Abstract
Metabolism is the critical basis for mammalian physiological functions. The systematic metabolic characteristics of major organ development during late gestation to adapt to postnatal environmental changes are still absent. Here, we detected metabolic patterns of the ICR mouse fetal organs, including the heart, stomach, liver, brain, and placenta, from embryonic days (E)15.5 to 19.5 using liquid chromatography-mass spectrometry combined with RNA sequencing and proteomics data. Our metabolic and multi-omics data showed that organs exhibited their unique metabolic characteristics during late gestation, and significant metabolic pattern transitions, especially the enhancement of digesting the fatty acids and proteins, occurred at the E16.5 to E18.5 stage. Additionally, we found the abundance of carnosine and histidine in the placenta may serve as a way to test their levels in the newborn brain in vitro. Our dataset provides a comprehensive metabolic landscape of mammalian organ development in late gestation.
Similar content being viewed by others
Introduction
The development of intrauterine embryos undergoes the cleavage stage in the fallopian tube, the blastocyst stage of implantation into the uterus, the gastrula stage, which initiates organogenesis, and the organ maturation stage at mid to late gestation. The development of embryos is strictly regulated, with genetic regulation, epigenetic remodeling, and material metabolism1,2. The metabolism plays an irreplaceable role and shows an inseparable relationship with gene expression, epigenetic regulation, and cell function specialization during tissue and organ development3,4. However, the metabolites cannot be completely predicted through genomes or epigenetics since the multiple feedback mechanisms. The metabolic characteristics of cleavage stage and mid-gestation embryo detected using mass spectrometry and other technology revealed the metabolomic profiles and support for the embryo development5,6,7,8, the dynamic metabolism of the fetal development in late gestation is still unclear.
The maturation of organs is the key process of fetal development during late gestation9,10. The transport of metabolites from mother to fetus through the placenta is the core support for embryonic development after implantation8. It was found that maternal hyperglycemia can cause metabolic changes in the fetal tissue development during mid-to-late gestation11, while a high-fat diet during pregnancy led to reduced bioavailability of serotonin in the fetal brains of male offspring12. These data highlighted the pivotal role of metabolic homeostasis in fetal intrauterine organ development and postnatal health.
The metabolism plays a critical role in supporting the physiologic function of different organs13,14,15,16,17,18. As pregnancy progresses, the fetal metabolic pattern gradually begins to adapt to organ development and the dramatic changes in the metabolic environment after birth. Maturity of heart development is accompanied by the enhancement of fatty acid oxidation19,20. The brain has unique branched-chain amino acid requirements at different developmental stages to meet the development of nerve cells21. Meanwhile, the fetus needs to prepare for the self-digestion of the milk to obtain energy, as a crucial metabolic conversion after birth22. The diverse role of metabolism in organ development urgently requires systematic investigation of metabolic alteration and adjustment with the various organ maturation in late gestation.
In this present study, we utilized untargeted metabolomics combined with targeted metabolomics and multi-omics data to create a metabolomic atlas of the main fetal organs in mice during late gestation. We observed a significant metabolic reprogramming in the fetus from E16.5 to E18.5, possibly in preparation for birth. This included an enhancement of the TCA cycle in the heart, as well as significant changes in fatty acid metabolism in the liver, stomach, and brain. Moreover, we found that the brain and placenta shared partially similar metabolic patterns, and identified that carnosine and histidine may serve as biomarkers for in vitro diagnostics of newborns. Our data established the metabolic landscape of the main organs in mice to better understand fetal development and metabolic preparation for birth during late gestation.
Results
Metabolic pattern and characteristics of fetal major organs during late gestation
The metabolic characteristics of different organs over development time are closely related to their respective physiologic functions over development time. To detect the metabolites of the major organs during late gestation, the fetal heart, brain, stomach, liver, and placenta were collected at E15.5, E16.5, E18.5, and E19.5, respectively. In total, 119 samples of these organs were collected and analyzed by liquid chromatography-mass spectrometry (LC-MS) for untargeted metabolomics, followed by metabolite identification and analysis (Fig. 1a). Quality control (QC) samples were prepared from mixed samples, which were used to reflect the overall quality of metabolomics data23. A total of 136 metabolites were identified (Supplementary Data 1). Additionally, 98% of the mass spectrometry feature peaks under positive and negative ion modes had a coefficient of variation less than 1, while the QC samples clustered significantly in the PCA score plot, indicating high quality and stability of our metabolomics data (Supplementary Fig. 1a, b).
a The workflow of this study (created with Biorender). b The metabolic pattern of organs by using PLS-DA analysis. Various scattered icons represent different tissue samples, and colors represent different developmental times. c Heatmap showing the identified 136 metabolites in 5 organs at E15.5, E16.5, E18.5, E19.5, with color indicating the expression level of the metabolites, red indicating higher expression level, and blue indicating lower expression level. d The log10(abundance) of energy related metabolites in 5 organs at E15.5, E16.5, E18.5 E19.5. e The log10(abundance) of 4-pyridoxic acid in 5 organs at E15.5, E16.5, E18.5 E19.5. The significance was analyzed by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). The sample sizes were: n = 5 (heart and stomach at E15.5), n = 7 (heart at E16.5), and n = 6 (all other groups). Error bars represent mean ± SD.
Using partial least squares-discriminant analysis (PLS-DA) based on identified metabolites, we were able to separate the different organs and observe independent metabolic patterns during late gestation. The placenta and brain were found to be discrete from other organs (Fig. 1b). Our findings showed that developmental time did not affect the overall metabolic characteristic of each organ, but rather impacted on the metabolic specialization within each organ (Fig. 1b, Supplementary Fig. 1c). This suggests that although various organs in late gestation were still developing, they had already undergone metabolic specialization. To further explore the pattern of each organ over development time, the abundance of identified metabolites was measured. The results showed that the most metabolites in the heart and stomach were relatively high, while the overall abundance of metabolites in the placenta and brain were low, declaring the significant metabolic difference among the organs, with the heart and stomach being metabolically active organs during late gestation (Fig. 1c). Moreover, the identified metabolites from different organs were mainly enriched in alanine, aspartate and glutamate metabolism, purine metabolism, TCA cycle, pantothenate and CoA biosynthesis and others (Supplementary Fig. 1d), suggesting the important role of these metabolic pathways in different organs during late gestation.
The differential metabolites across the organs and developmental times were identified by using PLS-DA and 60 metabolites (numbered from M1-M60) were screened (Supplementary Fig. 2a). We found some specific metabolites, including adenylsuccinic acid showed a higher overall abundance in the heart and a lower abundance in the placenta; L-asparagine was very low in other organs but abundant in the brain; Palmitoylcarnitine was relatively high in the placenta and stomach, but lower in the liver; The abundance of linoleic acid and orotic acid were relatively high in the stomach throughout late gestation, and lower in the brain and placenta (Supplementary Fig. 2a–c).
Moreover, the abundance of the differential metabolites showed sudden changes from E16.5 to E18.5 in each organ (Supplementary Fig. 2a), indicating the importance of this stage in the metabolic development of the fetus. Notably, the enrichment analysis of the differential metabolites showed impressive energy metabolism across all organs. Besides the pantothenate and CoA biosynthesis found in each organ, the metabolites in the heart were enriched in the glycolysis, the citrate cycle (TCA cycle), and the pentose phosphate pathway (PPP); The biosynthesis of unsaturated fatty acids was found to be involved in the stomach and liver; The pentose and glucuronate interconversions existed in the brain and placenta (Supplementary Fig. 3). The relevant metabolites in the metabolic pathways associated with energy metabolism mainly changed at the E16.5 to E18.5 stage, such as D-Glucose 6-phosphate (G6P) in glycolysis, D-ribulose 5-phosphate in PPP, succinic acid in the TCA cycle, L-aspartic acid in pantothenate and CoA biosynthesis, phosphorylcholine in glycerophospholipid metabolism, and linoleic acid related to biosynthesis of unsaturated fatty acids, suggesting the crucial role of organ energy metabolism during late gestation (Fig. 1d).
In addition, we also found the metabolism of various amino acids was a prominent feature among organs, while the vitamin B6 metabolism pathway, which is closely related to amino acid metabolism24, was enriched in each organ (Supplementary Fig. 3), indicating its effect in organ development. Of note, we found that 4-pyridoxic acid exhibited the same metabolic pattern over development time in different organs, especially increasing in abundance from E16.5 to E18.5 (Supplementary Fig. 2a, Fig. 1e). 4-pyridoxic acid is a decomposition metabolite of the vitamin B6 pathway25,26. The elevated 4-pyridoxic acid after E16.5 may serve as a marker for organ maturation around birth.
Fatty acids and proteins are the main components of milk, providing energy and nutrition for infants after birth27,28. Our data found the strengthening of energy and amino acid metabolism were the main metabolic characteristics in late gestation, which may establish a metabolic basis for the adaptation of the fetus to the postnatal environment, while the period from E16.5 to E18.5 is the decisive time window for the metabolic pattern transformation of fetal organ development.
The energy metabolism changes of the fetal heart at the E16.5 to E18.5 stage
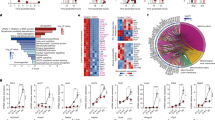
The heart is one of the most active organs for energy metabolism, which developed from the mesoderm. Metabolic homeostasis and reprogramming are crucial for heart development both during pregnancy and after birth8,29. The differential metabolites also showed the core role of energy metabolism in the heart, especially from E16.5 to E18.5 (Supplementary Fig. 2a, b). To further investigate the changes at this stage, orthogonal partial least squares-discriminant analysis (OPLS-DA) was applied and the heart samples were significantly separated between E16.5 and E18.5 (Fig. 2a). The top 20 differential metabolites with VIP values greater than 1 were used for KEGG enrichment analysis, and the results showed that these metabolites were mainly involved in PPP, glycolysis, biosynthesis of unsaturated fatty acids, TCA cycles and other energy related terms (Fig. 2b), indicating that the energy metabolism change was the major feature of the fetal heart at the E16.5 to E18.5 stage.
a OPLS-DA analysis of heart at E16.5 (n = 7) and E18.5 (n = 6). b The KEGG pathway enriched by the top 20 differential metabolites in heart from E16.5 (n = 7) to E18.5 (n = 6). The color of the bubble represents the value of −log10 (p-value). c Experimental design of multi-omics analysis (created with Biorender). Targeted metabolomics, stable isotope tracing, and transcriptome sequencing were applied to analyze the conversion process of heart from E16.5 to E18.5. d The abundance of cis-aconitic acid, malic acid, fumaric acid, succinic acid, glucose 1-phosphate, L-lactic acid in heart at E16.5 (n = 3) and E18.5 (n = 3). (*p < 0.05). e Comparison of fractional enrichment of glucose 6-phosphate, citric acid and fumaric acid through isotope tracing (n = 5 per group; *p < 0.05). f Heatmap of energy metabolism-related gene expression in the heart at E16.5 (n = 3) and E18.5 (n = 3). g Metabolic remodeling process in heart from E16.5 to E18.5 (created with Biorender). The metabolites showed in the box. Red represented upregulation in E18.5, and blue represented downregulation. The significance was analyzed by Student’s t-test, error bars represent mean ± SD.
To detect the detailed characteristics of heart energy metabolism from E16.5 to E18.5, we employed targeted metabolomics to identify the central carbon cycle metabolites (Fig. 2c). Total 51 targeted metabolites were quantified (Supplementary Fig. 4a, Supplementary Data 2). These metabolites also significantly separated the heart samples of E18.5 from E16.5 (Supplementary Fig. 4b). Among them, 27 upregulated and 24 downregulated metabolites were identified in E18.5 compared with E16.5 (Supplementary Fig. 4c). The changes in these metabolites showed that the most of the metabolites in the TCA cycle were upregulated in E18.5 (Supplementary Fig. 4d), including the cis-aconitic acid, malic acid, and fumaric acid (Fig. 2d), while many metabolites of other metabolic pathways were decreased, such as glucose 1-phosphate (G1P) and L-lactic acid in glycolysis and 6-phosphogluconic acid (6-PG) in PPP (Fig. 2d, Supplementary Fig. 4d). These results demonstrated that the metabolic remodeling between E16.5 and E18.5 was a transition from an active glycolytic pathway to an active TCA cycle, which may closely relate to heart development and prepare for the birth.
To further measure the energy metabolism transformation of the heart in vivo, 13C labeled glucose was injected to pregnant mice in vitro and observed the distribution of these labeled metabolites in the fetal heart at E16.5 and E18.5 (Supplementary Fig. 5a). The results showed that during the process of utilizing exogenous glucose, the carbon atom rearrangement process of G6P (M + 2) was significantly decreased and G6P (M + 6) was also decreased (p < 0.05), while the citric acid (M + 4) in the TCA cycle was increased at E18.5 (Fig. 2e, Supplementary Data 3). Although there was no significant difference in fumaric acid between the two groups, the carbon atom rearrangement process clearly increased at E18.5 (Fig. 2e). These stable isotope tracing results further suggest a weakened glycolysis process and a strengthened TCA cycle process in the fetal heart at the E16.5 to E18.5 stage.
To extend our exploration to the transcriptome level, we measured the gene expression of the heart at E16.5 and E18.5 using RNA-seq. There were 139 upregulated genes and 55 downregulated genes in the E18.5 group, and these genes were mainly involved in epithelial cell differentiation, cellular response to ATP and other processes (Supplementary Fig. 5b–d, Supplementary Data 4). We further analyzed the expression of energy metabolism-related genes, including G6pc2 and Galt in glycolysis, G6pdx, Pgd and Shpk in PPP, Pck2, Pdhx and Pdk2 in TCA cycle. We found that Pdhx and G6pc2 were upregulated, while others were downregulated at E18.5 (Fig. 2f, g), confirming the enhancement of the TCA cycle.
To date, combined with the multi-omics analysis, we depicted the conversion process from glycolysis to TCA at E16.5 to E18.5 stage in fetal heart (Fig. 2g). The metabolites G1P, G6P, and fructose 6-phosphate (F6P) were all decreased, mainly caused by the downregulation of Galt, which catalyzes the conversion of G1P30. The upregulation of G6pc2, which enhanced the hydrolysis of G6P, indicates that the glycolysis of the heart was downregulated at E18.5. Meanwhile, phosphoenolpyruvate (PEP) and pyruvic acid were accumulated, providing a source for the synthesis of acetyl-CoA in the TCA cycle. Subsequently, the upregulation of Pdhx and downregulation of Pdk2 promoted the conversion of pyruvic acid to acetyl CoA31, which increased the abundance of citric acid, α-Ketoglutaric acid, fumaric acid and malic acid, indicating the enhancement of TCA cycle. In addition, the 6-PG and sedhepyulose 7-phosphate (S7P) in the PPP pathway were reduced, and the related genes, including G6pdx, Pgd and Shpk, were also downregulated, demonstrating the decrease of PPP. This data revealed that energy metabolism is crucial for the development of the fetal heart in late gestation, and the TCA cycle was activated at E18.5 before birth.
Enhanced fatty acid metabolism in the liver and stomach during the E16.5 to E18.5 stage
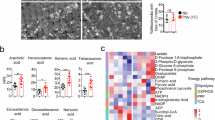
The digestive organ is one of the organ types with the greatest changes in functional environment before and after birth, as the body needs to convert transports nutrients from the placenta before birth to self-digest milk rich in fatty acids and protein after birth28. The stomach and liver are the main digestive organs developed from the endoderm, and it is currently unclear how they develop in late gestation to adapt to the new environment after birth in terms of metabolic function. The abundance of the main differential metabolites in the stomach was mainly increased at E18.5, while the metabolites in the liver were decreased (Supplementary Fig. 2a), suggesting the changes in organ metabolic function. OPLS-DA also showed that the samples from the stomach and liver at E16.5 and E18.5 had different metabolic patterns (Fig. 3a, b). To further investigate these changes in the stomach and liver, we screened the top 20 metabolites in these two digestive organs. The enrichment analysis showed the biosynthesis of unsaturated fatty acids was involved in both the stomach and liver (Fig. 3c, d), indicating fatty acid metabolism is an important characteristic of the developmental metabolism of the digestive organs. In addition, we found many fatty acids that are closely related to the generation and metabolism of unsaturated fatty acids showed relatively high levels in the stomach and liver. Among them, the abundances of linoleic acid, arachidonic acid, vaccenic acid and adrenic acid were higher in the stomach than those in other organs (Figs. 1d, 3e), and the 12S-HHT level was also relatively high in the stomach and liver (Fig. 3e). However, the abundances of these metabolites were reduced at E18.5, indicating that the maturation process of digestive function in late gestation is accompanied by the upregulation of fatty acid metabolism, especially the catabolism of fatty acids. Dephospho-CoA is an intermediate in pantothenate and CoA biosynthesis32, it increases sharply at E18.5 in the stomach, indicating that the metabolic activity of acetyl coenzyme may be enhanced (Fig. 3e). Notably, we found bilirubin, which can usually cause jaundice in newborns33, was highly abundant in the liver and was decreased from E18.5 (Fig. 3e), demonstrated that the fetal liver may start to decompose bilirubin at E18.5 and this metabolic dysfunction of bilirubin in liver may cause the neonatal jaundice after birth.
a, b OPLS-DA analysis of stomach (a) and liver (b) at E16.5 and E18.5 (n = 6 per group). c, d The KEGG enrichment of stomach (c) and liver (d). The pathways enriched by top 20 differential metabolites from E16.5 to E18.5. The color of the bubble represents the value of −log10 (p-value). e The log10(abundance) of characteristic metabolites at E15.5, E16.5, E18.5, E19.5 (The sample sizes were: n = 5 (heart and stomach at E15.5), n = 7 (heart at E16.5), and n = 6 (all other groups). f, g Volcano diagram of differential proteins in stomach (f) and liver (g) at E16.5 and E18.5 (n = 3 per group). h, i KEGG enrichment analysis of upregulated differential proteins in stomach (h) and liver (i) at E16.5 and E18.5. j The abundance of proteins in stomach and liver at E16.5 and E18.5 (n = 3 per group, only statistically significant (p < 0.05) p-values were displayed). k Schematic diagram of fatty acid metabolism pathway (created with Biorender). The upregulated proteins were marked by red oval shape. The significance was analyzed by Student’s t-test, error bars represent mean ± SD.
To further understand the metabolic fluctuations in these two organs during E16.5 to E18.5, we analyzed the data from reported proteomic studies on the stomach and liver of mice at E16.5 and E18.534,35. The protein pattern also showed a significant difference between E16.5 and E18.5 (Supplementary Fig. 6a, b). Compared with E16.5, most of the differential proteins in the stomach were upregulated in E18.5, including prochymosin (CYM), PGA5, and FABP5 (Fig. 3f). High levels of pepsinogen CYM, and pepsinogen enzyme PGA5 in the stomach play an important role in milk protein digestion, FABP5 binds to hydrophobic ligands such as long-chain fatty acids and plays a key role in lipid-related metabolic processes36. While most differential proteins in liver were also upregulated at E18.5 and the downregulated proteins were rare (Fig. 3g). Similar to FABP5 upregulated in the stomach, FABP1 upregulated in the liver also plays a critical role in the metabolism of fatty acids in the digestive system37. LDHA and ALDOB are mainly related to carbohydrate metabolism, and ASS1 was an important rate-limiting factor for the generation of arginine38, which was upregulated in the liver at E18.5. Enrichment analysis of upregulated proteins at E18.5 showed that the biosynthesis of amino acids, fatty acid degradation, TCA cycle, and others were all involved in both stomach and liver (Fig. 3h, i); these data suggest that the enhancement of the metabolism of protein and fatty acids in stomach and liver from E18.5.
The metabolic and protein data indicate that fatty acid metabolism and amino acid metabolism were enhanced in both the liver and stomach during the E16.5 to E18.5 stage. Most unsaturated fatty acids were downregulated at E18.5, such as linoleic acid and arachidonic acid (Figs. 1d, 3e), which may be due to their full utilization in oxidative phosphorylation processes. The key proteins involved in the β oxidation of fatty acids, including ACOT1, HADHA, HADHB and others, also showed upregulated at E18.5 in both the stomach and liver (Fig. 3j, k). The increased activity of proteins in fatty acid metabolism at E18.5 also indicates that the primary metabolic alterations in the digestive organs during this stage involve the oxidative phosphorylation of fatty acids. Combining metabolomics and proteomics data, we found that the E16.5 to E18.5 stage was a critical metabolic time window for the liver and stomach to possess certain catabolic functions, and the maturation process of this function is closely related to fatty acid oxidation (FAO) as well as amino acids, preparing for the function of digesting milk after birth (Fig. 3k).
Differences and commonalities in metabolic characteristics between the brain and placenta of the fetus
The brain is the center of the nervous system from the ectoderm, while the placenta from the extraembryonic tissue is the site of material exchange between mother and fetus; they are totally different organs in function and have their own metabolic characteristics39,40. However, we found differential metabolites from E15.5 to E19.5 mainly enriched in purine and pyrimidine metabolism in the brain and placenta (Supplementary Figs. 2a, 3d, 3e). The abundances of metabolites related to these two pathways in the brain and placenta were relatively low, especially in the placenta from E16.5 to E18.5, which may indicate that the brain and placenta gradually stabilize nucleic acids, coenzymes, and signaling molecules involved in purine and pyrimidine metabolism during late gestation (Supplementary Fig. 7a).
The top 20 differential metabolites of the brain and placenta between E16.5 and E18.5 were screened from OPLS-DA analysis (Fig. 4a, b). Pyrimidine metabolism was enriched in both organs, with the overall reduction of metabolites. Notably, we found the differential metabolites were mainly enriched in fatty acid metabolism terms in the brain (Fig. 4c, d). However, these metabolites significantly decreased at E18.5, but were rescued at E19.5, such as palmitoylcarnitine, tetradecanoylcanitine and palmitelaidic acid (Fig. 4e). The role of this sudden downregulation of fatty acid metabolism in the brain at E18.5 needs further investigation. Additionally, guanine in purine metabolism, glutathione and glycine in amino acid metabolism were significant differential metabolites of placenta between E16.5 and E18.5 (Fig. 4e). We found tetradecanoylcanitine and hexanoylcarnitine were also higher in the placenta (Fig. 4f). Previous studies have found that insufficient metabolic stability of acylcarnitine levels and FAO in the placenta during pregnancy may lead to spontaneous premature birth41, thus, the acylcarnitine may act as an important metabolic characteristic of placenta, especially in the late gestation.
a, b OPLS-DA analysis of brain (a) and placenta (b) at E16.5 and E18.5 (n = 6 per group). c, d The KEGG enrichment of brain (c) and placenta (d). The pathways enriched by top 20 differential metabolites from E16.5 to E18.5. The color of the bubble represents the value of −log10 (p-value). e The abundance of differential metabolites in brain and placenta (n = 6 per group). f The log10(abundance) of tetradecanoylcanitine and hexanoylcarnitine in different organs at different developmental times (The sample sizes were: n = 5 (heart and stomach at E15.5), n = 7 (heart at E16.5), and n = 6 (all other groups). g Venn diagram of metabolites with similar abundance between brain and placenta at different developmental stages. h KEGG enrichment analysis of screened similar metabolites between brain and placenta. The color of the bubble represents the value of −log10 (p-value). i The log10(abundance) of carnosine and histidine at E15.5, E16.5, E18.5 E19.5 (The sample sizes were: n = 5 (heart and stomach at E15.5), n = 7 (heart at E16.5), and n = 6 (all other groups). j, k The abundance of carnosine, histidine of brain (j) and placenta (k) at E15.5, E16.5, E18.5, E19.5 (n = 6 per group). l, m Quantitative comparison of carnosine (l) and histidine (m) in mother’s brain (MB) (n = 3), brain (n = 4) and placenta (n = 6) of newborn. The significance was analyzed by Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant). Error bars represent mean ± SD (e, f, i–k) or mean ± SEM (l, m).
Interestingly, we found that the brain and placenta showed partially similar metabolic patterns and metabolic pathways (Fig. 1b, Supplementary Figs. 2a, 3d, 3e). To further identify the common metabolic characteristics between the brain and placenta, we determined that the abundances of 12, 14, 22 and 15 metabolites were similar at different time points in the brain and placenta, respectively (Supplementary Fig. 7b). These metabolites were mainly enriched in the riboflavin metabolism, arginine and proline metabolism pathway (Supplementary Fig. 7c). Additionally, we further screened for common similar metabolites at different developmental times and conducted enrichment analysis on these metabolites, which mainly involve histidine metabolism (Fig. 4g, h). It is worth noting that we found carnosine and histidine in the histidine metabolism pathway had similar abundance in the brain and placenta at E18.5 and E19.5, suggesting the abundance of these two metabolites was stable in the brain and placenta after E18.5 (Fig. 4i–k). Carnosine can be converted to histidine, and then further converted to L-aspartate, ultimately entering the TCA cycle42,43 (Supplementary Fig. 7d). Carnosine is mainly enriched in brain and muscle, and carnosine, as well as histidine, are critical for maintaining brain function, playing antioxidant effects in the brain to protect neural development44. To further verify this data, we detect the abundance of carnosine and histidine in newborn brain and placenta. The result showed that the carnosine level was no significant difference among the newborn brain, placenta and the mother’s brain (MB), while the histidine was also no significant difference between the newborn brain and placenta, but higher than the MB (Fig. 4l, m, Supplementary Data 5). The data indicated that there was no significant difference in the levels of carnosine and histidine in the fetal brain and placenta around the time of delivery. Therefore, the levels of carnosine and histidine in the newborn placenta may be used to assess their levels in the newborn brain.
Discussion
The development of metabolic function is crucial for organ maturation in late gestation and for the fetus to adapt to the postnatal environment. In this study, we investigated the metabolic patterns of major organs developed from different germ layers in mice during late gestation. We also identified the metabolic characteristics of these organs as they developed over time. Untargeted metabolomics and multi-omics analysis demonstrated that the organs underwent significant metabolic transformation before birth, especially from E16.5 to E18.5. In this stage, organs acquire metabolic capacity that matches their physiological function, mainly represented as the enhanced oxidative phosphorylation and fatty acid metabolism in the heart, stomach, and liver, while the amino acid metabolism was also upregulated. The maturation of these metabolic functions prepares the main organs of the fetus to begin digesting fatty acids and proteins in milk after birth. Moreover, we found some common characteristics between the brain and placenta. The abundances of carnosine and histidine were similar in the brain and placenta of newborns, which may serve as an in vitro testing method for the carnosine and histidine levels in the brain of newborns.
Energy metabolism is the fundamental process for the organism. It is reported that long-chain acyl-CoA dehydrogenase increased in the placenta during late gestation, which converts long-chain fatty acids into medium-chain fatty acids and helps to more effectively utilize fatty acids as energy sources45, demonstrating the key role of FAO in energy metabolism in late gestation. The fat accumulated in maternal adipose tissue during early pregnancy can be used for placental metastasis in late gestation to meet the fetal demand for fatty acids45, emphasizing that abnormal FAO is closely associated with maternal and fetal complications during pregnancy. We found the fetal organs from different germ layers underwent a significant metabolic transition from E16.5 to 18.5 and the main characteristic was the change in energy metabolism (Fig. 5). At E18.5, TCA was enhanced in the heart, while unsaturated fatty acids such as linoleic acid and arachidonic acid in the liver and stomach were consumed and utilized for fatty acid beta-oxidation, as the fatty acid degradation related proteins were all upregulated in the liver and stomach. These metabolic characteristics involve fatty acid metabolism and oxidative phosphorylation, indicating that the organs are already prepared for a new energy metabolism pattern after birth.
The heart is a metabolically active organ that is constantly in the process of energy absorption and metabolism. It is reported that the way the heart utilizes energy is closely related to oxygen concentration. Before birth, the energy of heart mainly comes from glycolysis, and after birth, it mainly comes from oxidative phosphorylation29,46. This conversion of energy metabolism may start from mid gestation (E10.5-E13.5), when TCA cycle intermediates are beginning to be highly expressed in the heart, and the ratio of citric acid to pyruvate markers in the heart increases8. The heart undergoes rapid growth during mid-gestation and is about to adapt to an oxygen-rich environment after birth in late gestation, the TCA cycle was further enhanced, which may be due to increased mitochondrial activity in the heart8,47. Studies have also confirmed that inhibiting FAO can promote heart regeneration in adult mice19. We found that glycolytic metabolism was significantly weakened, and fatty acid metabolism was strengthened during E16.5 to E18.5 through metabolomics and transcriptomics in the heart, mostly due to the decrease of G6P and DHAP. G6P is located at the intersection of three different metabolic pathways, and its concentration changes are crucial for the function of the heart. Research has found a correlation between myocardial glucose metabolism disorders, intracellular G6P accumulation, and cardiac contractile dysfunction, and G6P levels are associated with mTOR activation and endoplasmic reticulum stress48. Our multi-omics research showed that the metabolic changes from glycolysis to oxidative phosphorylation, which affect heart function, and the heart adapts to the fetal environment mainly occurred from E16.5 to E18.5 before birth. This may also be an important manifestation of fetal heart maturation.
A critical change in the fetus after birth is the conversion of nutrients supplied by the mother through the placenta to the self-digestion of milk to obtain nutrients and energy. This requires digestive organs such as the stomach and liver to digest and metabolize proteins and fatty acids in milk49. The gastric proteasomes CYM and PGA5 during stomach development were highly expressed in late gestation in mice34. In this study, we found that CYM and PGA5 were significantly increased from E16.5 to E18.5, and the proteins of fatty acid metabolism in stomach and liver were all upregulated at E18.5. Meanwhile, the enzymatic hydrolysis of fatty acids and metabolite decomposition functions in the liver had matured, and the proteins related to the biosynthesis of unsaturated fatty acids and oxidative phosphorylation were upregulated in the stomach and liver. This demonstrates that the digestive function of the mouse stomach and liver begins to rapidly improve at E18.5. Although the abundance of arachidonic acid and linoleic acid was high in the stomach and liver compared to other organs (Figs. 1d, 3e), these metabolites decreased at E18.5, possibly due to continuous consumption into FAO. Notably, studies found that in newborns, jaundice is caused by the accumulation of unconjugated bilirubin in the blood and tissues33. Bilirubin is an important antioxidant in the body, and its metabolism is related to physiological functions. We found that bilirubin decreased from E16.5 to E18.5, indicating that the liver had begun to exert its decomposing function for bilirubin at this stage. The abnormal development of liver metabolic function during this stage may lead to the occurrence of jaundice in newborns.
The abundance of metabolites in the brain and placenta was relatively low compared to other organs, and the relative homeostasis of their metabolic levels may be related to the maturity of organ development. Embryonic neocortical neural precursor cells synthesize and secrete a large amount of lactic acid through anaerobic glycolysis, stimulating vascular growth and regulating mitochondrial morphology to maintain their symmetrical proliferation and division in the mid-gestation17. In our study, we found fatty acid metabolism-related metabolites, such as acylcarnitine, in the brain were significantly reduced at E18.5, but recovered at E19.5, the physiological effects of which were unknown. Moreover, the metabolism and oxidative capacity of fatty acids in the placenta may affect the rate and composition of lipids delivered to the fetus50. Our study found that the placenta was rich in acylcarnitine substances in late gestation, which may be a sustained supply of fetal lipid metabolism. Specially, we found that the abundance of carnosine and histidine was consistent in these two organs after E18.5. Carnosine is crucial for brain development and is closely related to the occurrence of various neurological diseases51. This finding may provide a new method for clinical evaluation of fetal brain development status through placental metabolite detection.
In addition, the metabolism of amino acids such as alanine, aspartate and glutamate was also increased during late gestation, which correlated with the enhancement of the oxidative phosphorylation in mitochondria (Fig. 5). Interestingly, we found that the abundance of 4-pyridoxic acid increased in all of organs at E18.5. 4-pyridoxic acid is the main metabolic decomposition product of pyridoxine in vitamin B6 metabolic pathway52, which is also strengthened in all organs, indicating an increase in the intake of pyridoxine in the body. These may be related to the enhanced metabolism of amino acids, lipids and carbohydrates, and prepare for metabolizing proteins in milk after birth.
It should be noted that there may be some potential limitations in this study. To enhance the robustness of our findings, we incorporated publicly available protein datasets from prior studies. However, the absence of appropriate experimental controls in these supplementary datasets may limit the interpretation of results, which represents a common challenge in multi-omics research integration. Future studies will incorporate molecular validation experiments (e.g., targeted proteomics) and transcriptomic or multi-omics cross-validation to further substantiate these findings. Furthermore, our study did not explore potential metabolic differences between sexes, which represents an important avenue for future research.
In summary, by quantifying the metabolic landscape and conducting multi-omics analysis, we have constructed the comprehensive and characteristic metabolic patterns of the main fetal organs developed from different germ layers in late gestation. The period from E16.5 to E18.5 is identified as the critical time window for the metabolic development of fetal organs. During this stage, the main organs undergo a transition in metabolic pattern, including energy metabolism and amino acid metabolism, to establish the metabolic basis for the fetus’s adaptation to the postnatal environment. Our multi-omics dataset provides a comprehensive metabolic landscape of mammalian organ development during late gestation.
Methods
Animals
All animal experiments were approved by the Tongji University Animal Research Committee (TJAA06420101), and we have complied with all relevant ethical regulations for animal use. All ICR mice were housed in a pathogen-free environment (temperature 20–26 °C, humidity 30–70%) and group housed in a standard 12 h:12 h light–dark cycle. Healthy 8–10-week-old, naive pregnant female mice undergo mating. The following morning, displaying vaginal plugs were identified as pregnant and recorded the gestation time.
Samples collection
All pregnant mice at E15.5, E16.5, E18.5, and E19.5 were euthanized by cervical dislocation following isoflurane anesthesia, with death confirmed by apnea and cardiac arrest. Then, cut out the uterus with microscopic scissors, rinsed with phosphate-buffered saline (PBS), first removed the placenta intact, then removed the mouse embryo, and rinsed with PBS. The brain, heart, liver, and stomach were removed and preserved.
Metabolomic analysis
For non-targeted metabolomics analysis, we collected at least 5 samples from distinct embryonic organs at varying developmental stages to ensure comprehensive coverage of metabolic profiles. Transferred the thawed samples of each tissue to a tissue grinder (WHEATON, 357538), and added 100 μL cooled water. After thorough grinding, added 400 μL of chloroform-methanol (1:1) mixed solution, carefully ground the mixture, and transferred to a new tube. Then vortexed the tube for 30 s to mix it thoroughly. Centrifuged at 13,000×g at 4 °C for 20 min, transferred the upper layer to a new tube. Prepared quality control samples (QC) by uniformly mixing 5 μL of each sample for quality control of omics data. All samples were dried with nitrogen.
50 μL water-methanol (1:1) mixed solution was added to each dried sample, and vortexed and mixed for 20 s, and centrifuged at 13,000×g for 15 min at 4 °C. The solution after high-speed centrifugation was transferred to an injection bottle containing an inner liner for testing. Instrument information: Ultimate 3000 (UPLC, Thermo Fisher)-timsTOF (Bruker). Chromatographic column: Waters BEH C18 (100 mm * 2.1 mm), injection volume: 3 μL, flow rate: 0.4 mL/min, column temperature: 40 °C. The mobile phase consists of 0.05% formic acid water (A) and 0.05% acetonitrile (B). The mass spectrometry parameters: ion source type: ESI ion, polarity: positive/negative, capillary tube: 4500 volts (+)/3500 volts (-), nebulizer: 2.0 Bar, dry gas: 8.0 L/min, drying temperature: 200 °C, calibration solution: sodium formate, quality scanning range: 20–1050, scanning mode: automatic MS/MS. To minimize batch-to-batch variation, all metabolomic analyses were performed in a single experimental batch under identical analytical conditions. Before sample analysis, the QC sample was injected six times and then injected at regular intervals (every 10 samples) to monitor the stability of the system. QC samples refer to composite samples made by mixing 5 μL of each sample.
The raw MS data were first converted to MzML files using RawConverter (Scripps Research Institute) and then subjected to peak picking (mz diff = 20 ppm) and grouping (bw = 5, mz window = 0.025, minfrac = 0.2) with MS-DIL software (version 3.0) based on the endogenous metabolite database in HMDB. The assigned metabolites were further annotated by matching the isotopic abundance ratio (12C/13C approximately 1.003) and MS/MS spectra information. All experimental raw data were publicly available through the Metabolights database (accession number MTBLS12560).
Quantitative analysis of targeted metabolites in the heart
Merging 20 mg of heart samples at the same stage, and preparing 3 biological replicate samples at each stage. Adding two small steel balls and 500 μL extraction solution (methanol-water, 3:1 volume ratio) to each sample, vortexing for 30 s, mixing well, and grinding at 40 Hz for 4 min. Ultrasound under ice water bath conditions for 5 min, and the grinding ultrasonic steps were repeated three times. After grinding, the sample was left to stand at −40 °C for 1 h, centrifuged at 4 °C and 12,000 rpm for 15 min. 400 μL of the supernatant was transferred to an EP tube and vacuum spin dried. Then, 150 μL of pure water was added to re-dissolve the sample, which was then filtered, and the supernatant was taken to an injection bottle for LC-MS analysis.
Accurately weighed the corresponding amount of standard substance into a 10 mL volumetric flask and prepared 10 mmol/L of standard stock solution separately. Taken the corresponding amount of standard stock solution into a 10 mL volumetric flask, prepared a mixed standard solution, and sequentially diluted the standard solution to obtain a series of calibration solutions (the list of targeted metabolites shown in Supplementary Data 2). This experiment used Thermo Scientific Dionex ICS-6000 HPIC (Thermo Scientific) high-efficiency ion chromatograph with a protective column of AG11-HC RFIC, 2 × 50 mm, The separation column is AS11-HC RFIC (2 × 250 mm). The A-phase of ion chromatography was a 100 mM NaOH aqueous solution, and phase D was pure water. Add methanol containing 2 mM acetic acid with a flow rate of 0.15 mL/min after the column. The temperature of the column incubator is 30 °C, the temperature of the sample tray was set to 4 °C, and the injection volume was 5 μL. Mass spectrometry analysis used 6500 Qtrap mass spectrometer for Multiple Reaction Monitoring (MRM). The ion source parameters were as follows: capillary voltage = −4500 V, source temperature = 450 °C, gas1 = 45 psi, gas2 = 45 psi, CUR = 30 psi. After optimizing the MRM parameters, selected the ion pair with the best response for quantitative analysis. Following this, 56 metabolites were extracted from the original data, and individual metabolites were filtered for further analysis. After preprocessing, a total of 51 metabolites were retained in the experimental sample.
Pregnant mouse infusions
In this study, labeled isotopic reagents were administered through the tail vein of mice. The labeling solution consisted of 2.11 mg of 13C glucose (D-Glucose, U-13C6, Cambridge Isotopes, CLM1396-1) dissolved per gram of pregnant mouse weight in 650 μL of physiological saline. Before undergoing in vivo labeling, pregnant mice were fasted for 8 h. Labeling method: After pre-anesthesia (Isoflurane, Reward) of pregnant mice, a respiratory anesthesia machine was placed on the mouth of the pregnant mice, and the pregnant mice were continuously anesthetized with a low flow rate. The isotope labeling solution was injected through the tail vein. Infusion rate: 120 μL/min: 1 min, 150 μL/h: 3.5 h, with a total infusion of approximately 650 μL (experimental schematic shown in the Supplementary Fig. 5a). After about 3.5 h of infusion, it was stopped immediately, and the fetal heart was dissected. Collected 5 labeled samples in each group for metabolite extraction and performed metabolomics analysis. The labeled metabolites and isotopologue distributions were extracted using EI-Maven software, and the accucor R packages were applied to correct the natural abundance of labeled metabolites. Then, the percentage of labeled metabolites out of all labeled forms will be calculated to assess the relative changes of labeled metabolites. The significant differences between E16.5 and E18.5 fetal hearts were analyzed by Student’s t-test.
RNA-seq
Three heart samples were collected in E16.5 and E18.5, respectively. Extract total RNA from the sample using Trizol reagent (Thermo Fisher, 15596018). Use Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent) to analyze the total RNA content and purity in the sample, and construct a sequencing library using high-quality RNA samples with RIN numbers >7.0. After extracting the total RNA, purify the mRNA from the total RNA (5 μg) for two rounds of purification. After purification, the mRNA fragments are divided into short segments at high temperature. Then, the cleaved RNA fragments are reverse transcribed using reverse transcriptase to generate cDNA, which is then used for E. coli DNA polymerase I (NEB, m0209), RNase H (NEB, m0297) and dUTP solution (Thermo Fisher, R0133) to synthesize U-labeled double-stranded DNA. Use magnetic beads to select fragment sizes. PCR amplification of the connecting product was performed under the following conditions: initial denaturation at 95 °C for 3 min, 8 times of denaturation at 98 °C for 15 s, annealing at 60 °C for 15 s, extension at 72 °C for 30 s, and finally, extension at 72 °C for 5 min. The average insertion fragment size of the final cDNA library is 300 ± 50 bp, and the library was sequenced by using Illumina Novaseq™ 6000 underwent paired-end mode. Differential expression genes (DEGs) analysis was performed between two groups using DESeq2 software (and by edgeR between two samples). The genes with the parameter of false discovery rate (FDR) below 0.05 and absolute log2 fold change ≥1 were considered differentially expressed genes. DEGs were then subjected to enrichment analysis of Gene Ontology (GO) functions and KEGG pathways. The raw RNA-seq data from heart tissues in this study have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE299066.
Quantitative analysis of carnosine and histidine in newborns
We collected placenta and brain samples from newborn mice, as well as brain tissue samples from pregnant mice. After washing the sample with PBS, add PBS according to a weight to volume ratio of 1:9. Homogenized the tissue, centrifuged and collected the supernatant for testing. Used the mice carnosine ELISA kit instruction (SAIPEISEN BIOLOGY) and mice histidine ELISA kit instruction (SAIPEISEN BIOLOGY) for analysis and detection.
Statistics and reproducibility
To account for potential metabolite concentration variations due to tissue weight differences, we performed weight-based normalization by adjusting metabolite peak intensities relative to organ weight using the sample-specific normalization approach in MetaboAnalyst 5.0 tool (https://www.metaboanalyst.ca/). This enabled the expression of metabolite quantities per milligram of tissue. The metabolite matrix was subjected to log10 transformation to eliminate heterogeneity between samples. The Pareto scaling was performed to reduce the relative importance of large values. Then, the PCA is used to determine the distribution between QC and organizational samples to assess the quality of metabolomics data. The PLS-DA was performed to determine the distribution characteristics and metabolic differences among multiple tissues, while OPLS-DA was applied to determine the metabolic differences between two sample groups. These analyses are achieved through MetaboAnalyst.
The differential metabolites during developmental processes were determined based on the variable important in projection (VIP) values from PLS-DA analysis among multiple groups and OPLS-DA analysis among two groups (select the top 20 metabolites with the highest VIP values). Meanwhile, the pathway enrichment analysis of metabolites was conducted by MetaboAnalyst to identify the metabolic pathways that exhibit significant changes during organ development. In addition, differential proteins were screened (fold change ≥ 2, p < 0.05, VIP > 1) of stomach and liver proteomic data in the E16.5 and E18.5 from the published data35, and the biological pathway was analyzed by GO enrichment.
Statistical significance was calculated using an unpaired two-tailed Student’s t-test using Prism 10 (GraphPad Prism, San Diego, CA). The visualization boxplot and pathway enrichment results were performed using the ggplot2 R packages (version 4.3.2). Data presented as individual values include mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). Significance representation was *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. The sample sizes indicated represent independent biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The metabolomics raw data have been deposited in the Metabolights database (accession number MTBLS12560, https://www.ebi.ac.uk/metabolights/MTBLS12560). The raw RNA-seq data from heart tissues in this study have been deposited in the Gene Expression Omnibus (accession number GSE299066). All source data in the main figures can be found in Supplementary Data.
References
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
He, P. et al. The changing mouse embryo transcriptome at whole tissue and single-cell resolution. Nature 583, 760–767 (2020).
Diaz-Cuadros, M. et al. Metabolic regulation of species-specific developmental rates. Nature 613, 550–557 (2023).
Zhang, J., Nuebel, E., Daley, G. Q., Koehler, C. M. & Teitell, M. A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11, 589–595 (2012).
Zhao, J. et al. Dynamic metabolism during early mammalian embryogenesis. Development 150, dev202148 (2023).
Zhao, J. et al. Metabolic remodelling during early mouse embryo development. Nat. Metab. 3, 1372–1384 (2021).
Zhang, L. et al. Low-input lipidomics reveals lipid metabolism remodelling during early mammalian embryo development. Nat. Cell Biol. 26, 278–293 (2024).
Solmonson, A. et al. Compartmentalized metabolism supports midgestation mammalian development. Nature 604, 349–353 (2022).
Rabaglino, M. B., Keller-Wood, M. & Wood, C. E. Transcriptomics of the late gestation ovine fetal brain: modeling the co-expression of immune marker genes. BMC Genomics 15, 1001 (2014).
Agnew, M. E., Slack, J., Stefanovski, D., Linton, J. K. & Sertich, P. L. Sonographic appearance of the late gestation equine fetal intestine. Theriogenology 138, 121–126 (2019).
Perez-Ramirez, C. A. et al. Atlas of fetal metabolism during mid-to-late gestation and diabetic pregnancy. Cell 187, 204–215.e214 (2024).
Ceasrine, A. M. et al. Maternal diet disrupts the placenta-brain axis in a sex-specific manner. Nat. Metab. 4, 1732–1745 (2022).
Miao, H. et al. Lipidome atlas of the developing heart uncovers dynamic membrane lipid attributes underlying cardiac structural and metabolic maturation. Research 2022, 0006 (2022).
Iruretagoyena, J. I. et al. Metabolic gene profile in early human fetal heart development. Mol. Hum. Reprod. 20, 690–700 (2014).
Aye, I., Aiken, C. E., Charnock-Jones, D. S. & Smith, G. C. S. Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am. J. Obstet. Gynecol. 226, S928–s944 (2022).
Bulusu, V. et al. Spatiotemporal analysis of a glycolytic activity gradient linked to mouse embryo mesoderm development. Dev. Cell 40, 331–341.e334 (2017).
Dong, X. et al. Metabolic lactate production coordinates vasculature development and progenitor behavior in the developing mouse neocortex. Nat. Neurosci. 25, 865–875 (2022).
Ivy, J. R. et al. Glucocorticoids regulate mitochondrial fatty acid oxidation in fetal cardiomyocytes. J. Physiol. 599, 4901–4924 (2021).
Li, X. et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 622, 619–626 (2023).
Gu, Y. et al. Multi-omics profiling visualizes dynamics of cardiac development and functions. Cell Rep. 41, 111891 (2022).
Knaus, L. S. et al. Large neutral amino acid levels tune perinatal neuronal excitability and survival. Cell 186, 1950–1967.e1925 (2023).
Mosca, F. & Giannì, M. L. Human milk: composition and health benefits. Pediatr. Med. Chir. 39, 155 (2017).
Broeckling, C. D. et al. Current practices in LC-MS untargeted metabolomics: a scoping review on the use of pooled quality control samples. Anal. Chem. 95, 18645–18654 (2023).
Akiyama, T. et al. Pyridoxal 5’-phosphate, pyridoxal, and 4-pyridoxic acid in the paired serum and cerebrospinal fluid of children. Clin. Chim. Acta 472, 118–122 (2017).
Obeid, R., Geisel, J. & Nix, W. A. 4-Pyridoxic acid/pyridoxine ratio in patients with type 2 diabetes is related to global cardiovascular risk scores. Diagnostics 9, 28 (2019).
Ulvik, A. et al. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 100, 250–255 (2014).
Demmelmair, H. & Koletzko, B. Lipids in human milk. Best. Pract. Res. Clin. Endocrinol. Metab. 32, 57–68 (2018).
Andreas, N. J., Kampmann, B. & Mehring Le-Doare, K. Human breast milk: a review on its composition and bioactivity. Early Hum. Dev. 91, 629–635 (2015).
Chen, X. et al. Metabolic reprogramming: a byproduct or a driver of cardiomyocyte proliferation? Circulation 149, 1598–1610 (2024).
Kumar, S. U. et al. An extensive computational approach to analyze and characterize the functional mutations in the galactose-1-phosphate uridyl transferase (GALT) protein responsible for classical galactosemia. Comput. Biol. Med. 117, 103583 (2020).
Wang, C. et al. Targeting PDK2 rescues stress-induced impaired brain energy metabolism. Mol. Psychiatry 28, 4138–4150 (2023).
He, W., Berthiaume, J. M., Previs, S., Kasumov, T. & Zhang, G. F. Ischemia promotes acyl-CoAs dephosphorylation and propionyl-CoA accumulation. Metabolomics 19, 12 (2023).
Hansen, T. W. R., Wong, R. J. & Stevenson, D. K. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol. Rev. 100, 1291–1346 (2020).
Li, X. et al. A time-resolved multi-omic atlas of the developing mouse stomach. Nat. Commun. 9, 4910 (2018).
Gong, T. et al. A time-resolved multi-omic atlas of the developing mouse liver. Genome Res. 30, 263–275 (2020).
Guo, Y. et al. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 12, 7094 (2021).
Yan, T. et al. Intestinal peroxisome proliferator-activated receptor α-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology 77, 239–255 (2023).
Lin, R. et al. CLOCK acetylates ASS1 to drive circadian rhythm of ureagenesis. Mol. Cell 68, 198–209.e196 (2017).
Barros, L. F., Ruminot, I., Sandoval, P. Y. & San Martín, A. Enlightening brain energy metabolism. Neurobiol. Dis. 184, 106211 (2023).
Vaughan, O. R. & Fowden, A. L. Placental metabolism: substrate requirements and the response to stress. Reprod. Domest. Anim. 51, 25–35 (2016).
Elshenawy, S. et al. The metabolomic signature of the placenta in spontaneous preterm birth. Int. J. Mol. Sci. 21, 1043 (2020).
Brosnan, M. E. & Brosnan, J. T. Histidine metabolism and function. J. Nutr. 150, 2570s–2575s (2020).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Berezhnoy, D. S. et al. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids 51, 139–150 (2019).
Bartha, J. L., Bugatto, F., Fernandez-Deudero, A., Fernandez-Macias, R. & Perdomo, G. Tissue specific expression of human fatty acid oxidation enzyme genes in late pregnancy. Lipids Health Dis. 15, 200 (2016).
Tan, C. M. J. & Lewandowski, A. J. The transitional heart: from early embryonic and fetal development to neonatal life. Fetal Diagn. Ther. 47, 373–386 (2020).
Sakamoto, T. et al. A critical role for estrogen-related receptor signaling in cardiac maturation. Circ. Res. 126, 1685–1702 (2020).
Kundu, B. K. et al. Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis. Cardiology 130, 211–220 (2015).
He, X., McClorry, S., Hernell, O., Lönnerdal, B. & Slupsky, C. M. Digestion of human milk fat in healthy infants. Nutr. Res. 83, 15–29 (2020).
Powell, T. L. et al. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 158861 (2021).
Boldyrev, A. A., Aldini, G. & Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 93, 1803–1845 (2013).
Ueland, P. M., McCann, A., Midttun, Ø & Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 53, 10–27 (2017).
Acknowledgements
This research was funded by the National Natural Science Foundation of China (82271644, 82273465), Natural Science Foundation of Shanghai (24ZR1471400), Special Project of Family Planning (20JSZ01), Funding for health services, Military Medical Research Project (BKJ21J007), Natural Science Foundation of Hebei (H2024206423). The workflow, experimental design and metabolic pathway diagrams in the figures were created in BioRender.
Author information
Authors and Affiliations
Contributions
Jinfeng Xue, Hongli Yan and Zhigang Xue designed and supervised the study. Chanyi Li, Wuping Liu, Xiaodong Li, Bianling Xu and Jing Xu collected and prepared the sample. Chanyi Li and Wuping Liu designed the study and performed computational analyses. Chanyi Li, Wuping Liu and Jinfeng Xue wrote the manuscript. Bo Lv and Ning Yi participated in drafting the manuscript and experimental design. Jiaying Qin performed statistical analysis and participated in drafting the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editors: Zheng-Jiang Zhu and Joao Valente.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Liu, W., Li, X. et al. Metabolomic landscape of fetal organ development during late gestation in mice. Commun Biol 8, 1423 (2025). https://doi.org/10.1038/s42003-025-08820-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08820-3