Abstract
Microbes in cold seep water columns are essential for methane sequestration and biogeochemical cycling, yet their structures and ecological functions, particularly at the bottom water interface (BWI), are poorly understood. Here, we performed metagenomic analyses to explore the microbial biodiversity and functions at the F-site cold seep in the South China Sea. Functional stratification revealed that photosynthetic autotrophs dominate surface zones, heterotrophs are prevalent in mesopelagic zones, and chemosynthetic bacteria are abundant at the BWI. We obtained 377 metagenome-assembled-genomes (MAGs) and constructed genome-scale metabolic models to unveil metabolic interactions facilitating the coupling of carbon, nitrogen, and sulfur among microbes, particularly at the BWI. Notably, methanotrophic bacteria with diverse metabolic capabilities distributed from the BWI zone to the deep mesopelagic regions, highlighting the broader influence of methane. In conclusion, our findings reveal a high degree of heterogeneity in the composition and function of microorganisms across the F-site cold seep water column. Our study also sheds light on the ecological interactions and environmental gradients that shape these microbial communities.
Similar content being viewed by others
Introduction
Cold seeps are ecosystems that occur along continental slopes of the world’s oceans. In these areas, geological processes release fluids rich in hydrogen sulfide, methane, and other hydrocarbons from the Earth’s crust. These fluids provide the necessary nutrients and chemical energy to support diverse life forms1. At the bottom-water interface (BWI), the cold seep fluid continuously shapes the local habitat, altering the local environmental characteristics and profoundly impacting the area’s biological composition, especially the microbial community. The high level of productivity in the interface zone can nourish the thriving cold seep community and result in unique landforms such as mussel beds and tube-worm beaches2.
Cold seep fluids are rich in hydrocarbons, and microbial metabolism in these environments is influenced by the availability of essential elements like nitrogen, sulfur, and phosphate. While sharp vertical geochemical gradients in cold seeps have been detected by in situ Raman detectors3, it is still unclear to what extent seepage activity can influence the biotic community in the water column. The distribution and heterogeneity characteristics of microbial communities in cold seep water columns determine the efficiency of methane filtering and element turnover4. Understanding how microorganisms adapt to this chemical-skewed environment is critical for understanding their roles in geochemical recycling.
While microbes in cold seep sediments can consume most hydrocarbons (Jørgensen and Boetius, 2007; Joye, 2020), a substantial amount of methane can still escape into the water, forming bubble flumes that can reach tens5 to hundreds of meters high6. It is estimated that around seven million metric tons of methane (CH₄) are emitted into the atmosphere from submarine seepages worldwide every year7,8. Consequently, hydrocarbons and other reduced materials above seepages influence microbiome community structures9. At the same time, microbes capture methane and affect local carbon cycles4,10. Therefore, the characteristics and functions of microorganisms in cold seep water columns are not only important biological indicators of cold seep activities but also crucial for assessing the potential risk of methane escaping into the atmosphere5. However, there is only limited information available in the literature.
The coupling of biogeochemical recycling by diverse microbes is a key feature of cold seeps11,12,13. Notably, methane-oxidizing bacteria (MOB) and sulfur-oxidizing bacteria (SOB) exhibit functional versatility, engaging in methane oxidation, nitrate reduction, and the oxidation of reductive sulfur compounds under oxygen-limited conditions, which enhances energy conservation14,15,16,17. Some microbes also use methane to drive nitrogen fixation, increasing ecosystem productivity in nutrient-poor areas18. Metabolism in microbiomes is often a collective process where different species complement each other through a network of metabolic interactions19. These synergistic interactions can improve the usage and turnover of elements, benefiting the entire community 20. For example, the study of ectosymbiont communities associated with squat lobsters revealed that interactions between MOB and SOB fostered nutrient cycling and supported ecosystem productivity 21. However, the mechanisms by which microbes in the water column above seepage, particularly at the BWI, adapt and collaborate to overcome nutrient limitations and sustain their metabolic processes remain unclear.
The cold seeps in the southwest Taiwan Strait are among the most actively studied areas in the South China Sea2, particularly the Formosa Ridge (F-site) and Haima cold seep, which support vibrant ecosystems22,23,24. Extensive studies have already been conducted on their physicochemical properties23 and community structures25, making them vital for studying microbiological complexity and global geochemical cycling. While microbial community structures in these cold seeps have been examined, most studies focus on sediments26,27,28,29,30,31, with fewer addressing the water columns. A comparative study on bottom-water prokaryotic communities above the Haima cold seep revealed distinct bottom-water bacterial communities at methane seeps with various seepage intensities9, and prokaryotic species coexistence was influenced by local environmental heterogeneity in the water column4. However, we still lack a comprehensive understanding of how vertical environmental heterogeneity in the cold seep water column influences microbial community compositions and their ecological functions.
In this study, we used a high-throughput water sampling device to accurately filter seawater at specific depths and positions, enabling us to obtain high-quality DNA for metagenomic sequencing. This effort allowed us to investigate the metabolic potential of microbes in the water column above the seepage area and to identify their biodiversity and functions. Metabolic models of metagenome-assembled genomes (MAGs) also provided insights into their cooperative interactions and potential contributions to the ecosystem.
Results and discussion
A stratified microbial community shaped by environmental factors
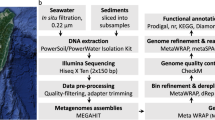
Samples were collected from the cold seep at F-site for environmental parameters detection and metagenomic analysis (Fig. 1A and Supplementary Table 1). The BWI layer samples filtered in situ at the seepage (BWI-0) and 50 cm above the seepage (BWI-50 cm) (Fig. 1B), and seawater samples at various depths (30, 65, 100, 200, 600, 800, 1000, and 1100; designated as SEEP-30 m to SEEP-1100 m) were collected and filtered on deck.
A Regional map showing the location of the F-site cold seep area at in the northeastern continental slope of the South China Sea, constructed with GeoMapApp (www.geomapapp.org). B The high-throughput sampling device is equipped with the ROV FAXIAN and in situ working underwater. A meter stick measurement was used to accurately determine vertical height from the seepage. C A schematic overview of metagenomic analysis was performed in this study.
Key environmental parameters of the seawater samples were detected in situ or onboard (Fig. 2A and Supplementary Table 2). The temperature dropped with increasing depth, from 30.50 °C at the surface to 3.59 °C at the BWI, and the halocline was found between 30 to 100 m depth. Dissolved oxygen (DO) peaked at 30 m (6.20 mg/L) and declined to about 3 mg/L at the interface. Methane (CH₄) dropped sharply from 387.20 nmol/L at the BWI-0 to less than 10 nmol/L at 50 m from the bottom (SEEP-1100 m). The nitrate (NO3−) concentration escalated with water depth, increasing from 0.13 μmol/L at the surface to 27.45 μmol/L at 1100 m depth, then dropping to 22.33 μmol/L near the bottom layer. The concentration of ammonium (NH4+) was relatively stable at the mesopelagic zone but steeply increased from 2.12 μmol/L at a depth of 1100 m to 5.12 μmol/L at the bottom layer. Notably, nitrite (NO2−) increased sharply to 3.22 μmol/L in the interfacial layer. These results indicate a significant bio-coupled nitrogen transformation in the seepage, underscoring the importance of in situ sampling. Despite previous research on cold seep water4,6,9, the geochemical properties of the interface layer are still poorly understood. Previous results based on in situ Raman observations show that methane and sulfide concentrations in the interface layer may be significantly underestimated32. Our results also indicate that the chemical environments at the BWI have undergone significant changes, possibly owing to active microbiological processes.
A Key geochemical factors in the water column. CH4 denotes methane; DO, dissolved oxygen; NO2−, nitrite; NH4+, ammonium. Temperature, salinity, DO, and CH4 were detected in situ by sensors. Nitrate, nitrite, and ammonium nutrients were determined onboard by a QuAAtro continuous flow analyzer. B Bar chart of the relative abundances of class-level taxa in samples of cold seep water columns. C Heat map based on the 20 most abundant orders of the samples. The log10 value of the taxa read number is indicated by the color gradient from blue (low) to red (high). D Redundancy analysis (RDA) of environmental factors influencing microbial community composition. Circles represent the microbial community at each water layer of cold seep columns, and the arrows represent individual environmental parameters. BWI-50 cm could not be accurately measured in situ and is not shown. DO dissolved oxygen, T temperature, S salinity, CH4 methane, NO2− nitrite, NO3− nitrate, NH4+ ammonium.
Based on metagenomic analysis (Fig. 1C), a total of 7736 Normalized Taxonomic Units (NTU) classification labels were obtained using phyloFlash33. Proteobacteria (Alphaproteobacteria and Gammaproteobacteria) were abundant in all samples (accounting for 28.2%–60.1%), especially in the water column between 200 and 800 m depths, a pattern that was also documented in the Haima cold seep in a previous study4 (Supplementary Table 3). Based on the class-level taxonomic composition, the water column at the seepage could be subdivided into three partitions: the BWI (including BWI-0 and BWI-50cm), the mesopelagic zone (SEEP-200 m, SEEP-600 m, SEEP-800 m, and SEEP-1000 m), and the surface zone (SEEP-100 m, SEEP-65 m, and SEEP-30 m) (Fig. 2B). Among the 20 most abundant orders (Fig. 2C and Supplementary Table 4), chemosynthetic microbes, including MOB of Methylococcales, SOB of Thiotrichales and Campylobacterales clearly dominated the BWI (BWI-0 and BWI-50 cm) of the seepage. Heterotrophic Bacteroidales also showed high abundance at the BWI and likely obtain energy and nutrients by breaking down organic matter34,35. Within the mesopelagic zone, the orders Nitrosopumilales, SAR11, Marinimicrobia SAR406, and Pseudomonadales were dominant. In the euphotic layers, SAR11 was the most dominant lineage, accounting for more than 20% of the samples at a depth of 30 m, followed by Synechococcales and Pseudomonadales (Supplementary Table 4).
Redundancy analysis (RDA) was performed to identify environmental factors that may shape the microbial community across the cold seep water column (Fig. 2D). The two main axes accounted for 82.9% of the variance in microbial community composition. Samples from the BWI positively correlated with CH4, NO2−, and NH4+. Temperature and DO were the most important explanatory variables for surface sites. A coupled conversion of carbon and nitrogen suggested a strong link between these biogeochemical cycles at the BWI. Consistent with previous studies4, the vertical geochemical gradients in the water column dictated microbial abundance, highlighting the role of environmental selection in shaping microbial communities.
Our dataset is generally comparable to other deep-sea water column studies, including datasets from the Black Sea36, the Mediterranean Sea37,38, and the Malaspina Gene DataBase35. The microbial communities at the BWI were distinct from those of the cold seep sediments but contained some signatures of these habitats39,40. Chemosynthetic bacteria, the hallmark microbe of the surface sediments, were also predominant lineages at the BWI. The co-occurrence of heterotrophic microbes with high abundance suggests that organic carbon is utilized efficiently, possibly by public goods sharing (see ref. 21). Although all were fueled mainly by methane, the F-site seepage community structure differed significantly from those at the Haima cold seeps4,9, which lacked chemosynthetic bacteria at the BWI, possibly owing to the different chemical compositions at the F-site. These results underscore the influence of specific environmental factors on microbial community composition in cold seep ecosystems.
Microbial co-occurrence networks in different water zones of cold seep
To further explore the interaction between the dominant microbes in different water cold seep zones, we carried out a network analysis of the most abundant 600 NTUs (relative abundance >0.01%) (Fig. 3). The constructed network comprised 548 nodes and 13,016 edges, with an overall network connectivity of 0.087, suggesting a relatively dense arrangement of connections among the species. The network’s modularity was 0.589, much higher than that of the random network (0.088), indicating that the nodes in the network tend to form clusters. The dominant species formed six main modules (Module 1 to Module 6) across all water samples (Fig. 3A). Three of these modules correspond to the dominant microbial groups in distinct regions, as evidenced by the relative abundance shown in the Fig. 3B heat map.
A Module 1 contained 198 nodes and 7068 edges, Module 2 contained 146 nodes and 3387 edges, and Module 3 contained 118 internal nodes and 2254 edges. Each node is colored by different phyla. The size of each node is proportional to the number of connections. Edges represent co-occurrence relationships that were consistent at the 0.75 correlation level and false discovery rate P < 0.05. B The heat map shows each module’s relative microbial community abundance in different samples. C Taxonomic composition of microbes in each module at the order level.
Module 1 revealed the co-occurrence of surface water dominant taxa, including photosynthetic Synechococcales, SAR11 bacteria, MGII archaea, Pseudomonadales, Actinomarinales, and Rhodospirillales. Module 2 is formed by the microbes of mesopelagic zones, such as Nitrosopumilales, Pseudomonadales, UBA10353, and SAR202 clade as the dominant taxa (Fig. 3C). More connections were found between Module 2 and Module 1 than between Module 2 and Module 3, suggesting that mesopelagic zones may be influenced marine snow from surface zones. Module 3 is formed by the co-occurrence networks of microbes at the BWI, especially Campylobacterales, Flavobacteriales, Thiotrichales, and Methylococcales (Fig. 3C). These potential interactions suggest their possible cooperation in metabolic processes, such as ammonia oxidation and sulfide detoxification reported in squat lobsters21. Previous research highlighted a close relationship between chemosynthetic microorganisms and heterotrophic taxa41. Heterotrophic microorganisms may survive on the leakage of organic molecules or establish symbiotic relationships with autotrophic species. This may constitute a cost-effective strategy for microorganisms to persist in the dark ocean; however, its extent in the global deep ocean is unknown35.
Ecological niche differentiation of Methylococcales in the cold seep water column
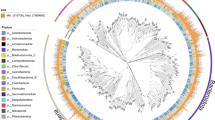
We constructed MAGs from the metagenomic data and investigated the relative abundance of key microbial groups across different water layers. After trimming, dereplication, and assembly, the contigs were clustered into 377 MAGs (average nucleotide identity, ANI, ≤95%, completeness ≥70%, and contamination ≤20%), covering 355 bacterial and 22 archaeal genomes from 33 known or candidate phyla. Their average completeness and contamination were 82.38% and 4.32%, respectively (Fig. 4A and Supplementary Table 5). There were 92 high-quality MAGs, with completeness ≥90% and contamination ≤10%. The 377 MAGs exhibited significant taxonomic novelty, with more than 81% of archaeal MAGs and 76% of bacterial MAGs representing unnamed species.
A A phylogenomic tree derived from 377 MAGs based on 23,826 orthologous genes found by OrthoFinder. Their size, completeness, contamination, and relative abundance in each sample are indicated by different colors. Among the MAGs, the phyla Proteobacteria (n = 163, containing 84 Gammaproteobacteria and 27 Alphaproteobacteria), Actinobacteriota (n = 27), and Bacteroidota (n = 24) were most in bacteria, while Thermoplasmatota (n = 21) predominated in archaea. At a 95% ANI new species threshold, the MAGs show significant taxonomic novelty, with more than 81% of archaeal MAGs and 76% of bacterial MAGs representing new species, and 199/377 without a corresponding reference genome. The red branches represent Methylococcales, Thiotrichales, Nitrosococcales, Campylobacterales, and Bacteroidales; orders with higher abundance at the BWI. B Heat map illustrating the relative abundance of 18 Methylococcales MAGs across different sample sites, and the presence of the key genes involved in methylotrophic metabolism. The MAGs are labeled by their respective genera. Notably, the genus UBA1147 is classified under the family UBA2278, while all other genera belong to the family Methylomonadaceae. pmo particulate methane monooxygenase, mmo methane monooxygenase, mdh methanol dehydrogenase, xoxF lanthanide-dependent methanol dehydrogenase, hxl 3-hexulose-6-phosphate synthase, fae 5,6,7,8-tetrahydromethanopterin hydro-lyase, mtd methylenetetrahydromethanopterin dehydrogenase, mch methenyltetrahydromethanopterin cyclohydrolase.
Regarding the relative abundance of the MAGs, it is evident that most microorganisms occupy unique ecological niches. For instance, the microbes from orders Methylococcales, Thiotrichales, Nitrosococcales, Campylobacterales, and Bacteroidales had a significantly higher abundance at BWI than in other layers. Previous studies revealed that Methylococcales are generally found at much lower abundance or not detected in common open ocean environments42,43,44,45. In contrast, in cold seeps or mud volcanoes, methane generally impacts the water column at depths of 50–100 m from the seafloor5,38,42,46. Similarly, our metagenomic analysis revealed a significant presence of Methylococcales at the BWI and mesopelagic layers deeper than 800 m, with genera dominating distinct layers (Fig. 4B). At the BWI, the primary genus identified within order Methylococcales was an unclassified member of QPIN01, also known as Marine Methylotrophic Group 2. This genus has been associated with microbial communities in bottom waters near methane spill sites and as biosymbionts, indicating a preference for environments with high methane concentrations40. In the mesopelagic zone, around 800 m depth, an unidentified genus UBA1147 (IheB2-23) presented as the dominant Methylococcales species, which contained the particulate methane monooxygenase genes, along with genes necessary for formaldehyde assimilation (Fig. 4B). UBA1147 MAGs with potential methylotrophic capabilities have also been identified in methane seeps in the southeastern Mediterranean Sea and the South China Sea47,48. Our findings further highlight the ongoing influence of methane seeping from the seafloor, which can reach more than 500 m49. The stratification of Methylococcales according to methane concentration gradients in marine environments reflects their diverse metabolic strategies and ecological niches, allowing for efficient methane aerobic oxidation and methane filtering.
The metabolic potential and interaction of water column microbes
The gene set enrichment analysis (GSEA) enrichment analysis in the surface zone revealed high gene abundances for processes such as photosynthesis, fatty acid and steroid biosynthesis pathways, secondary metabolite production, and lipid utilization (Supplementary Tables 6 and 7). This suggests that photosynthetic functions and heterotrophic bacteria are crucial at the surface layers. In the mesopelagic zone, there was a notable increase in genes for organic carbon degradation and a corresponding decrease in genes for autotrophic carbon fixation, indicating a shift toward heterotrophic microbial communities that are especially evident at depths of 800 to 1000 m. In the BWI zone, there was a notable abundance of Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology terms (KOs) related to hydrocarbon metabolism (particularly genes for CH₄ oxidation, alkene and aromatic compound degradation, and short-chain olefin oxidation), aligning with high hydrocarbons input fluxes in the seepage (Fig. 5A and detailed in Supplementary Fig. 1, Supplementary Tables 6 and 7). Additionally, significant primary productivity is indicated by a high abundance of genes associated with inorganic CO2 fixation pathways, particularly the reverse citrate cycle (rTCA) and Wood-Ljungdahl (WL) pathways, along with hydrogenase and sulfur oxidation pathways (SOX system) contributing to carbon sequestration. The over-representation of nitrogen metabolism suggests active nitrogen cycling processes, including nitrogen fixation, highlighting carbon-nitrogen coupling at the BWI layer, which is crucial for substrate utilization rate in cold seeps20,47,48. Enriched pathways for flagellar assembly and biofilm formation indicate the coexistence of biofilms and free-living forms, while abundant genes related to infection processes suggest extensive microbial interactions with invertebrates due to common symbiotic relationships3,50,51.
A Relative abundance of mentalism-associated genes in each sample. Relative abundance in each sample is in Table S8. B The metabolic potential of major MAGs. The leftmost column shows the relative abundance of MAGs in each order. The upper triangles represent the maximum module completeness value among all the MAGs of the order, suggesting the highest metabolic potential of microbes within that order, while the lower triangles display the median completeness of the MAGs of the order, indicating the typical metabolic potential of most microbes in that order. Information on the modules is in Table S9. COX cytochrome C oxidase, CBB Calvin-Benson-Bassham cycle, rTCA reverse citrate cycle, FA fatty acid, DNRA dissimilatory nitrate reduction to ammonium, SOX sulfur-oxidizing system, DSR dissimilatory sulfite reduction.
The potential metabolic processes of each MAG reveal clear overlaps among dominant MAGs in the surface and mesopelagic zones (Fig. 5B). Despite relatively high oxygen levels, many taxa in the surface and mesopelagic zones have lost oxygen oxidoreductase in the respiratory chain, preventing them from using oxygen as an electron acceptor. In the surface zone, only MAGs belonging to the order PCC-6307 (in the phylum Cyanobacteria) possessed a complete photosystem I (M00163) and partial Calvin-Benson-Bassham cycle (M00166), confirming their photoautotrophic status. However, other taxa (such as Poseidoniales and Actinomarinales) contained genes for light-driven proton pumps but lacked carbon-fixing modules, indicating their involvement in photoheterotrophic processes. They may partially utilize light energy to produce ATP for other cellular functions52.
Compared to upper water volumes, the BWI harbors a unique chemoautotrophic community that derives energy from reducing compounds released by seeping fluids (Fig. 5B). This process is likely primarily carried out by Methylococcales, which possess a complete methane oxidation pathway within their MAGs (M00174, methanotroph). As observed in the taxonomic compositions and heat map of key metabolic genes, MOB were found to have relatively high abundance at the BWI and mesopelagic layers deeper than 800 m (Figs. 2, 4B and 5A). However, the completeness of modules for sulfur oxidation and nitrate respiration in Methylococcales MAGs in mesopelagic layer was much lower than those in the BWI zones (Fig. 5B). This suggests that the coupling of nitrate reduction with methane and sulfide oxidation is a complex interplay of biogeochemical processes, and that this coupling is more prevalent in the BWI zones than in mesopelagic and surface waters. Additionally, Nitrosococcales participate in the methylotrophic process, possibly relying on methanol leaked as an intermediate from Methylococcales21. Sulfide oxidation coupled with carbon dioxide fixation appears common, mainly conducted by SOB members of Thiotrichales and Campylobacterales, utilizing the CBB plus reverse dissimilatory sulfite reductase (DSR) and rTCA plus SOX modules, respectively. Another interesting result in the MAGs from the BWI zone was their metabolic potential. For example, complete sulfur-oxidizing modules were found in MAGs belonging to Methylococcales, known as methanotrophic bacteria. They can also utilize nitrate through denitrification and dissimilatory nitrate reduction to ammonium (DNRA) and oxygen as electron acceptors. This versatility was also present in MAGs from various dominant taxa at the BWI zone. For instance, the genes sqr (sulfide:quinone oxidoreductase) and fccB (sulfide dehydrogenase [flavocytochrome c] flavoprotein chain) were widespread across the genomes of dominant taxa such as Bacteroidales, Flavobacteriales, Pseudomonadales, and Burkholderia, suggesting that these heterotrophic bacteria possess significant metabolic versatility in environments rich in sulfur compounds, potentially providing them with a competitive advantage.
Potential metabolic interactions and dependencies within communities
Based on the genome-scale metabolic modeling derived from MAGs (metaGEMs) construction, the competitive and cooperative potential of microbial populations inhabiting different water layers was predicted using the species metabolic interaction analysis (SMETANA)53,54, measured by metabolic resource overlap (MRO) and metabolic interaction potential (MIP) scores (Fig. 6A). Generally, the MRO scores were higher in the SEEP-100 m and SEEP-200 m communities than in other layers, exhibiting high nutrient requirement similarities (competition). In contrast, MIP scores were highest in the communities associated with the BWI zone (BWI-0 and BWI-50 cm), indicating a stronger metabolic relationship in the seepage compared to the mesopelagic and surface zones.
A The MRO and MIP interactions are analyzed based on different community sizes. MRO (metabolic resource overlap) quantifies competition, the similarity of the nutritional requirements between all species in a community. The smaller the MRO, the more complementary the microbial metabolisms are to each other. MIP (metabolic interaction potential) quantifies cooperation, the number of metabolites that can be exchanged among the community members to decrease their dependency on the abiotic environment. Alluvial diagrams show metabolic compounds exchanged between main MAGs in surface (B), mesopelagic (C), and BWI (D) of the cold seep water columns. The left column on the diagram represents the metabolic substrate donor orders, and the right column represents the receiver orders. The colors are used only to distinguish the distinct components of the alluvial diagrams.
Alluvial diagrams illustrate (Fig. 6C, D) the metabolic interactions among microbes in the surface, mesopelagic, and BWI water layers at the seepage. Distinct dependencies on simple compounds, particularly small organic molecules and inorganic ions (e.g., PO₄³−, Fe³⁺, O₂, NO₂−, NH₄⁺, H₂S), are exchanged across these layers. In the surface layer (Fig. 6B), Poseidoniales and Acidimicrobiales are major donors of inorganic nutrients such as PO₄³−, Fe³⁺, and H₂S, while Pseudomonadales and Marinimicrobia contribute small organic molecules. In the mesopelagic zone (Fig. 6C), key donor groups such as Acidimicrobiales, Marinimicrobia, and Enterobacteriales provide metabolites like PO₄³− and H₂S to other groups. The BWI zone’s metabolic landscape is simpler, with fewer substrates and stronger interactions among specific microbial groups, especially MOB and SOB (Fig. 6D). Thiotrichales donate thiosulfate and sulfur compounds, while Methylococcales donate and receive substrates like NO₂− and O₂. This zone reflects the stable metabolic environment typical of cold seep habitats. H₂S exchange decreased significantly in the BWI, likely due to its high availability.
Microbiome metabolism is essential for vital ecosystem functions, as individual members often perform only partial substrate catabolism or lack full metabolic capabilities for growth. Instead, they complement each other by exchanging metabolic intermediates and cellular building blocks to achieve collective metabolism19. At the BWI, elemental coupling results from imbalances fostering metabolic complementarity among dominant microbes, particularly in carbon, nitrogen, and sulfur cycling 20. MAGs and metaGEMs analysis suggest a possible cooperation between diverse nitrogen metabolism and the oxidation of reducing agents such as methane (Figs. 5A and 6). In the MAGs of MOB and other prevalent bacteria, nitrate reduction serves as an electron acceptor, facilitating methane consumption in seep fluids. This integrated process is also observed in MOB from anoxic lakes14,15,55, ensuring sustained methane filtration under hypoxic conditions. In our study, the distinct metabolic profiles observed across different water zones illustrate the complex ecological dynamics shaped by nutrient availability and environmental conditions. Notably, the intricate metabolic exchanges highlight a synergistic relationship between MOB and SOB, fostering a resilient and efficient nutrient cycling system.
In conclusion, our study contributes to the knowledge of microbial community structure and their ecological function in cold seeps. We present a unified picture of the vertical stratification and the important metabolic roles of microbial communities in the water columns of the F-site cold seep in the South China Sea (Fig. 7). The vertical structure of these communities is characterized by distinct ecological roles: an autotrophic community dominates the surface and BWI, and heterotrophic bacteria dominate mesopelagic water columns. A total of 377 MAGs, representing 33 phyla, revealed a rich diversity of taxa, including chemosynthetic bacteria that utilize methane as substrates, indicating a potential for carbon sequestration through the CBB or rTCA cycle. In addition, alternative energy sources like sulfide and hydrogen at the BWI water layer highlight the metabolic versatility and close cooperation between these microbes. The coupling of nitrate reduction with methane and sulfide oxidation suggests a complex interplay of biogeochemical processes. The presence of aerobic methane oxidation in mesopelagic waters, alongside the dominance of heterotrophic bacteria, underscores the community’s adaptability. Ours is the first of, hopefully, many studies to characterize the microbial diversity of the world’s cold seeps.
The environmental parameters of the seawater samples were measured either in situ or onboard (Fig. 2A and Supplementary Table 2). The metabolic potential and cooperation in the surface zone, mesopelagic zone, and the BMI were inferred from the functional modules of MAGs (Fig. 5). The microbial groups with the highest abundance participating in each process are indicated by yellow circles. The numbers within the yellow circles indicate the following: 1. Rhodobacterales; 2. Cyanobacteria; 3. Rhodobacterales and Pseudomonadales; 4. Pseudomonadales; 5. SAR324; 6. SAR324, Pseudomonadales, Pesosphaerales and Rhodobacterales; 7. Rhodobacterales and Enterobacterales; 8. Pseudomonadales and Enterobacterales; 9. Methylococcales; 10. Enterobacterales; 11. Acidimicrobiales, Nitrososphaerales and Pedospharales; 12. Nitrosococcales; 13. Pseudomonadales and Thiotricales; 14. Campylobacterales and Thiotricales; 15. Thiotricales; 16. Campylobacterales and Methylococcales; 17. Campylobacterales, Methylococcales, Thiotricales, Bacteroidales, and Flavobacteriales; 18. Campylobacterales; 19. Burkholderiales, Nitrososphaerales, and Thiotricales; 20. Flavobacteriales and Bacteroidales; 21. Bacteroidales and Flavobacteriales.
Materials and methods
Sample collection
Samples were collected from the cold seep at F-site (22.12°N and 119.28°E, at ~1150 m water depth) during the single scientific cruise of the Research Vessel KEXUE from May to June 2020 (Fig. 1A and Supplementary Table 1). The BWI layer samples were in situ filtered (0.22 µm pore size, Millipore, USA) at the venting seepage (BWI-0) and 50 cm above the seepage (BWI-50 cm, measured by a meter ruler) using a high-throughput sampling device attached to the ROV FAXIAN (Fig. 1B). Seawater samples at various depths (30, 65, 100, 200, 600, 800, 1000, and 1100 m; designated as SEEP-30m to SEEP-1100m) were collected using a Niskin sampler and filtered on deck with a 0.22 µm pore size filter (Millipore, USA). Approximately 100 L of water was filtered in each sampling layer, and the filters were stored at −80 °C until DNA extraction.
Environmental parameters detection
Key environmental parameters of the seawater samples—temperature, salinity, DO, and methane—were measured in situ by our mini-sensor system as soon as sampling 23. Temperature, salinity, DO, and dissolved methane of surface and mesopelagic water layers were measured using an SBE 911 conductivity-temperature-depth (CTD) sensor equipped with an SBE 43 DO sensor (Sea-Bird Electronics, USA) and a CONTROS HydroC CH4 sensor (Kongsberg Gruppen, Norway). At the BWI, the ROV manipulator arm accurately held a multi-sensor system at the seepage, which included an SBE 25 plus CTD (Sea-Bird Electronics, USA), a miniaturized RINKO I DO probe (JFE Advantech, Japan), and a CONTROS HydroC® CH4 sensor (Kongsberg Gruppen, Norway). Due to the heaviness of the mini-sensor system, the manipulator arm of the ROV had difficulty maintaining a stable position at the 50 cm height above the seepage. As a result, we did not obtain reliable and stable values for the in situ measurement of the sample BWI-50cm. The concentrations of NH4+, NO3−, and NO2− in the seawater were determined onboard using a QuAAtro continuous flow analyzer (SEAL Analytical, Germany).
DNA extraction and metagenomic sequencing
The schematic overview of metagenomic analysis is shown in Fig. 1C. The genomic DNA from the 0.22 μm filters was extracted using the PowerWater DNA Isolation Kit (QIAGEN, China). The DNA samples were examined using gel electrophoresis and quantified with the Qubit dsDNA Assay Kit (Life Technologies, USA). Metagenomic sequencing was performed at Novogene (Tianjin, China) on the Illumina HiSeq X Ten platform (Illumina, USA). The raw data was preprocessed using fastp to remove low-quality (Q15) or ambiguous bases (5%).
Microbial diversity and interaction network
To reconstruct the microbial community, the PhyloFlash v3.3b2 pipeline was employed to analyze the small-subunit (SSU) rRNA using the Silva database v138.133. Distance-based RDA was used to explore the relationships between microbial communities and environmental factors at the seep, as the axis lengths of DCA were <4.056. The results were visualized by ImageGP 2 (https://www.bic.ac.cn/BIC)57. The microbial interaction network was constructed using ggClusterNet58.
Genome binning, taxonomic identification, and quantitation
SPAdes v3 was used for assembly with k-mers of 21, 33, 55, 71, 91, and 10159, using the meta option and discarding short contigs (less than 1000 bp). Genomes were then binned based on their tetranucleotide frequency, differential coverage, GC content, as well as codon usage, using MaxBin 2.060, MetaBAT 261, CONCOCT62, VAMB63, and GroopM64. The binning results were integrated with the MetaWrap refinement pipeline65, based on completeness and contamination assessed by CheckM66. The resulting bin sets were aggregated and dereplicated at 95% ANI using dRep67, retaining MAGs with completeness >70% and contamination <20%. Data were aligned back to non-redundant MAGs using minimap2, and RPKG_minimap.py obtained relative quantification (RPKG) for MAGs in each sample50. Z-scores were used to standardize values and assess deviations from the mean, facilitating the drawing of the RPKG.
Taxonomic identification and phylogeny
The taxonomy of the MAGs was determined using the GTDB-Tk toolkit v2.1.068,69. SSU sequences were verified against the Silva dataset by aligning them with reference genomes using SINA v1.2.170. OrthoFinder identified orthologs of predicted proteins in each MAG (similarity 0.4, sequence alignment ratio of at least 0.7)71. Homologous gene pairs with over four members from at least three MAGs were retained for gene trees, which were aligned with PRANK72 and pruned using TrimAI73. Gene trees were built with RAxML’s CAT + GTR model74, and ASTRAL-MP was used to construct the species tree, calculating node support with the LPP algorithm75. The tree was visualized on the iTOL web server (https://itol.embl.de/).
Gene prediction, annotation, and abundance calculations
To compare the potential metabolic abilities among different water layers, the relative abundance of key genes in each sample was analyzed. DIAMOND BlastX was used to align the clean data back to the KEGG database (similarity > 40%, reads coverage > 70%, and E value < 1 × 10−6)76,77. The number of KOs in each sample was counted, and the R package “tidyverse” was used for classification and normalization78. GSEA with the ClusterProfiler software identified the top pathways and modules for each sample79,80.
For microbial functional modules, coding regions of the MAGs were predicted using the Protist Genome Annotation Process81. The predicted genes were searched against the nr and KEGG databases using DIAMOND BLAST76,77 and the eggnog database with eggNOG mapper82. For less organized gene families in KEGG, we downloaded relevant HMM models and used hmmsearch implemented in HMMER83. Hydrogenase and CAZy annotations were analyzed using HydDB84 and run_dbCAN85, respectively. The completeness of each module was evaluated with the Python script module_completeness.py (https://github.com/CODRbio/StrainSybionts_mussel). It assesses the presence of defined orthologs from a KEGG module definition file against gene presence data—where each ortholog is marked as present (1) or absent (0)—and computes a completeness score as the ratio of present orthologs to the total assessed, resulting in a value between 0 and 1.
To infer potential metabolic competition and cooperation between microbes, we performed species metabolic interaction analysis. The metaGEMs were reconstructed using CarveMe v1.5.086. The species metabolic interaction analysis (SMETANA v.1.0) tool, which employs a constraint-based community modeling approach, assessed the extent of resource competition and metabolic interdependencies within the identified co-occurring communities. This involved estimating MRO and MIP from the reconstructed metaGEMs53,54. The mathematical formulations for MIP and MRO are detailed in the original publication53, and the tool is implemented as a standalone Python package (https://github.com/cdanielmachado/smetana). Detailed metabolic interactions for different zones were predicted using SteadierCom (https://github.com/cdanielmachado/SteadierCom).
Statistics and reproducibility
As this study involves the analysis of in situ environmental probes, no statistical analysis was performed beyond basic quality checks. A total of 10 samples were sequenced, with one sample collected per depth (each sample was ~100 L). No technical replicates were sequenced. All procedures were conducted following standardized protocols to ensure consistency and reproducibility. The sample number and experimental design are explicitly described in the Materials and Methods section to maintain transparency.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article. The raw sequences for 10 samples are available on NCBI GenBank (SRA) under the accession numbers SRR29288225–SRR29288234 under the BioProject accession number PRJNA1115057. The genome sequence of the MAGs and Supplementary Tables 5–9 are available through FigShare (https://figshare.com/s/7c2b18317b341f8126b4).
References
Sibuet, M. & Olu, K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep Sea Res. Part II Top. Stud. Oceanogr. 45, 517–567 (1998).
Feng, D. et al. Cold seep systems in the South China Sea: an overview. J. Asian Earth Sci. 168, 3–16 (2018).
Wang, B. et al. Seabed features associated with cold seep activity at the Formosa Ridge, South China Sea: Integrated application of high-resolution acoustic data and photomosaic images. Deep Sea Res. Part I Oceanogr. Res. Pap. 177, 103622 (2021).
Huang, Y. et al. Community assemblages and species coexistence of prokaryotes controlled by local environmental heterogeneity in a cold seep water column. Sci. Total Environ. 868, 161725 (2023).
Hansman, R. L., Thurber, A. R., Levin, L. A. & Aluwihare, L. I. Methane fates in the benthos and water column at cold seep sites along the continental margin of Central and North America. Deep Sea Res. Part I Oceanogr. Res. Pap. 120, 122–131 (2017).
Bayon, G. et al. Evidence for intense REE scavenging at cold seeps from the Niger Delta margin. Earth Planet. Sci. Lett. 312, 443–452 (2011).
Saunois, M. et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 12, 1561–1623 (2020).
Etiope, G., Ciotoli, G., Schwietzke, S. & Schoell, M. Gridded maps of geological methane emissions and their isotopic signature. Earth Syst. Sci. Data 11, 1–22 (2019).
Li, X. P. et al. Distinct bottom-water bacterial communities at methane seeps with various seepage intensities in Haima, South China Sea. Front. Mar. Sci. 8, https://doi.org/10.3389/fmars.2021.753952 (2021).
Fischer, D. et al. Interaction between hydrocarbon seepage, chemosynthetic communities, and bottom water redox at cold seeps of the Makran accretionary prism: insights from habitat-specific pore water sampling and modeling. Biogeosciences 9, 2013–2031 (2012).
Arshad, A. et al. A metagenomics-based metabolic model of nitrate-dependent anaerobic oxidation of methane by Methanoperedens-like archaea. Front. Microbiol. 6, 1423 (2015).
Chen, Y. et al. Diversity of anaerobic methane oxidizers in the cold seep sediments of the Okinawa Trough. Front. Microbiol. 13, https://doi.org/10.3389/fmicb.2022.819187 (2022).
Kleindienst, S. et al. Diverse sulfate-reducing bacteria of the Desulfosarcina/Desulfococcus clade are the key alkane degraders at marine seeps. ISME J. 8, 2029–2044 (2014).
van Grinsven, S. et al. Methane oxidation in anoxic lake water stimulated by nitrate and sulfate addition. Environ. Microbiol. 22, 766–782 (2020).
Kits, K. D., Klotz, M. G. & Stein, L. Y. Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ. Microbiol. 17, 3219–3232 (2015).
Sun, Q. L. et al. High-throughput sequencing reveals a potentially novel sulfurovum species dominating the microbial communities of the seawater-sediment interface of a deep-sea cold seep in South China Sea. Microorganisms 8, 687 (2020).
Zhang, J. et al. A novel bacterial thiosulfate oxidation pathway provides a new clue about the formation of zero-valent sulfur in deep sea. ISME J. 14, 2261–2274 (2020).
Dong, X. et al. Phylogenetically and catabolically diverse diazotrophs reside in deep-sea cold seep sediments. Nat. Commun. 13, 4885 (2022).
Huelsmann, M., Schubert, O. T. & Ackermann, M. A framework for understanding collective microbiome metabolism. Nat. Microbiol. 9, 3097–3109 (2024).
Joye, S. B. The Geology and Biogeochemistry of Hydrocarbon Seeps. Annu. Rev. Earth Planet. Sci. 48, 205–231 (2020).
Xu, Z. et al. Metabolism interactions promote the overall functioning of the episymbiotic chemosynthetic community of Shinkaia crosnieri of Cold Seeps. mSystems 7, e0032022 (2022).
Liang, Q. Y. et al. Authigenic carbonates from newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluid sources, formation environments, and seepage dynamics. Deep Sea Res. Part I Oceanogr. Res. Pap.124, 31–41 (2017).
Cao, L. et al. In situ detection of the fine scale heterogeneity of active cold seep environment of the Formosa Ridge, the South China Sea. J. Mar. Syst. 218, 103530 (2021).
He, X. et al. Same (sea) bed different dreams: Biological community structure of the Haima seep reveals distinct biogeographic affinities. Innov. Geosci. 1, 100019 (2023).
Wang, H. N., Fu, X. X., Zhao, C. Q., Luan, Z. D. & Li, C. L. A deep learning model to recognize and quantitatively analyze cold seep substrates and the dominant associated species. Front. Mar. Sci. 8, 775433 (2021).
Cui, H. et al. Microbial diversity of two cold seep systems in gas hydrate-bearing sediments in the South China Sea. Mar. Environ. Res. 144, 230–239 (2019).
Zhang, T. et al. Active anaerobic archaeal methanotrophs in recently emerged cold seeps of northern South China Sea. Front. Microbiol. 11, 612135 (2020).
Feng, J. X. et al. Methane source and turnover in the shallow sediments to the west of Haima cold seeps on the northwestern slope of the South China Sea. Geofluids 2019, 1010824 (2019).
Jiang, Q., Jing, H., Liu, H. & Du, M. Biogeographic distributions of microbial communities associated with anaerobic methane oxidation in the surface sediments of deep-sea cold seeps in the South China Sea. Front. Microbiol. 13, 1060206 (2022).
Niu, M., Fan, X., Zhuang, G., Liang, Q. & Wang, F. Methane-metabolizing microbial communities in sediments of the Haima cold seep area, northwest slope of the South China Sea. FEMS Microbiol. Ecol. 93, fix101 (2017).
Lu, R. et al. Asgard archaea in the haima cold seep: Spatial distribution and genomic insights. Deep Sea Res. Part I Oceanogr. Res. Pap. 170, 103489 (2021).
Du, Z. et al. In situ Raman quantitative detection of the cold seep vents and fluids in the chemosynthetic communities in the South China Sea. Geochem. Geophys. Geosyst. 19, 2049–2061 (2018).
Gruber-Vodicka, H. R., Seah, B. K. B. & Pruesse, E. phyloFlash: rapid small-subunit rrna profiling and targeted assembly from metagenomes. mSystems 5, e00920-20 (2020).
Fernandez-Gomez, B. et al. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J. 7, 1026–1037 (2013).
Acinas, S. G. et al. Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities. Commun. Biol. 4, 604 (2021).
Cabello-Yeves, P. J. et al. The microbiome of the Black Sea water column analyzed by shotgun and genome centric metagenomics. Environ. Microbiome 16, 5 (2021).
Haro-Moreno, J. M. et al. Fine metagenomic profile of the Mediterranean stratified and mixed water columns revealed by assembly and recruitment. Microbiome 6, 128 (2018).
Techtmann, S. M. et al. The unique chemistry of Eastern Mediterranean water masses selects for distinct microbial communities by depth. PLoS ONE 10, e0120605 (2015).
Zhang, Y. et al. Microbial diversity in cold seep sediments from the northern South China Sea. Geosci. Front. 3, 301–316 (2012).
Ruff, S. E. et al. Global dispersion and local diversification of the methane seep microbiome. Proc. Natl. Acad. Sci. USA 112, 4015–4020 (2015).
Gibson, R., Atkinson, R. & Gordon, J. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanogr. Mar. Biol. Annu. Rev. 43, 1–46 (2005).
Zhang, Y., Jing, H. & Peng, X. Vertical shifts of particle-attached and free-living prokaryotes in the water column above the cold seeps of the South China Sea. Mar. Pollut. Bull. 156, 111230 (2020).
Li, J. et al. Variation in abundance and community structure of particle-attached and free-living bacteria in the South China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 122, 64–73 (2015).
Salazar, G. et al. Gene expression changes and community turnover differentially shape the global ocean metatranscriptome. Cell 179, 1068–1083.e1021 (2019).
Nunoura, T. et al. Distribution and niche separation of planktonic microbial communities in the water columns from the surface to the hadal waters of the Japan Trench under the Eutrophic Ocean. Front. Microbiol. 7, 1641 (2016).
Peketi, A. et al. First record of cold-seep induced enhanced water column methane concentrations from the EEZ of India. J. Earth Syst. Sci. 130, 179 (2021).
Mau, S. et al. Methane seeps and independent methane plumes in the South China Sea offshore Taiwan. Front. Mar. Sci. 7, https://doi.org/10.3389/fmars.2020.00543 (2020).
Sisma-Ventura, G. et al. Cold seeps alter the near-bottom biogeochemistry in the ultraoligotrophic Southeastern Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 183, 103744 (2022).
Mao, S.-H. et al. Aerobic oxidation of methane significantly reduces global diffusive methane emissions from shallow marine waters. Nat. Commun. 13, 7309 (2022).
Sun, Y. et al. Mosaic environment-driven evolution of the deep-sea mussel Gigantidas platifrons bacterial endosymbiont. Microbiome 11, 253 (2023).
Osman, E. O. & Weinnig, A. M. Microbiomes and obligate symbiosis of deep-sea animals. Annu. Rev. Anim. Biosci. 10, 151–176 (2022).
Martinez-Garcia, M. et al. High-throughput single-cell sequencing identifies photoheterotrophs and chemoautotrophs in freshwater bacterioplankton. ISME J. 6, 113–123 (2012).
Zelezniak, A. et al. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci. USA 112, 6449–6454 (2015).
Machado, D. et al. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 5, 195–203 (2021).
Padilla, C. C. et al. Metagenomic binning recovers a transcriptionally active gammaproteobacterium linking methanotrophy to partial denitrification in an anoxic oxygen minimum Zone. Front. Mar. Sci. 4, https://doi.org/10.3389/fmars.2017.00023 (2017).
Oksanen, J. et al. Vegan: Community Ecology Package. R Package Version 2.2-1. Vol. 2, 1–2 (2015).
Chen, T. et al. ImageGP 2 for enhanced data visualization and reproducible analysis in biomedical research. iMeta 3, e239 (2024).
Wen, T. et al. ggClusterNet: an R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 1, e32, (2022).
Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017).
Wu, Y. W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2016).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146 (2014).
Nissen, J. N. et al. Improved metagenome binning and assembly using deep variational autoencoders. Nat. Biotechnol. 39, 555–560 (2021).
Imelfort, M. et al. GroopM: an automated tool for the recovery of population genomes from related metagenomes. PeerJ 2, e603 (2014).
Uritskiy, G. V., DiRuggiero, J. & Taylor, J. MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6, 158 (2018).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics https://doi.org/10.1093/bioinformatics/btz848 (2019).
Parks, D. H. et al. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat. Biotechnol. 38, 1079–1086 (2020).
Pruesse, E., Peplies, J. & Glockner, F. O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28, 1823–1829 (2012).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Loytynoja, A. Phylogeny-Aware Alignment with PRANK and PAGAN. Methods Mol. Biol. 2231, 17–37 (2021).
Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Zhang, C., Scornavacca, C., Molloy, E. K. & Mirarab, S. ASTRAL-Pro: Quartet-Based Species-Tree Inference despite Paralogy. Mol. Biol. Evol. 37, 3292–3307 (2020).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Wickham, H., Averick, M. & Bryan, J. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Tatusova, T. et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624 (2016).
Cantalapiedra, C. P., Hernandez-Plaza, A., Letunic, I., Bork, P. & Huerta-Cepas, J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 38, 5825–5829 (2021).
Murali, R. et al. Diversity and evolution of nitric oxide reduction in bacteria and archaea. Proc. Natl. Acad. Sci. USA 121, e2316422121 (2024).
Sondergaard, D., Pedersen, C. N. & Greening, C. HydDB: A web tool for hydrogenase classification and analysis. Sci. Rep. 6, 34212 (2016).
Zheng, J. et al. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 51, W115–W121 (2023).
Machado, D., Andrejev, S., Tramontano, M. & Patil, K. R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 46, 7542–7553 (2018).
Acknowledgements
We acknowledge the support of the Research Vessel KEXUE of the National Major Science and Technology Infrastructure from the Chinese Academy of Sciences (CAS) and Canter for Ocean Mega-Science, CAS. We are especially grateful to the pilots and crew of ROV FAXIAN. This work was supported by National Natural Science Foundation of China (42030407), Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2022QNLM030004-3), National Natural Science Foundation of China (42106100 and 42076091), and State Key Laboratory of Microbial Technology Open Projects Fund (M2023-10).
Author information
Authors and Affiliations
Contributions
Y. Liu, H. Zhang, M. Wang, I. Seim, and C. Li. designed the research. Y. Liu, H. Zhang, L. Cao, L. Fu, and C. Lian performed sample collection. L. Cao and C. Lian performed the environmental parameters detection. Y. Liu, H. Zhang, L. Fu, Y. Guo, and Z. Zhong performed the bioinformatic analyses. Y. Liu and H. Zhang wrote the original draft. I. Seim, M. Wang and C. Li reviewed and edited this manuscript. All authors read, amended, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Tobias Goris.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Zhang, H., Cao, L. et al. Methane filtration and metabolic cooperation of microbial communities in cold seep water columns from South China Sea. Commun Biol 8, 1052 (2025). https://doi.org/10.1038/s42003-025-08471-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08471-4