Abstract
Aortic aneurysm and dissection (AAD) are severe cardiovascular conditions that carry significant risks and currently lack an effective treatment. Ursodeoxycholic acid (UDCA) can delay the onset of various metabolic diseases such as type 2 diabetes and obesity. However, it remains unclear whether UDCA can delay the onset of AAD. We demonstrated pathological activation of the intestinal Farnesoid X receptor (FXR) in both AAD patients and AAD mice. UDCA significantly suppressed intestinal FXR activation. In vivo experiments demonstrated that knockdown of intestinal FXR in mice using adeno-associated virus (AAV) reduced the incidence of AAD, reduced macrophage infiltration, and improved extracellular matrix degradation. In vitro experiments demonstrated that activating intestinal FXR increases the expression of ceramide synthase 2 (Cers2), which participates in the de novo synthesis of ceramides, and promotes the release of C20 ceramide. C20 ceramide promotes extracellular matrix metalloproteinases (MMPs) release from macrophages, leading to extracellular matrix degradation and contributing to AAD development. Our findings highlight the role of UDCA in reducing AAD incidence, revealing the therapeutic potential of the intestinal FXR/Cers2 axis against AAD.

Similar content being viewed by others
Introduction
Aortic aneurysm and dissection (AAD) is a condition characterized by the separation of the true and false lumens within the aortic wall1,2. This pathological process occurs when blood from the aortic lumen dissects into the aortic media, leading to the separation and subsequent expansion of the media along the longitudinal axis of the aorta3. AAD is relatively common, with an incidence ranging between 2% and 8%4. Despite its prevalence, the underlying mechanisms driving AAD remain poorly understood, and effective pharmacological treatments are not available. As a result, there is a pressing need to investigate potential early warning systems and therapeutic approaches for AAD.
The Farnesoid X Receptor (FXR) is a ligand-activated nuclear receptor with significant roles in regulating cholesterol and bile acid metabolism5,6. Recent studies have shown that FXR is also involved in the development of various cardiovascular diseases7,8. For example, Sheng et al. demonstrated that activation of FXR significantly reduces liver lipid levels and bile acid concentrations, while promoting the excretion of cholesterol via feces, thereby inhibiting the development of atherosclerosis9. Additionally, Xia et al. found that FXR activation improves cardiac remodeling and dysfunction following myocardial infarction (MI)10. Importantly, hypertension is a well-established risk factor for AAD, and studies have shown that FXR can regulate blood pressure by modulating the Renin-Angiotensin-Aldosterone System (RAAS)11,12,13. Given these roles of FXR in regulating cardiovascular processes, it is plausible that FXR could also influence the pathogenesis of AAD. However, a direct link between FXR activation and AAD has not been clearly established. Our study aims to elucidate the role of FXR in AAD pathology and explore whether its regulation could reduce the occurrence or severity of AAD.
Elevated circulating ceramide levels are recognized as a significant risk factor for cardiovascular diseases14. Ceramides, a class of bioactive sphingolipids, are cytotoxic to various cell types and contribute to the pathogenesis of myocardial infarction, coronary artery disease, and aortic aneurysm14,15. Clinical evidence consistently demonstrates a strong association between elevated ceramide levels and adverse cardiovascular events, including AAD16. Ceramide metabolism occurs through three primary pathways: sphingomyelinase, de novo synthesis, and salvage pathways. Inhibition of ceramide synthesis has been shown to delay the onset and progression of chronic metabolic diseases, presenting potential therapeutic opportunities for conditions such as atherosclerosis and insulin resistance17. Notably, FXR has recently been implicated in the regulation of ceramide metabolism. Gonzalez et al. showed that intestinal FXR promotes ceramide biosynthesis in the intestine, leading to elevated circulating ceramide levels and contributing to metabolic disorders such as hepatic steatosis and gluconeogenesis18,19. This process may exacerbate cardiovascular diseases, including atherosclerosis and potentially AAD. Thus, the relationship between FXR and ceramide metabolism is critical in understanding AAD progression.
In this study, we identified prominent activation of intestinal FXR in both AAD patients and AAD mouse models. This activation leads to upregulation of Ceramide Synthase 2 (Cers2), which promotes the de novo synthesis of C20 ceramides and their subsequent release into the bloodstream, thereby exacerbating AAD progression. Furthermore, our study demonstrates that intestinal FXR acts as a cis-regulator of Cers2. Inhibition of intestinal FXR by Ursodeoxycholic acid (UDCA) effectively suppresses Cers2 expression, reduces circulating ceramide levels, stabilizes the vasculature, and attenuates AAD progression. These findings underscore the complex interplay between FXR and ceramide metabolism in the pathogenesis of AAD, suggesting that targeting FXR could represent a novel therapeutic strategy for managing this condition.
This study aims to clarify the role of FXR in regulating ceramide metabolism and its contribution to AAD pathology. By understanding this connection, we hope to identify new therapeutic approaches to prevent or treat AAD. A diagram illustrating the proposed hypothesis and underlying mechanisms is provided to help visualize the relationship between FXR, ceramide metabolism, and AAD progression (Fig. 1A).
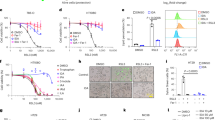
A Validation process of this study flowchart. B Serum expression levels of ursodeoxycholic acid. (n = 7, per group). C Mouse serum expression levels of bile acid. (n = 10, per group). D Serum FGF19, MMP2, MMP9 levels in normal person, AA patients and AD patients (n = 30 per group). E–G ELISA results of FGF15, MMP2, and MMP9 during the 4-week BAPN-induced AAD process. (n = 10. per group). H Hepatic FXR and SHP mRNA expression in the AAD model mice induced by BAPN. (n = 6, per group). I Intestinal FXR, FGF15 and SHP mRNA expression in the AAD model mice induced by BAPN. (n = 6, per group). J Co-immunoprecipitation results from the intestine of AAD mice. Date in (E–H) and I are shown as the mean ± SEM. Statistical analysis was performed using two-tailed unpaired Student’s t test. Data in B and C are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
The intestinal FXR activation is involved in the occurrence of AAD
To elucidate the relationship between intestinal farnesoid X receptor (FXR) signaling and aortic aneurysm and dissection (AAD), we analyzed serum samples from AAD patients and mice. ursodeoxycholic acid (UDCA)20, a natural FXR inhibitor, was significantly reduced in both AAD patients and AAD mice compared to controls (Fig. 1B, C and Supplementary Fig. 1A–I). We then examined Fibroblast Growth Factor 19 (FGF19), a key FXR downstream molecule21, and found its serum levels were elevated in AAD patients, along with increased matrix metalloproteinase2 (MMP2) and matrix metalloproteinase9 (MMP9) levels (Fig. 1D). FGF19 positively correlated with MMP2 and MMP9 expression (Supplementary Fig. 2A, B). In β-aminopropionitrile (BAPN)-induced AAD models, Fibroblast Growth Factor 15 (FGF15), MMP2, and MMP9 levels increased with prolonged induction time (Fig. 1E–G). At the end of the 4th week of modeling, when the mice reached 8 weeks of age, we validated the activation of FXR in the liver and intestine. We found that intestinal FXR promoted the occurrence of AAD, rather than hepatic FXR. (Fig. 1H–J). Similar findings were observed in AngiotensinII (AngII)-induced AAD models (Supplementary Fig. 1J–N). Immunohistochemistry (IHC) and immunofluorescence (IF) assays on human AAD tissues revealed significant clusters of differentiation 68 (CD68)+ macrophage infiltration, elevated MMP2 and MMP9 expression, and Fibronectin 1 (FN1) degradation (Supplementary Fig. 2C–G). Notably, FXR expression in aortic tissues showed no difference between AAD patients/mice and controls (Supplementary Fig. 2H, I). Collectively, our study highlights intestinal FXR’s role in AAD pathogenesis, emphasizing CD68+ macrophage infiltration, increased MMP2/MMP9 expression, and FN1 degradation in AAD tissues.
UDCA reduces the occurrence of AAD
we observed that intestinal FXR becomes activated during the onset of AAD. To investigate whether inhibiting intestinal FXR activation could prevent AAD, we administered the intestinal FXR antagonist UDCA or a vector control to mice and established an AAD model using BAPN and AngII induction (Supplementary Fig. 3A)22,23. UDCA treatment significantly reduced the incidence of AAD (from 72.5% to 30%) and mortality (from 47.5% to 15%) in mice. Furthermore, we demonstrated that intestinal FXR activation increased with modeling time, accompanied by a notable upregulation of MMP2 and MMP9 expression. UDCA specifically inhibited intestinal FXR without impacting hepatic FXR activation (Fig. 2D–H). Histopathological analysis, including Hematoxylin and Eosin (H&E) staining, Masson staining, and Verhoeff-Van Gieson (EVG) staining, revealed that UDCA significantly improved aortic pathology (Fig. 2I–K). We analyzed the relative expression levels of AAD-related markers. The mRNA levels of macrophage markers (inducible nitric oxide synthase, Inos; tumor necrosis factor-alpha, TNF-α; prostaglandin-endoperoxide synthase 2, Ptgs2; interleukin-1 beta, IL-1β and interleukin-6, IL-6) were significantly reduced in the aortas of BAPN + UDCA-treated mice compared to BAPN-treated mice24. However, no significant differences were observed in the mRNA levels of VSMC phenotypic transformation genes (smooth muscle actin, SMA and smooth suscle protein 22 alpha, SM22α), adhesion molecules (intercellular adhesion molecule, Icam and vascular cell adhesion molecule, Vcam), or the endothelial cell marker keratinocyte-derived Receptor (Kdr) between the two groups (Supplementary Fig. 3B–D)25,26. A positive correlation was identified between serum FGF15 levels and the expression of serum MMP2 and MMP9 (Supplementary Fig. 3E, F). Circulating inflammatory cytokine levels were significantly lower in BAPN + UDCA-treated mice compared to BAPN-treated mice (Supplementary Fig. 3G, H). UDCA treatment reduced the expression levels of MMP2 and MMP9 in aortic tissues, decreased the infiltration of F4/80-positive macrophages, and maintained the stability of the vascular extracellular matrix (Fig. 2L–P). Notably, UDCA treatment did not affect hepatic, renal, or cardiac function in mice (Supplementary Fig. 3I–K and Fig. 2Q).
A–D Three-week-old male mice were administered with 0.25% BAPN for 30 days with or without UDCA (50 mg/kg/d) and then infused with saline or angiotensin II (1000 ng/kg/min) for 3 days. A Representative morphology of the aortas from different groups of mice. B Survival curve and number at risk (log-rank test). C Incidence of AAD in different groups of mice. (n = 40; chi-square test). D qRT-PCR was performed to validate FXR activation in mouse liver tissue. (n = 6, per group). E The activation status of FXR in mouse intestine was determined by qRT-PCR. (n = 6, per group). F–H Serum levels of FGF15, MMP2, and MMP9 were measured by ELISA. (n = 10, per group). I HE staining, EVG staining, and Masson staining of the aorta (n = 6 from each group). J Statistical chart of the maximum aortic diameter in mice (n = 6 from each group). K Analysis of elastic fiber degradation. (n = 6, per group) (L–N) Immunohistochemical results of MMP2 and MMP9 and the analysis of immunohistochemical (n = 7 per group). O, P Immunofluorescence results show F4/80 (+) cell rate and the expression of COL1 and FN1 and the quantitative analysis. F4/80 (red) in aortas. COL1 (red) in aortas. FN1 (red) in aortas. Nuclei were counterstained with DAPI (blue). (n = 7 per group). Q Hepatic function analysis was performed in mice, and the results are presented. (n = 8, per group). Data in (D–H, J, K, M, N, P,Q) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars: 200 μm and 50 μm.
In a second AAD model, UDCA administration reduced the incidence of AAD in ApoE−/− mice infused with AngII for 28 days. UDCA’s protective effects were primarily mediated through anti-inflammatory properties, as evidenced by improved aortic quality, reduced MMP2 and MMP9 expression in perivascular tissues, and decreased macrophage infiltration, while maintaining vascular stability (Supplementary Figs. 4, 5).
The intestinal FXR promotes intestinal-derived C20 ceramide secretion through Cers2
Recent studies have shown that intestinal FXR promotes the secretion of intestinal-derived ceramides, contributing to Non-Alcoholic Fatty Liver Disease (NAFLD), obesity, insulin resistance, and atherosclerosis19,27,28. Wu et al. reported that FXR activation upregulates sphingomyelin phosphodiesterase 3 (Smpd3), a key enzyme in ceramide synthesis, leading to increased ceramide release and atherosclerosis19. Based on these findings, we hypothesize that FXR activation may contribute to AAD by enhancing intestinal-derived ceramide secretion.
To investigate this, we performed untargeted lipidomics analysis on mouse ileum and found significantly increased C20 ceramide content in a BAPN-induced AAD mouse model, along with sphingolipid metabolic pathway perturbations (Fig. 3A, B and Supplementary Fig. 6A). Ultra-High Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS) analysis of serum samples from AAD patients and mice showed significantly higher C20 ceramide levels in AAD patients compared to healthy individuals, and in AAD mice compared to normal mice (Fig. 3C, D and Supplementary Fig. 6B–G).
A Differential lipid heatmap of ileal tissue. (n = 6 per group). B Kyoto Encyclopedia of Genes and Genomes (KEGG) Bubble Chart for inter group differential metabolites (n = 6 per group). C Quantification of serum ceramides in normal mice, AAD mice and UDCA mice (n = 8 per group). D Pearson’s correlation analysis between serum FGF15 and C20 ceramide levels in mice. E–G Detection results of ceramide synthase expression in the AAD model induced by BAPN. (n = 6, per group). H–J Detection results of ceramide synthase expression in the AAD model induced by AngII. (n = 6, per group) (K–L) Western blot results confirm that treatment with the FXR agonist GW4064 (10 μM) or DMSO enhances the expression of Cers2 in primary enterocytes (n = 4 per group). M Motif analysis shows FXR putatively bound to Cers2 motif. N Chip-qPCR was performed in mice treated with GW4064 (80 mg/kg/d) or DMSO (n = 3 per group). O Results of the dual-luciferase assay (n = 3 per group). Data in L and N are shown as the mean ± SEM. Statistical analysis was performed using two-tailed unpaired Student’s t test. Data in (C, E–J, O) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001).
To explore FXR-mediated ceramide synthesis mechanisms, we performed Quantitative Real-Time Polymerase Chain Reaction (qPCR) on enzymes involved in ceramide synthesis and metabolism in mouse intestines29. Ceramide synthase 2 (Cers2), a key enzyme in ceramide synthesis, was highly expressed in the intestines of BAPN-induced AAD mice, and UDCA inhibited Cers2 expression. Similar results were observed in the AngII-induced AAD mouse model, further supporting Cers2’s role in AAD development. (Fig. 3E–J).
To determine if FXR directly regulates Cers2, we isolated primary enterocytes and treated them with the FXR agonist GW4064. FXR activation significantly promoted Cers2 expression (Fig. 3K, L, Supplementary Fig. 7A–D and Supplementary Fig. 8A–C). FXR knockdown in organoids using lentivirus showed that FXR is essential for GW4064-induced Cers2 expression (Supplementary Fig. 8D–F).
To confirm FXR’s direct interaction with the Cers2 promoter, we performed Chromatin Immunoprecipitation Sequencing (ChIP-seq) on mice treated with GW4064 (80 mg/kg/day for 1 week) (Supplementary Fig. 8G)30. Results showed FXR binds to the Cers2 promoter, promoting its expression (Fig. 3M and Supplementary Fig. 8H–J). Chromatin Immunoprecipitation Quantitative Polymerase Chain Reaction (ChIP-qPCR) and dual-luciferase assays confirmed FXR directly regulates Cers2 expression through transcriptional activity (Fig. 3N, O).
In summary, our results demonstrate that intestinal FXR promotes Cers2 expression, increasing intestinal-derived ceramide levels. These findings provide new insights into AAD development mechanisms and highlight the potential therapeutic targeting of the FXR-Cers2-ceramide axis for AAD treatment (Supplementary Fig. 9A).
Supplementing C20 ceramide can eliminate the beneficial effect of UDCA
To confirm the protective role of UDCA in reducing the release of intestinal-derived C20 ceramide, rescue experiments were conducted. UDCA alone, UDCA combined with C20 ceramide, or a vehicle control was administered in mice with a BAPN/AngII-induced AAD model. UDCA significantly reduced mortality and the incidence of AAD, but this protective effect was abolished by C20 ceramide administration, negating UDCA’s benefits (Fig. 4A–C). C20 ceramide supplementation did not affect intestinal FXR activation but significantly increased serum MMP2 and MMP9 levels (Fig. 4D–F). Exogenous C20 ceramide worsened vascular lesions (Supplementary Fig. 10A, B) and increased inflammatory cytokines, MMP2, and MMP9 expression in the aorta (Fig. 4G, H and Supplementary Fig. 10C, D). Mice treated with UDCA and C20 showed significant infiltration of F4/80-positive macrophages in aorta, along with degradation of Collagen Type I (COL1) and FN1 (Supplementary Fig. 10E, F).
A–D Three-week-old male mice were administered with 0.25% BAPN and UDCA (50 mg/kg/d) for 30 days with or without C20 (10 mg/kg/d) and then infused with saline or angiotensin II (1000 ng/kg/min) for 3 days. A Representative morphology of the aortas from different groups of mice. B Survival curve and number at risk (log-rank test). C Incidence of AAD in different groups of mice. (n = 40; chi-square test). D–F Serum levels of FGF15, MMP2, and MMP9 were measured by ELISA. (n = 10, per group). G, H The ELISA results indicate the levels of inflammatory cytokines. (n = 6, per group). Data in (D, E–H) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001).
Inhibiting the synthesis of ceramides can reduce the occurrence of AAD
Given that ceramide exacerbates AAD (Fig. 4), we hypothesized that inhibiting ceramide de novo synthesis might reduce AAD incidence. To test this, we used Myriocin (Myr), an established ceramide synthesis inhibitor31. Administering Myr to mice significantly reduced AAD incidence (from 75% to 25%) and mortality (from 50% to 20%, Fig. 5A–C), while improving vascular quality (Fig. 5D, E). To verify ceramide’s role in inflammation via macrophage activation, we analyzed aortic MMP2 and MMP9 expression, F4/80-positive macrophage infiltration, and COL1 and FN1 expression (Fig. 5F–I). These results showed that elevated ceramide activates macrophages, leading to increased MMP2 and MMP9 release, which degrades COL1 and FN1, reduces vascular stability, and ultimately promotes AAD occurrence.
A–D Three-week-old male mice were administered with 0.25% BAPN for 30 days with or without Myr (0.5 mg/kg/d) and then infused with saline or angiotensin II (1000 ng/kg/min) for 3 days. A Representative diagram of mouse aorta. B Incident of AAD. (n = 20; chi-square test). C Survival curve and number at risk (log-rank test). D HE staining, EVG staining, and Masson staining of the aorta (n = 6 from each group). E Statistical chart of the maximum aortic diameter in mice and analysis of elastic fiber degradation. (n = 6, per group). F, G Immunohistochemical results and analysis of MMP2 and MMP9 in the aorta. (n = 5, per group). H, I Immunofluorescence results show F4/80 (+) cell rate and the expression of COL1 and FN1 and the immunofluorescence quantitative analysis. F4/80 (red) in aortas. COL1 (red) in aortas. FN1 (red) in aortas. Nuclei were counterstained with DAPI (blue). (n = 5 per group). Data in (E, G, I) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars: 200 μm and 50 μm.
Specific knockout of intestinal FXR can reduce the occurrence of AAD
To enhance the persuasiveness of our conclusion, we used adeno-associated virus (AAV) to specifically reduce intestinal FXR expression, as described by Wu et al.19. Due to age-related limitations in the BAPN-AAD model, we adopted the AngII-induced AAD model in ApoE−/− mice. 4-week-old ApoE−/− mice were employed, and AAV was used to reduce intestinal FXR expression. To evaluate the knockdown efficiency, six mice were sacrificed weekly for analysis, confirming specific intestinal FXR knockdown and generating AAV-sh-FXR-ApoE−/− (sh-FXR+AngII) mice (Supplementary Fig. 11A).
At 8 weeks of age, we induced AAD (Fig. 6A and Supplementary Fig. 11A, B). In another part of AAV-sh-FXR-ApoE−/− mice, we administered C20 ceramide followed by AngII infusion for 30 days, named (sh-FXR+AngII+C20). Sacrificing the mice after 4 weeks. Based on results from (Fig. 3), we investigated FXR’s role in Cers2 expression. Our findings showed that specific knockout of intestinal FXR reduced Cers2 expression, while C20 ceramide supplementation did not promote Cers2 expression, suggesting FXR regulates Cers2 through transcriptional activity (Supplementary Fig. 11C–F).
A–C Eight-week-old ApoE−/− male mice were infused with saline or angiotensin II (1000 ng/kg/min) for 30 days with or without C20 ceramide (10 mg/kg/d). AAV-vector-ApoE−/−+ angiotensin II, n = 20; AAV-sh-FXR-ApoE−/−+ angiotensin II, n = 20; AAV-sh-FXR-ApoE−/−+ angiotensin II + C20 ceramide, n = 20. A Diagram of viral intervention in mice and AAD model creation. B AAD incidence rate (n = 20; chi-square test). C Representative diagram of mouse aorta. D–F Serum levels of FGF15, MMP2, and MMP9 were measured by ELISA. (n = 10, per group). G, H Representative images of aortic sections subjected to HE staining, EVG staining, and Masson’s trichrome staining and a quantitative analysis of the maximum aortic diameter and analysis of elastic fiber degradation in mice (n = 6 per group). I, J Immunohistochemical results and analysis of MMP2 and MMP9 in the aorta. (n = 5, per group). K, L F4/80 (+) cell rate and the expression of FN1 in aortic tissue and the quantification of immunofluorescence. F4/80 (red) in aortas.FN1 (red) in aortas. Nuclei were counterstained with DAPI (blue). (n = 5 per group). Data in (D–F, H, J, L) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars: 200 μm and 50 μm.
The incidence of AAD was lower in sh-FXR+AngII mice compared to AAV-vector-ApoE−/− (Vector+AngII) mice. Additionally, C20 ceramide supplementation eliminated the protective effects of intestinal FXR knockout (Fig. 6B, C). Knockdown of intestinal FXR decreased serum inflammatory cytokines and MMP2/MMP9 expression, while C20 ceramide increased these levels without activating FXR in the intestine (Fig. 6D–F and Supplementary Fig. 11G, H).
The aortic vascular quality in sh-FXR+AngII mice was significantly better than in Vector+AngII and sh-FXR+AngII+C20. Elastic fibers in the aorta of Vector+AngII mice were disordered (Fig. 6G, H). IHC and IF on aortic tissues showed lower MMP2 and MMP9 expression in sh-FXR+AngII mice (Fig. 6I, J). Vector+AngII mice exhibited significant F4/80 (+) macrophage infiltration and FN1 degradation. Exogenous C20 ceramide reversed the vascular protective effects of intestinal FXR knockout (Fig. 6K, L).
C20 ceramide can stimulate the release of MMP2 and MMP9 from bone marrow-derived macrophages
Phenotypic transformation of vascular smooth muscle cell (VSMC), increased adhesion molecule expression in endothelial cell (EC), and inflammatory transformation of macrophages are key pathological processes in AAD. C20 ceramide was found to play a pivotal role in AAD. We stimulated VSMC, EC, and bone marrow-derived macrophage (BMDM) with C20 ceramide. Results showed it did not induce VSMC phenotypic transformation or upregulate adhesion molecules in EC, but it triggered inflammatory transformation in BMDM (Fig. 7A–D and Supplementary Fig. 12A, B). Further, pre-treating BMDM with C20 ceramide before LPS stimulation significantly elevated inflammatory-related gene expression (Fig. 7A–D and Supplementary Fig. 12A, B).
A qPCR results related to vascular smooth muscle cell (VSMC) phenotypic transformation. (n = 6, per group). B qPCR Results for Endothelial Cell (EC) adhesion molecules. (n = 6 per group). C, D INOS, TNF-α, Ptgs2, IL-1β, IL-6 mRNA levels in BMDM (n = 6, per group). E MMP2 and MMP9 mRNA levels in BMDM (n = 6, per group). F Expression of MMP2 and MMP9 in cell culture supernatant. (n = 6, per group). G Cell co-culture mode diagram. H, I INOS, TNF-α, Ptgs2, IL-1β, IL-6 mRNA levels in BMDM treated with GW4064 (10 μM) or DMSO (n = 6 per group). J MMP2 and MMP9 mRNA levels in BMDM treated with GW4064 (10 μM) or DMSO (n = 6, per group). K Expression of MMP2 and MMP9 in cell culture supernatant treated with GW4064 (10 μM) or DMSO. (n = 6, per group). Date in (A, B) are shown as the mean ± SEM. Statistical analysis was performed using two-tailed unpaired Student’s t test. Data in (C–F, H–K) are shown as the mean ± SEM. Statistical analysis was performed using a one-way anova test with Dunnett’s multiple comparisons posttest. (*p < 0.05, **p < 0.01, ***p < 0.001).
MMP-mediated ECM degradation is critical in AAD, with MMP2 and MMP9 primarily released from macrophages, VSMC, and fibroblast. We explored whether C20 ceramide influences this process by stimulating BMDM, VSMC, and fibroblast. C20 ceramide promoted robust MMP2 and MMP9 release from these cells (Fig. 7E, F, Supplementary Fig. 13A and Supplementary Fig. 14A).
To investigate FXR activation’s role in regulating Cers2, we treated primary enterocytes with the FXR agonist GW4064. FXR activation promoted Cers2 expression, which induced macrophage inflammatory transformation and increased MMP2/MMP9 secretion (Fig. 7G–K and Supplementary Fig. 12C, D). Additionally, FXR activation promoted Cers2 expression, leading VSMC and fibroblast to release large amounts of MMP2 and MMP9 (Supplementary Fig. 13B, C and Supplementary Fig. 14B, C).
Discussion
Aortic aneurysm and dissection (AAD) is a life-threatening condition characterized by a high mortality rate and poor prognosis32. Therefore, it is imperative to explore potential therapeutic approaches for the treatment of AAD. The activation of the farnesoid X receptor (FXR) has emerged as a pivotal mechanism in the pathogenesis of AAD. In this study, we conducted targeted bile acid profiling on serum samples from AAD patients and mice. We found that ursodeoxycholic acid (UDCA) levels were significantly reduced in both. These findings suggest that activating intestinal FXR may promote AAD occurrence. Exogenous UDCA supplementation reduced AAD incidence in various mouse models without apparent adverse effects, highlighting its potential therapeutic use in treating AAD. Our study also demonstrates that FXR activation leads to increased ceramide synthesis, particularly the production of C20 ceramides, which in turn triggers the release of matrix metalloproteinases (MMP2 and MMP9) through the enzyme ceramide synthase 2 (Cers2). UDCA also enhances vascular tolerance to rapid blood pressure fluctuations. While this ceramide-mediated pathway offers a novel insight into FXR’s role in AAD, it is essential to explore alternative pathways that might link FXR activation to MMP upregulation. Elucidating these mechanisms is crucial for developing targeted therapeutic strategies to mitigate AAD progression.
In addition to its effects on macrophages, ceramide may also exert influences on cardiac myocytes and neurons within blood vessels. In cardiac cells, C20 ceramide has been shown to modulate calcium handling, which is critical for maintaining proper cardiac rhythm and contraction. Dysregulation of ceramide levels, including C20, has been linked to cardiac dysfunction in conditions such as heart failure and ischemia. Moreover, ceramides can induce apoptosis in cardiac cells, potentially contributing to myocardial injury under pathological conditions33. In neurons, C20 ceramide plays a dual role in cellular function. It can modulate ion channel activity, such as sodium and potassium channels, which are essential for neuronal excitability and neurotransmission34.
Beyond the ceramide-mediated pathway, inflammatory cytokines and oxidative stress are likely to play significant roles in connecting FXR activation to MMP upregulation. Pro-inflammatory cytokines, such as TNF-α and IL-6, are well-documented to be elevated in the aortic wall during AAD35. These cytokines may interact with FXR signaling, either synergistically or independently, to enhance MMP expression. For instance, TNF-α is known to induce MMP production by activating the mitogen-activated protein kinase (MAPK) pathway, while IL-6 can potentiate inflammatory responses by upregulating the expression of pro-inflammatory genes36. The interplay between these cytokines and FXR signaling could exacerbate aortic wall degradation by creating a synergistic effect on MMP activity. Furthermore, oxidative stress, a well-established factor in vascular diseases, may serve as a critical mediator between FXR activation and MMP upregulation. Reactive oxygen species (ROS) are known to activate redox-sensitive pathways that lead to the expression of MMPs37. Specifically, ROS can enhance the activity of transcription factors such as NF-κB and AP-1, which are key regulators of MMP genes38. The interplay between inflammatory cytokines and oxidative stress further complicates the mechanisms linking FXR activation to AAD progression. Inflammatory cytokines can induce the production of ROS through various cellular pathways, creating a feed-forward loop that amplifies oxidative stress. Similarly, ROS can enhance the expression of pro-inflammatory cytokines by activating specific signaling pathways, such as the MAPK and NF-κB pathways39,40,41,42. The identification of FXR as a key regulator of ceramide-induced MMP upregulation opens promising avenues for therapeutic intervention in AAD. FXR inhibitors represent a logical strategy for mitigating AAD by targeting the root cause of ceramide-driven MMP expression. Although there are no ongoing clinical trials specifically investigating FXR inhibitors for aortic diseases, several trials are currently exploring their efficacy in other conditions, such as non-alcoholic steatohepatitis (NASH) and cholestatic liver diseases43,44,45,46. These trials provide valuable insights into the safety and tolerability of FXR inhibitors, which could be extrapolated to their potential use in AAD. This suggests that UDCA, already approved for the treatment of certain liver diseases, could be repurposed for AAD. Future clinical trials could be designed to evaluate the efficacy of UDCA in reducing the incidence or progression of AAD in high-risk populations. Additionally, identifying biomarkers such as circulating ceramide levels could help stratify patients who may benefit most from FXR-targeted therapies.
Another critical area of exploration is the identification of non-invasive biomarkers for early diagnosis and monitoring of AAD. Circulating levels of ceramides, inflammatory cytokines, and ROS could serve as potential biomarkers to identify patients at high risk of AAD progression47,48,49. These biomarkers could also be used to assess the response to FXR-targeted therapies, enabling personalized treatment approaches. Moreover, the development of combination therapies targeting multiple pathways involved in AAD pathogenesis holds great promise. For example, combining FXR inhibitors with antioxidants or anti-inflammatory agents could address both oxidative stress and inflammation, leading to a more robust therapeutic effect. These combination approaches would require careful evaluation to ensure their safety and efficacy, but they represent a promising direction for future research.
In conclusion, while our study highlights the critical role of the FXR-ceramide axis in AAD, a deeper understanding of alternative pathways involving inflammatory cytokines, oxidative stress, and their cross-talk is essential to fully elucidate the mechanisms driving aortic pathology. Translating these findings into clinical therapies will require rigorous investigation of FXR inhibitors and UDCA, alongside the identification of biomarkers for patient stratification. Additionally, exploring the therapeutic potential of combination therapies and upstream targets will broaden the scope of treatment options for AAD (Graphical Abstract).
Methods
Human samples
We collected discarded aortas from patients with AAD after aortic replacement in the Fujian Medical University Union Hospital. Patients are treated immediately after clinical symptoms appear, and surgery is usually performed on the same day or the next day. AAD was diagnosed by clinical history, physical examination, and CT. Normal thoracic aortic tissue was obtained from heart transplant organ donors. None of these patients had cardiovascular disease, and their blood vessels served as controls. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved and conducted under the guidance of the Ethics Committee of Fujian Medical University Union Hospital (No. 2019-36). All subjects signed written informed consent. During the surgical procedure, dissected arteries were collected, and some tissues were immediately stored at -80°C for subsequent experiments. The remaining tissues were placed in 4% paraformaldehyde for pathological staining.
Study population
All patients over the age of 18 who experienced at least one of the following symptoms within the past 14 days were included: chest, abdominal, or back pain; syncope; and signs or symptoms of perfusion deficit. Patients with a history of aortic trauma, pseudoaneurysm, or infectious diseases were excluded from the study. The diagnosis was confirmed through imaging, surgical visualization, or autopsy. Healthy control subjects, recruited from communities affiliated with Fujian Medical University Union Hospital, were free from significant systemic diseases, including ischemic heart disease, cancer, pulmonary disease, and infectious diseases. Serum samples were collected from healthy controls and AAD patients to determine the serum metabonomic characteristics and the diagnostic performance of ceramides in distinguishing aortic diseases (Supplementary Table 3).
Mice
In this study, the experimental protocol was approved by the Ethical Review Committee of Fujian Medical University Union Hospital and accompanied by animal welfare and ethical requirements (IACUC FJMU 2025-0037). Establishment of the AAD mouse model using a combination of β-aminopropionitrile (BAPN) (HY-Y1750, MCE, Shanghai, China) and Angiotensin II AngII (HY-13948, MCE, Shanghai, China). In short, 3-week-old C57BL/6 J mice were given water containing 0.25% BAPN for 4 weeks and the water was changed every other day. As for UDCA (50 mg/kg/d, HY-13771A, MCE, Shanghai, China), it was administered via gavage. In our animal experiment with BAPN + UDCA + C20 ceramide, during a 4-week intervention with C20 ceramide, mice received intraperitoneal injection of C20 ceramide (10 mg/kg/d) once a day. Four weeks later, AngII (1000 ng/kg/min) was administered to mice three times daily using a syringe, with an interval of one hour between each injection, for three consecutive days to induce AAD. Mice were anaesthetized by isoflurane inhalation (induction dose: 3%5%, maintenance dose: 1–2%; Harvey-bio, Beijing, China). To obtain aortas, mice were euthanized by CO2 inhalation.
In our second AAD animal model, we used AngII to induce the AAD mouse model. ApoE −/− male mice were infused with AngII (1000 ng/kg/min) for 30 days using a mini osmotic pump (Model 2004, Alzet, California, USA) at 8 weeks of age to induce AAD. And UDCA (50 mg/kg/d) is administered by gavage.
To knock down FXR in the ileum, mice received a Adeno-associated-virus (AAV) intervention as described previously19. AAV-m-FXR(5′to3′:(RNA)-GCCAUGUACAGAUUCUCGUAGAAUU) and negative control vector (AAV-GFP) were purchased from Shanghai Hanbio Biotechnology. In short, lift the small intestine 6–8 centimeters from the distal end of the cecum and tie it with clips at both ends to limit the virus’s outward flow and intestinal fluid’s inward flow. Then, 0.2 mL of AAV-GFP or AAV-m-FXR (Hanbio Biotechnology, Shanghai, China) expressing mismatched sequences was administered via insulin needle. After 30 minutes, rinse the intestine with physiological saline and remove the clamp. Finally, suture the incision with 10-0 suture and inject Ang II (1000 ng/kg/min) after 3 weeks. In a 4-week C20 ceramide administration model, We obtained 3-week-old ApoE−/− mice, and at 4 weeks of age, these ApoE−/− mice underwent AAV intervention. Four weeks later (i.e., when the AAV-sh-FXR-ApoE−/− mice reached 8 weeks of age), intraperitoneal injections of C20 ceramide (10 mg/kg/day) were administered daily. FXR expression was detected in ApoE−/− mice at 8 weeks of age. Mice were anaesthetized by isoflurane inhalation (induction dose: 3%5%, maintenance dose: 1–2%; Harvey-bio, Beijing, China). To obtain aortas, mice were euthanized by CO2 inhalation. To evaluate myriocin’s efficacy in suppressing the progression of AAD, we established a BAPN-induced AAD mouse model using C57BL/6 mice. The subjects were treated with myriocin (Myr) (HY-N6798, MCE, Shanghai, China) (0.5 mg/kg/d) through intraperitoneal injection for 28 consecutive days.
Organoid culture, lentiviral transduction, and treatment
Ileum were isolated from ApoE–/- mice, dissected, and washed with Dulbecco’s PBS 12 times. Then, the fragments were incubated with Gentle Cell Dissociation Reagent (STEMCELL Technologies, Vancouver, Canada) to segregate the crypts and villi from the intestinal basement membrane. After centrifugation, the crypts were isolated and resuspended in a 1:1 mixture of Matrigel (Corning, New York, USA) and IntestiCult organoid growth medium (OGM) (STEMCELL Technologies, Vancouver, Canada) at a density of 6000 crypts/mL. Place a 50 μL droplet containing 300 crypts into the center of each well of a preheated 24-well plate to form a dome. After the dome solidifies, add 750 μL of OGM to each well. The crypts were cultured at 37°C and 5% CO2, and the culture medium was replaced every 3 days. For organoid lentiviral transduction, organoids were harvested and placed into 15 mL tubes. After centrifugation, HBLV-m-FXR (1 × 106 PFU/100 crypts) was added. HBLV -m- FXR (5′to3′: (RNA)-GCCAUGUACAGAUUCUCGUAGAAUU). The organoid virus mixture was placed in a 37 °C incubator for 1 h for transduction. Finally, the organoids are plated and cultured as described above. To test the protein level of Cers2, the organoids were cultured in OGM for 7 days. Then, the organoids were cultured with GW4064 (10 μM) or 0.05% DMSO for 3 days before being harvested for RNA and protein extraction.
Primary enterocytes
First euthanize the mice, then immerse them in a beaker containing 75% ethanol, transfer them to a super-clean workbench, and fix the mice. Open the mouse abdominal cavity, remove the ileum tissue, remove other tissues such as the mesentery, longitudinally open the ileum, repeatedly clean the intestinal contents, and cut the intestine into 1mm3 tissue fragments. Then place the tissue fragments into a centrifuge tube containing PBS (PB180327, Procell, Wuhan, China) centrifuge 50 g for 3 min, and repeat this step 3 times. Then add trypsin (PB180225, Procell, Wuhan, China) and digest in a 37 °C water bath shaker for 25 min. Repeatedly blow until the liquid becomes turbid, let it stand at room temperature for 1 minute, take the supernatant and centrifuge 100 g for 5 min, then discard the supernatant, add complete culture medium and resuspend. Centrifuge 100 g for 5 min, wash 3 times, and retain the cell precipitate.
Bone Marrow-Derived Macrophages
Primary mouse Bone Marrow-Derived Macrophages (BMDM) were cultured as previously described50. In short, BMDM is isolated from the femur and tibia of C57BL/6 J. And then cultured on Petri dishes in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% Hepes, 1% sodium pyruvate plus 10 ng/ml recombinant macrophage colony-stimulating factor (416-ML-010, R&D Systems, Shanghai, China). Differentiated cells were refed on day 3. On day 7, differentiated BMDMs were collected, counted, and replated in complete RPMI 1640 medium in culture plates. After an overnight incubation, BMDMs were treated accordingly.
Histology
AAD is characterized by a tear in the intimal layer and disruption of the medial layer of the aortic wall, typically resulting from a combination of hemodynamic stress and structural abnormalities. This condition permits blood infiltration, leading to separation of the aortic wall layers. During the experimental process, deceased mice presented with hemorrhagic clots filling the thoracic cavity, completely encasing the heart, which indicated the occurrence and rupture of AAD. The aortas and intestine were harvested and preserved in 4% paraformaldehyde for 48 h. For the mice that completed the 28-day modeling period, the aortas were similarly excised and fixed in 4% paraformaldehyde for 48 h following euthanasia. The specimens were then embedded in paraffin, sectioned into 4 μm-thick slices, and prepared for histopathological analysis. For Hematoxylin-eosin (H&E) staining, the paraffin-embedded sections underwent deparaffinization and rehydration. Hematoxylin staining was applied for 2 minutes, followed by a brief immersion in hydrochloric acid ethanol solution for 1 second to remove excessive hematoxylin. Subsequently, eosin staining was performed for 30 seconds, after which the sections were rinsed with distilled water. The slides were then subjected to the standard dehydration and sealing protocol. For Verhoeff-Van Gieson staining (EVG) staining, the paraffin sections were dewaxed to hydration and stained with EVG (R20391–3, Yuanye Bio-technology, Guangzhou, China). For Masson staining, the paraffin sections were dewaxed to hydration and stained with Masson trichrome (G1340, Solarbio Life Science, Beijing, China). For intestine, tissues were fixed in paraformaldehyde (4%, PFA) and sectioned (5 μm) using a Vibratome (Leica VT12000S).
Measurement of aortic diameter
The maximum aortic diameter in the representative images of each group of mice was measured using ImageJ version 1.8.0 software, based on the scale bar.
Immunofluorescence and immunohistochemistry
To prepare paraffin sections, human and mouse aorta samples underwent a dehydration process, followed by paraffin embedding and sectioning into thin slices. Following standard histological procedures, the sections were subjected to deparaffinization and antigen retrieval. Subsequently, immunohistochemical staining was conducted utilizing the UltraSensitive SP IHC Kit (KIT-9710, MXB, Fuzhou, China), which is specifically designed for high-sensitivity detection of mouse or rabbit primary antibodies. Anti-MMP2 antibody (10373-2-AP, Proteintech, Wuhan, China), anti-MMP9 antibody (10375-2-AP, Proteintech, Wuhan, China) were used to detect protein expression in the aorta tissues. Images were captured at 200× with an Olympus immunofluorescence microscope. Quantification of the relative intensity of protein staining was performed by automated image analysis in five randomly chosen 40× fields for each sample. The immunohistochemical analysis followed the formula for average optical density, calculated as IOD divided by the area of positive pixels. This method reflects the average depth of positive signals, using the intensity of the positive signals to evaluate their strength.
For immunofluorescence analysis of tissues, aortic and intestinal tissue paraffin blocks were obtained and sectioned into 5 μm slices. Primary antibodies anti-F4/80 antibody (30325S, Cell signaling technology, Massachusetts, USA), anti-COL1A11 antibody(72026S, Cell signaling technology, Massachusetts, USA), anti-FN1 antibody(ab2413, Abcam, Massachusetts, USA), anti-Cers2 antibody(ab315452, Abcam, Massachusetts, USA), anti-CD68 antibody(ab303565, Abcam, Massachusetts, USA) were incubated overnight at 4 °C. The next day, corresponding species-specific fluorescent secondary antibodies (Alexa Fluor 488 and tetramethylrhodamine) were incubated for 2 h at room temperature. DAPI solution (C0065, Solarbio Life Science, Beijing, China) was then added to stain the nuclei for 10 min. The slides were then sealed with an antifade mounting medium (36307ES08, Yeasen, Shanghai, China) to prevent fading and maintain tissue integrity. Immunofluorescence images were acquired using a fluorescence microscope. ImageJ software was employed to quantify the average fluorescence intensity of protein expression in each image, with all analyses conducted under consistent parameters to ensure comparability.
RNA extraction, reverse transcription and RT-QPCR
RNA was extracted by TRIzol reagent (Invitrogen, New York, USA). The reverse transcription process follows the instructions of the reverse transcription reagent (RR037A, Takala, Tokyo, Japan). RT-QPCR also follows the relevant reagent instructions (R011, Takala, Tokyo, Japan). The target gene primers are shown in (Supplementary Table 1).
Cell culture
Mouse primary enterocytes and BMDM were isolated from the mouse body using the separation method described before. Cultivating primary enterocytes using complete culture medium (CM-M045, Procell, Wuhan, China). Vascular smooth muscle cell (VSMC; CP-M076, Procell, Wuhan, China) were cultured in vascular smooth muscle cell complete medium (CM-M076, Procell, Wuhan, China). Fibroblasts (CP-M077, Procell, Wuhan, China) were cultured in fibroblast complete medium (CM-M077, Procell, Wuhan, China). Vascular endothelial cells (BNCC317467, BNCC, Henan, China) were cultured in vascular endothelial cell complete medium (BNCC368023, BNCC, Henan, China). To investigate the functional effects of C20 ceramide on macrophages, vascular smooth muscle cell (VSMC), fibroblast and endothelial cell (EC), we utilized C20 ceramide (860520 P, Sigma-Aldrich, Darmstadt, Germany) to directly stimulate bone marrow-derived macrophages (BMDMs), VSMC, fibroblast and EC. Harvest BMDM, VSMC, EC and fibroblast after 1 day and perform RNA extraction. For C20 ceramide pre-stimulation BMDM experiment. We pre-stimulated BMDM with C20 ceramide (100 ng/ml) for 1 h, then discarded the culture medium and switched to LPS (100 ng/ml, HY-D1056, MCE, Shanghai, China) to stimulate BMDM. However, for the control group, LPS (100 ng/ml) was directly used to stimulate BMDM. Harvest BMDM in 1 days. For cell co-culture experiments, We first transferred primary enterocytes in the subventricular layer of Transwell (05522012, Corning, New York, USA). When the cell density reached 70–80%, we stimulated the primary enterocytes with GW4064 (10 μmmol, HY-50108, MCE, Shanghai, China) or 0.05% DMSO for 2 days. Meanwhile, BMDM, VSMC, vascular endothelial cell and fibroblast was administered to the upper layer of the Transwell transfer chamber. Primary enterocytes were pretreated with the FXR agonist GW4064 (10 μM) for 48 hours prior to co-culture with BMDM. Following co-culture, RNA was isolated from BMDM. For analysis of ceramide metabolism enzymes, primary enterocytes were initially plated in 6-well plates. After the cell density reached 70% -80%, primary enterocytes were stimulated with GW4064 (10 μM). The vehicle group received DMSO (HY-Y0320, MCE, Shanghai, China).
Western blot analysis
Ileal and aortic tissue segments were mechanically homogenized using RIPA buffer supplemented with protease and phosphatase inhibitors. Following protein extraction, samples underwent SDS-PAGE separation and electrophoretic transfer to PVDF membranes. Membranes were subjected to overnight incubation with primary antibodies at 4 °C.
Chip-seq
C57BL/6 mice were fed FXR agonist GW4064 (80 mg/kg/d) for Chip seq. ChIP DNA fragments accompanied with the FXR antibody were amplified using the Illumina ChIP seq DNA Sample Prep Kit (San Diego, California, USA). DNA libraries were sequenced on a Genome Analyzer II by Illumina Sequencing Services. After sequencing, data files were processed through Genome Analyzer Pipeline Software (Illumina) and mapped to the genome. Enriched intervals, referred to as peak values, were defined when a given region appeared more than 20 times (a conservative threshold was arbitrarily set at >20). The distribution of FXR binding sites relative to the transcription start site (TSS) was analyzed by JMP 7.0. Analysis was done to determine the average peak value of FXR binding as well as to determine the total number of FXR binding events in relation to distance of site from a gene TSS.
Chip-qPCR
ChIP assays were performed on mice intestines (n = 4) using EZ CHIP KIT (Millipore, Temecula, Canada) following a similar protocol as described above. qPCR reactions were carried out using Maxima SYBR Green (Fermentas, Glen Burnie, Maryland, USA).
Enzyme-linked immunosorbent assay
The serum of AAD patients comes from before surgery. Mice blood were taken from the inner eye canthus for five consecutive weeks and plasma was obtained by centrifugation. Plasma levels of FGF19/15, MMP2, MMP9, TNF-α, IL-12, MCP-1, IL-10, IL-6, IFN-γ are measured by enzyme-linked immunosorbent assay (ELISA). All samples were tested without any dilution.
Ceramide analysis
Obtain blood from normal mice and AAD mice, then centrifuge 900 g for 10 min to obtain mouse serum. The composition and concentration of ceramide were analyzed using ultra-high performance liquid chromatography-mass spectrometry (UPLC-MS) as previously described19.
Bile acid analysis
Serum samples were prepared using a precipitation method. Chlorpropamide (Sigma-Aldrich, Cat# C1290) was added as an internal standard for the quantification of bile acid levels. The bile acid concentrations in the supernatants were determined using an UPLC/Synapt G2-Si QTOF MS system (Waters Corp., Milford, MA) equipped with an ESI source for detection. Chromatographic separation was performed on an Acquity BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm, Waters Corp.), with the column temperature maintained at 45 °C and a flow rate of 0.4 ml/min. The mobile phase consisted of a mixture of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B), with a gradient elution program applied. MS detection was conducted in negative ion mode, and the mass range was set to m/z 50–850. For accurate identification of bile acid metabolites, standards for all bile acids were used to validate the LC-MS findings.
Co-immunoprecipitation (Co-IP)
Briefly, Ileum tissues from normal mice and AAD mice were collected and lysated in IP lysis buffer with 6 the addition of PMSF (protease inhibitor, ST505, Beyotime, China). All procedures were carefully executed on ice. The resulting supernatants were incubated with agarose-conjugated anti-RXR (21218-1-AP Proteintech, WuHan, China) or IgG (AC005, ABclonal, Wuhan, China) overnight at 4 °C. The immunoprecipitated beads were then washed three times, eluted with 1X loading buffer (P0015A, Beyotime, China), and boiled at 99 °C for 5 min. Upon the removal of beads, these samples were subjected to Western blot analysis and probed with the anti-FXR (72105S Cell signaling technology, MA, USA).
Lipidomics analysis
Firstly, euthanize the mice, and then obtain the mouse ileum tissue. Then, sequencing analysis was performed on the mouse ileum tissue. The analysis was conducted by Hangzhou Lianchuang Biotechnology Co., Ltd. (Hangzhou, China).
Cell transient transfections
The small interfering RNA against Cers2 (SiCers2) and the negative controls were obtained from Shanghai Hanbio Biotechnology. For siRNA experiments, SiCers2(#1 5′-GGAACAGAUCAUCCACCAUtt-3′; #2 5′-GCAUUGCCUCUGAUGUCAAtt-3′) was transfected into primary enterocytes. Transient transfection was performed using Lipofectamine 3000 according to the product instructions.
Luciferase assay
HCT116 cells (ATCC, CCL-247) were transfected with mouse FXR/RXR expression vectors, pGL4.27 luciferase plasmids and a Renilla luciferase control vector (pRL-luciferase, Promega) using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). After 24 h, the cells were exposed to DMSO or 10 μM GW4064. Luciferase assays were performed using a dual-luciferase assay system (Promega).
Statistics and reproducibility
We used GraphPad Prism software (version 8.0) to analyze the data without understanding the grouping situation. All experimental data are reported as the mean ± SEM. Two-sided Student’s t tests, Mann-Whitney U test, one-way ANOVA with Tukey’s post hoc test, Kruskal-Wallis test followed by Dunn’s test, or Pearson’s correlation analysis were applied for the mouse and clinical human samples. Normality testing was conducted using the Shapiro–Wilk test. For data following a normal distribution, two-group comparisons were analyzed using the unpaired t-test, while multiple-group comparisons were evaluated via one-way ANOVA, assuming homogeneity of variances. In cases where data did not conform to a normal distribution, non-parametric tests were employed: the Mann–Whitney U test for two-group comparisons and the Kruskal–Wallis test for multiple-group comparisons. Post hoc analyses were performed using Dunn’s multiple comparisons test for the Kruskal–Wallis test and Tukey’s multiple comparisons test for the one-way ANOVA to adjust for multiple comparisons. All data points in the bar graphs represent actual values from individual biological samples, with no data points excluded. The incidence of AAD across groups was analyzed using the chi-square test, and survival curves were compared using the log-rank test. Statistical significance was defined as P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The ChIPseq data have been deposited in Gene Expression Ominubus (GEO) with accession numbers GSE280869. The source data for the graphs analyzed in this study can be found in Supplementary data 1. The full blot images for all western blot analyses are included in Supplementary Fig. 15. All other data are available from the corresponding author.
References
Erbel, R. et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2873–2926 (2014).
Cui, H. et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur. Heart J. 42, 4373–4385 (2021).
Ohira, S., Cameron, D. E., Lansman, S. L. & Spielvogel, D. Complex Bentall Operation: Clinical Pearls to Standardize the Procedure. Ann. Thorac. Surg. 119, 744–754 (2025).
Rylski, B., Schilling, O. & Czerny, M. Acute aortic dissection: evidence, uncertainties, and future therapies. Eur. Heart J. 44, 813–821 (2023).
Jia, W., Xie, G. & Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15, 111–128 (2018).
Thibaut, M. M. & Bindels, L. B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med 28, 223–236 (2022).
Vítek, L. Bile Acids in the Treatment of Cardiometabolic Diseases. Ann. Hepatol. 16, s43–s52 (2017).
Mayerhofer, C. C. K. et al. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail 23, 666–671 (2017).
Sheng, Y., et al. Dan-shen Yin promotes bile acid metabolism and excretion to prevent atherosclerosis via activating FXR/BSEP signaling pathway. J. Ethnopharmacol. 330, 118209 (2024).
Xia, Y. et al. Adiponectin determines farnesoid X receptor agonism-mediated cardioprotection against post-infarction remodelling and dysfunction. Cardiovasc Res 114, 1335–1349 (2018).
Levi, M. Role of Bile Acid-Regulated Nuclear Receptor FXR and G Protein-Coupled Receptor TGR5 in Regulation of Cardiorenal Syndrome (Cardiovascular Disease and Chronic Kidney Disease). Hypertension 67, 1080–1084 (2016).
Guo, Y., Luo, T., Xie, G. & Zhang, X. Bile acid receptors and renal regulation of water homeostasis. Front Physiol. 14, 1322288 (2023).
Herman-Edelstein, M., Weinstein, T. & Levi, M. Bile acid receptors and the kidney. Curr. Opin. Nephrol. Hypertens. 27, 56–62 (2018).
Choi, R. H., Tatum, S. M., Symons, J. D., Summers, S. A. & Holland, W. L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 18, 701–711 (2021).
Laaksonen, R. et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016).
Zhang, X. et al. Alkaline ceramidase 1-mediated platelet ceramide catabolism mitigates vascular inflammation and abdominal aortic aneurysm formation. Nat. Cardiovasc Res 2, 1173–1189 (2023).
Chaurasia, B. & Summers, S. A. Ceramides in Metabolism: Key Lipotoxic Players. Annu Rev. Physiol. 83, 303–330 (2021).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest 125, 386–402 (2015).
Wu, Q., et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Invest 131, e142865 (2021).
Chung, S. W. et al. Additional fibrate treatment in UDCA-refractory PBC patients. Liver Int 39, 1776–1785 (2019).
Girisa, S. Targeting Farnesoid X receptor (FXR) for developing novel therapeutics against cancer. Mol Bio Med 2, 21 (2001).
Martorell, S. et al. Vitamin D Receptor Activation Reduces Angiotensin-II-Induced Dissecting Abdominal Aortic Aneurysm in Apolipoprotein E-Knockout Mice. Arterioscler Thromb. Vasc. Biol. 36, 1587–1597 (2016).
Li, R. et al. EZH2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection. Cell Death Dis. 9, 180 (2018).
Lian, G. et al. Macrophage metabolic reprogramming aggravates aortic dissection through the HIF1α-ADAM17 pathway✰. EBioMedicine 49, 291–304 (2019).
Zhuang, X., et al. Hepatic Abnormal Secretion of Apolipoprotein C3 Promotes Inflammation in Aortic Dissection. J. Am. Heart Assoc. 14, e037172 (2025).
Al-Rifai, R. et al. JAK2V617F mutation drives vascular resident macrophages toward a pathogenic phenotype and promotes dissecting aortic aneurysm. Nat. Commun. 13, 6592 (2022).
Jiang, C., et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Xie, C. et al. An Intestinal Farnesoid X Receptor-Ceramide Signaling Axis Modulates Hepatic Gluconeogenesis in Mice. Diabetes 66, 613–626 (2017).
Yang, H., et al. Metabolomic Profile Reveals That Ceramide Metabolic Disturbance Plays an Important Role in Thoracic Aortic Dissection. Front Cardiovasc Med 9, 826861 (2022).
Jungwirth, E., Panzitt, K., Marschall, H.-U., Thallinger, G. G. & Wagner, M. Meta-analysis and Consolidation of Farnesoid X Receptor Chromatin Immunoprecipitation Sequencing Data Across Different Species and Conditions. Hepatol. Commun. 5, 1721–1736 (2021).
Cutler, R. G. & Mattson, M. P. Sphingomyelin and ceramide as regulators of development and lifespan. Mech. Ageing Dev. 122, 895–908 (2001).
Wang, Y. et al. Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 25, 901 (2024).
Levin, M. C., Andersson, L. & Borén, J. Cardiomyocytes, sphingolipids and cardio myotoxicity. Curr. Opin. Lipido. 34, 180–188 (2023).
Norman, E., Cutler, R. G., Flannery, R., Wang, Y. & Mattson, M. P. Plasma membrane sphingomyelin hydrolysis increases hippocampal neuron excitability by sphingosine-1-phosphate mediated mechanisms. J. Neurochem 114, 430–439 (2010).
Wang, Y. et al. ACKR1hiECs Promote Aortic Dissection Through Adjusting Macrophage Behavior. Circ. Res 136, 211–228 (2025).
Zhang, M. et al. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 14, 100223 (2022).
Wang, Y. et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 11, 1069–1082 (2019).
Ma, J.-D. et al. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res. Ther. 21, 153 (2019).
Zhou, F. et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 9, 973–985 (2019).
An, D., et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol. 46, 102088 (2021).
Zhang, B. et al. Circadian Rhythm-Dependent Therapy by Composite Targeted Polyphenol Nanoparticles for Myocardial Ischemia-Reperfusion Injury. ACS Nano 18, 28154–28169 (2024).
Wang, J. et al. Mitochondria-engine with self-regulation to restore degenerated intervertebral disc cells via bioenergetic robust hydrogel design. Bioact. Mater. 40, 1–18 (2024).
Hirschfield, G. M. et al. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J. Hepatol. 74, 321–329 (2021).
Loomba, R. et al. Multicenter Validation of Association Between Decline in MRI-PDFF and Histologic Response in NASH. Hepatology 72, 1219–1229 (2020).
Payne, T. et al. A Double-Blind, Randomized, Placebo-Controlled Trial of Ursodeoxycholic Acid (UDCA) in Parkinson’s Disease. Mov. Disord. 38, 1493–1502 (2023).
Schattenberg, J. M. et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 74, 1344–1354 (2021).
Cai, D., Sun, C., Murashita, T., Que, X. & Chen, S.-Y. ADAR1 Non-Editing Function in Macrophage Activation and Abdominal Aortic Aneurysm. Circ. Res 132, e78–e93 (2023).
Zhou, X. et al. Identification of Lysophosphatidylcholines and Sphingolipids as Potential Biomarkers for Acute Aortic Dissection via Serum Metabolomics. Eur. J. Vasc. Endovasc. Surg. 57, 434–441 (2019).
Zhang, C. et al. Targeted Inhibition of Matrix Metalloproteinase-8 Prevents Aortic Dissection in a Murine Model. Cells 11, 3218 (2022).
Xu, C. et al. CTRP6 promotes the macrophage inflammatory response, and its deficiency attenuates LPS-induced inflammation. J. Biol. Chem. 300, 105566 (2024).
Acknowledgements
This research was sponsored by the Fujian Provincial Special Reserve Talents Fund (2021-25), Fujian Medical University Startup Fund for scientific research (2022QH2019), Joint Funds for the innovation of science and Technology, Fujian province (2020Y9067) and (2023CZ004) and the National Natural Science Foundation of China (U2005202) (82370470) and (82241209). The Chip-seq detections and analysis were performed by Seqhealth Technology Co. LTD (Wuhan, China), and we deeply thank Yihang Wei from this company for bioinformatic analysis. In addition, the authors thank LC-Bio Technology Co. LTD. Hangzhou, China, especially Shuaitong Chen, for technical assistance with the metabolomics analysis.
Author information
Authors and Affiliations
Contributions
Zhaofeng Zhang, Linfeng Xie and Xinfan Lin: conceptualisation, data curation, formal analysis, writing-original draft, writing-review&editing, Jian He, Jiakang Li and Xinghui Zhuang: data curation, formal analysis, investigation, methodology, Yuling Xie, Lele Tang and Rumei Xie: resources, software, supervision, validation, Qingsong Wu, Zhihuang Qiu and Liangwan Chen: conceptualisation, funding acquisition, project administration, writing, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of Fujian Medical University Union Hospital (No.XH2023-028) and complies with the Helsinki Declaration.
Peer review
Peer review information
Communications Biology thanks Mitra Esfandiarei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Jesmond Dalli and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Xie, L., Lin, X. et al. Ursodeoxycholic acid alleviates aortic aneurysm and dissection through the intestinal farnesoid X receptor/ceramide synthase 2 axis. Commun Biol 8, 1009 (2025). https://doi.org/10.1038/s42003-025-08403-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08403-2