Abstract
Macromolecular complexes anchored at one cell pole have previously been utilized to achieve synthetic asymmetric cell division in Escherichia coli. In nature, however, robust asymmetric cell division often relies on gradients established between two opposing poles. Here, we present a second-generation synthetic genetic circuit that enables two distinct scaffolding proteins to localize at opposite poles in E. coli. By engineering a PodJ fragment as the second scaffold and integrating SpmX, a negative regulator of PodJ condensation, as a direct chimera with PopZ, we effectively confined PopZ and PodJ to opposite poles within E. coli. Additionally, we developed a neural network-based classification method to identify these synthetic dual-pole cells, further advancing the synthetic biology toolkit for programmable cell differentiation.
Similar content being viewed by others
Introduction
Synthetic biology aims to advance foundational biological research and enable the construction of genetic systems from the ground up. Early work in this field has focused on achieving temporal regulation, such as oscillators1 and memory2, and creating multicellular patterns3. Beyond these initial goals, synthetic biology has expanded to explore spatial regulation, dynamic pattern formation, and cellular organization4. By abstracting biological modules and reassembling them in new contexts, researchers have begun to investigate how complex behaviors can emerge from modular genetic parts. This approach has also been applied to the study of cell polarization5,6 and, more recently, asymmetric cell division7,8,9,10,11.
The aquatic bacterium Caulobacter crescentus, while possessing a relatively simple cellular architecture compared to multicellular organisms, harbors a highly coordinated and complex regulatory network governing cell polarity and differentiation. It has thus served as a valuable model for studying spatial organization in bacteria. A key molecular player in establishing polarity in C. crescentus is the scaffold protein PopZ. Initially identified as a polar organizing factor, PopZ self-assembles into a polar matrix that anchors key cell cycle regulators and chromosomal loci at the cell poles12,13,14. This spatial localization is essential for asymmetric division and faithful chromosome segregation12,13,14. Further studies have revealed that PopZ is an intrinsically disordered protein15 capable of forming biomolecular condensates through phase separation16, providing a mechanistic basis for its ability to organize diverse client proteins and create membrane-less compartments within the bacterial cytoplasm. These properties underscore PopZ’s role as a dynamic and adaptable scaffold for spatial organization in bacterial cells.
Importantly, core components of the C. crescentus polarity machinery, particularly PopZ, have been successfully transferred and functionally reconstituted in Escherichia coli, a bacterium that lacks intrinsic polarity and typically undergoes symmetric division. In heterologous contexts, PopZ retains its ability to form discrete polar foci13,14, demonstrating the portability and modularity of polarity systems across species. Building on these insights, PopZ and other regulatory modules have been repurposed to enable synthetic spatial control beyond native C. crescentus7,8,9,10 (Fig. 1a). These components have been used as synthetic platforms to program spatial signaling and induce differentiation in E. coli7,8,9,10 (Fig. 1a). Synthetic differentiation can occur at later divisions when the septum functions as a physical barrier, enabling compartmentalization of a localized source and its downstream gradient7,8 (Fig. 1b). Differentiation at the first cell division requires additional mechanisms to restrict effector diffusion and establish intracellular asymmetry prior to septation9 (Fig. 1c).
a A graphical representation of synthetic polarity and the induction of asymmetric cell division in E. coli. In these systems, scaffold proteins, such as PopZ8,9,10 or ParB7 from C. crescentus, are introduced into E. coli, where they form signaling hubs to localize enzymes or plasmid DNA. This results in asymmetric division and subsequent cellular differentiation. b Differentiation at the second or later divisions7,8. When the division septum forms, it serves as a physical barrier to compartmentalize the localized signaling source. Differentiation (i.e. differences in reporter fluorescence) becomes evident in subsequent divisions. c Differentiation at the first cell division occurs when effector diffusion is restricted, for example, through pole-targeting mechanisms9. d A graphical depiction of the PopZ–PodJ two-pole system and neural network-based classification, used to select cells with PopZ and PodJ located at distinct cell poles.
Maintaining intracellular asymmetry in micron-scale cells poses significant biophysical and engineering challenges. In reconstitution experiments, a localized source coupled with limited diffusion can generate protein gradients, though the resulting patterns often exhibit considerable heterogeneity9. One strategy to improve spatial precision involves restricting gradient formation to a short-lived, modified form of the protein, such as a phosphorylated or dimerized intermediate, with a lifetime much shorter than that of the unmodified species17. In natural systems such as C. crescentus, robust and sustained gradients typically rely on both a localized source and a spatially confined sink at the opposite pole18,19,20,21,22. Such sinks can be mediated by targeted enzymatic degradation or sequestration, reinforcing and maintaining intracellular asymmetry18,19,20,21,22.
Inspired by this natural principle of dual-pole organization, we build upon the native mechanisms of C. crescentus by expanding our previous first-generation system, which utilizes PopZ as the organizing hub for the localized source9. Specifically, we introduce a second scaffold positioned at the pole opposite to PopZ in E. coli (Fig. 1d), aiming to mimic the source-sink architecture found in nature. While binary threshold-based classification was effective for profiling PopZ localization in the first-generation system9, it is insufficient for distinguishing more complex phenotypes involving dual-pole configurations. To address this limitation, we enhanced our image analysis pipeline by incorporating a neural network model capable of reliably classifying synthetic dual-pole E. coli cells (Fig. 1d). We envision that this second-generation platform will enable more precise spatial control over synthetic differentiation and, when combined with microfluidic sorting technologies, facilitate the targeted selection of functionally differentiated cells.
Results
Selecting a PodJ variant as the second scaffold
In C. crescentus, the PopZ-SpmX-DivJ complex and the PodJ scaffold recruit primary histidine kinases to the stalked cell pole (old pole)23 and the swarmer cell pole (new pole)24,25,26, respectively. We hypothesized that PodJ could serve as an effective second scaffold and sought to identify a truncated, smaller version of PodJ that retains its essential functional elements. PodJ is a transmembrane protein with a cytoplasmic N-terminus consisting of a coiled-coil (CC; 1-6) region followed by an intrinsically disordered region (IDR), as well as a periplasmic C-terminus involved in pilus biogenesis27 (Fig. 2a, top). The periplasmic domain is dispensable for PodJ’s localization at the cell pole26. Recent findings indicate that PodJ forms biomolecular condensates through phase separation both in vitro and in vivo28. Domain analysis using truncation variants has shown that either the CC4-6 domain or the IDR alone is sufficient to drive PodJ phase separation in vitro, with the CC1-3 domain facilitating condensate formation by adding structural rigidity28. Additionally, PodJ has been shown to interact directly with PopZ via the CC4-6 domain, both in vitro and in vivo29.
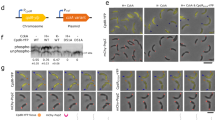
a Domain organization of the PodJ variants tested in this study (from top to bottom: full-length PodJ, PodJCC1−3, IDR, TM, and PodJCC1−3, IDR). “CC”, “IDR,” and “TM” denote the coiled-coil region, intrinsically disordered region, and transmembrane domain, respectively. b Circuit diagrams of the sfGFP-fused PodJ variants expressed from the IPTG-inducible Ptac promoter. c Images showing the bipolar localization pattern of sfGFP-fused PodJ variants, induced by 250 μM IPTG at 37 °C for 1 h. Scale bars: 1 μm. d Circuit diagrams illustrating the co-expression of sfGFP-PodJ variants with mRFP-PopZ. The sfGFP-PodJ variants were expressed under the Ptac promoter, while mRFP-PopZ was expressed from the Anderson promoter J23116. e Images showing mRFP-PopZ expression patterns with or without co-expressed sfGFP-PodJ variants. sfGFP-PodJ variants were induced by 500 μM IPTG at 28 °C for 2 h. Scale bars: 1 µm. f Quantification of PodJ variant co-localization with PopZ in (e). n = 60, 79, and 74 for cells expressing PodJ, PodJCC1−3, IDR, TM, and PodJCC1−3, IDR, respectively. The center line represents the median, box limits indicate the upper and lower quartiles, and whiskers extend to 1.5× the interquartile range. Statistical differences were determined using a two-tailed Student’s t test; **** indicates p < 0.0001.
We therefore designed a PodJ truncation based on the following rationale: (1) excluding the periplasmic region, which is unnecessary for PodJ’s polar localization26 and phase separation28; (2) removing the CC4-6 domain to prevent interaction with PopZ29, as PopZ and PodJ must localize to opposite cell poles; and (3) retaining the CC1-3 domain and IDR to maximize PodJ’s ability to form polar clusters28. To evaluate these modifications, we created fluorescent fusions with two PodJ variants: one with (PodJCC1-3,IDR,TM) and one without (PodJCC1-3,IDR) the transmembrane domain (Fig. 2a, b and Supplementary Fig. 1 for membrane domain prediction), with full-length PodJ as a control for polar cluster formation and PopZ interaction (Fig. 2a, b).
To express mRFP-PopZ in E. coli, we used the medium-strength Anderson promoter J231169, while sfGFP-PodJ variants were expressed from the IPTG-inducible promoter Ptac (expression levels quantified in Supplementary Fig. 2, with J23116 as a baseline). As expected, full-length PodJ exhibited bipolar localization in E. coli (Fig. 2c, left column), consistent with previous reports28. PopZ alone localized unipolarly (Fig. 2e, left column). When full-length PodJ was co-expressed with PopZ at modest expression levels, a large fraction of PodJ became unipolar and strongly co-localized with PopZ, confirming their direct interaction (Fig. 2d, top; Fig. 2e, second column; quantified in Fig. 2f by Pearson correlation). Varying the expression level of PodJ relative to PopZ modulated PodJ localization patterns (Supplementary Fig. 3). At higher PodJ:PopZ ratios, we observed increased bipolar PodJ localization, suggesting that polarity outcomes are sensitive to stoichiometric balance (Supplementary Fig. 3). Both truncation variants, PodJCC1-3,IDR,TM and PodJCC1-3,IDR, also formed polar clusters (Fig. 2c, middle and right columns) but did not co-localize with PopZ when co-expressed (Fig. 2e, f), validating the successful removal of the PopZ interaction domain. As soluble, membrane-less variants offer greater versatility in applications (noting that the prior PopZ module is also cytoplasmic9), we selected PodJCC1-3,IDR as the second scaffold for further characterization.
Similar to PopZ9, PodJCC1-3,IDR displayed a mostly diffuse pattern at low heterologous expression levels (Supplementary Fig. 4). The phenotypes were initially mixed, with occasional mid-cell or unipolar foci, but upon reaching a certain expression threshold, a significant proportion of PodJCC1-3,IDR clusters shifted to unipolar or bipolar (Supplementary Fig. 4). At this saturating concentration, PodJCC1-3,IDR maintained its localization when co-expressed with PopZ (Fig. 2e). PodJCC1-3,IDR and PopZ appeared as distinct foci with clear spatial separation (Supplementary Fig. 5), suggesting that there is no residual PopZ-PodJ interaction and supporting the use of PodJCC1-3,IDR as the second scaffold.
SpmX promotes PodJ localization to cell poles opposite PopZ
Since co-expression of PopZ and PodJCC1-3,IDR did not lead to their spontaneous localization at opposite cell poles, we again turned to natural mechanisms in C. crescentus for insights. In C. crescentus, the scaffold protein SpmX plays a key role in dispersing PodJ from the old cell pole30. SpmX, an integral membrane protein with an IDR31, has been shown to disrupt PodJ localization in E. coli when both are membrane-associated28. SpmX can also impair PodJ phase separation in vitro when both are membrane-less28.
We hypothesized that a membrane-less SpmX variant (SpmXΔTM, Fig. 3a and Supplementary Fig. 6 for membrane domain prediction) could also affect the subcellular accumulation of PodJCC1-3,IDR, which lacks the CC4-6 domain (Fig. 2a, bottom). Indeed, in the presence of SpmXΔTM, PodJCC1-3,IDR clusters at the cell poles were disrupted compared to the mRFP control (Fig. 3b, c), as also evident from fluorescence intensity profiles along the long axis of the cell (Fig. 3c, right, showing representative cells displaying both SpmXΔTM and PodJCC1-3,IDR signals; Fig. 3d, showing population-level quantification of PodJCC1-3,IDR). This confirms that SpmXΔTM negatively affects PodJCC1-3,IDR condensate formation.
a Domain organization of full-length SpmX and its transmembrane domain-truncated variant, SpmXΔTM. “IDR” denotes the intrinsically disordered region, and “TM” denotes the transmembrane domain. b Circuit diagrams illustrating the co-expression of sfGFP-PodJCC1−3, IDR with either mRFP (top) or mRFP-SpmXΔTM (bottom). sfGFP-PodJCC1−3, IDR was expressed under the control of the Ptac promoter, while mRFP and mRFP-SpmXΔTM were expressed from the AHL-inducible Plux promoter. c Images showing sfGFP-PodJCC1−3, IDR co-expression with either mRFP or mRFP-SpmXΔTM. mRFP or mRFP-SpmXΔTM were expressed under the control of the Plux promoter, and sfGFP-PodJCC1−3, IDR was expressed under the control of the Ptac promoter. Induction was performed at 28 °C with 10 μM AHL for 3 h, followed by 200 μM IPTG for 2 h. Scale bars: 1 µm. Fluorescence intensity profiles of sfGFP and mRFP along the long axis of the cell for the representative cell are shown on the right. d Fluorescence intensity (sfGFP) profiles along the long axis of the cell, analyzed from (c), with sfGFP-PodJCC1−3, IDR co-expressed with either mRFP (top) or mRFP-SpmXΔTM (bottom). Solid lines and colored belts represent the average intensity and standard deviation, respectively. n = 62 and 64 cells for mRFP and mRFP-SpmXΔTM co-expression, respectively.
Next, we tested whether the local presence of SpmXΔTM might impede PodJCC1-3,IDR foci formation in PopZ’s proximity. To bypass the complex spatiotemporal regulation in the native C. crescentus context30, we devised a direct translational fusion of SpmXΔTM to mRFP-PopZ, referred to as S’RP (Fig. 4a). When SpmXΔTM was anchored by PopZ, its localization changed from diffuse to unipolar foci (Supplementary Fig. 7). S’RP and PodJCC1-3,IDR were then co-expressed in E. coli, and de novo PodJCC1-3,IDR foci formation was monitored via live-cell imaging (Fig. 4b). In the absence of SpmXΔTM (Fig. 4a, top), PodJCC1-3,IDR foci typically first appeared near PopZ (Fig. 4b, left, early time points), sometimes becoming bipolar at later stages in certain cells (Fig. 4b, left, late time points). When SpmXΔTM was fused to PopZ (Fig. 4a, bottom), PodJCC1-3,IDR foci instead initially appeared at the opposite cell pole (Fig. 4b, right). The fraction of cells with PodJCC1-3,IDR localized opposite to PopZ increased substantially, from an average of 5.83% to 19.33%, upon introduction of SpmXΔTM (Fig. 4c and Supplementary Fig. 8). We also tested whether polarity could persist beyond the first generation and whether daughter cells could inherit distinct foci. To test this, we used arabinose-inducible promoter pBAD instead of the constitutive Anderson promoter to express PopZ (Supplementary Fig. 9). Indeed, PopZ and PodJCC1-3,IDR foci were inherited by distinct sibling cells and persisted until the onset of the second cell division (Supplementary Fig. 9), suggesting that PopZ and PodJCC1-3,IDR can serve as robust polarity hub to program downstream differentiation.
a Circuit diagrams illustrating co-expression of sfGFP-PodJCC1−3, IDR with either mRFP-PopZ (top) or the translational fusion SpmXΔTM-mRFP-PopZ (bottom). sfGFP-PodJCC1−3, IDR was expressed under the control of the Ptac promoter, while mRFP-PopZ and SpmXΔTM-mRFP-PopZ were expressed from the Anderson promoter J23116. b Time-lapse imaging of sfGFP-PodJCC1−3, IDR co-expressed with either mRFP-PopZ (left) or SpmXΔTM-mRFP-PopZ (right). Expression of sfGFP-PodJCC1−3, IDR was induced with 500 μM IPTG from the Ptac promoter at 28 °C, starting at the onset of imaging (0 min). Scale bars: 1 μm. c Proportion of cells with PodJ localized opposite to PopZ for two groups: sfGFP-PodJCC1−3, IDR co-expressed with either mRFP-PopZ (RP) or SpmXΔTM-mRFP-PopZ (S’RP), measured at the 60-min time point in (b). Data are presented as mean ± s.d. (n = 3 experiments). d Opposite pole localization of PodJ using SpmX expressed separately (non-fused) from PopZ. Circuit diagrams are shown on the left; the proportion of cells with PodJ localized at poles opposite to PopZ is shown on the right. Data are presented as mean ± s.d. (n = 3 experiments).
We also tested whether opposite-pole localization can still be achieved when PopZ and SpmXΔTM are expressed as separate proteins. Indeed, although at a lower frequency, the presence of a separate SpmXΔTM copy still promoted opposite-pole localization (an average of 9.3%, compared to 19.33% for the direct fusion; Fig. 4d and Supplementary Fig. 10). We further tested whether increasing the stoichiometry of SpmXΔTM could enhance dispersal of PodJCC1-3,IDR foci near PopZ by constructing a tandem repeat fusion with an additional copy of SpmXΔTM (Supplementary Fig. 11). While the extra SpmXΔTM appeared to improve clearance of residual PodJCC1-3,IDR signal by delaying PodJCC1-3,IDR accumulation near PopZ (Supplementary Figs. 11a–c and 12), it also reduced detectable PodJCC1-3,IDR foci levels, as indicated by a decrease in average fluorescence (Supplementary Fig. 11d).
Collectively, our data demonstrate that SpmXΔTM effectively disrupts the polar localization of PodJCC1-3,IDR in E. coli. SpmXΔTM facilitates local dispersal of PodJCC1-3,IDR condensates, promoting the formation of cells with PodJCC1-3,IDR localized at poles opposite to PopZ, whether through direct fusion or recruitment. Achieving a balanced stoichiometry is critical to maximizing opposite-pole localization while preserving PodJCC1-3,IDR foci integrity and abundance.
Classification of synthetic dual-pole E. coli cells through Otsu preprocessing and deep learning
Cells can exhibit significant phenotypic heterogeneity, possibly due to variability in gene expression32. A binary threshold-based approach, commonly referred to as Otsu’s method, was previously employed to develop a rule-based polarity classifier for the first-generation system9. Given that the fraction of cells with PodJ localized at poles opposite to PopZ also varies, a primary goal of the second-generation platform was to develop a new analysis pipeline capable of identifying synthetic dual-pole E. coli cells.
We first tested whether the Otsu-based classifier could be adapted to analyze the polar localization of both scaffold proteins. A labeled image dataset (n = 6875) was constructed using cells co-expressing S’RP and PodJCC1-3,IDR (Fig. 4a, bottom; representative images shown in Fig. 5a). In brief, Otsu’s method, applied to cell segmentation outputs from Oufti33, converted individual E. coli images into one-dimensional Boolean vectors based on calculated intensity thresholds (see “Methods” for details). Each cell was divided into three regions: left, middle, and right (L, M, and R, respectively; Fig. 5b). A region was labeled “T” (true) if it contained consecutive 1’s, or “F” (false) otherwise (Fig. 5b), enabling cell polarity classification based on a predefined truth table (Fig. 5b; algorithm in Supplementary Fig. 14).
a Representative images of the four phenotypic classes for co-expression of SpmXΔTM-mRFP-PopZ with sfGFP-PodJCC1−3, IDR. SpmXΔTM-mRFP-PopZ was expressed from the Anderson promoter J23116, whereas sfGFP-PodJCC1−3, IDR was expressed from the Ptac promoter and induced with 500 µM IPTG for 1 h at 28 °C. Only cells displaying unipolar SpmXΔTM-mRFP-PopZ patterns were shown. Scale bars: 1 μm. b Schematic representation for classification using Otsu’s method (details in “Methods”). Top: Division of the vector into three segments: left (L), middle (M), and right (R). Bottom: truth table for classification with predefined features. c Representative images for: (top) mRFP-PopZ accurately classified as “unipolar,” (middle) sfGFP-PodJCC1−3, IDR misclassified as “unipolar” due to weak fluorescence in one focus, and (bottom) sfGFP-PodJCC1−3, IDR misclassified as “diffuse” due to weak unipolar fluorescence. Scale bars: 1 µm. d Classification accuracies for PopZ and PodJ using Otsu’s method. The table compares classification results based on the presence of different consecutive entries of 1’s. Three consecutive 1’s were selected as they provide the highest accuracy for PopZ classification.
The Otsu-based polarity classifier excelled in classifying PopZ status, achieving an accuracy of over 90% based on consecutive 1’s (Fig. 5d; Fig. 5c, top, for a representative accurate classification; Supplementary Fig. 13a for additional images and converted vectors). This high accuracy is attributed to PopZ’s strong oligomerization propensity, which causes small foci to coalesce into a single unipolar focus with minimal cytosolic background. In contrast, Otsu’s method was less effective in classifying PodJ polarity, achieving an accuracy close to 80% due to PodJ’s more complex phenotypes and strong cytosolic background (Fig. 5d). We identified two primary phenotypes leading to false classifications: (1) bipolar PodJ cells with substantially higher intensity at one pole, where the weaker signal at the other pole fell below the threshold, causing a false unipolar classification (Fig. 5c, middle, for a representative false classification; Supplementary Fig. 13b for additional images and converted vectors); and (2) unipolar PodJ cells with weak foci intensity only slightly above background, where the threshold was too low, resulting in most entries exceeding it and the cell being falsely classified as diffused (Fig. 5c, bottom, for a representative false classification; Supplementary Fig. 13c for additional images and converted vectors).
Given the success of deep learning-based image classification in both clinical practice and cell biology34, we revised our classification pipeline to include a neural network model (Fig. 6a; details in “Methods”; algorithm in Supplementary Fig. 15). Briefly, Otsu’s method was used as a data preprocessing step to select unipolar PopZ cells for further classification, applying a criterion of three consecutive 1’s that achieved an accuracy of 96.1% (Fig. 5d). The fluorescence profiles of the two oligomers were normalized for intensity and length, then concatenated into one-dimensional vectors to serve as the input layer. We employed a fully connected neural network with five hidden layers, using dropout to improve generalization (Fig. 6a; details in “Methods”). The output layer was a four-entry vector, with each entry representing a class of PodJ phenotypes: bipolar, same pole as PopZ, opposite pole to PopZ, and others (i.e., diffused, mid-cell, or multiple foci). This method achieved a classification accuracy of 91.9% (95% CI: 89.3–93.5%; Fig. 6b for confusion matrix), demonstrating a 10.2% improvement over the Otsu-based classifier (81.7%, Supplementary Fig. 16).
a Illustration of the workflow for classification using a neural network model. Co-expression of SpmXΔTM-mRFP-PopZ with sfGFP-PodJCC1−3, IDR was used to construct a dataset containing 6875 single-cell images. A total of 6179 unipolar PopZ images were selected using Otsu’s method in the data-preprocessing step. The images were then processed using Oufti to generate two one-dimensional vectors for PopZ (red fluorescence) and PodJ (green fluorescence) signals. Both vectors were normalized for intensity (to [0, 256]) and length (to 80 entries), then concatenated to form a 160-entry input vector for the neural network model. The model includes four hidden layers, with a 25% dropout applied to the first two layers. The output layer contains 4 neurons, corresponding to four phenotypic classes: bipolar PodJ, PodJ located at the same pole as PopZ, PodJ located at the opposite pole from PopZ, and others (i.e., cells with complex PodJ phenotypes, including mid-cell foci, multiple foci, and dispersed fluorescence). b Accuracy of multiclass classification, assessed using a confusion matrix. The overall model accuracy was 91.5%, with a 95% confidence interval ranging from 89.3% to 93.7%. The accuracies for the four phenotypic classes were: bipolar (91.8%; 146/159), same pole (95.2%; 160/168), opposite pole (93.1%; 149/160), and others (84.7%; 111/131).
Discussion
Bimolecular condensation of scaffold proteins has recently emerged as a central mechanism in asymmetric cell division16,28,31,35. Building on our previously developed single-pole module9, this study introduces a novel class of synthetic dual-pole E. coli cells by positioning a second scaffold at the pole opposite to PopZ. Drawing from prior research on C. crescentus28,29, we identified key elements in PodJ and its regulator, SpmX. While PodJ and SpmX are membrane-associated in their native context, our synthetic platform is designed to operate entirely in the cytosol, allowing the scaffolds to form membrane-less biocatalytic compartments23,25,28, an architecture that may simplify future purification and engineering efforts.
The PopZ and PodJ scaffolds are inherited by distinct daughter cells and remain sufficiently stable to serve as initial polarity cues (Fig. 4b, right column; Supplementary Fig. 9). Additional regulatory reinforcement is required to maintain long-term differentiation. In C. crescentus, this is achieved through a transcriptional regulatory network centered on the master regulator CtrA19. In synthetic systems, sustained differentiation can be achieved through the incorporation of positive feedback loops36 or memory circuits2 to ensure inheritance across generations. An application of such synthetic differentiation is to enable division of labor within microbial consortia, for example, in cooperative chemical production37. In systems involving multiplexed computation with layered logic gates38, differentiated sibling cells can function independently as sensors and actuators. These distinct lineages can further coordinate through mechanisms such as quorum sensing, thereby distributing functional roles and alleviating the metabolic burden that may arise from overloading the translational capacity of a single cell39.
In C. crescentus, cell pole remodeling during swarmer-to-stalked cell differentiation requires precise spatial and temporal regulation40. PopZ binds to both PodJ and SpmX, and SpmX expression coincides with PodJ condensate dispersal at the old cell pole23,41. Knowledge to date indicates that SpmX impairs PodJ condensate dynamics and stability28, leading to local PodJ dispersal and a compositional shift from a PodJ-rich signaling hub to one dominated by SpmX23. In our synthetic design, we removed the PopZ-interacting domain of PodJ and introduced SpmXΔTM as a chimeric fusion to PopZ. This resulted in a marked increase in cells with PodJ localized opposite to PopZ when SpmXΔTM was present (Fig. 4a–c). Similar local PodJ dispersal was also observed when PopZ and SpmXΔTM were expressed as separate proteins (Fig. 4d). PodJ clearance could be modestly enhanced, albeit with reduced overall PodJ abundance, by including an additional copy of SpmXΔTM (Supplementary Figs. 11 and 12). Additional native partners may also contribute to pole remodeling within C. crescentus. Since SpmX was identified through a small candidate screen28, a broader screen could help uncover other negative regulators of PodJ condensation, potentially improving synthetic dual-pole E. coli cell rational design.
Otsu’s thresholding method classifies PopZ with impressive accuracy but is less effective for PodJ, which forms weaker foci and has a lower signal-to-noise ratio. To address this, we integrated a supervised learning module into our platform, achieving an overall classification accuracy exceeding 90% (Fig. 6b and Supplementary Fig. 16). For data preprocessing, Oufti33 and Otsu’s method were used to reduce the neural network model’s input to a single dimension. We implemented a moderately deep, fully connected neural network with dropout layers to minimize neuron count and parameter load, thereby minimizing the risk of overfitting. Future improvements in generalization may be achieved through domain-adaptation techniques. More advanced architectures, such as convolutional neural networks, could further enhance phenotype classification, particularly for weakly condensed scaffolds, especially if higher-dimensional imaging data is used as direct input. We anticipate that this classification framework is scalable and amenable to integration with microfluidic-based cell sorting platforms, enabling the efficient enrichment of synthetic dual-pole E. coli cells from heterogeneous populations.
Methods
Bacterial strains and growth conditions
We used Escherichia coli strain DH5α throughout this study, a recombination-deficient strain due to the deletion of the recA1 gene. Its genotype is: endA1 recA1 relA1 gyrA96 hsdR17(rk−, mk+) phoA supE44 thi-1 Δ(lacZYA-argF)U169 Φ80 Δ(lacZ)M15 F−.
Depending on the induction conditions required for subsequent experiments, cells were grown overnight in LB medium supplemented with appropriate antibiotics at either 28 °C or 37 °C. The cultures were then diluted 20-fold and grown in 4 mL of fresh LB medium until reaching an optical density (OD600) of 0.3–0.4. At this stage, cells were either induced for expression studies or sampled for microscopy. Chloramphenicol was used at a concentration of 35 µg/mL for plasmid maintenance and selection.
Plasmid circuit construction
The circuits used in this study were constructed following the BioBrick standard assembly. Briefly, DNA fragments encoding proteins and promoters were standardized with flanking cloning sites (prefix: EcoRI and XbaI; suffix: SpeI and PstI) and then combined to create larger circuits using a unified protocol. Protein fusions and truncations were primarily generated through Gibson Assembly. All circuits were cloned into the high-copy-number plasmid pSB1C3 as single plasmids and sequenced between the prefix and suffix using Sanger sequencing (Genomics, Taiwan).
The PopZ coding sequence was amplified from the Caulobacter crescentus genome. An mRFP-PopZ fusion protein was generated by attaching mRFP to the N-terminus of PopZ, as the C-terminus contains an oligomerization domain. The mRFP-PopZ construct was then cloned into a BioBrick plasmid under the control of the Anderson promoter for regulated expression.
The PodJ coding sequence was isolated from the C. crescentus genome, and truncated variants of PodJ were generated. One variant, designated PodJCC1−3, IDR, TM, contained the 1–3 coiled-coil region (residues 1–249), the intrinsically disordered region (residues 431–642), and the transmembrane domain (residues 643–702). A second variant, PodJCC1−3, IDR, lacked the transmembrane domain. sfGFP was fused to the N-terminus of each PodJ variant, which was then expressed under the control of the Ptac promoter BioBrick (containing one copy of LacI) for inducible expression.
The SpmX coding sequence was isolated from the C. crescentus genome, and residues 1–350 were amplified to produce SpmXΔTM, which lacks the transmembrane domain. mRFP was fused to the N-terminus of SpmXΔTM, and the fusion protein was expressed using the Plux BioBrick (containing one copy of LuxR) to assess its effect on PodJ subcellular localization. Additionally, mRFP-PopZ was fused to the C-terminus of SpmXΔTM via the amino acid linker “FHTGKAS” (N to C-term) to examine whether SpmXΔTM promotes PodJ foci formation at opposite cell poles when fused to PopZ. To enhance PodJ foci disruption further, an additional SpmXΔTM was fused to the N-terminus of SpmXΔTM-mRFP-PopZ using the amino acid linker “KLDAEFLL” (N to C-term). These SpmXΔTM fusions were subsequently expressed using the Anderson promoter J23116 BioBrick.
Microscopy
Cells were sandwiched between agarose pads for imaging (1% agarose in distilled water for snapshot experiments and in LB for time-lapse experiments) and placed in 8-well μ-Slides (iBidi). Phase contrast and epifluorescence images were acquired using a Zeiss AxioObserver Z1 inverted microscope equipped with an EC Plan-Neofluar 100×/1.30 NA objective (Carl Zeiss), an ORCA-Flash4.0 V3 Digital CMOS camera C13440-20CU (Hamamatsu), and an X-Cite 120Q excitation light source (Excelitas). The microscope was operated with Zen software. Depending on the liquid culture conditions, snapshot experiments were performed at either 28 °C or 37 °C, while time-lapse experiments were conducted at 28 °C. All experiments were performed in a humidified chamber.
Quantification of the fluorescent images
Outlines and fluorescence intensity profiles of individual cells were extracted from cell lists generated by Oufti33. Briefly, profiles along the cell’s long axis were exported as one-dimensional vectors and analyzed using custom Python scripts. Fluorescence intensity was calculated as the total fluorescence normalized by cell length. The degree of co-localization was quantified using Pearson’s correlation coefficient between the two fluorescence channels.
Phenotype classification using Otsu’s thresholding method
Fluorescence profiles along the cells’ long axis were exported as one-dimensional vectors using Oufti33. The vector entries were normalized to a [0, 256] scale by setting the maximum entry to 256 and adjusting the remaining entries proportionally. Otsu’s method was then applied to determine the optimal threshold that maximizes interclass variance between the two classes. This threshold converted the analog vector entries into Boolean values, with entries below the threshold marked as 0 and those above as 1. The vector was subsequently divided into three regions, and a rule-based classification was developed using a truth table, where “T” represents the presence of consecutive 1’s in a region (otherwise, “F”; Fig. 5b). The label definition for cells co-expressing PopZ and PodJ is shown in Supplementary Fig. 16 (left panel). Supplementary Fig. 14 provides the pseudocode.
Data preprocessing and augmentation for the neural network model
The Otsu-based classifier was used as a preprocessing step to select E. coli cells expressing the construct with unipolar PopZ foci, resulting in 6179 cells after preprocessing. For each fluorescent channel (PopZ and PodJ), fluorescence profiles along the cell’s long axis were exported as one-dimensional vectors using Oufti33. These vectors were intensity-normalized as described previously. Additionally, each vector was adjusted to a standardized length of 80 entries: if the original length exceeded 80, entries were randomly removed; if shorter, new entries were randomly inserted between adjacent entries, using the mean of the two values. For each cell, the model input was a concatenated one-dimensional vector comprising both PopZ and PodJ vectors, totaling 160 entries. Data augmentation was applied by flipping the vectors and adding random noise.
Neural network architecture and training configurations
We developed a neural network model inspired by fully connected layer architectures for learning-based classification. The model begins with an initial fully connected layer that accepts input vectors of size 160. This is followed by four fully connected hidden layers: the first expands the representation to 128 neurons, and the second maintains this size. To enhance generalization and reduce overfitting, we applied dropout regularization with a rate of 0.25 after the first and second layers. The third layer reduces the dimensionality to 64 neurons, and the fourth compresses it further to 32 neurons. Each layer uses a rectified linear unit (ReLU) activation function. The output layer consists of a fully connected layer that maps the encoded features to four output classes corresponding to the dataset labels. The complete model contains 47,588 trainable parameters.
The dataset was split into training (80%), validation (10%), and testing (10%) sets, with consistent hyperparameters across all experiments. We trained the model using the Adam optimizer with a batch size of 64 for a total of 200 epochs, computing the loss with cross-entropy loss at each epoch. The initial learning rate was set to 0.001, and the model was evaluated every five epochs by tracking both training and validation accuracies. Any improvement in validation accuracy triggered a save of the model weights, preserving the best-performing model. The highest validation accuracy achieved during training was recorded, and the best model was subsequently evaluated on the test set. Supplementary Fig. 15 provides the pseudocode.
Statistics and reproducibility
All statistical analyses were performed using custom Python scripts9. A two-tailed t-test was used to assess differences between the two groups, with statistical significance set at p < 0.05. All experiments were conducted with at least three biologically independent samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data from this work are provided in Supplementary Data 1. Plasmids have been deposited to Addgene with ID numbers 241364 and 241365.
Code availability
The classification program is available at: https://github.com/Karasu744/Ecoli_polarity_FC_model.
References
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Basu, S., Gerchman, Y., Collins, C. H., Arnold, F. H. & Weiss, R. A synthetic multicellular system for programmed pattern formation. Nature 434, 1130–1134 (2005).
Khalil, A. S. & Collins, J. J. Synthetic biology: applications come of age. Nat. Rev. Genet. 11, 367–379 (2010).
Chau, A. H., Walter, J. M., Gerardin, J., Tang, C. & Lim, W. A. Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 151, 320–332 (2012).
Altschuler, S. J., Angenent, S. B., Wang, Y. & Wu, L. F. On the spontaneous emergence of cell polarity. Nature 454, 886–889 (2008).
Molinari, S. et al. A synthetic system for asymmetric cell division in Escherichia coli. Nat. Chem. Biol. 15, 917–924 (2019).
Mushnikov, N. V., Fomicheva, A., Gomelsky, M. & Bowman, G. R. Inducible asymmetric cell division and cell differentiation in a bacterium. Nat. Chem. Biol. 15, 925–931 (2019).
Lin, D. W. et al. Construction of intracellular asymmetry and asymmetric division in Escherichia coli. Nat. Commun. 12, 888 (2021).
Hong, J. C. et al. Localized proteolysis for the construction of intracellular asymmetry in Escherichia coli. ACS Synth. Biol. 10, 1830–1836 (2021).
Ma, H. R. & You, L. Polar-opposite fates. Nat. Chem. Biol. 15, 850–852 (2019).
Ptacin, J. L. et al. Bacterial scaffold directs pole-specific centromere segregation. Proc. Natl. Acad. Sci. USA 111, E2046–E2055 (2014).
Bowman, G. R. et al. A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell 134, 945–955 (2008).
Ebersbach, G., Briegel, A., Jensen, G. J. & Jacobs-Wagner, C. A self-associating protein critical for chromosome attachment, division, and polar organization in caulobacter. Cell 134, 956–968 (2008).
Holmes, J. A. et al. Caulobacter PopZ forms an intrinsically disordered hub in organizing bacterial cell poles. Proc. Natl. Acad. Sci. USA 113, 12490–12495 (2016).
Lasker, K. et al. The material properties of a bacterial-derived biomolecular condensate tune biological function in natural and synthetic systems. Nat. Commun. 13, 5643 (2022).
van Teeseling, M. C. F. & Thanbichler, M. Generating asymmetry in a changing environment: cell cycle regulation in dimorphic alphaproteobacteria. Biol. Chem. 401, 1349–1363 (2020).
Iniesta, A. A., McGrath, P. T., Reisenauer, A., McAdams, H. H. & Shapiro, L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc. Natl. Acad. Sci. USA 103, 10935–10940 (2006).
Laub, M. T., Shapiro, L. & McAdams, H. H. Systems biology of Caulobacter. Annu. Rev. Genet. 41, 429–441 (2007).
Angelastro, P. S., Sliusarenko, O. & Jacobs-Wagner, C. Polar localization of the CckA histidine kinase and cell cycle periodicity of the essential master regulator CtrA in Caulobacter crescentus. J. Bacteriol. 192, 539–552 (2010).
McGrath, P. T., Iniesta, A. A., Ryan, K. R., Shapiro, L. & McAdams, H. H. A dynamically localized protease complex and a polar specificity factor control a cell cycle master regulator. Cell 124, 535–547 (2006).
Lasker, K. et al. Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in Caulobacter crescentus. Nat. Microbiol. 5, 418–429 (2020).
Perez, A. M. et al. A localized complex of two protein oligomers controls the orientation of cell polarity. mBio 8, e02238–16 (2017).
Hinz, A. J., Larson, D. E., Smith, C. S. & Brun, Y. V. The Caulobacter crescentus polar organelle development protein PodJ is differentially localized and is required for polar targeting of the PleC development regulator. Mol. Microbiol. 47, 929–941 (2003).
Zhang, C., Zhao, W., Duvall, S. W., Kowallis, K. A. & Childers, W. S. Regulation of the activity of the bacterial histidine kinase PleC by the scaffolding protein PodJ. J. Biol. Chem. 298, 101683 (2022).
Viollier, P. H., Sternheim, N. & Shapiro, L. Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc. Natl. Acad. Sci. USA 99, 13831–13836 (2002).
Lawler, M. L., Larson, D. E., Hinz, A. J., Klein, D. & Brun, Y. V. Dissection of functional domains of the polar localization factor PodJ in Caulobacter crescentus. Mol. Microbiol. 59, 301–316 (2006).
Tan, W. et al. Phase separation modulates the assembly and dynamics of a polarity-related scaffold-signaling hub. Nat. Commun. 13, 7181 (2022).
Lu, N. et al. Scaffold-scaffold interaction facilitates cell polarity development in Caulobacter crescentus. mBio 14, e0321822 (2023).
Zhao, W. et al. A circuit of protein-protein regulatory interactions enables polarity establishment in a bacterium. Preprint at bioRxiv https://doi.org/10.1101/503250 (2018).
Saurabh, S. et al. ATP-responsive biomolecular condensates tune bacterial kinase signaling. Sci. Adv. 8, eabm6570 (2022).
Elowitz, M. B., Levine, A. J., Siggia, E. D. & Swain, P. S. Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002).
Paintdakhi, A. et al. Oufti: an integrated software package for high-accuracy, high-throughput quantitative microscopy analysis. Mol. Microbiol. 99, 767–777 (2016).
Greener, J. G., Kandathil, S. M., Moffat, L. & Jones, D. T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 23, 40–55 (2022).
Wen, W. Phase separation in asymmetric cell division. Biochemistry 59, 47–56 (2020).
Cagatay, T., Turcotte, M., Elowitz, M. B., Garcia-Ojalvo, J. & Suel, G. M. Architecture-dependent noise discriminates functionally analogous differentiation circuits. Cell 139, 512–522 (2009).
Honjo, H. et al. Synthetic microbial consortium with specific roles designated by genetic circuits for cooperative chemical production. Metab. Eng. 55, 268–275 (2019).
Moon, T. S., Lou, C., Tamsir, A., Stanton, B. C. & Voigt, C. A. Genetic programs constructed from layered logic gates in single cells. Nature 491, 249–253 (2012).
Ceroni, F., Algar, R., Stan, G. B. & Ellis, T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat. Methods 12, 415–418 (2015).
Govers, S. K. & Jacobs-Wagner, C. Caulobacter crescentus: model system extraordinaire. Curr. Biol. 30, R1151–R1158 (2020).
Schrader, J. M. et al. Dynamic translation regulation in Caulobacter cell cycle control. Proc. Natl. Acad. Sci. USA 113, E6859–E6867 (2016).
Acknowledgements
We would like to thank Lucy Shapiro (Stanford, USA) for the generous gift of the Caulobacter strain. We thank Technology Commons at the College of Life Science, National Taiwan University, for the use of general equipment such as plate readers; Hao-Chun Fan and Chia-Wen Shih for their experimental assistance. This work was funded by the National Science and Technology Council (110-2311-B-002-007-MY3; 111-2326-B-002-008-MY3).
Author information
Authors and Affiliations
Contributions
T.-W.C., I.-J.H., Y.-C.H., S.-H.W., J.-C.H., and T.-R.S. contributed to project development, including cloning, imaging experiments, quantification, and labeling images for the classification model. Y.-H.K. and T.-W.C. constructed the classification model and performed the analysis. J.-H.R.J. and H.-C.H. advised on the classification model. H.-C.H. guided research strategies and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Aylin Bircan, David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chou, TW., Huang, IJ., Hsu, YC. et al. Construction and phenotypic classification of synthetic dual-pole Escherichia coli cells. Commun Biol 8, 1078 (2025). https://doi.org/10.1038/s42003-025-08495-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08495-w