Abstract
Telomere length (TL) is important for maintaining the individual health of a species. Recent studies shows that in vitro fertilization therapy can drastically reduce TL in offspring, however, the underlying molecular mechanism remains unknown. Sirt6 is a NAD+-dependent epigenetic regulator that has recently been found to play an important role in maintaining telomere stability. Here, we report that NAD+ deficiency in in vitro-cultured blastocysts impairs Sirt6 function, triggering telomere shortening of the inner cell mass and possibly affecting newborns. This phenotype could be effectively mitigated by supplementation with nicotinamide mononucleotide (NAD+ precursor) during in vitro culture, while it could not be achieved in Sirt6 conditional knockout embryos. mtROS accumulation and epigenetic modifications may also be involved in this process. Our results reveal the mechanism by which in vitro culture induces telomere shortening in preimplantation embryos, providing a potential target for improving in vitro culture conditions.
Similar content being viewed by others
Introduction
Approximately 10 million infants have now been born globally via assisted reproductive technology (ART) since 19781 with delivery rates of 2–6% in Europe, 2% in the United States, and 1% in China2,3,4. The vast majority of ART offspring appear to be healthy, however, associated small, but worrying, safety concerns have been discovered in this population, with higher incidences of congenital anomalies, preterm birth, and low birth weight5,6,7. Several studies have shown that distinct growth patterns in ART-conceived children potentially linked to the development of metabolic diseases later in life8. More recently, Wang et al. have shown that blastocyst transfer via ART may be associated with offspring telomere shortening in leukocytes—a biomarker of reduced healthspan9. The study suggests that extended in vitro culture conditions may be responsible for the shorter telomere length (TL) in preimplantation embryos, while the exact mechanism remains largely unclear, and no effective interventions currently exist to mitigate this risk.
The Developmental Origins of Health and Disease (DOHaD) theory proposes that fetal developmental conditions can establish lifelong disease susceptibilities in offspring10. Preimplantation embryos demonstrate increased sensitivity to environmental perturbations during in vitro culture, which compromises blastocyst formation and alters gene expression profiles and metabolic activity11,12,13. Furthermore, previous studies have shown that compared to in vivo development, in vitro fertilization (IVF) disrupts critical biological processes across multiple species (mice, cattle, pigs, etc), including genetic information processing, energy and amino acid metabolism, and cytoskeletal organization14. The mechanism underlying the sensitivity of embryos to in vitro culture could involve a complex of interacting processes composed of epigenetic, metabolic, and cellular components11.
Telomeres are located at the ends of chromosomes to maintain genomic stability15. Various risk factors may all lead to an increase in oxidative stress and chronic inflammation, which can contribute to telomere shortening16,17,18. Cellular oxidative stress, which occurs when the quantity of pro-oxidant molecules in an organism exceeds that of antioxidants, is a significant current process contributing to telomere shortening19, primarily through elevated metabolic rate, inflammation or toxin exposure20. Furthermore, recent reports have highlighted the interactions of telomere length (TL) with key metabolic pathways in cells21,22. On the basis of this biochemical evidence, some studies have proposed the “metabolic telomere depletion hypothesis” in which, during energy shortages, cells catabolize telomeres to generate nucleotides for survival in resource-limited environments23. Studies have shown that various factors affecting an individual’s energy balance can lead to the upregulation of metabolic mediators in animals, which can affect TL through several possible biochemical pathways24.
Nutrient deficiency triggers glucocorticoid/AMP-activated kinase (AMPK) signaling that downregulates telomere maintenance enzymes and proteins via rapamycin inhibition25. Mitochondrial ATP depletion reduces nucleotide synthesis, accelerating telomere attrition26. CD38 overexpression disrupts NAD+ metabolism, thereby disrupting the Sirtuin 1 (SIRT1)-dependent mitochondrial axis to increase reactive oxygen species (ROS) and causing poly (ADP-ribozuse) polymerase (PARP)-related genome repair deficiencies to accelerate telomere dysfunction27. Furthermore, as an NAD+-dependent histone H3 lysine 9 (H3K9) deacetylase, Sirtuin 6 (SIRT6) maintains telomeric chromatin structure and genomic integrity28. Previous studies found that Sirt6-deficient mice exhibit genomic instability and metabolic dysregulation and die at around 4 weeks of age29, while transgenic mice overexpressing Sirt6 have a longer lifespan than wild-type mice30. Growing evidence suggests that SIRT6 plays a crucial role in suppressing genomic damage, maintaining telomere integrity, promoting DNA repair, sustaining metabolic homeostasis, and alleviating oxidative stress31. While oocyte SIRT6 deficiency through in vitro interference experiments leads to early embryonic development arrest and telomere dysfunction in mice32, its involvement in in vitro culture-induced telomere shortening remains unelucidated—a key knowledge gap our study addresses.
Therefore in this study, using mouse models, we demonstrate that in vitro culture induces telomere shortening in blastocyst inner cell masses (ICMs) via NAD+ depletion impairing Sirt6 function, further increase mtROS accumulation. And this telomere shorting may spread to offspring. Furthermore, offspring methylation analyses identify transient hypermethylation in Sirt6 promoter regions for the offspring born from in vitro blastocyst transfer, suggesting that epigenetics modifications may be involved in telomere regulation. Overall, this study pioneers in elucidating the NAD+-Sirt6 axis as the central mechanism underlying in vitro culture-induced telomere shortening, providing a potential target for optimizing culture conditions and a theoretical framework for mitigating long-term health risks in ART-conceived offspring.
Results
Telomere shortening, DNA damage and elevated oxidative stress in blastocysts cultured in vitro
Figure 1a depicts the morphology of mouse blastocysts that developed in vivo and were cultured in vitro. We first compared the relative TL between in vivo and in vitro blastocysts via quantitative PCR (qPCR) (Fig. 1b). As shown in the Fig. 1b, TL was significantly lower in vitro (0.71 ± 0.47, n = 34) than in vivo (1.16 ± 0.53, n = 34) (In vivo vs. In vitro, p = 0.0005). To further define TL at different sites, we evaluated the ICM and Trophectoderm (TE) cell independently (Fig. 1c), and found that the ICM of in vitro blastocysts (0.70 ± 0.39, n = 30) had a considerably shorter TL than did the ICM of in vivo blastocysts (1.11 ± 0.70, n = 30) (In vivo vs. In vitro, p = 0.0067), the same was true for TE cell (In vivo vs. In vitro, p = 0.0071). We further analyzed the TL of embryos at different developmental stages and found no significant difference in TL between the in vivo and in vitro groups prior to the eight-cell stage (In vivo vs. In vitro, p < 0.05), however, post-eight-cell stage, TL differences between the two groups became increasingly apparent (Morula: In vivo vs. In vitro, p = 0.0409; Blastocyst: In vivo vs. In vitro, p = 0.0070; Hatching-blastocyst: In vivo vs. In vitro, p = 0.0010) (Supplementary Fig. 1a, b).
a Created in BioRender. Li, D. (2025) https://BioRender.com/0tdenun. Overview of the experimental design. Blastocysts in vivo or in vitro were collected at embryo days (E) 3.5 and (E) 4.5, respectively. Representative images of embryonic development in vivo and in vitro are shown. Scale bars, 50 µm. b Comparison of telomere length between in vivo (n = 34) and in vitro (n = 34) blastocysts. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. c Comparison of the telomere length of the ICM and TE between in vivo (n = 30; n = 31) and in vitro (n = 30; n = 29) blastocysts. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. d Representative images of in vivo and in vitro blastocysts stained with DAPI (blue) for DNA, Oct4 (green) for ICM lineage and by TUNEL for fragmented DNA (red). Scale bars, 20 µM. e Quantification of cell numbers in blastocysts (ICM/TE/total) in vivo (n = 28) and in vitro (n = 25). f The percentage of apoptotic cells for blastocysts (ICM/TE/total) in vivo (n = 28) and in vitro (n = 25), defined as blastocysts (ICM/TE/total) in vivo and in vitro with TUNEL-positive nuclei divided by DAPI-positive nuclei. g Comparison of apoptosis rates in ICM/TE between in vivo (n = 28) and in vitro (n = 25) blastocysts. h Representative images of in vivo and in vitro blastocysts labeled with the mitochondrial superoxide indicator MitoSox Red (Red) and Hoechst-33342 (blue) for DNA, and comparison of corrected total fluorescence intensity between in vivo (n = 17) and in vitro (n = 19) blastocysts. Scale bars, 20 µM. All the data are expressed as the median (interquartile range, IQR) and means ± standard deviations (SDs). *P < 0.05;**P < 0.01;***P < 0.001; ns: not significant, P > 0.05 (Student’s t test). ICM inner cell mass, TE Trophectoderm.
Telomeres are composed of repeated G-rich sequences and specific binding proteins that work together to stabilize chromosomal ends. These structures are closely associated with apoptosis33,34. To evaluate whether the lineage-specific cell apoptosis exists, the in vivo and in vitro blastocysts were respectively subjected to differential cell staining and TUNEL staining, showing that compared with the in vivo blastocysts, the number of ICM (17.04 ± 7.33, n = 28 vs. 13.72 ± 3.66, n = 25, p = 0.0461), TE (31.50 ± 4.45, n = 28 vs. 28.24 ± 6.35, n = 25, p = 0.0336) and total cell (48.54 ± 10.14, n = 28 vs. 41.96 ± 9.38, n = 25, p = 0.0182) in the in vitro blastocysts significantly decreased, while the apoptotic rate significantly increased (In vivo vs. In vitro: 10.94 ± 7.74, n = 28 vs. 19.75 ± 10.55, n = 25 in the ICM, p = 0.0010; 4.66 ± 4.34, n = 28 vs. 8.48 ± 5.04, n = 25 in the TE, p = 0.0047; 6.62 ± 4.89, n = 28 vs. 12.00 ± 6.23, n = 25 in the total cell, p = 0.0009) (Fig. 1d–f), although the ICM:TE ratio of apoptotic rate did not differ between the two groups (1.88 ± 1.84, n = 28 vs. 2.11 ± 1.57, n = 25, p = 0.6183) (Fig. 1g). To further confirm the increase in DNA damage, on one hand, we evaluated DNA damage through γH2A.X staining and confirmed the presence of significantly increased DNA damage foci in in vitro cultured-blastocysts (Supplementary Fig. 2a, b); On the other hand, the activation of the p53 pathway also plays an important role, as evidenced by the increased mRNA expression of Trp53, Cdkn1a, Pmaip1, and Trp53bp1 in in vitro-cultured blastocysts. However, when the p53 pathway is inhibited, the expression shows no difference compared with the in vivo group (Supplementary Fig. 2c).
Considering that mitochondria are the main sites of reactive oxygen species (ROS) production, which is an up-regulation in response to cellular stress35. By using MitoSOX (which reflects mitochondrial superoxide production) to track mitochondria in blastocysts, it was found that compared with the in vivo group (0.97 ± 0.08, n = 17), blastocysts in the in vitro group (1.12 ± 0.14, n = 19) exhibited significantly elevated mitochondrial ROS (mtROS) (In vivo vs. In vitro, p = 0.0008) (Fig. 1h). Furthermore, by analyzing the mtROS during preimplantation embryo development, it could be observed that a significant increase in oxidative stress mainly occurs during the blastocyst formation stage and persists until the hatching-blastocyst stage (Supplementary Fig. 1c, d). Collectively, these findings demonstrate that in vitro culture leads to telomere shortening, DNA damage accumulation, and mitochondrial oxidative stress in blastocysts.
Postnatal offspring born from in vitro blastocyst trasfer displayed shorter telomere length one week after birth
We then tracked the change in TL after birth by transferring in vivo and in vitro blastocysts into the uteri of pseudopregnant ICR females. In the in vivo and in vitro groups, 13 (21.67%, 13/60) and 6 (7.89%, 6/76) living F1 pups were respectively delivered (Supplementary Fig. 3a), which were born healthily and respired normally (Fig. 2a). The in vitro group exhibited a significantly higher proportion of male offspring (4/6, 66.67%) compared to the in vivo group (6/13, 46.15%) (Fig. 2b and Supplementary Fig. 3a). Furthermore, the pups were born one day earlier in the in vitro group than in the in vivo group (Supplementary Fig. 3a). Next, tail samples of pups were collected every two weeks after birth to measure TL, and body weight was monitored. The results showed that the TL of F1 pups born from in vitro blastocyst trasfer (0.84 ± 0.15,n = 6) was significantly shorter than that of those born in vivo (1.01 ± 0.26, n = 13) at one week after birth (In vivo vs. In vitro, p = 0.0217), although there was no statistically significant difference in TL between the in vivo and in vitro groups after three weeks (In vivo vs. In vitro, p > 0.05) (Fig. 2d). Furthermore, the body weights of the F1 pups in the two groups were all within the normal range for the mice at the early growth stage (before 5 weeks), whereas at the late growth stage (after 5 weeks), the body weights of the F1 pups in the in vitro group tended to increase compared with those in the in vivo group (Fig. 2c). Grouping analysis by sex revealed that particularly male pups in the in vitro group presented the same trend of weight gain (Supplementary Fig. 3c), with male pups showing greater telomere shortening (0.80 ± 0.15, n = 4) at 1 week after birth than did those in the in vivo group (1.05 ± 0.28, n = 6) (In vivo vs. In vitro, p = 0.0195) (Supplementary Fig. 3e).
a Representative image of F1 live births by uterus transplantation of the blastocysts in vivo or in vitro. b Sex ratio pie chart of mouse F1 pups in the in vivo (n = 13) and in vitro (n = 6) groups. c Body weights of mouse F1 offspring monitored at 1 week, 3weeks, 5weeks, 7 weeks, 9 weeks, 11 weeks of age in the in vivo (n = 13) and in vitro (n = 6) groups. d Relative telomere length of mouse F1 pups in the in vitro (n = 13) and in vitro (n = 6) groups at 1 week, 3 weeks, 5 weeks, 7 weeks, 9 weeks, 11 weeks of age, respectively. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. e Body weights of mouse F2 offspring (self-mating of F1 females with F1 males within each group) monitored at 1 week, 3weeks, 5weeks, 7 weeks, 9 weeks, 11 weeks of age in the in vivo (n = 24) and in vitro (n = 20) groups. f Relative telomere length of mouse F2 pups in the in vivo (n = 24) and in vitro (n = 20) groups at 1 week, 3 weeks, 5 weeks, 7 weeks, 9 weeks, 11 weeks of age, respectively. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. All the data are expressed as the median (interquartile range, IQR) and means ± standard deviations (SDs). * P < 0.05; ****P < 0.0001; ns: not significant, P > 0.05 (Two-way ANOVA).
We subsequently obtained the F2 generation through F1 inbreeding (Supplementary Fig. 4a). Unlike the F1 generation, we found a significant increase in the proportion of female offspring born in the in vitro group (13/20, 65%) compared with the in vivo group (11/24, 45.83%) (Supplementary Fig. 4b). Then, we measured the TL of F2 generation newborn mice. Similarly, F2 cubs from the in vitro group (0.73 ± 0.21, n = 20) displayed a different growth pattern and had significantly shorter TL than did F2 cubs from the in vivo group (0.98 ± 0.25, n = 24) at one week after birth (In vivo vs. In vitro, p < 0.0001) (Fig. 2e, f). We also found that the growth pattern and TL of F2 generation female pups was more significantly affected in vitro (0.66 ± 0.20, n = 13) than in vivo (0.87 ± 0.19, n = 11) (In vivo vs. In vitro, p = 0.0147) (Supplementary Fig. 4d, f). Overall, these results indicate that offspring produced following blastocyst transfer in vitro exhibit shorter TL one week after birth, which could have intergenerational effects and be gradually recovered later.
Profiles of differentially expressed mRNAs in blastocysts in vivo and in vitro
To investigate the mechanism of telomere shortening caused by in vitro culture, we collected inner cell masses from blastocyst stage embryos for sequencing. The sequencing study was divided into two groups: in vivo fertilization and development as the control (A, in vivo group), and in vivo fertilization and in vitro culture (B, in vitro group). Following high-throughput sequencing, the heatmap revealed hierarchical clustering of distinct mRNA expression profiles within the two groups (Fig. 3a). Using differentially expressed gene sets, a hierarchical clustering analysis was performed, dividing the samples into two major clustering branches. Three replicate samples from the in vivo group were self-clustered into one branch, whereas three samples from the in vitro group were clustered into another branch. During the differential analysis of mRNAs, 644 differentially expressed genes (DEGs) were identified between the in vivo and in vitro groups, among which the percentages of upregulated and downregulated genes were 56.5% (364/644) and 43.5% (280/644), respectively (Fig. 3e). Each dot on the volcano plot represents an mRNA. The blue dots on the left and red dots on the right represent downregulated and upregulated mRNAs with significant differences, respectively, whilst the gray dots represent mRNAs that are expressed with no significant differences. We subsequently conducted KEGG and GO analyses on DEGs (Fig. 3b, c). KEGG analysis revealed that 49 of the 644 genes were enriched in metabolic pathways (Fig. 3b). GO analysis revealed that these genes were mainly involved in intracellular anatomical structure (375 genes), metabolic processes (314 genes), organic substance metabolic processes (305 genes) and etc. (Fig. 3c). Together, these results highlight the critical function of metabolic regulation in in vitro embryo culture. Furthermore, we integrated the DEGs (644 genes) with the metabolism pathway-related genes (1642 genes) from KEGG database, and telomere-related genes (190 genes) identified based on GOTERM_BP_DIRECT: telomere maintenance (GO:0000723) (Fig. 3d). Sirt6 is an NAD+ dependent deacetylase that was previously identified as a critical gene in telomere and metabolism. Our RNA sequencing results demonstrated significant expression and fold changes in Sirt6 in the in vivo group compared with the in vitro group. Therefore, we hypothesized that Sirt6 may be associated with the telomere shortening of blastocysts induced by in vitro culture.
a Heatmap analysis of the in vivo and in vitro groups. Three biological replicates were performed for each group, and for each replicate, the inner cell mass of a single blastocyst was collected. Letters and numbers denote the ID of the inner cell mass of a specific blastocyst. b Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation with differentially expressed genes (DEGs) (|log2fold change | ≥1, P < 0.05) for in vivo versus in vitro blastocysts and enrichment analysis (c) Gene Ontology (GO)-based functional annotation with DEGs for in vivo versus in vitro blastocysts and enrichment analysis. The X-axis represents the rich factor corresponding to the pathway and GO term, and the Y-axis represents the name of the pathway and GO term. The size of the dot represents the number of DEGs enriched in each term. The color of the dot represents the enrichment score (defined as −Log10 P value). The smaller the enrichment score is, the closer the color is to blue. d The Venn diagram of DEGs (in vivo blastocysts versus in vitro blastocysts) as well as metabolism-related genes and telomere-related genes (e) Volcano map analysis of the in vivo and in vitro groups. The plot defines upregulated/downregulated/nonsignificantly expressed genes, with the arrow indicating the Sirt6 gene.
The effects of NAD+ dependent Sirt6 and epigenetic regulation on telomere maintenance in preimplantation embryos during in vitro culture
To dissect the potential relationship between Sirt6 and telomere shortening induced by in vitro culture, we first examined Sirt6 expression in the inner cell mass of mouse blastocysts in vivo and in vitro. Real-time quantitative PCR (RT-qPCR) analysis revealed that the relative mRNA level of Sirt6 in the in vitro group (0.43 ± 0.15, n = 21) was significantly lower than that in the in vivo group (0.95 ± 0.30, n = 30) (In vivo vs. In vitro, p < 0.0001) (Fig. 4a). In line with these findings, immunofluorescence analysis revealed an approximately 50% decrease in the intensity of Sirt6 fluorescent signals detected in the inner cell mass of mouse blastocysts in vitro (0.57 ± 0.04, n = 37) (In vivo vs. In vitro, p < 0.0001) (Fig. 4b, c). We further analyzed the expression of Sirt6 in embryos at different developmental stages, which showed that the significant decrease of Sirt6 expression mainly initiated at the blastocyst stage (Supplementary Fig. 5a). To clarify the subcellular localization of Sirt6, we found that Sirt6 is mainly expressed in the ICM and the nucleus (Supplementary Fig. 5b–d). Furthermore, we found that the expression of Tert and Terc in blastocysts was significantly increased (Supplementary Fig. 5e, f), which might be a reactive upregulation in response to telomere shortening. These data indicate that decreased expression of Sirt6 may result in shorter telomeres of in vitro blastocysts. Previous studies using in vitro models have shown that the activity of the SIRT6 promoter is regulated by transcription factors and methylation levels, with high methylation inhibiting its expression36,37. Therefore, through whole-genome methylation analysis, we found that the methylation level of the Sirt6 promoter region in one-week-old F1 mice born from in vitro blastocyst transfer (68.02 ± 3.95, n = 3) was significantly higher than that in the in vivo group (56.61 ± 2.94, n = 3) (In vivo vs. In vitro, p = 0.016). However, there was no significant difference in the methylation level of the Sirt6 promoter region between the two groups at seven weeks (In vivo vs. In vitro: 74.76 ± 3.51, n = 3 vs. 63.91 ± 6.89, n = 3, p = 0.4128) (Supplementary Fig. 3b). These findings suggest that in vitro culture may cause an increase in methylation levels in the Sirt6 promoter region, which could affect Sirt6 expression and consequently affect telomere homeostasis.
a RT-qPCR analysis of Sirt6 mRNA in the inner cell masses of blastocysts in vivo (n = 30) and in vitro (n = 21). Blastocysts derived from the in vivo group or in vitro group were collected to evaluate Sirt6 expression. Compared with those in vivo, Sirt6 mRNA levels were lower in blastocysts in vitro. b Representative images of in vivo and in vitro blastocysts stained with antibodies against Sirt6 (red) and Oct4 (green), and co‐stained with DAPI for DNA (blue). The red box indicates the inner cell mass, and there is a 2.5× magnification for inner cell mass. Scale bars, 20 µm. c Quantification of Sirt6 fluorescence intensity in the in vivo (n = 26) group or in vitro (n = 37) group. d Comparion of the NAD+/NADH ratio between the in vivo and in vitro groups, n represents the number of experiments, each of which requires approximately 35 blastocysts (e) Representative images of the In vivo, In vitro+DMSO (0.1%), In vitro+Sirt6 inhibitor (10 µM), In vitro+Sirt6 inhibitor (20 µM), In vitro+NMN(10 µM), and In vitro + NMN(10 µM)+Sirt6 inhibitor (20 µM) blastocyst groups. Scale bars, 50 μm. f Comparison of telomere length in In vivo (n = 21), In vitro+DMSO (0.1%) (n = 30), In vitro+Sirt6 inhibitor (10 µM) (n = 15), In vitro+Sirt6 inhibitor (20 µM) (n = 19), In vitro+NMN (10 µM) (n = 32), and In vitro+NMN (10 µM)+Sirt6 inhibitor (20uM) (n = 22) blastocysts. Relative telomere length is expressed as a T/R ratio determined by pqPCR analysis. All the data are expressed as the means ± standard deviations (SDs). *P < 0.05; ****P < 0.0001; ns not significant, P > 0.05 (Student’s t test; one way ANOVA). DMSO dimethyl sulfoxide, NMN nicotinamide mononucleotide.
Considering that Sirt6 activity and expression could be affected by the NAD+ level, we further evaluated the ratio of NAD+/NADH throughout mouse blastocyst development in vivo and in vitro, and found that the ratio of NAD+/NADH in in vitro mouse blastocysts (0.61 ± 0.11, n = 3) was much lower than that in in vivo mouse blastocysts (1.02 ± 0.18, n = 3) (In vivo vs. In vitro, p = 0.0265) (Fig. 4d). Therefore, we speculated that a decrease in the NAD+/NADH ratio during in vitro growth would lead to impaired function of Sirt6 and thus result in telomere shortening in vitro. To determine whether the telomere shortening phenotype of blastocysts in vitro is related to the functional loss of Sirt6 induced by the decrease in the NAD+ level, we added different concentrations of a Sirt6 inhibitor (JYQ-42) at the eight-cell stage to inhibit its function and subsequently examined its embryonic development (Supplementary Fig. 6a). We discovered that the TL of blastocysts decreased as the inhibitor concentration increased (DMSO: 0.85 ± 0.40, n = 15; 1 µM: 0.84 ± 0.34, n = 12; 5 µM: 0.67 ± 0.27, n = 11; 10 µM: 0.47 ± 0.22, n = 12; 20 µM: 0.31 ± 0.13, n = 17) (Supplementary Fig. 6b). Moreover, we treated embryos at the eight-cell stage with nicotinamide mononucleotide (NMN), the precursor of NAD+ biosynthesis, and discovered that the TL (1.15 ± 0.48, n = 32) was restored to the same level as that of the blastocyst in vivo (1.18 ± 0.56, n = 21) (In vivo vs. In vitro, p > 0.9999), but the TL (0.62 ± 0.28, n = 22) was significantly reduced to that of untreated blastocysts in vitro (0.84 ± 0.32, n = 30) after the addition of high concentrations of Sirt6 inhibitors (In vivo vs. In vitro, p = 0.3149) (Fig. 4e, f). Taken together, these results suggest that the reduction in NAD+ levels during the preimplantation stage of mouse embryo development affects telomere maintenance primarily by impairing the function of Sirt6.
Oocyte-specific Sirt6 knockout mice exhibited shorter telomere length
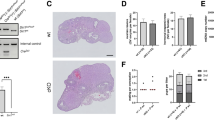
The upregulation of NAD+ could restore the TL of blastocysts in vitro, whereas Sirt6 suppression led to a shorter TL, implying that Sirt6, which is dependent on NAD+ regulation, plays a role in the regulation of the TL of preimplantation embryos. Considering that NAD+ may affect TL regulation through multiple pathways38, we generated oocyte-specific Sirt6 knockout mice to further validate that Sirt6 causes telomere shortening in in vitro cultured-blastocysts (Fig. 5a). Since Sirt6(-/-) mice die shortly after weaning29, we generated oocyte-specific Sirt6 knockout mice using ZP3-Cre to overcome this early postnatal lethality. We first identified the genotype of Sirt6 conditional knockout (CKO) mice, and observed that using a primer pair of P1 and P2, homozygous mice could produce a 444 bp fragment, whereas wild-type (WT) mice obtained a 390 bp fragment. And homozygous mice were able to obtain a 429 bp fragment while WT mice were unable to obtain a band when a primer pair of P3 and P4 was used (Fig. 5b). Furthermore, we performed RT-qPCR analyses on Sirt6 CKO mice, respectively. Compared with that in WT ovaries (1.01 ± 0.21, n = 3), the level of Sirt6 mRNA in CKO ovaries (0.08 ± 0.03, n = 3) decreased to <10% (WT vs. CKO, p = 0.0015) (Fig. 5c). Our study of 4-month-old mice revealed significant differences in TL between Sirt6 CKO and WT mice. We discovered that the TL of CKO mice (0.70 ± 0.23, n = 4) was significantly shorter than that of WT mice (1.12 ± 0.26,n = 8) when the mouse tail genome was tested (WT vs. CKO, p = 0.0206) (Fig. 5d). Then, we compared the TL between different tissues of CKO mice and WT mice, and the results showed that CKO mice had considerably shorter telomeres than WT mice did. In particular, lung and whole blood tissues were significantly different (WT vs. CKO: 2.61 ± 1.62, n = 3 vs. 0.92 ± 0.35, n = 3 in the lung, p = 0.0355; 3.48 ± 3.29, n = 3 vs.1.67 ± 0.23, n = 3 in the blood, p = 0.0250) (Fig. 5e).
a Created in BioRender. Li, D. (2025) https://BioRender.com/1uvlgww. Generation of oocyte-specific Sirt6 knockout mice. The conditional mutant allele of Sirt6 with loxP sites in introns 1 and 3 is deleted during zp3-Cre-mediated recombination. b PCR genotyping of Sirt6 CKO mice. Sirt6 wild type and conditional alleles were amplified using the primers P1: 5-gctaatgggaacgagaccaa-3 and P2: 5-acccacctctctcccctaaa-3. The PCR product of the WT allele is about 390 bp and that of the conditional allele is 444 bp, respectively. The zp3-Cre alleles are amplified using the primers P3: 5-cagatgaggtttgaggccacag-3 and P4: 5-gcgaacatcttcaggttctgc-3. The PCR product of the zp3-Cre allele is approximately 429 bp. c RT- qPCR analysis of Sirt6 expression in the ovaries of CKO (n = 3) and WT (n = 3) mice. The Sirt6 mRNA level reduced to <10% in the CKO ovary compared to control ovary. d Comparison of telomere length in tails of WT (n = 8) and CKO (n = 4) mice. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. e Comparison of mouse telomere length in different organs. WT‐Heart (n = 3) versus CKO‐Heart (n = 3), WT‐Lung (n = 3) versus CKO‐Lung (n = 3), WT‐Brain (n = 3) versus CKO‐Brain (n = 3), WT‐Liver (n = 3) versus CKO‐Liver (n = 3), WT‐Spleen (n = 3) versus CKO‐Spleen (n = 3), WT‐Muscle (n = 3) versus CKO‐Muscle (n = 3), WT‐Ovary (n = 3) versus CKO‐Ovary (n = 3), WT‐Kidney (n = 3) versus CKO‐Kidney (n = 3), and WT‐Blood (n = 3) versus CKO‐Blood (n = 3). Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. All the data are expressed as the means ± standard deviations (SDs). *P < 0.05;**P < 0.01 (Student’s t test). WT wild type, CKO conditional knockout.
Early embryonic development of oocyte-specific Sirt6 knockout mice is characterized by delayed development and shorter telomeres
To study the early embryonic development of Sirt6 CKO mice and its impact on the TL of embryos, as well as whether NAD+ regulates the TL of preimplantation embryos through Sirt6, we performed ICSI on WT and CKO mouse MII oocytes, and then cultured fertilized oocytes with or without NMN. The embryonic development of the preimplantation embryos in both groups was assessed with and without the administration of NMN (Fig. 6a). As shown in Fig. 6a, CKO or CKO + NMN mouse oocyte-derived embryos presented delayed development (red asterisk) compared with the WT group. In terms of the blastocyst formation rate, we noted that the incidence in the CKO (52.4%, n = 21) and CKO + NMN groups (52.4%, n = 21) was significantly lower than that in the WT group (89.6%,n = 48) (WT vs. CKO, p < 0.001; WT vs. CKO + NMN, p < 0.001), although its cleavage capacity remained unaffected (WT vs. CKO: 90.6%, n = 53 vs. 84%, n = 25, p = 0.3970; WT vs. CKO + NMN: 90.6%, n = 53 vs. 84%, n = 25, p = 0.3970) (Fig. 6b, c). These observations suggest that when Sirt6 is deficient in oocytes, the developmental potential of early embryos is compromised and cannot be restored even in the presence of NAD+. Figure 6d shows that the TL of mature oocytes in CKO mice (0.30 ± 0.23, n = 18) was considerably shorter than that in WT mice (0.90 ± 0.68, n = 23) (WT vs. CKO, p = 0.0010) (Fig. 6d). Consistent with this concept, the TL of blastocysts derived from the oocytes of CKO mice (0.52 ± 0.19, n = 11) was significantly shorter than that of WT mice (0.91 ± 0.37, n = 26) (WT vs. CKO, p = 0.0340). When NMN was added, the TL in the WT group (1.24 ± 0.52, n = 24) dramatically increased (WT vs. WT + NMN, p = 0.0203). The TL in the CKO group (0.53 ± 0.21, n = 11) was not substantially different from that previously reported (CKO vs. CKO + NMN, p > 0.9999), but was much shorter than that in the WT group (WT vs. CKO + NMN, p = 0.0400) (Fig. 6e). Taken together, these results suggest that oocyte Sirt6 is critical for maintaining early embryonic telomere function and genome integrity in the presence of NAD+, which determines developmental capacity.
a Representative images of the WT, WT + NMN (10 µm), CKO and CKO + NMN (10 µm) blastocyst groups. Red asterisks indicate the developmental delay. Scale bars, 50 µm. b Quantification of cleavage rates of mouse embryos in the WT (n = 53), WT + NMN (10 µm) (n = 51), CKO (n = 25) and CKO + NMN (10 µm) (n = 25) groups. c Quantification of blastocyst formation rates of mouse embryos in the WT (n = 48), WT + NMN (10 µm) (n = 46), CKO (n = 21) and CKO + NMN (10 µm) (n = 21) groups. d Comparison of telomere length in WT oocytes (n = 23) and CKO oocytes (n = 18). Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. e Comparison of telomere length in WT (n = 26), WT + NMN (10 µm) (n = 24), CKO (n = 11) and CKO + NMN (10 µm) (n = 11) blastocysts. Relative telomere length is expressed as a T/R ratio determined by qPCR analysis. All the data are expressed as means ± standard deviations (SDs). *P < 0.05;**P < 0.01; ****P < 0.0001; ns: not significant, P > 0.05 (Chi-square test; Student’s t test; One-way ANOVA). WT wild type; CKO conditional knockout;NMN nicotinamide mononucleotide.
Discussion
Previously, there were evidences that ART therapy was associated with poor short-term outcomes and an increased risk of metabolic diseases later in life5,6,7,8. Few studies have investigated the long-term health consequences of ART therapy. A study of 2022 found that children who were born from blastocyst embryo transfer ART cycles had shorter TL, implying that ART therapy may be associated with age-related traits9. This study also showed that extended in vitro culture may affect telomere maintenance, leading to telomere shortening. However, the exact mechanism has yet to be fully elucidated. In this study, we explored the possible mechanisms that contribute to telomere shortening of embryos at the blastocyst stage and progeny caused by in vitro culture, and discovered that NAD+-dependent Sirt6 depletion led to telomere shortening in preimplantation embryos induced by in vitro culture, and that NMN, a precursor of NAD+, could rescue this phenotype. Furthermore, mtROS accumulation and epigenetic modifications may also be involved in this process. To our knowledge, our study is the first to elucidate the mechanism by which in vitro culture causes telomere shortening in preimplantation embryos and provides a potential target for improving in vitro culture conditions.
Given prior findings showing telomere shortening in offspring born from blastocyst embryo transfer, we sought to determine whether changes in TL had already occurred in the preimplantation embryos. Therefore, in our study, we conducted separate verifications from various dimensions. On the one hand, we investigated the difference in TL between in vivo and in vitro embryos at the blastocyst stage (Fig. 1b). Furthermore, the dynamic change of TL across developmental stages revealed stage-specific responses to in vitro culture conditions. While no significant TL differences were detected between in vivo- and in vitro-derived embryos during pre-compaction stages (1C to 8 C), a progressive TL reduction became statistically significant (p < 0.05) in in vitro cultured embryos starting from the morula stage (Fig. S1b), demonstrating that in vitro culture conditions predominantly interfere with telomere maintenance during post-morula development. On the other hand, to clarify whether telomere shortening has specific effects on different cell lineages (ICM vs. TE) in blastocysts, we further analyzed the TL of the ICM (which develops into fetal tissues and organs) and TE (which develop into the fetal placenta), and found that the TL of both the ICM and TE in in vitro-cultured blastocysts was significantly reduced (P < 0.01) (Fig. 1c). Previous studies have shown that short telomeres can trigger a DNA damage response, leading to cellular senescence or apoptotic cell death38,39. By co-localizing TUNEL staining with lineage markers, we found that although there was no significant difference in the apoptosis distribution between different cell lineages (ICM vs. TE) (Fig. 1g), in vitro culture led to a significant increase in the apoptosis rate of blastocysts (P < 0.001) (Fig. 1f). This finding was highly consistent with the accumulation of the DNA damage marker γH2A.X (Fig. S2b) and the activation of the p53 - p21 pathway (Fig. S2c). Our study also found delayed development and compromised proliferative capacity in both ICM and TE lineages of in vitro-cultured blastocysts compared with their in vivo counterparts (P < 0.05) (Fig. 1e), as previously reported in studies demonstrating that in vitro culture could delay early development and reduce total cell counts during blastulation11,40.
Preimplantation development is characterized by highly coordinated physiological and epigenetic changes that occur as the fertilized oocyte develops into a blastocyst. As the embryo progresses from the fallopian tube to the uterus, the differential availability of nutrients, oxygen, and growth signals can fulfill the various energy demands that support this transition41. However, this dynamic metabolic environment can be lost with in vitro culture42. The in vitro culture environment fails to simulate the dynamic metabolic microenvironment in vivo, resulting in metabolic differences that in turn affect telomere maintenance mechanism and increase telomere attrition. In our study, we used metabolic gene sets, telomere gene sets and differentially expressed genes of ICMs between the in vivo and in vitro groups to create a Wayne map (Fig. 3d), and found that Sirt6 may be related to telomere shortening in in vitro-cultured blastocysts. Moreover, we found the significant downregulation of Sirt6 expression begins at the blastocyst stage (Fig. S5a), which coincides with the stage of accelerated accumulation of mtROS (Fig. S1d). The enrichment of Sirt6 in the ICM and the nucleus suggests its protective role in maintaining stemness and genomic stability (Fig. S5c-d). It has been reported that SIRT6 deficiency can affect the structure of telomere chromatin, leading to telomere dysfunction, and thus triggering cell aging28. SIRT6 is an NAD+-dependent deacetylase that has been shown to modulate the acetylation levels of H3K56 and H3K9 in cells during mitosis28,43. When SIRT6 deacetylase is absent in human telomeres, elevated levels of H3K9 acetylation are observed, frequently resulting in telomere dysfunction28. Previous studies have shown that oxidative stress can lead to the inhibition of SIRT6 function44. Conversely, SIRT6 alleviates oxidative stress by activating multiple signaling pathways45. Notably, SIRT6 deficiency has been shown to significantly impair antioxidant capacity, leading to intracellular ROS accumulation. Concurrently, the compensatory upregulation of Tert/Terc during blastocyst development indicates an increased demand for telomere repair, demonstrating the disruption of telomere homeostasis, which may originate from ROS mediated oxidative damage (Fig. S5e–f).
Given the established role of NAD+ in regulating the expression and activity of Sirt6 and coordinating the antioxidant defense system, we hypothesize that NAD+ is a key differentiating factor during embryonic development in vivo and in vitro. For this purpose, we compared the NAD+ levels of in vivo and in vitro generated blastocysts, and the results revealed that the NAD+ levels were lower in blastocysts in vitro than in control blastocysts (Fig. 4d). Subsequently, we tested whether supplementing NMN, the precursor of NAD+, could restore its TL, and the results revealed that the currently commonly used conditions of in vitro culture could not allow the embryos to produce enough NAD+ to maintain the function of Sirt6. First, a lack of NAD+ may lead to the impaired expression of Sirt646,47, second, a lack of NAD+ may directly weaken the activity of Sirt6. To determine whether NMN can maintain telomeres through Sirt6, we further verified the role of Sirt6 by using inhibitors (Fig. 4f and Fig. S6b) and a specific gene knockout mouse model (Fig. 5a), respectively. These findings revealed that in the absence of Sirt6 function, other pathways affected by NMN were unable to play a normal role in repairing, highlighting the importance of Sirt6. Together, these data strongly suggest that NAD+-dependent Sirt6 is critical for telomere maintenance during preimplantation embryogenesis in mice.
To further confirm whether the changes in TL in preimplantation embryos were carried over to the offspring, we performed uterine transfer of in vivo and in vitro blastocyst embryos and followed up postnatally, and we found consistent results in two successive generations (F1 and F2). The birth rate of F1 generation pups in vitro was substantially lower than that in vivo (Fig. S2a), suggesting the impaired development potential of preimplantation embryos in vitro. TL measurements in F1/F2 mice showed significantly shorter TL one week after birth in vitro than in vivo, but the difference gradually decreased with the increasing week age, and no significant difference in TL was detected between the two groups thereafter (Fig. 2d, f), which differed from the results of previous study9, possibly due to inconsistent culture conditions and different cell types. These results indicated that telomere shortening induced by changes in the metabolic state or stress response in vitro could have a further impact on the offspring, which could be maintained during successive generations of mating, although it could be recovered later. We speculate that since primordial germ cells differentiate and remain in a static state at an early stage of postimplantation, telomerase activity is inhibited, so the telomeres in the generated germ cells are not effectively repaired, and the TL of somatic cells can be restored due to the short-term reactivation of telomerase48,49. Epigenetic modifications may also play an important role in it. Research has found that ART-derived individuals exhibit global DNA methylation alterations, imprinted gene methylation profile shifts, and associated gene expression changes, compared to naturally conceived offspring50. Notably, Novakovic et al. demonstrated that ART-induced differential methylation patterns are transient and non-persistent into adulthood51. Building on this, our whole-genome methylation analysis revealed transient hypermethylation at the Sirt6 promoter in one-week-old offspring derived from in vitro blastocysts, which normalized by 7 weeks (Fig. S3b). In vitro models confirmed the suppressive role of high DNA methylation on Sirt6 transcription36,37. As for whether there are similar epigenetic effects in mouse embryos, methylation sequencing of embryos at different stages is still needed to verify in the future. Furthermore, changes in the growth pattern of the offspring after birth further suggest that early changes in telomere length may affect the metabolic pattern of the offspring in additional ways52,53. However, the above findings still need to be verified through further research. Collectively, these findings indicate that Sirt6 also plays an important role in maintaining telomere integrity during offspring development.
In this study, we obtained fertilized oocytes through in vivo fertilization to verify that they were fertilized in their natural environment, and then cultured them in vitro to determine whether changes in TL were connected to the in vitro embryo culture conditions. Considering the impact of metabolic differences between in vivo and in vitro environments on TL, our study focused on the relationship between telomere and metabolism in embryo culture, exploring the relevant mechanisms by which in vitro culture affects the telomere dynamics of preimplantation embryos. We found that the NAD+-dependent Sirt6 conditional depletion may be an important mechanism of telomere shortening in blastocysts induced by in vitro culture. Previous studies have shown that telomere regulation depends on the expression of Sirt6 at the molecular and cellular levels47, which has NAD+-dependent histone deacetylase activity, leading to chromatin compression. NAD+ is a cofactor required for the redox reaction, and its level changes with changes in nutrients and stress54,55. These findings suggest that Sirt6 may act as a nutritional and metabolic sensor, transmitting nuclear changes at the NAD+/NADH ratio to alter transcription and genomic stability54. Additionally, the accumulation of mtROS in blastocysts may also accelerate telomere shortening. In our study, we found that the disorder of NAD+ metabolic and the accumulation of mtROS in embryos during in vitro culture are related to telomere shortening, indicating the important role of mitochondrial function in telomere maintenance during embryonic development. Recent studies have shown a close relationship between TL and mitochondria in embryos56,57,58. More importantly, our study may identify a possible target for enhancing in vitro culture conditions, indicating that NMN, the precursor of NAD+, can alleviate the occurrence of short TL in blastocysts cultured in vitro. Moreover, the study of offspring has further focused our attention on the field of individual and intergenerational health, which provides convenience for the long-term health observation of offspring. However, the limitation of this study is that the regulation of telomere stability by Sirt6 may be mediated through epigenetic modifications at telomeric regions, and the existing in vitro telomerase detection system is difficult to fully simulate the physiological environment of telomere repair in blastocysts. Therefore, the telomerase activity was not directly detected in this study.
In summary, our results suggest that in vitro culture leads to insufficient NAD+ to maintain Sirt6 function, thus affecting telomere maintenance and may have an impact on newborn offspring, which indicates that NAD+-dependent Sirt6 is crucial for telomere maintenance in preimplantation embryos. Meanwhile, the accumulation of mtROS and epigenetic alterations may also play important roles in this process. Moreover, we propose that NMN, a precursor of NAD+, can attenuate the phenotype of telomere shortening in preimplantation embryos caused by in vitro culture. Understanding the mechanism of telomere shortening caused by in vitro culture can help guide the optimization of culture conditions, thereby improving the success rate of ART therapy and progeny safety. Nevertheless, direct application of these results to humans requires rigorous validation, as interspecies divergence in telomeric regulation and existing compensatory networks could limit their therapeutic relevance. Longitudinal human studies are still needed in the future for further exploration.
Methods
Animal preparation
F0 female mice (C57BL/6, ~4–6 weeks) and F0 male mice (ICR, ~10–12 weeks) were provided by the JSJ Laboratory Animal Co. Ltd (Shanghai, PRC) and maintained in a room with a controlled light cycle (12 L:12D). All animals were maintained and treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All studies adhered to procedures that are consistent with the Institutional Animal Care or Research Ethics Committee of Shanghai Jiao Tong University School of Medicine (Approval number 20161207). F0 females were superovulated by an intraperitoneal injection of 10 IU pregnant mare serum gonadotrophin (PMSG; Ningbo No. 2 Hormone Factory, China), followed 48 h later by an intraperitoneal injection of 10 IU human chorionic gonadotrophin (hCG; Ningbo No. 2 Hormone Factory, China). F0 females were naturally mated with F0 males after the hCG injection.
In vivo or in vitro embryo collection
As illustrated in Fig. 1a, all superovulated F0 female mice were plugged vaginally after mating and randomly divided into the two groups (in vivo or in vitro). After in vivo development, blastocysts (in vivo group) were collected by flushing each uterus of the plugged females with modified human tubal fluid media supplemented with 10% serum substitute supplement (mHTF-SSS; Irvine Scientific, USA). For the in vitro group, at 19–21 h post-hCG treatment, in vivo fertilized zygotes were collected from oviducts and cumulus cells were removed via digestion with hyaluronidase (H4272; Sigma-Aldrich) for 3–5 min. The zygotes were rinsed with mHTF-SSS, and placed in 20 μl drops of synthetic oviductal medium enriched with potassium (KSOM; MR-107, Millipore, Germany) covered with paraffin oil, and then equilibrated overnight in an incubator at 37 °C and 5% CO2. The zygotes were grown to the blastocyst stage at 37 °C with 5% CO2 atmosphere in vitro. The blastocysts in both groups were chosen on the basis of their developmental status and morphology. In detail, well-developed late-cavitating blastocysts were collected 96–100 and 106–112 h post-hCG for the in vivo and in vitro groups, respectively, based on prior descriptions of in vivo and in vitro blastocyst development59. Besides, embryos at different developmental stages were collected at precisely set time points according to the time after hCG injection. The 2-cell, 4-cell, 8-cell, morula, and hatching blastocyst developed in vivo were collected at 24–30 h, 36–48 h, 50–60 h, 72–84 h, and 100–110 h, respectively, after hCG injection. Conversely, the corresponding stages cultured in vitro were harvested at 30–36 h, 48–54 h, 60–72 h, 84–96 h, and 112–120 h post-hCG injection, respectively. Notably, ovulation induction and in vivo fertilization were performed in both the in vivo and in vitro groups to rule out any impact of these processes on oocyte and embryo quality. These processes were carried out according to standard and well-accepted procedures. All the technical procedures were performed by skilled technicians under strictly controlled conditions and optimized environments.
Separation of the ICM and TE
The blastocyst was initially artificially crinkled. After the blastocyst collapsed, the zona pellucida was pierced with a laser, and the trophoblast cells were absorbed through the pore by a biopsy needle and extended outward to elongate. The trophoblast cells at the outer edge of the zona pellucida were then hit with a laser once or twice before being mechanically separated. Thus, the trophoblast cells can be completely isolated from the inner cell mass.
Apoptosis analysis
At the blastocyst stage, the embryos were transferred to phosphate‐buffered saline (PBS) supplemented with 2% paraformaldehyde for fixation followed by permeabilization in 0.25% Triton X-100 at 37 °C for 20 min. Apoptotic nuclei were identified using the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay, employing the In Situ Cell Death Detection Kit (C1089;Beyotime). According to the manufacturer’s protocol, fixed embryos were incubated in the TUNEL reaction medium for 1 h at 37 °C in the dark. Following the cessation of the reaction, the embryos underwent a washing procedure and were subsequently immersed in a 4’,6‐diamidino‐2‐phenylindole (DAPI; Vector Laboratories) solution before being mounted onto slides. The quantification of apoptotic nuclei and the total number of nuclei was then performed using a confocal microscope (Olympus).
MitoSOX Red staining
Zygotes and embryos were collected and incubated at 37 °C under 5% CO2 for 30 min in calcium/magnesium-supplemented HBSS buffer containing 60 µg/ml BSA, 1 mg/ml PVP, 5 µM MitoSOX Red (M36008; Invitrogen), and 1 µg/ml Hoechst 33342 (C1027;Beyotime). Following incubation, samples were washed three times with washing buffer and subsequently mounted in pre-warmed HBSS (containing BSA, PVP, as above) for confocal imaging using an Olympu microscope. Fluorescence intensity quantification was performed with ImageJ software, where total fluorescence values were calculated and normalized against background signals to generate corrected total fluorescence values.
Mouse intrauterine embryo transfer
In the in vivo group, pseudopregnant female ICR mice (10–12 weeks old) were mated with vasectomized males 2.5 days prior to embryo transfer. In the in vitro group, female ICR mice (10–12 weeks old) were employed as pseudopregnant moms and mated with vasectomized ICR males 3.5 days before embryo transfer. Surrogated ICR mice were selected based on the presence of a vaginal plug and then anesthetized. The uterine horn was removed through the back. Thirty blastocysts from the in vivo or in vitro group were transferred to a single recipient, respectively. The surrogate mother mice were housed in a clean cage with sufficient water and high‐fat chow for 19–21 days before their F1 pups were born. After birth, F1 pups were weighed and clipped every two weeks until they reached 11 weeks of age (1, 3, 5, 7, 9 and 11 weeks accordingly). F1 pups were weaned after the third week, and females and males were placed separately. At 12 weeks of age, each group generated the F2 generation by putting male and female offspring together to form a couple, with the tail removed and weighed every two weeks until 11 weeks of age (1, 3, 5, 7, 9 and 11 weeks accordingly) after birth.
qPCR for telomere measurement
According to the published protocol60, a single-cell TL assay was used to measure TL in mouse embryos. Initially, single-cell genomic DNA was obtained by adding 2 μL 2 × lysis buffer (100 mM Tris·HCl pH 7.4, 300 mM NaCl, 0.8 mM EDTA, 2% NP-40, and 5 mM DTT) to a 0.2 ml PCR tube, and heated at 95 °C for 10 min. Then, a pre-amplification step was performed before quantitative PCR (qPCR). Briefly, Pre-PCR was performed using Phanta Flash Super-Fidelity DNA Polymerase. The reactions were set up by aliquoting 18 µL of master mix into the 0.2 mL PCR tubes each with 2 µL single-cell genomic DNA. Each reaction was set up with by 10 µL 2×Phanta Flash Master Mix, 1 µL each of telomere forward and reverse primer (10 µM), and 1 µL each of reference gene forward and reverse primer (10 µM) and enough water to make up a 20 µL final volume. Thermal cycler reaction conditions were set at 98 °C for 30 s followed by 16 cycles of 98 °C for 10 s, 60 °C annealing for 5 s and extension at 72 °C for 10 s, with a final extension for 1 min at 72 °C. PCR products were purified following the protocol of the purification kit (D4014; Zymo Research) and were eluted in 100 μL of double distilled water. A Bio-Rad thermocycler (CFX system test) was used with the same primers and reaction conditions of 95 °C for 10 min followed by 40 cycles of data collection at 95 °C for 15 s, 60 °C annealing for 30 s and 72 °C extension for 30 s along with 80 cycles of melting curves from 60 °C to 95 °C. After thermal cycling was completed, the CFX manager software was used to generate standard curves and Ct values for telomere signals and reference gene signals. The relative TL of individual cell was calculated by comparing the values of telomere (T) and reference gene (R) by the ΔΔCT method.
Total genomic DNA was isolated from tissue samples using the Blood/Cell/Tissue Genomic DNA Extraction Kit (DP304; TIANGEN BIOTECH) following the manufacturer’s instructions. The concentration of DNA was measured via Nanodrop (Thermo Fisher Scientific), using 10-fold diluted aliquots. For TL analysis, mouse DNA samples were adjusted to a working concentration of 4 ng/μL. The relative average TL was determined by qPCR. The sequences of primers used for qPCR are presented in Table S1.
RNA sequencing (RNA-seq)
A single inner cell mass of a blastocyst-stage embryo was pooled for each treatment group (in vivo and in vitro) and three independent replicates for each treatment procedure were collected. The in vivo and in vitro groups were named groups A and B, respectively, for subsequent sequencing. RNA extraction was carried out using a total RNA microextraction kit (Zymo Research). cDNA was synthesized using SuperScript II Reverse Transcriptase (18064014; Thermo Fisher) and amplified using the KAPA HiFi HotStart ReadyMix (2601; KAPA). Library preparation was performed using the TruePrep DNA Library Prep Kit V2 for Illumina (TD202; Vazyme Biotech, Nanjing, China) according to the manufacturer’s instructions. All the libraries were sequenced using an Illumina NovaSeq 6000 instrument from Genergy Biotechnology Co., Ltd. (Shanghai, China). The raw data were processed by Skewer (v0.2.2), and the data quality was assessed by FastQC v0.11.2. The sequencing reads had a length of 2 × 150 bp. The gene expression level was calculated as fragments per kilobase of transcript per million mapped reads (FPKM). DESeq2 was used to screen differentially expressed genes (DEGs) among the different groups (P < 0.05 and |log2-fold change | ≥ 1). Gene Ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted through the DAVID Bioinformatics Resources and OmicShare CloudTools, respectively. Moreover, the raw RNA-seq data have been deposited in the Gene Expression Omnibus database (GEO: GSE280684) of National Center for Biotechnology Information (NCBI).
Methylation analyses
DNA methylome preparation: The genomic DNA was fragmented by sonication, followed by end-repair, adenine tailing, and adapter ligation. Then, according to the manufacturer’s instructions, the genomic DNA was subjected to bisulfite treatment using the EZ DNA Methylation Kit (D5001; Zymo Research), and subsequent PCR amplification was carried out.
Quality control and alignment: First, Trimmomatic (version 0.33) was used to remove adapters and low-quality bases from all bisulfite sequencing reads. Next, the paired-end reads that passed quality control were mapped to the mouse reference genome (GRCm39) in paired-end mode using bismark (version 0.16.3). The single-end reads that passed quality control were mapped to the reference genome in single-end mode. Then, Samtools and Picard were used to sort the reads and remove PCR duplicate reads.
DNA methylation level analysis: Before determining the DNA methylation status, all sequencing reads containing incompletely converted non-CpG cytosines were removed. To extract the methylation status of cytosines, bismark_methylation_extractor was applied to both paired-end and single-end reads. For each CpG site, the methylation level was determined by the ratio of the number of methylated cytosines in the reads covering that CpG to the total number of reads covering that CpG. The DNA methylation level of the Sirt6 gene promoter region was determined by the average DNA methylation level of all covered CpG sites. Moreover, the raw methylation sequencing data have been deposited in the Gene Expression Omnibus database (GEO: GSE299048) of National Center for Biotechnology Information (NCBI).
RT-qPCR
Each group of the zygotes and embryos was collected separately and performed according to the single-cell sequence specific amplification kit (P621; Vazyme Biotech, Nanjing, China). cDNA was amplified through 18 cycles of PCR. Subsequently, 10-fold diluted cDNA was used as the template for qPCR, which was performed with TB Green Premix Ex Taq (R420Q; Takara, Japan) on a QuantStudio 6-Flex Real-Time PCR System (Applied Biosystems, Life Technologies) according to the manufacturer’s instructions. Actin was used as an internal control. The primers used for RT-qPCR are presented in Table S1. Each gene was tested in triplicate. The ΔΔCT method was used to calculate the abundance of the transcripts.
Immunofluorescence staining
The mouse blastocysts were fixed with 2% paraformaldehyde in PBS for 60 min at room temperature, followed by permeabilization in 0.25% Triton X-100 for 20 min at room temperature. The embryos were then blocked with 1% bovine serum albumin (BSA) (A600332, 25 G; BBI) in PBS for 1 h at room temperature after washing for three times in washing solution (1% BSA in PBS). Primary antibody incubation was performed overnight at 4 °C. The primary antibodies include: monoclonal rabbit anti-SIRT6 (ab191385; Abcam), monoclonal mouse anti-γH2A.X (05-636, EMD Millipore), monoclonal rat anti-Oct3/4 with Alexa FluorTM 488 (53-5841-82, invitrogen). The next day, the embryos were washed in washing solution for three times and incubated with DyLight 594/647 conjugated goat anti‐rabbit or mouse IgG (H&L) antibodies (bs-0295D-BF594, bs-0296G-AF647; Bioss) for 1 h at room temperature, and then the nuclei were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (H-1200; Vector Laboratories). After staining, the samples were mounted with coverslips and imaged with an Olympus confocal microscope. In our study, all signals were standardized by the fluorescent intensity of DAPI and analyzed using the ImageJ software.
Measurement of NAD+ and NADH levels
NAD+ and NADH levels were determined using the NAD+/NADH Glo cycling assay (G9071; Promega, Madison, WI, USA). Embryos collected in 50 µl of PBS were lysed with 50 µl of 0.2 M NaOH containing 1% DTAB and incubated at room temperature for 10 min. The sample mixture was divided into two tubes (50 µl each). To test NAD+, 25 µl of 0.4 N HCl was added to the solution, which was heated at 60 °C for 15 min. After 10 min of incubation at room temperature, 25 µl of 0.5 M Trizma base (T1503; Sigma) was added to the samples. To measure the level of NADH, the sample mixture was heated to 60 °C for 15 min, then incubated for 10 min at room temperature, followed by the addition of 25 µl of 0.5 M Trizma base and 25 µl of 0.4 N HCl. The assay reagent was added to the sample mixture, which was incubated for 1 h prior to cycling; luminescence was then determined (Varioskan LUX; Thermo Fisher, Waltham, MA, USA).
Treatment with inhibitors and chemicals
For inhibitor treatment, we prepared solutions of Sirt6 inhibitor (JYQ-42) and Trp53 inhibitor (PTF-α; HY-15484, MedChemExpress) in dimethyl sulfoxide (DMSO). The embryos were subjected to in vitro culture in KSOM medium until they reached the morula stage, after which they were transferred to KSOM medium supplemented with inhibitor for subsequent detailed analysis. Correspondingly, 0.1% DMSO was included as a negative control. For the NAD+ supplementation treatment, NMN (N3501; Sigma) solutions were prepared in water. Solutions were diluted to yield a final concentration in KSOM medium as needed (NMN, 10 µM).
Sirt6 (flox/flox, zp3-cre) mouse model generation
Sirt6 (flox/flox, zp3-cre) mice were obtained from Cyagen Biosciences (Guangzhou, China). Briefly, the pZp3–cre plasmid was injected into mouse zygotes (strain FVB/N) to produce transgenic founder mice, and the Zp3–Cre mouse line was established. Then, the Zp3-Cre mice were subsequently mated with mice carrying a floxed Sirt6 gene, and female progeny with cre were selected for further study. To identify mouse genotypes, a PCR-based genotyping approach was employed using specific primer pairs. The primers used for genotype identification are presented in Table S1.
Mouse intracytoplasmic sperm injection (ICSI) and embryo culture
C57BL/6 female mice (~4–6 weeks old) were superovulated via intraperitoneal injection of 10 IU PMSG followed by 10 IU hCG 48 h later. Cumulus-oocyte complexes (COCs) were retrieved from oviducts in mHTF-SSS 14–16 h after hCG injection. The metaphase II (MII) oocytes were washed several times with hyaluronidase to remove cumulus cells. The MII oocytes were recovered in KSOM for 1 h in an incubator (37 °C, 5% CO2), after which ICSI was performed. Sperm were obtained from the cauda epididymis and vas deferens of C57BL/6 male mice (~8–10 weeks old). To gain sperm motility, they were allowed to swim for 30 min at 37 °C in mHTF-SSS. Afterwards, sperm decapitation and microjection of the sperm heads into MII oocytes were conducted in mHTF-SSS and 7% polyvinyl pyrrolidone (PVP) solution (Irvine Scientific) on an inverted microscope (Leica) equipped with a warm plate and a micromanipulation system (MM-89; Narishige, Japan) as previously described61. Finally, post-microinjection embryos were fully washed and cultured in KSOM at 37 °C and 5% CO2 continuously to observe embryonic development.
mRNA extraction and cDNA generation
Total RNA was isolated from ovarian tissue using TRIzol. The protocol followed was as stated in the TRIzol Reagent User Guide (Invitrogen). Briefly, following sample homogenization with TRIzol, RNA was isolated through precipitation, washing, and resuspension. The RNA concentration and quality were checked using a Thermo Scientific NanoDrop 2000. Corresponding cDNA libraries were generated using the HiScript III RT SuperMix for qPCR (+gDNA wiper) (R323; Vazyme Biotech, Nanjing, China).
Statistical methods
The statistical analyses were performed using the GraphPad Prism (version 9.5) and Statistical Package for Social Sciences (version 25.0; SPSS Inc., USA) and. Continuous variables were presented as mean ± SD or median (interquartile range, IQR). The normality of continuous variables was determined using the Shapiro–Wilk test. The variables were compared by Student’s t test or one‐way ANOVA with post hoc analysis (defining LSD as the method that aims to correct for multiple pairwise tests). A P value < 0.05 was considered statistically significant. Based on the estimated effect size (mean difference ± SD) of preliminary experiments on TL, the sample size was determined by power analysis (α = 0.05, power=0.8).
Data availability
RNA sequencing and methylation sequencing data used in this study has been deposited in the Gene Expression Omnibus Database (GEO: GSE280684; GSE299048) of the National Center for Biotechnology Information (NCBI) in the United States and are publicly accessible at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE280684 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE299048. All source data underlying the graphics and charts in the main figures have been included as supplementary data 1. Supplementary data have been uploaded to Figshare and can be accessed through the following link: https://doi.org/10.6084/m9.figshare.29178737. The full length original gel image is provided in Supplementary Fig. 7.
References
Steptoe, P. C. & Edwards, R. G. Birth after the reimplantation of a human embryo. Lancet 2, 366 (1978).
Berntsen, S. et al. The health of children conceived by ART: “the chicken or the egg?”. Hum. Reprod. Update 25, 137–158 (2019).
Bai, F. et al. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum. Reprod. 35, 446–452 (2020).
De Geyter, C. et al. 20 years of the European IVF-monitoring Consortium registry: what have we learned? A comparison with registries from two other regions. Hum. Reprod. 35, 2832–2849 (2020).
Schieve, L. A. et al. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N. Engl. J. Med. 346, 731–737 (2002).
Castillo, C. M. et al. Associations of IVF singleton birthweight and gestation with clinical treatment and laboratory factors: a multicentre cohort study. Hum. Reprod. 35, 2860–2870 (2020).
Sunde, A. et al. Time to take human embryo culture seriously. Hum. Reprod. 31, 2174–2182 (2016).
Hann, M. et al. The growth of assisted reproductive treatment-conceived children from birth to 5 years: a national cohort study. BMC Med. 16, 224 (2018).
Wang, C. et al. Leukocyte telomere length in children born following blastocyst-stage embryo transfer. Nat. Med. 28, 2646–2653 (2022).
Lane, M., Robker, R. L. & Robertson, S. A. Parenting from before conception. Science 345, 756–760 (2014).
Fleming, T. P. et al. The embryo and its future. Biol. Reprod. 71, 1046–1054 (2004).
Watkins, A. J. et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl. Acad. Sci. USA 104, 5449–5454 (2007).
Rinaudo, P. & Schultz, R. M. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 128, 301–311 (2004).
Ren, L. et al. Dynamic comparisons of high-resolution expression profiles highlighting mitochondria-related genes between in vivo and in vitro fertilized early mouse embryos. Hum. Reprod. 30, 2892–2911 (2015).
McElligott, R. & Wellinger, R. J. The terminal DNA structure of mammalian chromosomes. EMBO J. 16, 3705–3714 (1997).
Mongelli, A. et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int. J. Mol. Sci. 22, (2021).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 101, 17312–17315 (2004).
Cheng, F. et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 9, 117–126 (2021).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Monaghan, P., Metcalfe, N. B. & Torres, R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 (2009).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Rubtsova, M. P. et al. Telomere reprogramming and cellular metabolism: is there a link? Int. J. Mol. Sci. 25, (2024).
Casagrande, S. & Hau, M. Telomere attrition: metabolic regulation and signalling function? Biol. Lett. 15, 20180885 (2019).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Ungar, L., Harari, Y., Toren, A. & Kupiec, M. Tor complex 1 controls telomere length by affecting the level of Ku. Curr. Biol. 21, 2115–2120 (2011).
Mannherz, W. & Agarwal, S. Thymidine nucleotide metabolism controls human telomere length. Nat. Genet. 55, 568–580 (2023).
Sun, C. et al. Re-equilibration of imbalanced NAD metabolism ameliorates the impact of telomere dysfunction. EMBO J 39, e103420 (2020).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Kanfi, Y. et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221 (2012).
Guo, Z., Li, P., Ge, J. & Li, H. SIRT6 in aging, metabolism, inflammation and cardiovascular diseases. Aging Dis 13, 1787–1822 (2022).
Ge, J. et al. SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging 11, 1965–1976 (2019).
Counter, C. M. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921–1929 (1992).
Webb, C. J., Wu, Y. & Zakian, V. A. DNA repair at telomeres: keeping the ends intact. Cold Spring Harb. Perspect. Biol. 5, (2013).
Spinelli, J. B. & Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754 (2018).
Hong, J.-Y. et al. Coordinate regulation by transcription factors and DNA methylation in the core promoter region of SIRT6 in bovine adipocytes. Arch. Biochem. Biophys. 659, 1–12 (2018).
Scisciola, L. et al. New insight in molecular mechanisms regulating SIRT6 expression in diabetes: Hyperglycaemia effects on SIRT6 DNA methylation. J. Cell. Physiol. 236, 4604–4613 (2021).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Deng, Y., Chan, S. S. & Chang, S. Telomere dysfunction and tumour suppression: the senescence connection. Nat. Rev. Cancer 8, 450–458 (2008).
Aljahdali, A. et al. The duration of embryo culture after mouse IVF differentially affects cardiovascular and metabolic health in male offspring. Hum. Reprod. 35, 2497–2514 (2020).
Leese, H. J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 143, 417–427 (2012).
Feuer, S. & Rinaudo, P. Preimplantation stress and development. Birth Defects Res. C Embryo Today 96, 299–314 (2012).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Liu, R., Liu, H., Ha, Y., Tilton, R. G. & Zhang, W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res. Int. 2014, 902842 (2014).
Yang, Y. et al. SIRT6 protects vascular endothelial cells from angiotensin II-induced apoptosis and oxidative stress by promoting the activation of Nrf2/ARE signaling. Eur. J. Pharmacol. 859, 172516 (2019).
Kim, H.-S. et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab 12, 224–236 (2010).
Amano, H. et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab 29, 1274–1290.e9 (2019).
Jaskelioff, M. et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106 (2011).
Coussens, M. et al. Regulation and effects of modulation of telomerase reverse transcriptase expression in primordial germ cells during development. Biol. Reprod. 75, 785–791 (2006).
Choufani, S. et al. Impact of assisted reproduction, infertility, sex and paternal factors on the placental DNA methylome. Hum. Mol. Genet. 28, 372–385 (2019).
Novakovic, B. et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 10, 3922 (2019).
Tellechea, M. et al. Telomere length in the two extremes of abnormal fetal growth and the programming effect of maternal arterial hypertension. Sci. Rep. 5, 7869 (2015).
Sibert, N. T. et al. Cord blood telomere shortening associates with increased gestational age and birth weight in preterm neonates. Exp. Ther. Med. 21, 344 (2021).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Landry, J. et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl. Acad. Sci. USA. 97, 5807–5811 (2000).
Ito, J. et al. Paternal aging impacts mitochondrial DNA content and telomere length in mouse embryos. Mitochondrion 68, 105–113 (2023).
Inoue, Y. et al. Telomere length determines the mitochondrial copy number in blastocyst-stage embryos. Mitochondrion 77, 101887 (2024).
Winstanley, Y. E. et al. Telomere length in offspring is determined by mitochondrial-nuclear communication at fertilization. Nat. Commun. 16, 2527 (2025).
Giritharan, G. et al. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction 134, 63–72 (2007).
Wang, F. et al. Robust measurement of telomere length in single cells. Proc. Natl. Acad. Sci. USA 110, E1906–E1912 (2013).
Lyu, Q. F. et al. New technique for mouse oocyte injection via a modified holding pipette. Reprod. Biomed. Online 21, 663–666 (2010).
Acknowledgements
This work was supported by the Natural Science Foundation of Shanghai Municipal Science and Technology Commission (23ZR1437500 to Q.L.), and the National Natural Science Foundation of China (81901478 to W.L.), and the Cross Research Fund Project of the Ninth People’s Hospital Affiliated with Shanghai Jiao Tong University School of Medicine (JYJC202220 to Q.L.). The funders have no role in the research design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank the Assisted Reproductive Department and Laboratory Animal Center of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine for providing the platform and improving the convenience of performing the experiments. Biorender was used to create the schematics.
Author information
Authors and Affiliations
Contributions
Q.F.L., Y.P.K., X.F.L. and W.Z.L. conceived and designed these experiments. Q.F.L. and W.Z.L. supervised the implementation of the project. D.J.L., W.Z.L. and X.Y.L. conducted experiments. D.J.L., W.Z.L., X.Y.L., S.T.J., M.M. and Z.J.H., analyzed the data. K.B.L., W.N.Y., X.S., Y.F., H.B.W., M.R.Y. provided some technical support. L.W., L.S., H.L. helped explain the data. D.J.L. and W.Z.L. drafted the article. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Adult female and male mice were provided by the JSJ Laboratory Animal Co. Ltd (Shanghai, PRC) and Sirt6 (flox/flox, zp3-cre) mice were obtained from Cyagen Biosciences (Guangzhou, China). All animals were maintained and treated in accordance with the Guide for the Care and Use of Animals for Research Purposes. All studies adhered to procedures that are consistent with the Institutional Animal Care or Research Ethics Committee of Shanghai Jiao Tong University School of Medicine (Approval number 20161207).
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mitsutoshi Yamada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Wee-Wei Tee and Mengtan Xing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, D., Li, W., Liao, X. et al. NAD+-dependent Sirt6 is a key regulator involved in telomere shortening of in vitro-cultured preimplantation embryos. Commun Biol 8, 1275 (2025). https://doi.org/10.1038/s42003-025-08567-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08567-x