Abstract
Clathrin-mediated endocytosis (CME), the main mechanism for swift, selective protein uptake in eukaryotic cells, initiates with adaptor protein AP2 recruitment to the plasma membrane (PM). AP2 recognizes PM-associated PtdIns(4,5)P2 and protein cargo for internalization. Nonetheless, many aspects of this process remain unclear due to their in vivo complexity. Here, a thermodynamic and time-resolved structural analysis of AP2 binding to different biomimetic PM was undertaken under physiological conditions using a combination of neutron reflectometry, interfacial tensiometry and rheology, and atomic force microscopy. The resultant in vitro data replicated previous in vivo observations, as well as yielded biophysical insights into normal and aborted CME. The presence of cargo may not be pivotal for the “activating” conformational change of AP2. However, the presence of cargo extends AP2’s residence time on the membrane surface, due to slower on- and off-rates, thereby tentatively giving sufficient time for CME to proceed fully. Moreover, upon interaction with AP2, phospholipid lateral diffusion decreases markedly, inducing a gel phase attributed to creating a percolated network involving AP2 on the membrane, which could potentially serve as a mechanism for modulating subsequent clathrin binding. The subsequent clathrin polymerization at the membrane is dependent on the AP2’s clathrin binding sequence.

Similar content being viewed by others
Introduction
Clathrin-mediated endocytosis (CME) is the primary route for swift and selective uptake of proteins into the intracellular endosomal system of eukaryotes1. It is crucial to controlling the plasma membrane (PM) proteome and thus plays an essential role in defining the trans-membrane protein complement of a mammalian cell’s limiting membranelle2. It is therefore fundamental to cell nutrition, homeostasis, signaling and maintaining its interactions with the outside world including with other cells and the blood and immune systems. CME is also “highjacked” by many invading pathogens, including most viruses, for cellular entry and establishing infection3,4. Although CME has served as a conceptual paradigm for other transport carrier formation processes5, (reviewed in ref. 6) many aspects of its initiation and control, and the molecular mechanisms behind them, still remain unclear at a fine-scale molecular level despite much effort.
In order to initiate CME, it has been postulated that the transmembrane PM proteins to be internalized, designated as “cargo”, need first to be selected by association with specific CME adapter proteins7,8, of which the most abundant is AP2, deletion of which in mice is lethal9. Clathrin binding the PM-bound AP2 leads to the formation of clathrin-coated pits (CCPs)10, which become, after subsequent scission, clathrin-coated vesicles (CCVs) carrying the cargo intracellularly.
The AP2 hetero-tetramer localizes to the cargo-presenting PM through binding the PM-specific phospholipid, phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2)11,12,13. The presence of this phosphoinositide appears essential for CME initiation since clathrin assembly and CCV formation are disrupted when PtdIns(4,5)P2 levels are lowered14. Similarly, acute depletion of PtdIns(4,5)P2, either by a phosphatase or by inhibition of production with butanol, results in an almost complete loss of CCV formation15.
Conversely, cargo overexpression leads to an increase in productive CCPs16. Cargo is recognized through the binding of specific short linear amino-acid motifs present in their cytoplasmic domain. The AP2 complex recognizes two of the most commonly found internalization signals, the tyrosine YxxΦ motif (where Φ represents a hydrophobic residue) and the acidic dileucine [DE]xxxL[LI] motif17,18,19,20. ‘‘Snap shot’’ structural studies by X-ray crystallography and cryo-EM single particle reconstruction and tomography have shown that AP2 can adopt different quaternary conformations. In a closed (or locked) conformation, adopted in solution, binding sites for the tyrosine and dileucine recognition motifs are hidden21,22. In an open conformational state, favored when AP2 is ‘‘on-membrane’’ and bound at multiple sites to PtdIns(4,5)P2, its cargo binding sites likely become exposed13,23. Correspondingly, the clathrin binding site in the β2-hinge, which is occluded in the close-state conformation, is presumed rendered accessible on membrane binding based on protein: protein interaction and basic CCV reconstitution assays24. Extrapolating from these ‘‘static’’ molecular structures to in cell analysis of CME has proven insightful25,26,27, but these studies suffer from a lack of dynamic physical description and energetic analysis of the collective mechanisms and driving forces, which couple AP2 cargo assembly to membrane processes. Such in vitro approach may provide insights to why almost half of nascent CCPs fail to mature into cargo-loaded CCVs and are instead aborted, as determined by live-cell microscopy8,16,28.
In this work, characterization of bottom-up, minimal component in vitro PM model membranes, containing PtdIns(4,5)P2 and representative CME cargo, at room temperature and under physiological conditions, was followed by time-resolved biophysical measurements of these systems after addition of recombinant AP2. Low/high resolution specular neutron reflectometry (SNR) experiments were performed to study association kinetics and resultant quaternary structures of AP2 on different PM models. In parallel, the dynamic and equilibrium affinities of AP2 were determined by measurements of the membranes’ lateral pressure in the presence of increasing concentrations of AP2 using a Langmuir trough. The specific role of the lateral organization of lipids into domains, as well as the PtdIns(4,5)P2 function in promoting conformational change in AP2 necessary for clathrin binding, was probed by atomic force microscopy (AFM), coupled with molecular-level fluorescence measurements. These imaging techniques highlighted the formation of 2-dimensional molecular features on the PM in the presence of AP2. Finally, mechanical properties measurements identified an AP2-induced transition from fluid-like to gel-like behavior of the PM model membrane. The structural resistance against shear deformation emerging above a critical threshold was rationalized as a 2D percolation network in which the elastic behavior is only due to an entropic configurational contribution of AP2 and PtdIns(4,5)P2. The subsequent addition of clathrin results in further pressure and rheometric changes, indicating clathrin self-assembly at the membrane. The clathrin membrane interaction is dependent on AP2 having its clathrin binding sequence.
Finally, these studies point the way as to how to investigate other cellular transport and membrane remodeling processes. Their intracellular locations, typically on organelles deep within the eukaryotic cell, mean they are not amenable to contemporary high spatial and temporal resolution in cell techniques.
Results
Biophysical characteristics of the plasma membrane can be recreated in vitro
The PM inner leaflet of mammalian cells is in a fluid state, which can be mimicked using a mixture of anionic and zwitterionic phospholipids. A model PM membrane can be formed at the air / aqueous buffer interface (see Methods), using a floating Langmuir monolayer prepared from 1,2-dioleoil-sn-glycero-3-phosphoetanolamine (DOPE), 1,2-dioleoil-sn-glycero-3-phosphocoline (DOPC), anionic 1,2-dioleoil-sn-glycero-3-phospho-L-serine (DOPS) and cholesterol, and further enriched with PtdIns(4,5)P2, and synthetic lipo-peptides, representing cargo. This 4,5 phosphoinositide is a key marker molecule for PM, while the TGN38 and CD4 peptidolipids, that display the tyrosine and the dileucine motif respectively, were used as archetypical CME cargo11 (see Supplementary Note 1). All in vitro experiments were undertaken in physiological buffers24,29.
At room temperature (T = 21 ± 0.5 °C), the PM model membranes’ surface pressure (Π) – Area per molecule (A) isotherms, and the corresponding lateral compressibility, did not exhibit any phase transition, suggesting that these lipid monolayers are in a liquid expanded (LE) phase, which is considered equivalent to a lipid bilayer’s liquid-crystalline state (see Fig. S2). Importantly, the presence of either the CD4 or the TGN38 lipo-peptides did not alter the existence of the LE phase (Fig. S2).
The structure of the lipid monolayer orthogonal to the plane of the interface was determined by SNR analysis (see Supplementary Note 8). The PtdIns(4,5)P2-enriched monolayers could be effectively modeled as two layers (Fig. S10C). A layer containing the hydrophobic acyl chains is exposed to air, while the hydrophilic lipid headgroups layer is in contact with the aqueous buffer (Table S7). The neutron scattering length density (SLD) distribution yielded the thickness of the lipid headgroups layer as 10 ± 1 Å (Fig. S10B, D). In the presence of cargo, the CD4 and TGN38 peptides were found to be confined to the headgroups layer (Table S7), with a solvent penetration into the hydrophobic layer of less than 12% in all cases. Furthermore, the neutron data for the different membranes measured at surface pressure Π0 = 25 ± 0.5 mN/m) yielded a mean area per molecule similar to the value determined from their Π-A isotherms (Fig. S2) as well as consistent with a compact lipid bilayer30,31.
AP2 and PtdIns(4,5)P2 co-cluster under near-physiological conditions
The morphology of the AP2 interacting with free-standing, Langmuir lipid monolayers was studied by epifluorescence microscopy under near physiological buffer conditions (125 mM Potassium Acetate, pH 7.4), initially identified in the reconstitution of AP2-mediated clathrin polymerization24. In a dedicated Langmuir trough (See Methods and Supplementary Note 3), Alexa Fluor 488-labeled AP2 and TGN38-enriched PM model lipid monolayers doped with a trace amount of Bodipy-TMR labeled PtdIns(4,5)P2 were imaged (Fig. 1A, B). Fig. S6 shows the colocalization in presence of CD4 monolayers. In the absence of AP2, the fluorescent inositide formed submicrometer-sized clusters in the lipid monolayer (Fig. S7), which is likely due to the magnesium cations’ millimolar concentration in the HKM buffer32. In the presence of AP2, simultaneous colocalization in the lateral optical plane of PtdIns(4,5)P2 and AP2 demonstrates the preference binding of AP2 with PtdIns(4,5)P2. Since the lipid fluorophore is labeled in the acyl chains, the interaction between the lipid’s inositide headgroup and AP2 is expected to be unperturbed. As expected11, in the absence of PtdIns(4,5)P2, no AP2 is observed membrane bound (see Supplementary Note 6).
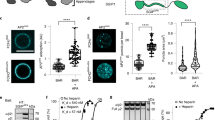
Simultaneous colocalization of AP2 and PtdIns(4,5)P2 clusters in Langmuir monolayers at the air water interface were reported by fluorescence images of TGN- PtdIns(4,5)P2 monolayer at the air/buffer interface labeled with 1 mol % Bodipy-TMR- PtdIns(4,5)P2 (A) interacting with AP2-labeled with Alexa Fluor 488 (B). Scale bars are 20 μm (main images in A, B) and 10 μm (corresponding insets). Transferred lipid monolayers immersed in buffer were examined by fluid-phase, tapping-mode AFM in absence (C) and presence (D) of AP2. E shows the thickness histograms of samples shown in (C, D) with straight lines being the corresponding Gaussian distributions, respectively. F Time-resolved AFM images shows AP2 yielded an increase of thickness and FWHM after injection until a plateau is reached when AP2 saturates the monolayer. Data points are represented as mean ± S.D. (n = 10). Individual data points can be found in the source data.
Tapping mode, liquid AFM investigations on Langmuir-Schaeffer supported TGN38-presenting PM model monolayers confirmed high surface coverage of the lipids, absence of defects ( <2% surface) and the lipidic components’ miscibility (Fig. 1C). The presence of ~2 nm high bright spots, which were identified as PtdIns(4,5)P2 clusters ( ≈ 8% surface), are in agreement with previous studies of biomimetic membranes33,34. The corresponding monolayer lipid thickness (Fig. 1E), is also in agreement with the above SNR analysis (30 ± 2 Å) (Fig. S10C and Table S2). Similar thicknesses were also found for CD4-enriched lipid monolayers (see Fig. S9 and Supplementary Note 7).
After addition of AP2, an increase in the bright spots’ size and thickness to ~10 nm is observed by AFM on TGN38-presenting PM model monolayers (Fig. 1D, E). A similar increment was also found on CD4-presenting monolayers (Fig. S9). Since in the absence of PtdIns(4,5)P2, such bright spots are not present (Fig. S8), these are likely associated to AP2 binding to PtdIns(4,5)P2 clusters, which structural studies show occurs at four different places per AP213. Time-resolved AFM shows the increase of the spot’s average thickness until a plateau is reached, which would correspond to the binding saturation of AP2 to the lipid monolayer (Fig. 1F). Similarly, in a Langmuir trough, after injection of AP2 into the bulk buffer underneath a PtdIns(4,5)P2-enriched lipid monolayer, an immediate and rapid increase in lipid lateral pressure is observed, followed by a slower increase until a plateau is reached (Fig. 2A).
A Increase in Surface Pressure (ΔΠ) as a function of time for different concentration of AP2 on lipid monolayers enriched in 10 wt. % PtdIns(4,5)P2 and TGN38 (2 wt. %). The initial surface pressure Π0 in all the cases has been fixed to 25 ± 1 mN/m similar to the lateral pressure in a plasma membrane leaflet. B Normalized surface pressure plotted against to the time elapsed after a lag period and fitted by a stretched exponential (straight lines) following Eq. 2. C Concentration dependence of the average relaxation ratio and a linear fit (straight lines). D Maximal increase in surface pressure ΔΠ∞ for lipid monolayers at different composition at Π0 = 25 ± 1 mN/m and a fixed concentration of AP2 of 500 nM. E Tensiometry binding analysis for AP2 recruited to a monolayer enriched in PtdIns(4,5)P2 and TGN38 or CD4 at different values of Π0. The increment in pressure, ΔΠ, is proportional to the amount of protein binding to the lipid monolayer at the air/water interface. Straight lines are fits to the experimental data obtained through the Hill-Langmuir relation (Eq. 1). F The increment in pressure, ΔΠ as a function of AP2 concentration for CD4 and TGN38 monolayers after subtracting PtdIns(4,5)P2 contribution. G Uppermost surface pressure increments due to the interaction of AP2 with a monolayer at Π0 = 25 ± 1 mN/m, at different concentration of PtdIns(4,5)P2. H Dissociation constants for PtdIns(4,5)P2 monolayers enriched in CD4 and TGN38 at different initial surface pressure. Bar diagrams in D,G,H are represented as mean ± S.D. (n = 5).
Equilibrium binding analysis of AP2 to a model PM shows synergy between PtdIns(4,5)P2 and cargo binding
The binding affinity of AP2 to the lipid monolayer can be directly determined through titration of surface pressure measurements (Fig. 2A). The analysis with a Langmuir adsorption model assumes the maximum increase in the lateral pressure ΔΠ∞, at constant surface area, is dependent on the fraction of AP2 (θ) interacting with the monolayer35 (see further details on Supplementary Note 3). The binding affinity of AP2 to the different lipid monolayers could therefore be determined from equilibrium analysis (Fig. 2E, Table 1),
where CAP2 is the bulk concentration of AP2, KD is the dissociation constant and n is the Hill coefficient36.
At the typical membrane lateral pressure Π0 = 25 ± 0.5 mN/m, the affinity of AP2 to a 10 wt. % PtdIns(4,5)P2 PM monolayer was KD = 5 ± 1 nM. The increase in concentration of PtdIns(4,5)P2 leads to an increase in ΔΠ∞ (Figs. 2G, S3) and can be rationalized by an increased in AP2 binding capacity of the membrane (as observed in Fig. 1A).
The addition of either TGN38 or CD4 cargo also yield further increases in lateral pressure ΔΠ∞, reflecting the synergy in binding AP2 from inositol phospholipids and from cargo present in the membrane (Figs. 2D, S3). At low AP2 concentration, binding curves to cargo-presenting monolayers mirror the binding curve for PtdIns(4,5)P2-only monolayer. However, as AP2 concentration increased, the presence of cargo led to higher equilibrium pressure changes. This biphasic binding behavior is observed at AP2 concentrations above 2 nM for TGN38, and 10 nM for CD4. Assuming independent binding sites for PtdIns(4,5)P2 and for cargo on AP2, the removal of the PtdIns(4,5)P2 binding effect results in an equilibrium-derived KD = 24.5 ± 1.6 nM and 75 ± 9 nM, for TGN38 and for CD4, respectively (Fig. 2F, Table 1). The lower affinity for TGN38 peptide to its binding site on AP2 determined in solution by ITC (2 μM) suggest lipid head groups also contribute to AP2 binding25. In support of this observation, AP2 has an overall weaker affinity to more densely packed PM model monolayers, which had larger initial pressure Π0 = 35 ± 0.5 mN/m (overall affinity weakening from KD = 15 ± 3 nM to 64 ± 10 nM and from 29.5 ± 5 nM to 93 ± 10 nM, for a TGN- and a CD4- enriched PM monolayer respectively) as shown Fig. 2H.
These affinities between AP2 and model PM appear to mirror the relative difference in affinities previously reported from SPR Biacore measurements on immobilized liposomes11. Although, the SPR-derived affinities were significantly weaker (311 nM for PtdIns(4,5)P2 and TGN-enriched liposomes, and 930 nM for PtdIns(4,5)P2 and CD4-enriched liposomes), this could be due to the higher salt concentration in the SPR buffer (250 mM NaCl), which would reduce any ionic interaction between PtdIns(4,5)P2 and AP2, in comparison to the more physiological buffer characterized here (125 mM Potassium Acetate).
Kinetic binding analysis shows that the presence of cargo significantly increases AP2 residence time on model PM
The binding affinity obtained by the Langmuir isotherms at equilibrium (steady state) were complemented by kinetic experiments to evaluate the temporal behavior of the association/dissociation of the AP2-lipid monolayer binding process (Fig. 2A). Under 70 nM AP2 solution concentration, the normalized kinetics, re-plotted in Fig. 2B, display an exponential trend, while for concentrations above 70 nM, the kinetics can be fitted by a stretched exponential expression based on rate distributions37 (see further details in Supplementary Note 3),
which are determined by the characteristic time constant τ and the stretched exponent β (0 < β < 1). The latter parameter controls the width of the corresponding rate distribution. At increasing AP2 concentrations, a decrease in both τ and β are observed (See Table S5). An average binding time <τ> was calculated from
Where Γ denotes the gamma function38. The associated relaxation rate kd = τ−1, increases linearly with AP2 concentration (Fig. 2C). Assuming a first-order kinetic reaction (see Supplementary Note 3), the apparent rate coefficient is given by
Where the apparent kon on rate and koff off rate take into account the combined effect of cargo and PtdIns(4,5)P2 binding.
The intercept and slope of a linear fit to the experimental values of <τ>−1 (Fig. 2C) yield the average backward and forward rate coefficients for AP2 association to PtdIns(4,5)P2-only membranes giving \(\langle {k}_{{off}}\rangle =5.6\pm 0.5\,{10}^{-4}{s}^{-1}\) and \(\left\langle {k}_{{on}}\right\rangle =7.9\pm 0.7\,{10}^{4}{M}^{-1}{s}^{-1}\), respectively. At 25 mN/m, the ratio \(\langle {k}_{{off}}\rangle /\langle {k}_{{on}}\rangle\) gives an average dissociation coefficient KD (7 ± 1 nM), which is similar to the corresponding value of (4.7 ± 0.5 nM) from the equilibrium analysis of the Langmuir isotherm (Fig. 2H).
In the presence of cargo, the affinities determined from both the kinetic and the equilibrium analysis of the Langmuir isotherm agree (12 ± 1 and 18 ± 3 nM in the presence of TGN38 and CD4, respectively) (Table 1). However, in comparison to cargo-less PM model membranes, the kinetic reaction slows down remarkably, with both the kon and koff rates reduced ~three-fold (see table). As a result, in vitro, the mean residence time of AP2 on the model membrane (1/koff) increases significantly from 1785 s (30 min) to 5555 s (90 min) and 8333 s (~140 min) in the presence of TGN38 and CD4 cargo, respectively, which is in line with the measured nanomolar KD. The cargo, therefore, not only stabilizes open conformation of AP211,13,24, but also its presence on the membrane. The cargo-driven increase in residence time at the membrane surface, we believe allows sufficient clathrin to bind and therefore productive nucleation of clathrin-coated pits to proceed. A similar effect was observed in cell by comparing wild type and mutant AP2 (the latter unable to bind specific cargo)23,26. Succinctly, if the AP2 membrane association is too fleeting, (for example due to the absence of cargo), CME is aborted: there is insufficient time for clathrin and/or other factors to be recruited to the growing CCP.
Similar agreement was also found between Kd calculated by the kinetic analysis and the Langmuir isotherm for the binding of CD4 and PtdIns(4,5)P2 monolayers. However, the non-exponential kinetics present at high bulk concentrations of AP2 would imply a more complex reaction scheme that cannot be adequately captured by average rate coefficients. This was also suggested by the analysis of AP2 liposome SPR “binding curves”11.
Time-resolved AP2 binding to model PM measured by SNR and by tensiometry agree
Time-resolved SNR was used to directly measure the AP2 concentration (Γ) associated to model PM (Supplementary Note 3 and Fig. S20). By comparing the surface pressure measured in parallel, increases in pressure and in SNR-derived membrane-bound protein concentration follow similar trends (Fig. 3A). A linear dependence ΔΠ ≈ Γ/Γ∞ ≈ ϕ, where ϕ represents surface coverage of AP2s, indicates that the protein recruited by the lipid monolayer directly contributes to the rise in lateral pressure. (Fig. 3B). Normalized plots of the recruitment of AP2 to different model PM are shown in Fig. 3G. After an induction period t < tlag, there is an increasing number of AP2 recruited to the membrane. The linear fit to the SNR data corresponds to a first-order binding reaction (See Fig. 3G and Supplementary Note 3). The values of binding time scale τ, reported in Fig. 3H, are in the same order, although slightly lower in the case of TGN38-monolayers, which were obtained from fitting the temporal evolution of the surface pressure data shown in Fig. 2B. Moreover, similar trends were observed by the temporal evolution of elliptically polarized light, which can be defined in terms of the ellipsometric angles that are proportional to the number of AP2 recruited to different PM monolayers per unit area (Fig. S5 and Supplementary Note 5).
A Normalized amount of AP2 protein (Γ/Γ∞, with Γ ∞ being the saturation concentration) interacting with a lipid monolayer enriched in PtdIns(4,5)P2 and TGN38 measured using time-resolved SNR in a restricted Q region and compared with the increase in surface pressure measured (ΔΠ). B Linear dependence between ΔΠ and the fraction coverage (ϕ = Γ/Γ∞). C The suggested transition mechanism from a fluid state to a percolated network state occurs as the coverage of AP2 on a PtdIns(4,5)P2-enriched membrane (ϕ) rises. D Time dependence of the interfacial shear viscoelastic moduli, divided in the elastic and viscous modulus (G’ and G´´, respectively). Data shows an increase in both G’ and G” after injecting AP2 in the subphase. E Re-plotted G’ and G” with respect to the surface coverage ϕ, revealing two differentiated regimes: a fluid-dominated state below ϕ = 0.73 and a gel-like state above this threshold that can be rationalized as percolated network according to (C). F G´ plotted as a function of the distance to the percolation threshold showing a power law exponent f = 2.3. G Comparative for monolayers enriched with and without different cargoes such as TGN38 and CD4. Straight lines are fits of the experimental data to obtain an average binding time plotted in (H). G”/G’ ratio of the different monolayers studied before and after the injection of AP2 is plotted in (I). J shows the values of G´ and G´´ for monolayers with different composition. The initial surface pressure Π0 in all the cases has been fixed to 25 ± 1 mN/m and the bulk concentration of the protein to 10 nM.
Fluid to solid-like transition in model PM monolayers on binding of AP2
In order to complement in situ measurements of lateral surface pressure, AP2 binding to model PM was monitored by interfacial shear rheological measurements (Methods and Supplementary Note 4). These data yielded the model PM lipid monolayer change in shear storage G’(t) and loss modulus G”(t) (Fig. 3D and Fig. S4). The former measures the amount of energy stored in the monolayer elastically, while the loss modulus G” accounts for energy lost as the monolayer returns to its original shape after deformation. The ratio of loss modulus to shear storage modulus (G”/G’) epitomizes the damping abilities of the monolayer.
Prior to AP2 injection, the different PtdIns(4,5)P2 enriched monolayers are in a fluid state, denoted by G” ≥ G’ (Fig. 3I, J). On injection of AP2, after an induction period t < tlag, both G’ and G” increase ~10,000 fold before reaching an equilibrium, at which G’ > G” indicates an elastic response that is characteristic of a solid-like regime. Moreover, in the presence of peptide-lipid cargo, the resultant moduli are further increased, with respect to PtdIns(4,5)P2 only monolayers, which may be rationalized by the increased number of AP2 recruited by the cargo-presenting PM monolayer. The higher shear modulus G’ would imply a drastic reduction in phospholipid mobility. The tightly packed lipid structure would tentatively generate strong intermolecular forces and resistance to shear deformation induced by clathrin polymerization.
The dependence of shear storage G’ and loss modulus G” with AP2 coverage is captured in Fig. 3E. Importantly, a critical surface threshold ϕc = 0.73 can be visualized when G’ = G”, which is associated with a randomly close packed distribution of proteins in 2D39. Beyond ϕc a disordered gel phase characterized by G’ > G” can be structurally hypothesized in terms of a percolation network40. This refers to a two-dimensional system where local molecular interactions between initially isolated AP2 on the membrane progressively lead to formation of a larger, continuous AP2 network across the membrane surface (as represented in Fig. 3C). Therefore, G’ can be described by a power law relation above the critical percolation concentration (ϕ − ϕc)f, with f = 2.3, which is typical of a protein network distribution41 (Fig. 3F). As a corollary, AP2 is able to create a solid but fragile connected network with a purely elastic modulus that is entropic in origin assuming excluded volume interactions42. This percolation network approach relies solely on a statistical physics framework of connected clusters, where network formation is solely influenced by molecular packing of AP2, and not by specific details of protein recruitment and reorganization. The connected network formed by AP2 could influence subsequent clathrin lattice assembly.
AP2 binds PM headgroups but embeds no further, as determined by low-resolution SNR
Due to the sub-micromolar binding affinity of AP2 to model PM monolayers, the SNR experimental set up was adapted for full-Qz range analysis to determine the structures of AP2 bound to lipid monolayers. Fig. S12 shows the reflectivity profiles measured as a function of Qz in two isotropic contrasts (38% D2O and 100% D2O, see methods), after the binding of AP2 to different model PM monolayers. Data modeling was performed by simultaneously fitting the two contrasts to get a single set of structural parameters (e.g., thickness, scattering length density, roughness and volume fraction), which also include the volume fraction of protein partitioning into different regions of the lipid monolayers (see Table S7 and Supplementary Note 8 for further details). The shape of AP2 was initially modeled as a series of stratified layers (See scheme in Fig. 4C). The SNR data from PtdIns(4,5)P2 presenting monolayer, in the presence and absence of different cargo peptides (CD4 and TGN38) indicated that in each case, AP2 lies primarily outside the membrane with a little insertion at the level of lipid headgroups ( ≈ 5 ± 1%), and a negligible presence in the acyl chain region (Table S8 and Fig. S13). A similar result was obtained by the SNR analysis of solid-supported bilayers (SLBs), composed of PtdIns(4,5)P2 enriched bilayers and CD4 cargo (Figs. S11, S21 and Supplementary Note 9). However, the volume fraction of protein bound to the membrane varied significantly between PM model membranes (Fig. 4A–C). In comparison to CD4, the presence of TGN38 leads to a larger fraction of AP2 bound to the membrane (Fig. 4A, B and Table S8). The smallest surface coverage was in the absence of cargo, on PtdIns(4,5)P2-only membrane (Fig. 4D). These SNR data agree with the measured relative increases in membrane lateral pressure and in shear moduli (Figs. 2 and 3).
The SNR-derived low-resolution slab models of membrane-bound AP2 show in the case of TGN38 and of CD4, total protein thickness is similar (91 ± 6 and 84 ± 6 Å respectively) (Table S8). AP2 would be oriented with its major axis parallel to the monolayer normal, as the protein’s maximum width is ~100 Å13, according to X-ray crystallography and electron tomography analysis. In the absence of cargo, the protein has reduced surface coverage and forms a more compact layer (63 ± 6 Å thickness). Although this width is smaller than the size determined by electron tomography23, and could therefore be a fitting artifact due to the low surface coverage, the compactness is in agreement with the in-plane AP2-PtdIns(4,5)P2 cluster formation visualized by AFM (Fig. 1D) and by epifluorescence microscopy (Fig. 1A, B).
AP2 binds in an open conformation to model PM in the presence and absence of cargo
The initial SNR data analysis of membrane-bound AP2 yielded a low-resolution slab model of constant SLD that is intrinsically linked to protein’s quaternary structure. Consequently, the preferred conformation and orientation of AP2 on the membrane can also be determined from the SNR data.
In order to identify the preferred orientation and position of AP2 with respect to the PM membrane, SLD profiles were calculated from atomic models of open, open+ and closed AP2 (based on energy minimized structures from PDB 2ax7, 6qh6 and from 2vgl, respectively). An ensemble of SLD profiles from 256 different orientations for each structure were generated with 5o increments in the Euler angles α and β (following an x-y-z extrinsic rotation scheme; the third Euler angle γ is not relevant as the SLD is plane-averaged in the z-direction). After each rotation, the structure’s distance with the membrane was optimized with the Sassie software by fitting the resultant theoretical and experimental SLD43 (Fig. S14 and Supplementary Note 8). In the final refinement step, the most favorable orientation for each protein conformation is fitted against the experimental reflectivity data (Fig. 5A, B and Fig. S13 for the CD4-presenting PM). The orientation shown in Fig. 5C provided the best fit, yielding the lowest χ² value compared to the alternative conformations and orientations presented in Figs. S13–S17. This corresponds to the open conformation, with the PtdIns(4,5)P2-binding sites on the μ2, α, and β2 subunits positioned consistently with those reported by other structural techniques44.
A Volume fraction profiles for open (PDB 2ax7) and close (PDB 2vgl) conformation of AP2. B Experimental SNR profile in NRM compared to a theoretical profile calculated for open (red, (C) conformation, based on the best possible orientation of the protein bound to the lipid monolayer. Heat map evaluating the different protein orientations for open, open+ and close conformations as a function of the α and β Euler angles for intrinsic rigid body rotations of AP2 are shown in Figures S15–S17, respectively. D Calculated volume fraction profiles corresponding to the preferred orientation of open conformation AP2 bound to PtdIns(4,5)P2-enriched monolayers in absence and presence of TGN38 or CD4 peptides. E Fractional surface coverage of AP2 for each orientation, derived from the analysis in (D) for CD4, TGN, and PtdIns(4,5)P2-enriched monolayers (presented as mean ± standard deviation (SD), n = 256). Individual data points can be found in the source data. F Violin plots show the distance to the interface, calculated by fitting the theoretical and experimental SLD values for each orientation of the protein in open conformation (PDB: 2xa7). The width of each violin represents the probability density of the data at different values. Embedded within each violin, a miniature box plot displays the median (central dot), with the box spanning the interquartile range (IQR, from the 25th to the 75th percentile), and whiskers extending to the most extreme data points within 1.5 times the IQR. (n = 256).
With regards to AP2 interacting with the different cargo-presenting PM model membranes, the calculated SLD profiles derived from open, open+26 and closed conformations were strikingly different, with the open conformation always having better fits than the other two (Fig. S18). In the most favorable scenario of an open AP2 conformation, the optimum position of the protein center-of-mass was found at a membrane head group distance of 12 ± 4 Å and 10 ± 4 Å, respectively for TGN38 and CD4, whereas in the PtdIns(4,5)P2 -only condition, AP2 is more closely embedded into the membrane headgroups region (Figs. 5F, S19). The protein’s orientation is consistent with the presentation of an open AP2’s PtdIns(4,5)P2 and cargo binding sites to the model PM (Fig. S18). The surface coverage of AP2 for TGN38- and CD4-presenting PM was 51% and 24% respectively, which agrees with tensiometry binding data (Fig. 5D, E). Analysis of the cargo-less model membranes identified a similar open conformation of AP2, although the surface coverage is reduced to 12% (Fig. 5E). These observations agree with cryo electron tomography studies that identified an open conformation of AP2 on membranes enriched in PtdIns(4,5)P2 with or without TGN38 peptido-lipids25,44. Besides, CD4 and TGN38 peptides used in this study are of similar length and volume fraction prior to AP2 binding. We therefore suggest that this similarity reduces the likelihood of peptide length significantly affecting the observed differences in AP2 proximity.
Evidence for AP2-clathrin interactions on the membrane
An important function for AP2 is to recruit clathrin to the cargo-presenting membrane. To characterize this interaction, clathrin binding was examined in the presence of AP2 variants, with and without its clathrin binding site (LLNLD).
In the absence of AP2, clathrin did not associate with the membrane. However, in the presence of AP2 containing the native clathrin binding site in its β2-hinge, clathrin could only bind after the AP2 had associated to the TGN cargo—and PtdIns(4,5)P2—presenting membrane (Fig. 6A, B). The clathrin binding sequence is therefore accessible when AP2 is bound to the membrane, indicating that AP2 is in an “open” conformation24. The removal of the clathrin binding sequence on the AP2 prevented clathrin from associating with the membrane (Fig. 6C). Time-resolved rheometry analysis indicated that the addition of clathrin to AP2-presenting membranes led to an increase in shear storage modulus G’, and decrease in loss modulus G”, yielding a ~ten-fold difference that is characteristic of a solid-like regime, which is an expected consequence of clathrin polymerization (Fig. 6E).
Clathrin-AP2 binding assay on TGN-presenting lipid monolayers: A Schematic Representation of the sequential AP2 (PDB ID: 2ax7, rendered using PyMOL) and clathrin binding on TGN-enriched lipid monolayers. B Tensiometry analysis (ΔΠ vs. time) of AP2 binding, followed by the addition of clathrin on lipid monolayers enriched in TGN. C Tensiometry control experiments: if clathrin is added first, no binding is observed, ΔΠ ≈ 0 (dark green curve), but when AP2 is introduced, binding is subsequently observed. AP2, missing its clathrin binding sequence can bind to the membrane, but no further increase of pressure (ΔΠ ≈ 0) is observed when clathrin is added (light green curve). D Transferred lipid monolayers immersed in buffer were examined by fluid-phase, tapping-mode AFM in presence of AP2 and clathrin showing CCPs. E Time dependence of the interfacial shear viscoelastic moduli (G’ and G). Data shows an increase in G’ due to clathrin binding (with respect to AP2). F In-plane averaged spatial distributions profiles of AP2 and Clathrin-AP2, expressed as volume fractions ϕ, in the direction orthogonal to the plane of the interface. Clathrin – AP2 profile does not show an increase in thickness but a remarkable change in volume occupancy, everything according to the scheme in (A).
NR analysis of cargo-presenting lipid monolayer indicated that clathrin binds to AP2-presenting membrane (Fig. 6F). Although it is not possible to distinguish AP2 and clathrin due to their similar SLD, binding was observed by an increase in protein surface coverage of the membrane from 30% to 58%, but not in overall protein thickness ( ~ 10 nm). AFM was able to visualize the self-association of clathrin on lipid bilayers to form assemblies similar in morphology to clathrin-coated pits (Fig. 6D). The protein thickness ( ~ 20 nm) was similar to that measured by cryo-election tomography (cryo-EM)25.
The observed difference in thickness between AFM/cryo-EM ( ~ 20 nm) and neutron reflectometry (NR, ~10 nm) for AP2-clathrin assemblies on PIP2-enriched monolayers likely arises from distinct technical factors inherent to each method. AFM and cryo-EM directly visualize maximal topographic height, capturing extended conformations of clathrin coats preserved in near-native (hydrated/vitrified) states, which may emphasize locally dense or fully assembled regions. In contrast, NR derives thickness from interfacial scattering length density profiles averaged over macroscopic areas, inherently integrating hydration, packing heterogeneity, and potential interpenetration of clathrin with AP2. The lower thickness obtained by NR may reflect incomplete lateral coverage of clathrin-AP2 networks or SLD averaging across sparse clathrin lattices. The increase in protein occupancy in the AP2 layer could consequently be due to an increase in AP2 bound to the membrane, due to an avidity effect by AP2 binding both the membrane and clathrin.
We further note that the NR data were collected using a Langmuir trough, where the lipid monolayer was experimentally constrained to be flat. In comparison, the increased height of assembly as measured by AFM and by tomography may be due to the membrane monolayer bending due to the clathrin self-assembly, as also observed by Ford and co-workers by electron microscopy45. The membrane curvature may result in the clathrin being able to associate though AP2 further away from the membrane.
This divergence shows the necessity of multimodal approaches: while AFM/cryo-EM resolve maximal structural dimensions, NR provides complementary insights into hydrated mass distribution and interfacial organization. Together, these data highlight the dynamic, context-dependent architecture of clathrin-coated assemblies, emphasizing the role of technique-specific sensitivities in interpreting membrane-protein interactions.
Discussion
CME is the primary mechanism through which eukaryotic cells selectively internalize proteins into the endosomal system. In solution, AP2 adopts a functionally closed (‘locked’) conformation, which precludes access to its cargo binding sites, to its clathrin binding LLNLD sequence in β2 and simultaneously prevents its four PtdIns(4,5)P2-binding sites from simultaneously engaging a planar PM44. Consequently, on membrane binding, a large-scale conformational reorientation of its subunits is required for full AP2 activation. Here, by using a battery of biophysical methods on bottom-up, minimal, in vitro membrane model systems, it was possible to address, under physiological conditions, the time-resolved recruitment and activation of AP2 by different models of the PM in a controlled environment. Synergistic interactions between the adapter protein and the membrane lead to each molecule adapting to the other.
With regards to the membrane, even though AP2 makes minimal lipid interactions, binding to the model PM monolayer induces a fluid-to-solid transition of the lipid leaflet, which is characterized by a larger viscosity and a finite shear elasticity. In contrast to cargo-less membrane, the increased binding of AP2 to cargo-presenting lipid monolayers leads to a drastic reduction in phospholipid mobility. Since AP2 binds specifically to PtdIns(4,5)P2, these rheometry data can be interpreted as AP2-induced clustering of PtdIns(4,5)P2, as previously observed with other PtdIns(4,5)P2 binding proteins46,47. As such this concentrating and clustering of PtdIns(4,5)P2 would increase the chance of productive CCV formation by attracting later stage acting PtdIns(4,5)P2-binding proteins, such as NECAP, SNX9/Amphiphysin and dynamin (the later needed for CCV necking and scission).
The percolation fraction ϕc refers to the critical density of AP2 molecules on the membrane surface needed to transition from isolated binding events to the formation of a fully connected network. Below ϕc = 0.73, where G’ = G”, the diffusion of AP2 and PtdIns(4,5)P2 is restricted to small, isolated liquid-phase domains (as observed in Fig. 2). Above ϕc = 0.73, the membrane transitions from a fluid to a solid state, which occurs independently from clathrin binding. This result clarifies an intriguing previous study with rheometry that had focused on concurrent addition of clathrin and their associated adapter proteins48. The AP2 - PtdIns(4,5)P2 cluster network would tentatively be better able to resist shear deformation induced by clathrin polymerization, and subsequent CCV formation. Indeed, here we show for the first time that the formation of these 2D entropic networks might be therefore closely related to the regulation of plasma membrane mechanisms to incorporate and polymerize clathrin molecules to build endocytic pits, which is observed in vitro by AFM and characterized by further changes in pressure and rheometry data.
With regards to AP2 adapter, the cargo not only stabilized the open (“active”) conformation of AP211,13,24, but also its presence on the plasma membrane. Intriguingly, our SNR structural data indicate that cargo containing either tyrosine Yxxφ motif and dileucine [DE]xxxL[LI] motifs may not play a critical role in AP2 “opening” conformational change, as interactions with PtdIns(4,5)P2-only PM is sufficient for full activation of AP2. This is in line with a recent tomography analysis, which had focused on carefully selected population of AP2 molecules binding to membranes, with and without TGN3825. However, in this current study, the inclusion of time-resolved kinetic data highlighted the AP2’s membrane surface dwell time, and resultant surface coverage, both significantly increase in the presence of cargo. We hypothesize the observed increases in AP2 residence time at the PM would allow sufficient time for clathrin to bind and therefore for productive nucleation of clathrin-coated pits to proceed. A similar effect was observed in vivo by comparing wild-type and mutant AP2 (the latter unable to bind specific cargo)23.
In conclusion, this in vitro analysis has yielded thermodynamic and structural insights into normal and aborted CME. It moreover provides proof-of-principle that other intracellular transport processes can be successfully studied using these experimental techniques and this conceptual framework developed in soft matter physics. This is particularly pertinent as the vast majority of these complex formation events occur deep within the cell and therefore are not compatible to characterization by current in cell techniques, like TIRF-SIM microscopy.
Methods
Lipids
1,2-dioleoil-sn-glycero-3-phosphoetanolamine (DOPE), 1,2-dioleoil-sn-glycero-3-phosphocoline (DOPC), 1,2-dioleoil-sn-glycero-3-phospho-L-serine (DOPS), Brain L-α-phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) and cholesterol (Chol), were purchased as powder from Avanti Polar Lipids (purity >99%, Alabaster, AL, USA). Stock solutions (1 mg/mL) of the lipid mixtures were prepared in chloroform stabilized with ethanol (purity 99.8%; Sigma-Aldrich, St. Louis, MO, USA). See Supplementary Note 1 for further details. D2O (99.9% of isotopical purity) was purchased from Sigma-Aldrich and used as received. HKT buffer was 25 mM HEPES, 125 mM Potassium Acetate and 1 mM DL-Dithiothreitol (DTT), pH (7.20 ± 0.05). HKM buffer was HKT buffer supplemented with 5 mM Magnesium Acetate.
Peptide-lipid conjugation
Peptides were synthesized with amino-terminal cysteines, and covalently conjugated to 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide11, also purchased from Avanti Polar Lipids (USA). The Yxxϕ-containing peptide TGN38 was derived from the cytoplasmic tail of TGN38 (CKVTRRPKASDYQRL) while the di-leucine peptide CD4 was derived from the phosphorylated cytoplasmic tail of CD4 (CHRRRQAERMS*QIKRLLSEK) (where * denotes phosphorylation) (Table S3 and Supplementary Note 2). The N-terminal cysteine was used for lipid coupling. Briefly, for covalent conjugation of a peptide to a synthetic lipid, 5 mmol peptide in 1 ml 10 mM MOPS-KOH pH 7.5, 50% DMF were mixed with an equal volume of lipid (1,2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide]) at 5 mg/ml in Chloroform and incubated for 2 h at 20 °C with end-over-end rotation. Coupling was blocked by addition of β-Mercaptoethanol to a final concentration of 10 mM and further incubation for 30 min. The lipid-linked peptide was collected from the organic phase after extraction with 2 ml Chloroform and 1 ml Methanol and dried under Nitrogen. The dried lipopeptide was resuspended in Chloroform/Methanol (2:1) at 5 mg/ml and stored at −20 °C.
Expression and purification of AP2
To produce recombinant AP2 proteins, bacterial expression and purification, were done as in refs. 13,24. The AP2 core lacks the α and β subunit hinges and appendages. AP2 FLAP lacks the α subunit hinge and appendage and therefore has the canonical clathrin-binding sequence present.
Briefly, recombinant AP2 proteins were prepared in 250 mM NaCl 2 mM DTT 10 mM Tris pH 8.7 according to previous protocols22. After induction with 0.2 mM IPTG followed by over-night expression at 22°C in BL21 plyS E. coli grown in 2TY media, cells were lysed using a cell disruptor (Constant Systems). AP2 were initially isolated with GST-beads and cleaved overnight with thrombin. The resultant protein was then isolated on NiNTA-beads, washed with buffer supplemented with 10 mM imidazole, and eluted from the beads with the same buffer supplemented with 300 mM imidazole. After size exclusion chromatography on a Superdex 200 column (GE Healthcare) in 250 mM NaCl 10 mM Tris pH 8.7 1 mM DTT, the AP-2 complexes were concentrated to >15 mg/ml with vivaspin concentrators, and flash frozen in 10% glycerol, until further use.
Expression and purification of Clathrin
Clathrin was purified from pig brain coated vesicle essentially as described previously by Dannhauser et al49. Briefly, clathrin from pig brain was isolated by size exclusion chromatography (Superose 6), ion-exchange chromatography (MonoQ) and hydroxyapatite chromatography. Clathrin was subsequently stored in 10 mM Tris–HCl, at pH 8.0.
Lipid monolayer and Langmuir trough experiments
A Langmuir trough (Kibron, Helsinki, Finland) was used to measure the surface pressure (Π) – area per molecule (A) isotherm of lipid monolayers as well as to determine the increase in pressure (ΔΠ) observed after the injection of the protein AP2 in the bulk phase. The variation of surface pressure was recorded using a Wilhelmy plate made of filter paper. Temperature was maintained at 21.5 ± 0.5 °C. Π was measured with an instrument accuracy of ±0.1 mN/m. Temporal evolution experiments were performed in five independent replicates (n = 5), and the standard deviation was consistently below the instrumental error. Further experimental details are given in the Supplementary Note 3.
Specular neutron reflectometry (SNR)
SNR elucidates the structure and composition of interfacial layers in the direction perpendicular to the plane of the interface. Experiments were performed on the time-of-flight reflectometer FIGARO at the ILL. Two different angles of incidence (θ1 = 0.6° and θ2 = 3.7°) and a wavelength resolution of 7% dλ/λ, yielding a momentum transfer, Qz = (4π/λ) sinθ, range from 0.007 to 0.25 Å−1 (the upper limit being limited by sample background) were used to perform the measurements and investigate the structure of the lipid monolayers upon AP2 interaction. Lipid monolayers were prepared in a Langmuir trough at a surface pressure of Π = 25 ± 1 mN/m. After SNR measurements of the lipid monolayer, AP2 was injected under the monolayer by a Hamilton syringe to a final bulk concentration of 10 nM. Two different isotopic solvent contrasts (100% D2O and 8.1% D2O (v/v %) respectively) were used for the characterization. Subsequent data analysis was performed using AuroreNR50 and Motofit51 software (see Supplementary Note 8). Optimization of model parameters was done by the combine use of a genetic algorithm for efficient search of the parameter space, and a Levenberg-Marquadt non-linear least-square algorithm for a final refinement of the fitting parameters. The quality of the fits was reported as reduced χ2. The uncertainties in structural parameters (e.g., layer thickness, volume occupancy) derive from the fitting process using the Parratt algorithm, which calculates confidence intervals via covariance matrix analysis of the least-squares optimization. These fitting-derived errors ( ± 1σ) were corroborated by triplicate experimental measurements, showing <5% variation in key parameters.
Imaging lipid monolayers by epifluorescence microscopy
Lipid monolayers were doped with 0.1 mol % BODIPY-TMR PtdIns(4,5)P2 and observed using an inverted bright-field microscope (Nikon Eclipse) with an extra-long working distance (WD 3.7–2.7 mm, NA 0.60) objective of 40× magnification. A CMOS camera (AVT Marlin F-131B) working at 30 frames per second was used to record a 160 × 120 μm field of view. Experimental limitations (drift motion, long-working distance and the diffraction limit of light) prevented an ideal visualization below the micron range.
Imaging supported lipid monolayers by liquid AFM
Supported lipid monolayers were prepared by transferring monolayers from the Langmuir trough onto glass coverslips using the Langmuir−Schaeffer method. AFM images of fluid-phase lipid monolayers were taken using a Cypher S microscope with a bluedrive photothermally excited cantilever Olympus OMCL-AC240TS (240 × 40 μm, large x width) working at a resonance frequency of 70 kHz (in air, and, approximately 24 kHz in liquid) and an elastic constant of 1.2 N/m. The images were taken with a scan speed of 1 Hz in tapping mode with 256 × 256 pixel resolution and 5 μm in size. Temperature and humidity conditions were stable. A droplet of HKM solution with an AP2 concentration was spread on the Langmuir-Schaeffer film of lipid, being visualized before droplet evaporation at a constant temperature. Thickness profiles were extracted from multiple regions across independently acquired images (n = 10) and fitted to a Gaussian function to determine both the mean thickness (reported as mean ± SD) and the full width at half maximum (FWHM), whose errors are not clearly visible in the plots due to their minimal values relative to the symbol size.
Interfacial shear rheology
The mechanical response of the monolayers under shear deformation was measured by means of a homemade magnetic microwire interfacial shear rheometer driven by a mobile magnetic trap. The experiments were oscillatory with a frequency of 0.5 rad/s. The shear strain applied to the monolayer remained consistently below 1% throughout the duration of the experiment, where it is expected to be operated within the linear regime. The subphase and surface drags on the microwire were decoupled by numerically solving the flow field at the shear channel, and the surface dynamic moduli, G’ and G”, were calculated from the surface velocity gradient at the contact line with the microwire52,53. Each measurement was repeated in triplicate (n = 3), with the standard deviation for each set of measurements consistently being lower than the instrumental error, which is ± 01 nN/m for shear dynamic moduli (see Supplementary Note 4, for more details).
Data analysis and visualization
Where appropriate, quantitative data are presented as mean ± standard deviation (SD). Data distributions were visualized using box and violin plots. All plots were generated using OriginPro 2021 (OriginLab Corporation, Northampton, MA, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw neutron scattering, tensiometry, and shear rheology data supporting this study are openly available on Zenodo at: https://doi.org/10.5281/zenodo.16386485. All additional raw data supporting the results of our study are available within the article and its supplementary materials. We confirm that all data analysis was performed using OriginPro 2021. All figures and quantitative analyses were generated using this software, ensuring the reproducibility of our results. The data analysis tools utilized are available upon reasonable request from the corresponding author, A.M.
References
Bitsikas, V., Corrêa, I. R. & Nichols, B. J. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife 2014, 1–26 (2014).
Kirchhausen, T., Owen, D. & Harrison, S. C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725 (2014).
Banerjee, I., Yamauchi, Y., Helenius, A. & Horvath, P. High-content analysis of sequential events during the early phase of influenza A virus infection. PLoS One 8, e68450 (2013).
Bayati, A., Kumar, R., Francis, V. & McPherson, P. S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 296, 100306 (2021).
Schmid, S. L., Sorkin, A. & Zerial, M. Endocytosis: past, present, and future. Cold Spring Harb. Perspect. Biol. 6, a022509 (2014).
Brodsky, F. M. Diversity of clathrin function: new tricks for an old protein. 10.1146/annurev-cellbio-101011-155716 28, 309–336 (2012).
Cocucci, E., Aguet, F., Boulant, S. & Kirchhausen, T. The first five seconds in the life of a clathrin-coated pit. Cell 150, 495–507 (2012).
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Mitsunari, T. et al. Clathrin adaptor AP-2 Is essential for early embryonal development. Mol. Cell Biol. 25, 9318–9323 (2005).
Motley, A., Bright, N. A., Seaman, M. N. J. & Robinson, M. S. Clathrin-mediated endocytosis in AP-2–depleted cells. J. Cell Biol. 162, 909–918 (2003).
Höning, S. et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol. Cell 18, 519–531 (2005).
Krauss, M. et al. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type I. J. Cell Biol. 162, 113–124 (2003).
Jackson, L. P. et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141, 1220–1229 (2010).
Cremona, O. & De Camilli, P. Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 114, 1041–1052 (2001).
Boucrot, E., Saffarian, S., Massol, R., Kirchhausen, T. & Ehrlich, M. Role of lipids and actin in the formation of clathrin-coated pits. Exp. Cell Res 312, 4036–4048 (2006).
Loerke, D. et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7, e1000057 (2009).
Kelly, B. T. & Owen, D. J. Endocytic sorting of transmembrane protein cargo. Curr. Opin. Cell Biol. 23, 404–412 (2011).
Kelly, B. T. et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976–979 (2008).
Ohno, H., Fournier, M.-C., Poy, G. & Bonifacino, J. S. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains*. J. Biol. Chem. 271, 29009–29015 (1996).
Owen, D. J. & Evans, P. R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science282, 1327–1332 (1998).
Kovtun, O., Dickson, V. K., Kelly, B. T., Owen, D. J. & Briggs, J. A. G. Architecture of the AP2/clathrin coat on the membranes of clathrin-coated vesicles. Sci. Adv. 6, eaba8381 (2024).
Collins, B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109, 523–535 (2002).
Kadlecova, Z. et al. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 216, 167–179 (2017).
Kelly, B. T. et al. AP2 controls clathrin polymerization with a membrane-activated switch. Science345, 459–463 (2014).
Zaccai, N. R. et al. FCHO controls AP2’s initiating role in endocytosis through a PtdIns(4,5)P2-dependent switch. Sci. Adv. 8, 2018 (2022).
Wrobel, A. G., et al. Temporal ordering in endocytic clathrin-coated vesicle formation via ap2 phosphorylation. Dev. Cell 50, 494–508 (2019).
Degroot, A. C. M., Gollapudi, S., Zhao, C., Lamonica, M. F. & Stachowiak, J. C. Weakly internalized receptors use coated vesicle heterogeneity to evade competition during endocytosis. Biochemistry 60, 2195–2205 (2021).
Ehrlich, M. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Pereira, D. et al. Engineering phosphatidylinositol-4,5-bisphosphate model membranes enriched in endocytic cargo: A neutron reflectometry, AFM and QCM-D structural study. Colloids Surf. B Biointerfaces 227, 113341 (2023).
Nagle, J. F. & Tristram-Nagle, S. Structure of lipid bilayers. Biochimica et. Biophysica Acta - Rev. Biomembranes 1469, 159–195 (2000).
Takamori, S. et al. Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006).
Carvalho, K., Ramos, L., Roy, C. & Picart, C. Giant unilamellar vesicles containing phosphatidylinositol(4,5)bisphosphate: characterization and functionality. Biophys. J. 95, 4348 (2008).
Ellenbroek, W. G. et al. Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. Biophys. J. 101, 2178–2184 (2011).
Santamaria, A., Carrascosa-Tejedor, J., Guzmán, E., Zaccai, N. R. & Maestro, A. Unravelling the orientation of the inositol-biphosphate ring and its dependence on Phosphatidylinositol 4,5-bisphosphate cluster formation in model membranes. J. Colloid. Interface Sci. 629, 785–795 (2022).
Santamaria, A. et al. Strikingly Different Roles of SARS-CoV-2 fusion peptides uncovered by neutron scattering. J. Am. Chem. Soc. 144, 2968–2979 (2022).
Weiss, J. N. The Hill equation revisited: uses and misuses. FASEB J. 11, 835–841 (1997).
Yang, H. et al. Protein conformational dynamics probed by single-molecule electron transfer. Science (1979) 302, 262–266 (2003).
Röcker, C., Pötzl, M., Zhang, F., Parak, W. J. & Nienhaus, G. U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 4, 577–580 (2009).
Berryman, J. G. Random close packing of hard spheres and disks. Phys. Rev. A27, 1053–1061 (1983).
Stauffer Dietrich and Coniglio, A. and A. M. Gelation and critical phenomena. in Polymer Networks (ed. Dušek, K.) 103–158 (Springer, 1982).
Corrigan, A. M. & Donald, A. M. Passive microrheology of solvent-induced fibrillar protein networks. Langmuir 25, 8599–8605 (2009).
Del Gado, E., De Arcangelis, L. & Coniglio, A. Elastic properties at the sol-gel transition. Europhys. Lett. 46, 288–294 (1999).
Curtis, J. E., Zhang, H. & Nanda, H. SLDMOL: A tool for the structural characterization of thermally disordered membrane proteins. Comput Phys. Commun. 185, 3010–3015 (2014).
Kovtun, O., Dickson, V. K., Kelly, B. T., Owen, D. J. & Briggs, J. A. G. Architecture of the AP2/clathrin coat on the membranes of clathrin-coated vesicles. Sci. Adv. 6, aba8381 (2020).
Ford, M. G. J. et al. Simultaneous binding of PtdIns (4,5) P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science291, 1051–1055 (2001).
Saarikangas, J. et al. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr. Biol. 19, 95–107 (2009).
Zhao, H. et al. Membrane-sculpting BAR domains generate stable lipid microdomains. Cell Rep. 4, 1213–1223 (2013).
Vandersarl, J. J. et al. Rheology and simulation of 2-dimensional clathrin protein network assembly. Soft. Matter 10, 6219–6227 (2014).
Dannhauser, P. N. & Ungewickell, E. J. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat. Cell Biol. 14, 634–639 (2012).
Gerelli, Y. Aurore: new software for neutron reflectivity data analysis. J. Appl Crystallogr. 49, 330–339 (2016).
Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl Crystallogr. 39, 273–276 (2006).
Sánchez-Puga, P., Tajuelo, J., Pastor, J. M. & Rubio, M. A. Flow field-based data analysis in interfacial shear rheometry. Adv. Colloid Interface Sci. 288, 102332 (2021).
Tajuelo, J., Pastor, J. M. & Rubio, M. A. A magnetic rod interfacial shear rheometer driven by a mobile magnetic trap. Cit. J. Rheol. 60, 1095 (2016).
Acknowledgements
The authors thank the Institut Laue-Langevin for the allocation of beamtime (https://doi.org/10.5291/ILL-DATA.8-02-780, https://doi.org/10.5291/ILL-DATA.8-02-795, and https://doi.org/10.5291/ILL-DATA.8-02-804) and the Partnership for Soft Condensed Matter (PSCM) for the lab support. A.M. acknowledges the financial support from The Wellcome Trust Institutional Strategic Support Fund (ISSF) for a Junior Interdisciplinary Fellowship; from MICIU under grant PID2021-129054NA-I00, the Department of Education of the Basque Government under grant PIBA-2023-1-0054 and from the IKUR Strategy under the collaboration agreement between Ikerbasque Foundation and Materials Physics Center. N.R.Z and D.J.O were supported by Wellcome Trust grant WT 207455/Z/17/Z awarded to D.J.O.
Author information
Authors and Affiliations
Contributions
A.M., N.R.Z., D.J.O. and P.C. conceived the study and planned the experiments. N.R.Z. and D.J.O. expressed and purified all proteins. A.M., N.R.Z., J.F.G.M., P.G. and R.C. performed neutron experiments. A.M, J.F.G.M., P.S.P., J.T., A.S., J.C.T., D.P. and B.T.K. carried out the rest of the experiments. AM processed all the experimental data and performed the analysis. M.A.R., I.M.M. and J.K provide resources and methodology. A.M., N.R.Z., D.J.O., and P.C. wrote the manuscript. All authors provide critical feedback and helped shape the research, analysis and manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Peer review
Peer review information
: Communications Biology thanks Richard Baker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Laura Rodríguez Pérez. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maestro, A., Zaccai, N.R., Gonzalez-Martinez, J.F. et al. Combined thermodynamic and time-resolved structural analysis of interactions between AP2 and biomimetic plasma membranes provides insights into clathrin-mediated endocytosis. Commun Biol 8, 1196 (2025). https://doi.org/10.1038/s42003-025-08597-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08597-5