534-545 (1997).
Hasenstaub, A. et al. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47, 423–435 (2005).
Elston, G. N., Benavides-Piccione, R., Elston, A., Manger, P. R. & DeFelipe, J. Pyramidal cells in prefrontal cortex of primates: marked differences in neuronal structure among species. Front. Neuroanat. 5, 2 (2011).
Boroujeni, K. B., anaie, Oemisch, M., Hassani, S. A. & Womelsdorf, T. Fast spiking interneuron activity in primate striatum tracks learning of attention cues. Proc. Natl Acad. Sci. 117, 18049–18058 (2020).
Dasilva, M. et al. Cell class-specific modulation of attentional signals by acetylcholine in macaque frontal eye field. Proc. Natl Acad. Sci. 116, 20180–20189 (2019).
Kvitsiani, D. et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366 (2013).
Yavorska, I. & Wehr, M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front. neural circuits 10, 76 (2016).
Jouhanneau, J.-S., Kremkow, J. & Poulet, J. F. Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 9, 1540 (2018).
Ghaderi, P., Marateb, H. R. & Safari, M.-S. Electrophysiological profiling of neocortical neural subtypes: a semi-supervised method applied to in vivo whole-cell patch-clamp data. Front. Neurosci. 12, 374322 (2018).
Lee, E. K. et al. PhysMAP-interpretable in vivo neuronal cell type identification using multi-modal analysis of electrophysiological data. BioRxiv 02, 582461 (2024).
Ma, W.-p. et al. Visual representations by cortical somatostatin inhibitory neurons—selective but with weak and delayed responses. J. Neurosci. 30, 14371–14379 (2010).
Tremblay, R., Lee, S. & Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292 (2016).
Urban-Ciecko, J. & Barth, A. L. Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 17, 401–409 (2016).
Yu, H. et al. In vivo cell-type and brain region classification via multimodal contrastive learning. bioRxiv 11, 622159 (2024).
Wei, Y. et al. Associations between in vitro, in vivo and in silico cell classes in mouse primary visual cortex. Nat. Commun. 14, 2344 (2023).
Jia, X. et al. High-density extracellular probes reveal dendritic backpropagation and facilitate neuron classification. J. Neurophysiol. 121, 1831–1847 (2019).
Paulk, A. C. et al. Large-scale neural recordings with single neuron resolution using Neuropixels probes in human cortex. Nat. Neurosci. 25, 252–263 (2022).
Sun, S. H. et al. Analysis of extracellular spike waveforms and associated receptive fields of neurons in cat primary visual cortex. J. Physiol. 599, 2211–2238 (2021).
Jung, Y. J. et al. Characterization of extracellular spike waveforms recorded in wallaby primary visual cortex. Front. Neurosci. 17, 1244952 (2023).
Soares, D. et al. Expression of Kv3. 1b potassium channel is widespread in macaque motor cortex pyramidal cells: A histological comparison between rat and macaque. J. Comp. Neurol. 525, 2164–2174 (2017).
Vigneswaran, G., Kraskov, A. & Lemon, R. N. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J. Neurosci. 31, 14235–14242 (2011).
Torres-Gomez, S. et al. Changes in the proportion of inhibitory interneuron types from sensory to executive areas of the primate neocortex: implications for the origins of working memory representations. Cereb. Cortex 30, 4544–4562 (2020).
Gentet, L. J. Functional diversity of supragranular GABAergic neurons in the barrel cortex. Front. neural circuits 6, 52 (2012).
Lemon, R. N., Baker, S. N. & Kraskov, A. Classification of cortical neurons by spike shape and the identification of pyramidal neurons. Cereb. cortex 31, 5131–5138 (2021).
Oram, M. W. Visual stimulation decorrelates neuronal activity. J. Neurophysiol. 105, 942–957 (2011).
Asaad, W. F., Rainer, G. & Miller, E. K. Task-specific neural activity in the primate prefrontal cortex. J. Neurophysiol. 84, 451–459 (2000).
White, I. M. & Wise, S. P. Rule-dependent neuronal activity in the prefrontal cortex. Exp. brain Res. 126, 315–335 (1999).
Rainer, G., Asaad, W. F. & Miller, E. K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393, 577–579 (1998).
Curtis, C. E. & Sprague, T. C. Persistent activity during working memory from front to back. Front. Neural Circuits 15, 696060 (2021).
Mozumder, R., Chung, S., Li, S. & Constantinidis, C. Contributions of narrow-and broad-spiking prefrontal and parietal neurons on working memory tasks. Front. Syst. Neurosci. 18, 1365622 (2024).
Ott, T. & Nieder, A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci. 23, 213–234 (2019).
Jacob, S. N., Ott, T. & Nieder, A. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J. Neurosci. 33, 13724–13734 (2013).
Vogel, P., Hahn, J., Duvarci, S. & Sigurdsson, T. Prefrontal pyramidal neurons are critical for all phases of working memory. Cell Rep. 39, (2022).
Cohen, M. R. & Newsome, W. T. Context-dependent changes in functional circuitry in visual area MT. Neuron 60, 162–173 (2008).
Clark, K. L. & Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. neural circuits 8, 33 (2014).
Warden, M. R. & Miller, E. K. Task-dependent changes in short-term memory in the prefrontal cortex. J. Neurosci. 30, 15801–15810 (2010).
Parto Dezfouli, M., Zarei, M., Constantinidis, C. & Daliri, M. R. Task-specific modulation of PFC activity for matching-rule governed decision-making. Brain Struct. Funct. 226, 443–455 (2021).
Abbas, A. I. et al. Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100, 926–939. e3 (2018).
Kim, D. et al. Distinct roles of parvalbumin-and somatostatin-expressing interneurons in working memory. Neuron 92, 902–915 (2016).
Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsaki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Shinomoto, S., Shima, K. & Tanji, J. Differences in spiking patterns among cortical neurons. Neural Comput. 15, 2823–2842 (2003).
Shinomoto, S. et al. Relating neuronal firing patterns to functional differentiation of cerebral cortex. PLoS computational Biol. 5, e1000433 (2009).
Shinomoto, S., Miyazaki, Y., Tamura, H. & Fujita, I. Regional and laminar differences in in vivo firing patterns of primate cortical neurons. J. Neurophysiol. 94, 567–575 (2005).
Meyer, T., Qi, X.-L. & Constantinidis, C. Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks. Cereb. cortex 17, i70–i76 (2007).
Author information
Authors and Affiliations
Contributions
A.A. and M.R.D. conceptualized the study design. C.C. designed the behavioral task and collected the data. A.A. conducted the data analysis, created the figures, and drafted the initial manuscript. A.A., C.C., and M.R.D. contributed to the manuscript revision and editing. M.R.D. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jacqueline Gottlieb and Jasmine Pan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asadi, A., Constantinidis, C. & Daliri, M.R. Functional role of cell classes in monkey prefrontal cortex after learning a working memory task. Commun Biol 8, 703 (2025). https://doi.org/10.1038/s42003-025-08142-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08142-4