Abstract
The solute carriers (SLC) superfamily comprises 66 families with more than 450 members. The Na+/\({{{{\rm{HCO}}}}}_{3}^{-}\) cotransporter NBCe1 (SLC4A4) of SLC4 family plays critical roles in intracellular pH regulation and transepithelial transport of fluid and electrolytes. Here, we explored the structural mechanisms of NBCe1-A regulation by two phosphorylation modules: P-loop in the amino-terminal domain and H-loop in the transmembrane domain. Mimic-phosphorylation of P-loop or H-loop substantially decreases NBCe1-A activity. Inhibition of NBCe1 by P-loop is abolished by mutations to specific basic residues in the fourth intracellular loop (IL4) in the carrier domain and IL3/IL6 in the scaffold. Inhibition by H-loop is abolished by specific mutations to IL3. We conclude that: (1) P-loop inactivates NBCe1-A by binding to the carrier and the scaffold; (2) H-loop blocks NBCe1-A by interacting with IL3 in the scaffold. Our findings have implications for studying the structural mechanisms for the regulation of other SLCs by phosphorylation.
Similar content being viewed by others
Introduction
The solute carriers (SLC) superfamily represents the second largest group of membrane proteins after the G-protein coupled receptors superfamily. In human, the SLC superfamily comprises up to 66 families with more than 450 members, accounting for ~50% of total membrane transporters and channels1,2. The SLCs play fundamental roles in cellular signaling and metabolism by catalyzing the transport of ions, nutrients, metabolites, and drug molecules across the plasma as well as cellular organelle membranes3,4. The SLCs utilize alternating-access mechanism for active substrate translocation, an idea originally conceptualized in 1950s–1960s5,6,7,8. The substrate-binding pocket in a transporter would never have simultaneous access to the intra- and extracellular aqueous environments to ensure the transport of substrates, a process that is usually uphill across the membranes.
In the past decade, many SLCs have been resolved for three-dimension (3D) structures9,10. The structural studies have provided considerable mechanistic insights into the transport by SLCs. Three alternating-access models have been recognized: rocker switch, rocking bundle, and elevator-like mechanism10,11. The transmembrane domain (TMD) of SLCs generally consists of two domains. The relative movement of the two domains controls the alternating access of the substrate-binding pocket to the inside and outside of cells. So far, little is known about the structural mechanisms of the regulation of SLCs by phosphorylation.
The SLC4 family consists of 10 members, including: (1) three Na+-independent anion exchangers AE1 − 3 (SLC4A1 − 3); (2) five Na+-dependent \({{{{\rm{HCO}}}}}_{3}^{-}\) transporters NBCe1 (SLC4A4), NBCe2 (SLC4A5), NBCn1 (SLC4A7), NBCn2 (SLC4A10), and NDCBE (SLC4A8); (3) two isolated members “AE4” (SLC4A9 which plays a key role in renal acid-base sensing12) and BTR1 (SLC4A11). The structure and function of SLC4 transporters has been extensively studied during the past decade13,14,15,16,18,19,20,21. The structural data support the idea that the SLC4s employ an elevator-like mechanism for ion translocation.
NBCe1 was the first Na+-dependent member of SLC4 family that was originally identified in renal proximal tubules22,23. NBCe1 is broadly expressed in many tissues throughout the body, playing essential roles in intracellular pH (pHi) regulation and systemic fluid/electrolyte homeostasis24,25. In mammals, SLC4A4 produces three major NBCe1 isoforms: NBCe1-A, -B, and -C. The basolateral membrane of renal proximal tubule expresses NBCe1-A (the dominant isoform) and NBCe1-B (the minor isoform)26,27. Here, NBCe1 (predominantly NBCe1-A) is responsible for the reclamation of ~80% of filtered \({{{{\rm{HCO}}}}}_{3}^{-}\), a house-keeping function of the kidney. Dysfunction of NBCe1 results in massive waste of \({{{{\rm{HCO}}}}}_{3}^{-}\) in the urine, causing severe metabolic acidosis24,28,29,30.
NBCe1 is regulated by many factors, such as phosphatidylinositol bisphosphate (PIP2), Ca2+, Cl−, angiotensin II, and interacting proteins (for review, see ref. 31). When expressed in heterologous system, the activity of NBCe1 is decreased by protein kinases, such as SPS1-related proline/alanine-rich kinase (SPAK), Ca2+-calmodulin-dependent protein kinase CaMKIIα, and PKC32,33,34, and stimulated by protein phosphatase PP135. These studies support the notion that phosphorylation generally down-regulates NBCe1 activity.
So far, a series of phosphosites have been identified in NBCe135,36,37,38,39,40. However, the molecular mechanism underlying the regulation of NBCe1 by phosphorylation remains unknown. In the present study, we identify two phosphorylation modules in NBCe1: P-loop in the amino-terminal domain (NTD) and H-loop in the TMD. Mimic-phosphorylation of P-loop or H-loop substantially decreases the activity of the renal isoform NBCe1-A. The inhibition of NBCe1-A by P-loop and H-loop is completely abolished by mutations to specific basic residues in the TMD of NBCe1-A.
Results
Identification of phosphosites in NBCe1
By mass-spectra analyses with native NBCe1 from rat brain as well as mouse NBCe1-A and NBCe1-B heterologously expressed in Xenopus oocytes, we identified a series of novel phosphosites in NBCe1 and also confirmed many that had previously been reported (Fig. 1a). Figure 1b summarizes the distribution of all known phosphorylation sites in NBCe1 (also see Table S1). Notably, the vast majority of the phosphosites are localized in the NTD and many are present in the TMD, indicating the importance of the NTD and TMD in the regulation of NBCe1. The presence of phosphosites in extracellular loops suggests that NBCe1 is affected by secreted protein kinases41,42.
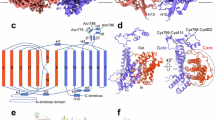
a Western blotting (left) and silver staining (right) of NBCe1 preparations. Shown here is an example of NBCe1 isolated from the lysate of rat brain by immunoprecipitation (IP). The band indicated by arrow in the right panel was excised for the identification of phosphosites in NBCe1. b Topological model showing the distribution of all known phosphosites in NBCe1. See Table S1 for the source of the phosphosites. Shown in blue lines are the two phosphorylation modules P-loop in NTD and H-loop (the fifth intracellular loop IL5) in TMD. Green cylinders represent the transmembrane α helices (TM) in the carrier domain. Blue cylinders indicate the TMs in the scaffold domain. The numbering of residues is based on human NBCe1. The numbering of the residues in the conserved regions is according to human NBCe1-B (NCBI accession no. NP_001091954.1). The numberings in red indicate the novel phosphosites identified in the present study. Also see Table S1. c Diagram showing the perfusion of Xenopus oocytes for electrophysiology recordings. The oocytes were first perfused with nominally \({{{{\rm{HCO}}}}}_{3}^{-}\)-free ND96 and then with a solution containing 5% CO2/33 mM \({{{{\rm{HCO}}}}}_{3}^{-}\). d Representative I-V curves of an oocyte expressing NBCe1-A. IND96 (blue) represents the gross current recorded in ND96. IHCO3 (purple) represents the gross current in CO2/\({{{{\rm{HCO}}}}}_{3}^{-}\) solution. The differential current (INBC, green) represents the net \({{{{\rm{HCO}}}}}_{3}^{-}\)-dependent component mediated by NBCe1-A. The slope conductance (GNBC) of INBC between ±40 mV was used as an index for NBCe1 activity.
In the present study, we investigated the regulation of NBCe1 by phosphorylation based on NBCe1-A which contains an auto-stimulatory domain at the Nt end. NBCe1 was expressed in Xenopus oocytes, a heterologous system widely used for studying the structure-function and functional regulation of membrane transporters. The activity of NBCe1 was determined by two-electrode voltage clamp. The oocyte was first perfused with nominally \({{{{\rm{HCO}}}}}_{3}^{-}\)-free ND96 solution and then with a solution containing 5% CO2/33 mM \({{{{\rm{HCO}}}}}_{3}^{-}\) (Fig. 1c). Upon the introduction of CO2/\({{{{\rm{HCO}}}}}_{3}^{-}\), CO2 fluxes into the cell by simple diffusion, causing the generation of H+ and \({{{{\rm{HCO}}}}}_{3}^{-}\). At the meantime, NBCe1 mediates the influx of Na+ and \({{{{\rm{HCO}}}}}_{3}^{-}\). Currents were recorded in both ND96 (IND96) and CO2/\({{{{\rm{HCO}}}}}_{3}^{-}\) solution (IHCO3). The IND96 of an oocyte expressing NBCe1 in ND96 presumably represents a \({{{{\rm{HCO}}}}}_{3}^{-}\)-independent Na+-leaking current via NBCe1, similar to the electroneutral NBCn143. INBC (the difference between IND96 and IHCO3) represents the component dependent on Na+ and \({{{{\rm{HCO}}}}}_{3}^{-}\) in IHCO3 mediated by NBCe1. Thus, the slope conductance (GNBC) of INBC provides an accurate measurement for the Na+/\({{{{\rm{H}}}}{{{\rm{C}}}}{{{\rm{O}}}}}_{3}^{-}\) cotransport activity of NBCe1. If NBCe1 is affected by a factor, e.g., a mutation, the change in GNBC would be almost solely contributed by the change in the Na+/\({{{{\rm{HCO}}}}}_{3}^{-}\) cotransport activity of NBCe1.
In the following of the article, we will focus on the characterization of the structural mechanisms of NBCe1 regulation by two specific phosphorylation modules: P-loop in the NTD and H-loop in the TMD.
Mimic-phosphorylation of P-loop decreases NBCe1-A activity
P-loop (phosphorylation loop, residues 230 − 262) belongs to a large flexible loop structure in the Nt-domain of NBCe1. P-loop is of interest in that it contains an alternative Cassette I and 12 potential phosphosites—residues of Ser and Thr (Fig. 2a, Fig. S1). Removal of Cassette I modestly stimulates NBCe1 activity44. Among the 12 Ser/Thr residues in P-loop, at least 9 have been identified with phosphorylation in previous studies35,36,37,39,40. The present study confirmed 4 of the 9 previously-identified phosphosites in P-loop. So far, no systematic investigation was available for the role of P-loop in NBCe1. The Muallem group showed that mimic phosphorylation of S232/S233/S235 by Asp substitution greatly reduced NBCe1-A activity35. Others examined the effect of mimic-phosphorylation of S245 or S255/S256/S257 on the function of NBCe1-B36,37.
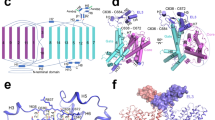
a 3D structure model of the NTD of NBCe1. The model of NBCe1 NTD was generated by homology modelling with the structure of NDCBE NTD (PDB: 5JHO) as template. P-loop (residues 230 − 262), which belongs to a large loop structure that is intrinsically-disordered in the NTD of NBCe1, contains 12 potential phosphosites (residues Ser and Thr) with two clusters in P-loopNt and P-loopCt. Highlighted in green is the optional Cassette I. b Mimic-phosphorylation of P-loop (P-loop12D) inhibits NBCe1-A activity. P-loop12A: 12 phosphosites replaced by Ala. P-loop12D: 12 phosphosites replaced by Asp. P-loop12A and P-loop12D have no significant effect on the relative expression levels of total (c) and surface (d) NBCe1-A in Xenopus oocytes. See Fig. S4a for full blots. e Mimic-phosphorylation of the middle portion of P-loop has no effect on NBCe1-A. f Maximal inhibition on NBCe1-A requires mimic-phosphorylation of both P-loopNt and P-loopCt. g Effect of P-loop deletions on NBCe1-A activity. Open bars: deletions based on NBCe1-AWT. Orange bars: deletions based on P-loop12D. Data are presented as mean ± SD. The numerals in the parentheses on the bars indicate the number of individual oocytes for activity data or the number of western blottings with independent pools of oocytes for expression data. One-way ANOVA followed by post hoc Dunnett’s multiple comparisons was used for statistical analysis. *p < 0.05; ****p < 0.0001, compared with NBCe1-AWT. ns not significant.
We presume that the phosphosites from residues 230 − 262 belong to a single regulatory module, which we refer to as P-loop. Based on wild-type (WT) NBCe1-A, we generated P-loop12D (all 12 Ser/Thr replaced by Asp in P-loop) and P-loop12E (12 Ser/Thr→Glu) for mimic phosphorylation, and P-loop12A (12 Ser/Thr→Ala) for mimic dephosphorylation of P-loop. While P-loop12A retains full activity, P-loop12D decreases the transport activity by ~80% compared to NBCe1-AWT (Fig. 2b). Similar results were obtained with P-loop12E (12 Ser/Thr→Glu; Fig. S2a). The downregulation of NBCe1-A by the mimic-phosphorylation of P-loop would be due to a disruption of the transport process, given that the mimic-phosphorylation of P-loop has no effect on NBCe1 expression in oocytes (Fig. 2c–d, Fig. S2b–c).
NBCe1-A contains an auto-stimulatory domain (ASD) at the Nt end. Removing ASD modestly decreases NBCe1-A activity44,45. In contrast to the ~80% decrease by P-loop12D for NBCe1 containing ASD, P-loop12D virtually inactivates NBCe1 lacking ASD, indicating that the presence of ASD reduces the inhibitory effect of P-loop on NBCe1-A (Fig. S3). It remains uncertain how the ASD interferes with the action of P-loop.
Compared to P-loop12D, partial mimic-phosphorylation of P-loop has lower effect on NBCe1-A (Fig. 2e–f). P-loop contains two clusters of phosphosites in the Nt- (P-loopNt) and Ct-portion (P-loopCt; Fig. 2a). The transport activity of construct “DDDDSSTDDDDD” with mimic-phosphorylation in both P-loopNt and P-loopCt is 70% lower than that of NBCe1-AWT (Fig. 2f). This is very close to the case of P-loop12D (construct “DDDDDDDDDDDD”), the activity of which is 80% lower than NBCe1-AWT. However, mimic-phosphorylation of just P-loopNt (“DDDDSSTTSSSS”) decreases NBCe1-A activity by 40%, and P-loopCt (“TSSSSSTDDDDD”) only 20%. Mimic-phosphorylation of the middle of P-loop (“TSSSDDDTSSSS”) has no significant effect on the activity of NBCe1-A. It is interesting that S245D inhibits the activity of NBCe1-B compared to S245A when heterologously expressed in HEK293 cells36. One likely explanation is that the mutation to S245 interferes with the phosphorylation of the other target sites in NBCe1-B in HEK293 cells. Together, our data suggest that full blockade of NBCe1-A requires the phosphorylation of both P-loopNt and P-loopCt.
Based on NBCe1-AWT, deletion of the middle portions (Δ236–244 or Δ236–253) or the entire P-loop (Δ230–262) modestly stimulates the activity of NBCe1-A (white bars, Fig. 2g). Compared to P-loop12D, Δ236–244 and Δ236–253 modestly stimulate NBCe1-A (orange bars), and of course Δ230–262 (white bar) fully activates NBCe1-A (Fig. 2g), corroborating the inhibitory effect of P-loop on NBCe1-A. It is interesting that deletion of the middle portion (Δ236–253) based on P-loop12D substantially reduces the magnitude of NBCe1-A inhibition by 34% (84−50% = 34%). Note that, mimic-phosphorylation of the middle portion of P-loop has little effect on NBCe1-A activity (Fig. 2e). These data together suggest that the length is important for P-loop to exhibit maximal effect on NBCe1-A.
Taking together, we conclude that P-loop is a regulatory module not essential for the normal function of NBCe1-A expressed in Xenopus oocytes. We propose that, upon phosphorylation, P-loop downregulates NBCe1-A activity by reducing its transport rate.
Effects of protein phosphatase and kinase on NBCe1-A activity
Previous studies show that NBCe1-A always elicits virtually full activity in oocytes44,45,46,47,48. Here, removal of P-loop just slightly enhances NBCe1-A activity (Fig. 2g). Presumably, P-loop is predominantly dephosphorylated by endogenous protein phosphatases, e.g., PP1 that is abundantly expressed in frog oocytes (Fig. 3a). Indeed, treatment of the oocytes with PP1 inhibitor calyculin decreases NBCe1-A activity by ~30% (Fig. 3b). On the other hand, expressing SPAK and/or CaMKIIα modestly decreases the activity of NBCe1-AWT, but not P-loop12A or P-loop12D (Fig. 3c–d), suggesting that SPAK and CaMKIIα affect the phosphorylation of P-loop. The effect of CaMKIIα on NBCe1-A is consistent with the previous study33. Interestingly, CaMKIIα and SPAK exhibit no additive effect on the activity of NBCe1-A (Fig. 3d). It is possible that the two kinases affect the same set of phosphosites in NBCe1-A when expressed in oocytes.
a Expression of endogenous protein phosphatase PP1 in Xenopus oocytes. Upper panel: oocytes of stages I − VI. Middle panel: Western blotting for PP1. Bottom a parallel gel stained with Coomassie brilliant blue to show the loading. See Fig. S4b for full blots. b Administration of PP1 inhibitor Calyculin inhibits NBCe1-A activity in oocytes. c, d Overexpressing SPAK and/or CaMKIIα has no effect on the activities of P-loop12A or P-lop12D. SPAKCA: constitutively-active SPAK containing mimic-phosphorylation mutations T243E and S383D. e SPAK significantly decreases the activity of NBCe1-A with mimic-phosphorylation at T254 and S256. f SPAK has no effect on NBCe1-A with mimic-(de)phosphorylation at S232, S233, and S235. The numerals in the parentheses on the bars indicate the number of individual oocytes. One-way ANOVA followed by post-hoc Dunnett’s multiple comparisons was used for statistical analyses for a, b. Student’s t-test was used for c, d, f. **p < 0.01; ***p < 0.001; ****p < 0.0001.
The effects of SPAK and CaMKIIα on NBCe1-A are qualitatively consistent with, but quantitatively much smaller than those of full mimic-phosphorylation of P-loop. One possibility is that, when expressed in Xenopus oocytes, these kinases affect just a subset of the phosphosites in the P-loop of NBCe1. In the present study, SPAK decreases the activity of P-loop2D (T254/S256 → DD), but not that of P-loop3D (S232/S233/S235 → DDD) (Fig. 3e–f). The data indicate that SPAK affects the phosphorylation of Ser232, Ser233, and Ser235 in P-loopNt, but presumably not T254 or S256 in P-loopCt.
P-loop blocks NBCe1-A by binding to the transmembrane domain
We generated the structural model of full-length NBCe1 by homology modelling with the cryo-EM structure of full-length AE1 (PDB accession no. 7TW2) as template (Fig. S5). NBCe1 TMD consists of a carrier domain and a scaffold domain. Right before TM1 in the carrier domain is H0, a helix that is about perpendicular to the transmembrane bundles (Fig. S6a–b). Structurally, H0 could be regarded as an integral part of the carrier domain. During the ion transport, the carrier domain slides back and forth along the scaffold, causing transition of NBCe1 conformation between outward-facing (OFS) and inward-facing states (IFS). Continuous ion translocation depends on the cyclic transition of NBCe1 between OFS and IFS.
What is the molecular mechanism underlying NBCe1-A inhibition by P-loop? A simple assumption is that P-loop, upon phosphorylation, directly interacts with TMD, therefore disrupting the conformation transition of NBCe1. Phosphorylation would increase the number of negative charges in P-loop. We hypothesize that P-loop blocks NBCe1-A by binding to specific target sites in TMD via electrostatic interaction.
Multiple structural elements in the intracellular side of NBCe1, such as H0 and the fourth intracellular loop (IL4) in the carrier, TM6-IL3 and IL6 in the scaffold, are particularly rich in basic residues (Fig. S6c). These structural elements could be positively charged under physiological pHi. If any of these structural elements serves as the binding target for P-loop in TMD, then mutation to the basic residues in this element would impair the interaction of P-loop with TMD, therefore decreasing the magnitude of NBCe1-A inhibition by P-loop.
We first examined the effect of IL4 in the carrier domain on P-loop (Fig. 4a–b). As shown in Fig. 4c, based on NBCe1-AWT, Ala-substitutions to the basic residues in IL4 modestly decrease the activity of NBCe1-A, e.g., IL4R808A,K809A vs IL4WT (white bars, with P-loopWT). Strikingly, the activity of P-loop12D/IL4R808A,K809A is not different from that of P-loopWT/IL4R808A,K809A (R808A,K809A: orange vs white bars, Fig. 4c). The results demonstrate that the mutations to R808 and K809 completely eliminate the inhibition of NBCe1-A by P-loop (Fig. 4d). Ala-mutation to H811 and K812 reduces by ~50% the magnitude of NBCe1-A inhibition by P-loop. The expressions of the mutants are normal in oocytes (Fig. S7a). Together, our data support the hypothesis that P-loop blocks NBCe1-A by interacting with IL4 in the carrier domain.
a Diagram showing the spatial arrangement of transmembrane α helices (TMs) in NBCe1 TMD (bottom view). Numbered circles represent the TMs. Yellow: TMs in the carrier domain. Green: TMs in the scaffold domain. b Localization of basic residues in IL4 and IL6. c Effect of mutations to basic residues in IL4 on the activity of NBCe1-A with either P-loopWT or P-loop12D. d Summary of the percentile inhibition of NBCe1-A by P-loop12D. The GNBC of individual oocytes contributed to the orange bars in panel c was subtracted from the mean of the corresponding control (open bar) and then divided by the mean of the control. The % difference represents the percentile inhibition of NBCe1-A by P-loop12D. e Effect of mutations to IL6 on the activity of NBCe1-A with either P-loopWT or P-loop12D. f Summary of percentile inhibition of NBCe1-A by P-loop12D. The figure was created based on the data contributed to e. Data are presented as mean ± SD. The numerals in the parentheses on the bars indicate the number of individual oocytes. ns not significant by student’s t-test. In d and f, one-way ANOVA followed by post-hoc Dunnett’s multiple comparisons was used for statistical analyses. **p < 0.01; ****p < 0.0001.
Similar analyses were performed with IL6 in the scaffold domain. As shown in Fig. 4e, based on NBCe1-AWT, Ala-mutations to the basic residues in IL6, except for K934/H935, have little or just mild effects on the transport activity of NBCe1-A (white bars, with P-loopWT). The expressions of the mutants are normal in oocytes (Figs. S7a, S8b). Note that, the activity of P-loop12D/IL6R925A/K927A is very close to that of P-loopWT/IL6R925A/K927A, demonstrating that Ala-mutations to R925 and K927 substantially reduce the inhibition of NBCe1-A by P-loop (Fig. 4e–f). The data are consistent with the hypothesis that P-loop blocks NBCe1-A by interaction with IL6 in the scaffold domain.
In the scaffold domain, TM6-IL3 (briefly referred to as “IL3” hereafter) contains multiple basic residues, particularly in the intracellular end of TM6 (Fig. 5a–b). IL3 is in close proximity to IL6. We hypothesize that, upon phosphorylation, P-loop also interacts with IL3. To test this hypothesis, we performed mutations to the basic residues in IL3. The expressions of the NBCe1 mutants are normal in oocytes (Fig. S8a). Based on NBCe1-AWT, Ala-mutations to K711 and K712 decrease the activity of NBCe1-A by ~62% (white bars, Fig. 5c), indicating that K711 and K712 play a critical role in the normal function of NBCe1. Notably, the activity of P-loop12D/IL3K711A,K712A is not different from that of P-loopWT/IL3K711A,K712A (orange bars, Fig. 5c), demonstrating that the Ala-mutation to K711 and K712 completely cancels the inhibition of NBCe1-A by P-loop (Fig. 5d). The Ala-substitution to K714 mildly reduces the magnitude of NBCe1-A inhibition by P-loop. Together, these data support the idea that IL3 is involved in the interaction with P-loop.
a Diagram showing the spatial arrangement of TMs in NBCe1 TMD (bottom view). Yellow: TMs in the carrier domain. Green: TMs in the scaffold domain. b Localization of basic residues in IL4 in the carrier (yellow), and IL3 and IL6 in the scaffold (green). c Effect of mutations to the basic residues in IL3 on the activity of NBCe1-A with either P-loopWT or P-loop12D. d Summary of percentile inhibition of NBCe1-A by P-loop12D. The figure was created based on the data contributed to c. e Proposed model for the mechanism of NBCe1-A inhibition by phosphorylation of P-loop. Data are presented as mean ± SD. The numerals in the parentheses on the bars indicate the number of individual oocytes. ns, not significant by two-tailed student’s t-test. In d, one-way ANOVA followed by post-hoc Dunnett’s multiple comparisons was used for statistical analyses. ****p < 0.0001.
Finally, mutational studies indicate that neither H0 nor IL5 is involved in the interaction of TMD with P-loop (Fig. S9).
Taken altogether, the above data supports the idea that, upon phosphorylation, P-loop inactivates NBCe1-A by simultaneously binding to IL4 in the carrier domain and IL3 and IL6 in the scaffold domain (Fig. 5e).
Mimic-phosphorylation of H-loop decreases NBCe1-A activity
H-loop corresponds to IL5 in the carrier domain. Structural studies on AE1, AE2, and SLC4A11 show that IL5 between TM10 and TM11 in the carrier domain adopts a β-hairpin loop structure14,15,16,17,20. In NBCe1, H-loop contains three phosphosites, i.e., T859, T861, and S862 (Fig. 6a–b).
a Diagram showing the arrangement of TMs in NBCe1 TMD (bottom view). Yellow: TMs in the carrier domain. Green: TMs in the scaffold domain. H-loop is shown in red. IL3 is shown in blue. b Side view of NBCe1 TMD (partial). Note that H-loop is spatially close to TM6-IL3. Mutations to H-loop have no significant effect on the relative expression levels of total (c) and surface (d) NBCe1-A in oocytes. H-loop3A: T859/T861/S862 substituted with Ala. H-loop3D: T859/T861/S862 substituted with Asp. See Fig. S4c for full blots. e Mimic-phosphorylation of H-loop inhibits NBCe1-A activity. f, g Effect of mutations to IL3 on the activity of NBCe1-A with either H-loopWT or H-loop3D. h Summary of percentile inhibition of NBCe1-A by H-loop. The figure was created based on the data contributed to f and g. i Proposed model for the mechanism of NBCe1-A inhibition by phosphorylation of H-loop. Data are presented as mean ± SD. The numerals in the parentheses on the bars indicate the number of individual oocytes for activity data or the number of western blottings with independent pools of oocytes for expression data. ns, not significant by two-tailed student’s t-test in f and g. One-way ANOVA followed by post hoc Dunnett’s multiple comparisons was used for statistical analyses in h. **p < 0.01; ****p < 0.0001.
Based on NBCe1-AWT, we generated H-loop3D (TTS → DDD) and H-loop3A (TTS → AAA) to mimic phosphorylation and dephosphorylation of H-loop, respectively. The expression of both H-loop3D and H-loop3A is similar to NBCe1-AWT (Fig. 6c–d). While H-loop3A retains normal activity, H-loop3D elicits just ~40% of activity compared to NBCe1-AWT (Fig. 6e). Our data show that: (1) H-loop plays a regulatory role in NBCe1-A; (2) mimic-phosphorylation of H-loop downregulates NBCe1 activity by perturbing the transport process.
H-loop blocks NBCe1-A by interaction with IL3 in the scaffold domain
What is the structural mechanism underlying the regulation of NBCe1 by H-loop? In NBCe1, H-loop contains two negatively-charged residues, i.e., E858 and E860 (Fig. 6b, Fig. S10). Phosphorylation would further increase the number of negative charges carried by H-loop. We noted that H-loop is adjacent to IL3 (including the intracellular end of TM6) in the scaffold domain. The basic residues in IL3 line up with H-loop along the cleft between the carrier and the scaffold (Fig. S10c–d). We presume that, upon phosphorylation, H-loop binds to IL3, thus disrupting the sliding of the carrier domain. If so, then mutations to the basic residues in IL3 would abolish the inhibition of NBCe1-A by H-loop.
We tested the above hypothesis by mutational analyses to IL3. The expressions of the mutants are normal in oocytes (Fig. S11). The mutant H-loopWT/IL3K711A,K712A elicits a transport activity ~60% lower than NBCe1-AWT (WT vs K711A,K712A, white bars, Fig. 6f), consistent with the observations in Fig. 5c—H-loopWT/IL3K711A,K71A is the same as P-loopWT/IL3K711A,K712A. The activity of H-loop3D/IL3K711A,K712A is not different from that of H-loopWT/IL3A711,A712 (K711A,K712A: orange vs white bars, Fig. 6f). Thus, the mutations to K711/K712 completely eliminate the inhibition of NBCe1-A by H-loop (Fig. 6h). Similarly, the Ala-mutations involving K714 entirely eliminate NBCe1-A inhibition by H-loop (Fig. 6g–h).
Taking together, we conclude that mimic phosphorylation of H-loop causes a local interaction between H-loop in the carrier domain and IL3 in the scaffold, resulting in NBCe1-A blockade (Fig. 6i).
Discussion
The SLCs are fundamentally distinct from conductive channels. While the opening of a channel allows continuous ion flux through the channel, continuous ion translocation by a transporter depends on the cyclic transition of its conformation between OFS and IFS. The TMD of SLCs generally contains two domains, e.g., the carrier domain and scaffold domain in the elevator-like SLC4s. The relative movement of the two domains controls the conformation transition of the transporter. In theory, any regulation would eventually affect the relative movement of the two domains on the molecular level, and thus the transport rate if the regulation does not change the transporter density in the membrane.
The transport of NBCe1 begins with the binding of substrate ions—first \({{{{\rm{HCO}}}}}_{3}^{-}\) and then Na+ 49. The substrate binding triggers a conformation transition of NBCe1 from OFS to IFS, followed by the release of the substrates into the cytosol. Finally, the conformation of NBCe1 returns back to OFS, getting ready for next cycle. The conformation transition depends on the sliding back and forth of the carrier along the scaffold driven by the electrochemical potential gradients of Na+/\({{{{\rm{HCO}}}}}_{3}^{-}\) (Fig. 7a).
a Conformation transition of NBCe1 during ion translocation. NBCe1 employs an elevator-like mechanism for ion translocation. The carrier domain (yellow) slides back and forth along the scaffold (green), causing the transition of NBCe1 between the outward-facing state (OFS) and the inward-facing state (IFS). b Inactivation of NBCe1-A by P-loop. Upon phosphorylation, P-loop simultaneously binds to IL4 in the carrier and IL3 and IL6 in the scaffold. This long-distance interaction disrupts the sliding of the carrier along the scaffold, causing NBCe1-A blockade. c Inactivation of NBCe1-A by H-loop. Upon phosphorylation, H-loop in the carrier binds to the IL3 in the scaffold. This local interaction interferes with the sliding of the carrier, therefore causing NBCe1-A blockade. d Hypothetical orientation of P-loop in its interaction with the TMD. P-loop contains two clusters of phosphosites in P-loopNt and P-loopCt. It is presumed that, upon phosphorylation, P-loopNt binds to IL4 in the carrier, whereas P-loopCt binds to IL3 and IL6 in the scaffold. See discussion in the main text.
The present study reports the structural mechanism of NBCe1 regulation by two phosphorylation modules: P-loop in the NTD and H-loop in the carrier domain of TMD. Most of the phosphosites in P-loop have been reported previously. The present study showed that these phosphosites actually belong to one single regulatory module, which we designated as P-loop. With the advantage of available structural models of SLC4 transporters, the present study provided a systematic investigation for the structural mechanisms underlying the regulations of NBCe1-A by these phosphorylation modules. Our findings indicate that the phosphorylation of P-loop or H-loop causes the inactivation of NBCe1 due to the interaction of these phosphorylation modules with specific target sites in the TMD.
Upon phosphorylation, P-loop simultaneously binds to IL4 in the carrier domain and IL3 and IL6 in the scaffold, interrupting the relative movement of the carrier along the scaffold, therefore causing NBCe1-A blockade (Fig. 7b). Upon phosphorylation, H-loop in the carrier domain binds to the adjacent IL3 in the scaffold, therefore interrupting the movement of the carrier relative to the scaffold, causing NBCe1-A blockade (Fig. 7c). It is well established that the SLC4 family transporters exist in dimers. The interaction between H-loop and TM6-IL3 is obviously intramolecular. That is, H-loop in the carrier domain would interact with the scaffold of the same monomer in NBCe1 dimer.
To our knowledge, our present study is the first such study on the structural mechanism of the regulation of SLC transporters by phosphorylation. The Na+-K+-Cl− cotransporters NKCC1 (SLC12A2) and NKCC2 (SLC12A1) of SLC12 family are known to be activated by phosphorylation of a regulatory module in the Nt50,51. In a structural study, it is presumed that, upon phosphorylation, the Nt regulatory module interacts with the large Ct domain, disrupting the dimerization of the Ct domain, thus causing the activation of NKCCs52. The cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel of the ATP-binding cassette transporter family, contains a regulatory R domain. Structural studies show that the R domain, upon dephosphorylation, interacts with the intracellular vestibule of the TMD, causing CFTR inactivation53. Phosphorylation of the R domain disrupts its interaction with the TMD, resulting in the dimerization of the nucleotide binding domain (NBD) and thus the activation of the channel54. It appears that the activation of both NKCC and CFTR by phosphorylation is associated with changes in the dimerization of specific structures, e.g., NBD in CFTR. It is intriguing whether the phosphorylation of P-loop or H-loop causes similar changes in the dimerization of the NTD in NBCe1.
The proposed simultaneous binding of P-loop to the carrier and the scaffold well explains the fact that mimic-phosphorylation of both P-loopNt and P-loopCt causes much greater inhibition of NBCe1-A than does mimic-phosphorylation of just P-loopNt or P-loopCt (Fig. 2f). Note that, P-loopNt and P-loopCt are separated by a peptide of 18 residues, which should be long enough to cover the region spanning from IL4 in the carrier to IL3/IL6 in the scaffold (Fig. 7d). Mimic-phosphorylated P-loopΔ236−253, in which P-loopCt follows right after P-loopNt without the connecting peptide, decreases NBCe1-A activity by only ~50%, in contrast to the ~84% decrease caused by the full-length P-loop12D in the same batch of oocytes (Fig. 2g). P-loop∆236−253 is likely too short to simultaneously bind to the carrier and the scaffold.
A question that arises is how P-loop is oriented regarding its interaction with the carrier and the scaffold. It is interesting to note that, the plane of the TMD dimer is about perpendicular to the plane of the NTD dimer in almost all reported 3D structures of full-length SLC4s, including AE1, AE2, and SLC4A1114,15,16,17,20. In the structural model of full-length NBCe1 dimer simulated based on the structure of AE1 with such an orientation, P-loopCt is in close proximity to IL6 in the scaffold domain (Fig. S5b). Recall that, upon mimic-phosphorylation, P-loopNt causes a greater inhibition in NBCe1-A than P-loopCt does (Fig. 2f). Note that, it is the carrier that moves back and forth for ion translocation. It is reasonable to speculate that P-loop binding to the carrier has a greater effect on the conformation transition of NBCe1 than does its binding to the scaffold. If the NTD remains dimerized, it would be reasonable to hypothesize that, upon phosphorylation, P-loopCt interacts with the scaffold domain, whereas P-loopNt reaches out to interact with the carrier (Fig. 7d). This hypothesis requires further testing by structural studies in future.
Is the interaction between P-loop and TMD intramolecular or intermolecular? Given the perpendicular orientation of the TMD plane relative to the NTD plane, the P-loop of subunit b is in close contact with the scaffold of subunit a in the model of NBCe1 dimer (Fig. S5b). If the NTD remains dimerized upon the phosphorylation of P-loop, it is possible that P-loop functions in an intermolecular manner. That is, the TMD of one subunit would be regulated by the P-loop of the other subunit in NBCe1 dimer. However, it is also possible that P-loop interacts with the TMD of the same monomer by an intramolecular manner if the phosphorylation of P-loop causes disruption of the dimer of NTD. Again, these hypotheses require further testing by structural studies in future.
H-loop likely plays dual roles in NBCe1. Structural studies on SLC4 members—including AE1, AE2, and SLC4A11—indicate that H-loop undergoes a large spatial displacement during the conformation transition from OFS to IFS14,15,16,17,20. In the model of NBCe1OFS simulated based on AE1OFS, H-loop adopts an orientation in close contact with IL3 in the scaffold domain (Fig. S12a–b). It is possible that H-loop has interaction with IL3 during the ion translocation in NBCe1. Such an interaction would cause the closing of the inner side of the ion pathway. In the model of NBCe1IFS simulated based on AE2IFS, H-loop reaches downward to interact with specific residues from the NTD (Fig. S12c–d). Such a displacement of H-loop would cause the opening of the inner side of the ion pathway. These structural data indicate that H-loop serves as a gating module in the SLC4s controlling the opening vs closing of the inner side of the ion pathway. The present study shows that H-loop serves as a regulatory module in NBCe1. Phosphorylation of H-loop could greatly enhance the interaction of H-loop with IL3 in the scaffold, locking the inner gate, thus causing NBCe1 inactivation.
The present study represents a major step forward for understanding the structural mechanisms underlying the regulation of NBCe1. Among the NBCe1 isoforms, NBCe1-B and -C can be inactivated by the autoinhibitory domain (AID) at the Nt end. The molecular mechanism underlying the regulation of NBCe1 by the AID has been extensively studied44,47,55,56,57. The present study discloses the structural mechanisms for the regulation of NBCe1-A by P-loop and H-loop. Although the present study focused on NBCe1-A, our findings could also be applicable to the AID-containing NBCe1-B and -C. However, compared to NBCe1-A, the regulation of NBCe1-B and -C by P-loop or H-loop could be more complicated due to the potential interaction between the AID and the phosphorylation modules.
Our findings may also have implication for understanding the regulation of other NBCs by phosphorylation. Although the primary sequences corresponding to the P-loop of NBCe1 are poorly conserved among the NBCs, they are all rich in Ser/Thr residues (Fig. S1). As far as H-loop is concerned, all three phosphosites are conserved in NBCe2, and two are conserved in the electroneutral NBCs (Fig. S13). It is possible that the other NBCs are also regulated by phosphorylation of P-loop or H-loop. Indeed, a search with a mouse phosphoproteomic database showed that phosphorylation has been identified for multiple residues in the P-loops of NBCn1 and NBCn2 (Fig. S14)58.
Finally, the present study could impact our understanding about the physiology and pathophysiology of acid-base transport in the kidney. NBCe1-A, the dominant NBCe1 isoform that plays a house-keeping function in the kidney, has been regarded to be constitutively active due to its full activity in heterologous systems. The present findings suggest that the molecular activity of NBCe1-A in the kidney could be tuned down by phosphorylation of P-loop or H-loop. Phosphorylation has been identified for specific residues in P-loop of NBCe1 in the kidney39,40. (see Table S1) A large body of literature indicates that the function of renal NBCe1 is modulated by diverse factors (for review, see ref. 25), such as angiotensin II59,60, parathyroid hormone61, glucocorticoids62, norepinephrine63, and thiazolidinedione64, and pathophysiological conditions, such as respiratory acidosis65, metabolic acidosis66,67, and hypokalemia68. It is possible that some of these regulations involve changes in the phosphorylation of NBCe1 in the kidney. For example, metabolic acidosis stimulates the Na+/\({{{{\rm{HCO}}}}}_{3}^{-}\) cotransport activity in the basolateral membrane of renal proximal tubules67,69,70. However, the protein expression of renal NBCe1 remains unchanged under metabolic acidosis71,72. It is intriguing whether metabolic acidosis causes adaptive changes in the phosphorylation of NBCe1 in the kidney.
Summary and perspectives
Based on mutational and functional analyses, the present study reveals the structural mechanisms underlying the inactivation of NBCe1-A by phosphorylation of P-loop and H-loop. The inactivation of NBCe1-A by P-loop and H-loop involves in the interaction of these phosphorylation modules with specific target sites in the TMD of NBCe1. These findings could impact our understanding of the regulation of systemic acid-base balance and also have implications in studying the structural mechanism for the regulation of transporters of the SLC superfamily by phosphorylation.
In what conformation—e.g., OFS or IFS—would NBCe1 be locked by P-loop upon phosphorylation? How would P-loop be oriented in its interaction with the TMD? In what conformation would NBCe1 be locked by H-loop upon phosphorylation? It requires further studies in future to address these questions.
Many phosphorylation sites have been identified in different isoforms of NBCe1 so far. The present study focuses on two specific structures, P-loop and H-loop in NBCe1-A, an isoform predominantly expressed in the proximal tubule. The phosphorylation of NBCe1 could be isoform and tissue specific. How is the phosphorylation of NBCe1 regulated in native tissues, e.g., the kidney under specific physiological and pathophysiological conditions? What are the molecular pathways responsible for the phosphorylation of P-loop and/or H-loop in NBCe1 in native tissues? The data in our present study indicate that protein kinases SPAK and CaMKIIα and protein phosphatase PP1 are involved in the modulation of the phosphorylation of P-loop in NBCe1 in Xenopus oocytes. Are these enzymes involved in the phosphorylation of NBCe1 in native tissues? To address these physiological questions requires further in vivo studies in future.
Methods
Ethical approval
Four female Sprague Dawley (SD) rats with ages of 8–9 weeks obtained from the Hubei Provincial Center for Disease Control (Wuhan, China) were anesthetized by subcutaneous injection of pentobarbital sodium (2% w/v, 0.5 ml per 100 g body weight) and sacrificed for brain tissue collection. Xenopus laevis (clawed frogs) were housed at 18 °C. The frogs were anesthetized with 0.2% tricaine (catalogue no. A5040, Sigma-Aldrich, St Louis, MO, USA) for oocyte preparation. All procedures for handling the animals were approved by the Institutional Animal Care and Use Committee at Huazhong University of Science and Technology. We have complied with all relevant ethical regulations for animal use.
Antibodies
Rabbit polyclonal antibody anti-NBCe1 was generated with peptide “NPDNGSPAMTHRNLTSSSL” and affinity-purified by Genscript (Nanjing, China). The anti-NBCe1 recognizes all major NBCe1 isoforms. Mouse anti-HA-tag was purchased from ABclonal (catalog no. AE008; ABclonal Technology, Wuhan, China). Mouse monoclonal antibody against EGFP was purchased from Proteintech (catalogue no. 66002. Proteintech, Wuhan, China). Rabbit anti-PP1Cβ was purchased from Abcam (catalogue no. Ab53315; Abcam, Shanghai, China). HRP-conjugated goat anti-rabbit IgG (catalogue no. A0218) and HRP-conjugated goat anti-mouse IgG (catalogue no. A0216) were purchased from Beyotime (Shanghai, China).
Molecular biology
Mouse NBCe1-A (accession no. HQ018820.1) and NBCe1-B (accession no. NM_018760.2), and rat CaMKIIα (accession no. NM_012920.1) and SPAK (accession no. NM_019362.1) were used in the present study. The constructs for expressing NBCe1-A and NBCe1-B in oocytes have been described previously44. Both NBCe1-A and NBCe1-B were tagged with enhanced green fluorescent protein (EGFP). The cDNAs of SPAK and CaMKIIα were cloned from rat tissues and then subcloned into pGH19 for heterologous expression in Xenopus oocytes. SPAK was tagged with HA (“YPYDVPDYA”) at the Ct. The constitutively-active SPAK contained two mutations T243E and S383D, mimicking-phosphorylation of SPAK73. Mutations in NBCe1 and SPAK were generated by regular molecular cloning approaches. cRNAs synthesis were performed with T7 mMessage mMachine Kit (catalogue no. AM1344, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Oocytes
For oocyte preparation, an ovarian lobule taken from a frog was cut into small pieces and separated by digestion in Ca2+-free NRS solution (in mM: 82 NaCl, 2 KCl, 20 MgCl2, 5 HEPES, pH7.5) containing 2 mg/mL collagenase (catalogue no. C0130, Sigma-Aldrich) for 90 min at room temperature. The oocytes were rinsed 4 times with NRS solution and then washed once with ND96 (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl, 5 HEPES, pH7.50) for 10 minutes. The oocytes at stages V − VI were selected for usage.
The oocytes were injected with cRNA by using Nanoject II Auto-Nanoliter Injector (Drummond Scientific Company, Broomall, PA, USA). For protein expression, the injected oocytes were incubated at 18 °C for 4–5 days in modified GibcoTM Leibovitz’s L-15 Medium (catalog no. 41300-039, Thermo Fisher Scientific) supplemented with 100 units/mL of penicillin and 100 μg/mL of streptomycin (catalog no. 15140122, Invitrogen, Carlsbad, CA, USA). The medium was adjusted to 200 mOsm osmolarity by dilution for usage with frog oocytes.
Phosphorylation identification by Mass-spectra analysis
Membrane proteins were prepared from rat brain tissues or Xenopus laevis oocytes heterologously expressing mouse NBCe1 by ultra-centrifugation. The brain tissues or Xenopus oocytes were homogenized in ice-cold protein isolation buffer (in mM: 7.5 NaH2PO4, 250 sucrose, 5 EDTA, 5 EGTA, pH 7.0) containing 1% protease inhibitor cocktail (Sigma-Aldrich, catalog no. P8340, St. Louis, MO, USA) and centrifuged at 3000 × g for 10 min at 4 °C. The resulting supernatant was ultracentrifugated at 100,000 × g for 60 min at 4 °C to pellet the membrane fraction. The membrane preparations were then resuspended in the protein isolation buffer. NBCe1 was isolated from the membrane preparations by immunoprecipitation with anti-NBCe1 (for usage with rat tissues) or anti-EGFP (for usage with Xenopus oocytes). The NBCe1 proteins were then separated by SDS-PAGE and silver-stained with Protein Stains K (catalogue no. C500021, Sangon Biotech, Shanghai, China). Mass-spectra analysis was performed by Beijing Protein Innovation Co., Ltd (Beijing, China) for the identification of phosphorylation in NBCe1.
Biotinylation assay and western blotting
Surface expression of NBCe1 in Xenopus oocytes was examined by biotinylation assay with PierceTM Cell Surface Protein Biotinylation and Isolation Kit (catalogue no. A44390, Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, 15 oocytes were pooled and washed twice with 3 mL of ice-cold PBS solution and incubated with 3 mL BupHTM PBS containing 0.24 mg/mL EZ-Link Sulfo-NHS-SS-Biotin for 30 min at room temperature. The cells were then washed for 2 × 5 min with TBS, and then disrupted in 200 μL of BupHTM TBS supplemented with 1% Triton X-100 plus 1% Protease Inhibitor Cocktail (catalogue no. P8340, Sigma-Aldrich). The crude lysate was centrifuged at 3000 g for 10 min at 4 °C. A small vial (40 μL) of the supernatant was saved, representing the ‘total proteins’. The rest of the supernatant was incubated with 100 μL of immobilized NeutrAvidinTM agarose resin for 30 min at room temperature and then centrifuged at 1000 g for 1 min at 4 °C. The resin was then washed 5 times with TBS and incubated with 100 μL Elution Buffer containing 50 mM dithiothreitol for 30 min at room temperature for the recovery of bound ‘surface proteins’.
For western blotting, the protein samples were separated by SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane (catalogue no. IPVH00010, Merck Millipore, Co. Cork, Ireland). The blot was blocked with 5% milk in TBST (1 mM Tris, 150 mM NaCl, 0.1% Tween20, pH7.4) for 1 h at room temperature, incubated with mouse anti-EGFP at a dilution of 1:10,000 in 1× TBST containing 1% milk, and then washed 5 times with 1× TBST. The blot was then incubated with goat anti-mouse secondary antibody conjugated with horseradish peroxidase (catalogue no. A0216, Beyotime, Shanghai, China) at a dilution of 1:10,000 in 1× TBST containing 1% milk. After 5 washes with 1×TBST, the blot was finally incubated with Super Signal West Pico PLUS Chemiluminescent Substrate (catalogue no. 34580, Thermo Scientific, Rockford, IL, USA). Protein bands were visualized by X-ray exposure. Densitometry was performed with ImageJ (National Institutes of Health, USA).
NBCe1 activity measurement by two-electrode voltage clamp
Two-electrode voltage clamp was performed with Oocyte Clamp OC-725 (Warner Instruments, Hamden, CT, USA). Microelectrodes were made from thin-walled borosilicate glass tubes (catalogue no. BF100-78-10, Sutter Instruments, Navato, CA, USA). The electrodes filled with 3 M KCl had a resistance of 0.5–2.0 MΩ. Data acquisition was performed with pCLAMP10.2 (Molecular Devices, San Jose, CA, USA).
For electrophysiological recordings, an oocyte was placed in a perfusion chamber and impaled with two microelectrodes, one for sensing the membrane potential (Vm) and the other for measuring the transmembrane current. The oocyte was equilibrated with nominally \({{{{\rm{HCO}}}}}_{3}^{-}\)-free standard ND96 solution and then perfused with 5%CO2/33 mM \({{{{\rm{HCO}}}}}_{3}^{-}\) solution (in mM: 66 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 mM HEPES, 33 NaHCO3, balanced with 5% CO2, pH7.50). The flow rate of perfusion was 3 mL/min. Voltage clamp was performed from −160 mV to +60 mV at a holding potential of −60 mV. Currents were acquired for oocytes both in ND96 (IND96) and CO2/\({{{{\rm{HCO}}}}}_{3}^{-}\) solution (IHCO3). The difference between IND96 and IHCO3 represented the \({{{{\rm{HCO}}}}}_{3}^{-}\)-dependent component mediated by NBCe1 (INBC).
Structural modelling
The NTD (residues 42–338) of mouse NBCe1-A (NCBI accession: HQ018820.1) was modeled using SWISS-MODEL with the crystal structure of the NTD of human NDCBE (PDB: 5JHO) as the template. The sequence of mouse NBCe1 NTD is 55% identical to human NDCBE NTD (NCBI: AAY79176). The resulting model of NBCe1 NTD had GMQE 0.71, QMEAN − 4.10, Cβ − 3.47, solvation −0.65, torsion −3.34, and MolProbity score 2.06.
To generate the structure models of full-length NBCe1-A, structural modeling was performed using SWISS-MODEL with the cryo-EM structures of AE1 (PDB ID: 7TW2) in OFS conformation and AE2 (PDB ID: 8JNJ) in IFS conformation as the templates, respectively. The sequence of mouse NBCe1-A is 41% identical to AE2 (NCBI accession number NP_001339271.1) and 39% identical to AE1 (NCBI accession number NP_000333.2), respectively. The model of NBCe1OFS had GMQE 0.53, QMEAN − 4.27, Cβ − 3.62, solvation −0.36, torsion −3.45, and MolProbity score 1.59. The model of NBCe1IFS had GMQE 0.49, QMEAN − 4.19, Cβ − 3.17, solvation −0.26, torsion −3.46, and MolProbity score 1.49.
Statistics and reproducibility
Data are presented as scatter plot with mean ± SD. Each data point for the activity (GNBC) of NBCe1 represented an individual oocyte. Each data points for the expression of NBCe1 represented a western blotting experiment with an independent pool of oocytes. Statistical analyses were performed by Graphpad Prism (GraphPad Software, Inc., Boston, MA, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Meixner, E. et al. A substrate-based ontology for human solute carriers. Mol. Syst. Biol. 16, e9652 (2020).
Dvorak, V. & Superti-Furga, G. Structural and functional annotation of solute carrier transporters: implication for drug discovery. Expert Opin. Drug Discov. 18, 1099–1115 (2023).
Lin, L., Yee, S. W., Kim, R. B. & Giacomini, K. M. SLC transporters as therapeutic targets: emerging opportunities. Nat. Rev. Drug Discov. 14, 543–560 (2015).
Hediger, M. A., Clemencon, B., Burrier, R. E. & Bruford, E. A. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol. Asp. Med. 34, 95–107 (2013).
Jardetzky, O. Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966).
Patlak, C. S. Contributions to the theory of active transport: II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull. Math. Biophys.19, 209–235 (1957).
Mitchell, P. A general theory of membrane transport from studies of bacteria. Nature 180, 134–136 (1957).
Jennings, M. L. Carriers, exchangers, and cotransporters in the first 100 years of the Journal of General Physiology. J. Gen. Physiol. 150, 1063–1080 (2018).
Shi, Y. G. Common folds and transport mechanisms of secondary active transporters. Annu. Rev. Biophys. 42, 51–72 (2013).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Beckstein, O. & Naughton, F. General principles of secondary active transporter function. Biophys. Rev. 3, 011307–011307 (2022).
Vitzthum, H. et al. The AE4 transporter mediates kidney acid-base sensing. Nat. Commun. 14, 3051 (2023).
Arakawa, T. et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350, 680–684 (2015).
Capper, M. J. et al. Substrate binding and inhibition of the anion exchanger 1 transporter. Nat. Struct. Mol. Biol. 30, 1495–1504 (2023).
Xia, X., Liu, S. & Zhou, Z. H. Structure, dynamics and assembly of the ankyrin complex on human red blood cell membrane. Nat. Struct. Mol. Biol. 29, 698–705 (2022).
Zhang, Q. et al. The structural basis of the pH-homeostasis mediated by the Cl-/HCO3- exchanger, AE2. Nat. Commun. 14, 1812 (2023).
Zhang, W. et al. Structural and functional insights into the lipid regulation of human anion exchanger 2. Nat. Commun. 15, 759 (2024).
Huynh, K. W. et al. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat. Commun. 9, 900 (2018).
Wang, W. G. et al. Cryo-EM structure of the sodium-driven chloride/bicarbonate exchanger NDCBE. Nat. Commun. 12, 5690 (2021).
Lu, Y. et al. Structural insights into the conformational changes of BTR1/SLC4A11 in complex with PIP2. Nat. Commun. 14, 6157 (2023).
Thurtle-Schmidt, B. H. & Stroud, R. M. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc. Natl. Acad. Sci. USA 113, 10542–10546 (2016).
Boron, W. F. & Boulpaep, E. L. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3- transport. J. Gen. Physiol. 81, 53–94 (1983).
Romero, M. F., Hediger, M. A., Boulpaep, E. L. & Boron, W. F. Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387, 409–413 (1997).
Holmberg, S. R., Sakamoto, Y., Kato, A. & Romero, M. F. The role of Na+-coupled bicarbonate transporters (NCBT) in health and disease. Pflüg. Arch.Eur. J. Physiol. 476, 479–503 (2024).
Parker, M. D. & Boron, W. F. The divergence, actions, roles, and relatives of sodium coupled bicarbonate transporters. Physiol. Rev. 93, 803–959 (2013).
Fang, L. et al. Expression of the B splice variant of NBCe1 (SLC4A4) in the mouse kidney. Am. J. Physiol.-Ren. 315, F417–F428 (2018).
Brady, C. T., Marshall, A., Zhang, C. & Parker, M. D. NBCe1-B/C-knockout mice exhibit an impaired respiratory response and an enhanced renal response to metabolic acidosis. Front. Physiol. 14, 1201034 (2023).
Igarashi, T. et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat. Genet. 23, 264–266 (1999).
Horita, S. et al. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J. Am. Soc. Nephrol. 16, 2270–2278 (2005).
Liu, Y. et al. Functional characterization of a novel SLC4A4 variant and uniparental isodisomy in proximal renal tubular acidosis patient. Biochem. Genet. 62, 2469–2481 (2024).
Thornell, I. M. & Bevensee, M. O. Regulators of Slc4 bicarbonate transporter activity. Front. Physiol. 6, 166 (2015).
Hong, J. H. et al. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3- cotransporters family. Proc. Natl. Acad. Sci. USA 110, 4105–4110 (2013).
Perry, C., Le, H. & Grichtchenko, I. I. ANG II and calmodulin/CaMKII regulate surface expression and functional activity of NBCe1 via separate means. Am. J. Physiol. Ren. Physiol. 293, F68–F77 (2007).
Perry, C., Blaine, J., Le, H. & Grichtchenko, I. I. PMA- and ANG II-induced PKC regulation of the renal Na+/HCO3- cotransporter (hkNBCe1). Am. J. Physiol. Ren. 290, F417–F427 (2006).
Vachel, L. et al. Modulation of Cl- signaling and ion transport by recruitment of kinases and phosphatases mediated by the regulatory protein IRBIT. Sci. Signal. 11, eaat5018 (2018).
Giannaki, M., Ludwig, C., Heermann, S. & Roussa, E. Regulation of electrogenic Na+/HCO3- cotransporter 1 (NBCe1) function and its dependence on m-TOR mediated phosphorylation of Ser245. J. Cell. Physiol. 237, 1372–1388 (2022).
Khakipoor, S. et al. Functional expression of electrogenic sodium bicarbonate cotransporter 1 (NBCe1) in mouse cortical astrocytes is dependent on S255-257 and regulated by mTOR. Glia 67, 2264–2278 (2019).
Gross, E. et al. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3- : Na+ stoichiometry from 3:1 to 2:1 in murine proximal tubule cells. J. Physiol. 537, 659–665 (2001).
Grahammer, F. et al. mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J. Am. Soc. Nephrol. 28, 230–241 (2017).
Feric, M., Zhao, B., Hoffert, J. D., Pisitkun, T. & Knepper, M. A. Large-scale phosphoproteomic analysis of membrane proteins in renal proximal and distal tubule. Am. J. Physiol. Cell Physiol. 300, C755–C770 (2011).
Gerson-Gurwitz, A. et al. Ancestral roles of the Fam20C family of secreted protein kinases revealed in C. elegans. J. Cell Biol. 218, 3795–3811 (2019).
Park, B. C., Reese, M. & Tagliabracci, V. S. Thinking outside of the cell: secreted protein kinases in bacteria, parasites, and mammals. IUBMB Life 71, 749–759 (2019).
Choi, I., Aalkjær, C., Boulpaep, E. L. & Boron, W. F. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405, 571–575 (2000).
Su, P. et al. IRBIT activates NBCe1-B by releasing the auto-inhibition module from the transmembrane domain. J. Physiol. 599, 1151–11 (2021).
McAlear, S. D., Liu, X., Williams, J. B., McNicholas-Bevensee, C. M. & Bevensee, M. O. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J. Gen. Physiol. 127, 639–658 (2006).
Chang, M. H., DiPiero, J., Sonnichsen, F. D. & Romero, M. F. Entry to “HCO3 tunnel” revealed by SLC4A4 human mutation and structural model. J. Biol. Chem. 283, 18402–18410 (2008).
Shirakabe, K. et al. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3– cotransporter 1 (pNBC1). Proc. Natl. Acad. Sci. USA 103, 9542–9547 (2006).
Moss, F. J. & Boron, W. F. Carbonic anhydrases enhance activity of endogenous Na-H exchangers and not the electrogenic Na/HCO3 cotransporter NBCe1-A, expressed in Xenopus oocytes. J. Physiol. 598, 5821–5856 (2020).
Wu, H. et al. Molecular insight into coordination sites for substrates and their coupling kinetics in Na+/HCO3- cotransporter NBCe1. J. Physiol. 600, 3083–3111 (2022).
Giménez, I. & Forbush, B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am. J. Physiol. Ren. 289, F1341–F1345 (2005).
Monette, M. Y. & Forbush, B. Regulatory activation is accompanied by movement in the C terminus of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 287, 2210–2220 (2012).
Zhang, S. et al. The structural basis of function and regulation of neuronal cotransporters NKCC1 and KCC2. Commun. Biol. 4, 226 (2021).
Liu, F., Zhang, Z., Csanady, L., Gadsby, D. C. & Chen, J. Molecular structure of the human CFTR ion channel. Cell 169, 85–95 (2017).
Zhang, Z., Liu, F. & Chen, J. Conformational changes of CFTR upon phosphorylation and ATP binding. Cell 170, 483–491 (2017).
Lee, S. K., Boron, W. F. & Parker, M. D. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: role of IRBIT vs. amino-terminal truncation. Am. J. Physiol. Cell Physiol. 302, C518–C526 (2012).
Lee, S. K. & Boron, W. F. Exploring the autoinhibitory domain of the electrogenic Na+/HCO3- transporter NBCe1-B, from residues 28 to 62. J. Physiol. 596, 3637–3653 (2018).
Gui, T. X. et al. Redox state of NAD modulates the activation of Na-bicarbonate cotransporter NBCe1-B via IRBIT and L-IRBIT. Sci. China Life Sci. 68, 1452–1462 (2025).
Huttlin, E. L. et al. A Tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Wang, T. & Chan, Y. L. Mechanism of angiotensin II action on proximal tubular transport. J. Pharmacol. Exp. Ther. 252, 689–695 (1990).
Horita, S. et al. Biphasic regulation of Na+-HCO3- cotransporter by angiotensin II type 1A receptor. Hypertension 40, 707–712 (2002).
Ruiz, O. S., Qiu, Y. Y., Wang, L. J. & Arruda, J. A. Regulation of the renal Na-HCO3 cotransporter: V. Mechanism of the inhibitory effect of parathyroid hormone. Kidney Int. 49, 396–402 (1996).
Ruiz, O. S., Wang, L. J., Pahlavan, P. & Arruda, J. A. Regulation of renal Na-HCO3 cotransporter: III. Presence and modulation by glucocorticoids in primary cultures of the proximal tubule. Kidney Int. 47, 1669–1676 (1995).
Sonalker, P. A., Tofovic, S. P., Bastacky, S. I. & Jackson, E. K. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin. Exp. Pharmacol. Physiol. 35, 594–600 (2008).
Endo, Y. et al. Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARγ-dependent nongenomic signaling. Cell Metab. 13, 550–561 (2011).
de Seigneux, S. et al. Renal compensation to chronic hypoxic hypercapnia: downregulation of pendrin and adaptation of the proximal tubule. Am. J. Physiol. Ren. 292, F1256–F1266 (2007).
Santella, R. N., Gennari, F. J. & Maddox, D. A. Metabolic acidosis stimulates bicarbonate reabsorption in the early proximal tubule. Am. J. Physiol. 257, F35–F42 (1989).
Preisig, P. A. & Alpern, R. J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO 3)3 symporter in the rat proximal convoluted tubule. J. Clin. Investig. 82, 1445–1453 (1988).
Soleimani, M., Bergman, J. A., Hosford, M. A. & McKinney, T. D. Potassium depletion increases luminal Na+/H+ exchange and basolateral Na+ : CO32- : HCO3- cotransport in rat renal cortex. J. Clin. Investig. 86, 1076–1083 (1990).
Soleimani, M., Bizal, G. L., McKinney, T. D. & Hattabaugh, Y. J. Effect of in vitro metabolic acidosis on luminal Na + /H + exchange and basolateral Na + :HCO 3 - cotransport in rabbit kidney proximal tubules. J. Clin. Investig. 90, 211–218 (1992).
Akiba, T., Rocco, V. K. & Warnock, D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J. Clin. Investig. 80, 308–315 (1987).
Kwon, T. H. et al. Chronic metabolic acidosis upregulates rat kidney Na-HCO3- cotransporters NBCn1 and NBC3 but not NBC1. Am. J. Physiol. Ren. 282, F341–F351 (2002).
Amlal, H., Chen, Q., Greeley, T., Pavelic, L. & Soleimani, M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 60, 1824–1836 (2001).
Gagnon, K. B. & Delpire, E. On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl- cotransport activity. Am. J. Physiol. Cell Physiol. 299, C614–C620 (2010).
Acknowledgements
This work was supported by grant 32271195 from NSFC as well as grants JCYJ20220530161011025 and GJHZ20240218114705011 from Shenzhen Science and Technology Innovation Commission.
Author information
Authors and Affiliations
Contributions
H.W., Y.L., and L.-M.C. designed the study and wrote the paper. H.W., X.F., M.W., T.G., M.F., M.Z., Z.H., and X.L. performed the experiments and data collection. H.W. and L.-M.C. analyzed the data and prepared the figures. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Silvia Dossena and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Janesh Kumar and Laura Rodríguez Pérez. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, H., Feng, X., Wang, M. et al. Inactivation mechanisms of Na+/HCO3− cotransporter NBCe1 by phosphorylation. Commun Biol 8, 1295 (2025). https://doi.org/10.1038/s42003-025-08713-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08713-5