Abstract
Edwardsiella piscicida T3SS protein EseJ plays a dual role. As a regulator, EseJ suppresses type 1 fimbriae, inhibiting macrophage apoptosis stimulated by FimH. As an effector, it promotes E. piscicida replication in host cells. In this study, the mechanism of EseJ in converting apoptotic cell rounding to pyroptotic cell rupture in murine macrophages was explored. Overexpressing EseJ in the ΔfimH strain suppresses pyroptosis in murine macrophages by deactivating the caspase-8 and NLRP3 inflammasome. This is evidenced by pretreatment with the pharmacological inhibitors z-IETD-fmk (a caspase-8 inhibitor) and MCC950 (an NLRP3 inhibitor), or by knocking down caspase-8 and NLRP3. This finding lends support to the idea that EseJ is involved in the inhibition of pyroptosis. Furthermore, EseJ translocation significantly increased the phosphorylation of TAK1, while reducing necroptosis, which is driven by the activation of receptor-interacting protein kinase 1 (RIPK1) and the phosphorylation of mixed lineage kinase domain-like (p-MLKL). Therefore, EseJ inhibits PANoptosis (pyroptosis, apoptosis and necroptosis) by activating TAK1 and disrupting the formation of the NLRP3-caspase-8-RIPK1 PANoptosome. Interestingly, E. piscicida is attenuated upon EseJ depletion, especially in the early phase after infection. These findings reveal a new way in which E. piscicida circumvents host cell death pathways, thereby increasing its virulence.
Similar content being viewed by others
Introduction
Edwardsiella piscicida PPD130/91, formerly known as Edwardsiella tarda PPD130/91, is a Gram-negative intracellular pathogen that causes haemorrhagic septicaemia in fish1,2. This bacterium is also emerging as an infectious agent in humans3,4. The adhesion, invasion and replication of E. piscicida in the host depend mainly on its type 1 fimbria, its type III secretion system (T3SS) and its type VI secretion system (T6SS)5,6,7,8.
Fimbriae are short, hair-like appendages on the surface of bacteria that mediate the adhesion of Gram-negative bacterial pathogens to host cells. They also trigger host cell signaling pathways that induce innate immune responses9,10. The Type 1 fimbriae play a key role in the adhesion and invasion of E. piscicida, and are negatively regulated by the T3SS protein EseJ5,11,12. The T3SS is a nanomachine located on the surface of bacteria through which specialized bacterial effectors are delivered directly into the host13. The assembly of the EscE-EsaG-EsaH complex and the secretion of the T3SS needle protein EsaG switch on the secretion or translocation of T3SS effectors in E. piscicida14. Once they are delivered to the host cytoplasm, the effectors initiate and maintain the infection process by manipulating the host cell’s biology15. The T3SS effector proteins EseG, EseJ, EseH, EseK, Trx2, and YfiD have been investigated in E. piscicida16,17,18,19,20,21. EseJ is unique among them in that it is a bifunctional protein, acting as both an effector and a regulator11,17. As an effector, EseJ promotes the replication of E. piscicida in vivo by inhibiting the production of reactive oxygen species (ROS) and by blocking endocytic trafficking to lysosomes17,22. As a regulator, EseJ suppresses the transcription of type 1 fimbriae, thereby reducing the adhesion and invasion of E. piscicida into host cells mediated by the FimH fimbrillum11. In addition, EseJ suppresses apoptosis by down-regulating type 1 fimbriae, which would otherwise stimulate apoptosis mainly via the extrinsic pathway by activating caspase-8 and caspase-323.
Bacterial infection typically induces cell death, which was initially thought to be a strategy for pathogens to eliminate host immune cells. Nevertheless, programmed cell death (PCD) is now widely accepted as a vital process in the host’s immune defence against invading pathogens24. The three PCD pathways are apoptosis, pyroptosis and necroptosis. Apoptosis is initiated by either caspase-8 or caspase-9, which both culminate in the activation of executioner caspase-325,26. Pyroptosis is stimulated by either caspase-1 or caspase-4/5/11. Both pathways lead to the cleavage of the gasdermin (GSDM) family of proteins, resulting in the formation of GSDM pores and the release of IL-1β or IL-1827,28. Necroptosis is another lytic cell death pathway initiated by the necroptosome. This contains the executioner of necroptosis, mixed lineage kinase domain-like (MLKL), as well as receptor-interacting protein kinase 1 and 3 (RIPK1 and RIPK3)29,30. During infection, many pathogens are capable of stimulating one or more of these three pathways of PCD. Yersinia and Salmonella can induce all three types of PCD, known as PANoptosis, in the same cell population31,32. PANoptosis is a distinct form of PCD involving inflammation. It is activated by specific triggers and regulated by the PANoptosome complex. The PANoptosome complex is a molecular scaffold that enables the simultaneous interaction of essential components of pyroptosis (the inflammasome adaptor protein ASC and the NLRP3 protein), apoptosis (the caspase-8 protein) and necroptosis (the RIPK1 and RIPK3 proteins)31,33,34,35. The molecular composition of the PANoptosome, as well as the phenotypic consequences of crosstalk and co-regulation in PANoptosis, depend on the stimuli present.
Caspase-8 was originally identified as the initiating caspase in extrinsic apoptosis. Active caspase-8 then transmits death signals to caspase-3, which subsequently activates the process of apoptotic cell death25. Although caspase-8 is known for its role in promoting apoptosis, it has also been associated with the initiation of necroptosis and pyroptosis36,37. Caspase-8 can cleave both RIPK1 and RIPK3, thereby preventing the initiation of necroptosis38. Caspase-8 can also cleave the pyroptosis executioner Gasdermin D (GSDMD) directly at aspartate 88 (D88)37. In addition, caspase-8 can interact with ASC, NLRP3, RIPK1, and RIPK3 and activate the PANoptosome to initiate PANoptosis. In addition, caspase-8 interacts with ASC, NLRP3, RIPK1, and RIPK3, activating the PANoptosome and initiating PANoptosis31,34. Thus, the critical function of caspase-8 in pyroptosis, apoptosis, and necroptosis (PANoptosis) is tightly controlled. Transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) has also been characterized as a regulator of PANoptosis, which is initiated under various conditions39,40. Pharmacological or pathogenic inhibition of TAK1 can promote PANoptotic cell death, which involves the following proteins: caspase-8, RIPK1, RIPK3, MLKL, NLRP3, caspase-1 and GSDMD31,41,42,43.
Bacterial T3SS effectors use various mechanisms to prevent the PANoptotic suicide response in host cells. The Yersinia T3SS effector YopJ inhibits TAK1 activity, thereby stimulating the death of inflammatory cells via the activation of the NLRP3 inflammasome, as well as the activation of caspases 1 and 3, and MLKL37,41,43. Yersinia induces PANoptosis by activating the PANoptosome, which contains RIPK1, RIPK3, caspase-8, ASC, FADD, and NLRP331. The Salmonella T3SS effector SopF inhibits both pyroptosis and apoptosis, promoting necroptosis instead by activating phosphoinositide-dependent protein kinase-1 (PDK1), which then phosphorylates p90 ribosomal S6 kinase (RSK). This process down-regulates the activation of caspase-832.
The wild-type strain of E. piscicida, but not the T3SS mutant, promotes the up regulation of anti-apoptotic genes in macrophages and zebrafish cells44,45. E. piscicida suppresses apoptosis via the EseJ protein by down regulating type 1 fimbriae23. Additionally, E. piscicida inhibits pyroptosis via the T6SS effector EvpP, while promoting it in a T3SS-dependent manner8,46. The T3SS effector that activates or inhibits pyroptosis remain(s) unclear in E. piscicida. Moreover, in our preliminary study, we repeatedly observed that apoptotic cell rounding at the initial stage post-infection was followed by pyroptotic cell rupturing in murine macrophages infected with the ΔeseJ strain. The way in which EseJ controls these two (or more) modes of cell death remains unclear. Through this study, we revealed that the T3SS-translocated protein EseJ suppresses pyroptosis by deactivating caspase-8 and impairing the assembly of the NLRP3 inflammasome. Furthermore, EseJ inhibits necroptosis and activates TAK1, which in turn inhibits PANoptosis by disrupting the caspase-8-NLRP3-RIPK1 PANoptosome.
Results
EseJ contributes to the virulence of E. piscicida, particularly during the early post-infection phase
EseJ promotes the replication of E. piscicida in eukaryotic cell lines by suppressing the release of ROS and lysosomal trafficking17,22. In order to investigate the contribution of EseJ to virulence in vivo, naïve blue gourami (wild-type) were injected intramuscularly with similar quantities of E. piscicida WT, ΔeseJ and ΔeseJΔfimA strains (approximately 2.48 × 105 CFU per fish per strain), and their survival curves were compared. Up to three days post infection (dpi), the ΔeseJ and ΔeseJΔfimA strains induced significantly higher cumulative survival rates than the wild-type (WT) strain. Although ΔeseJ-infected fish showed slightly increased survival compared to WT at 4–5 dpi, no differences were observed at 6–7 dpi. Throughout the infection period, the survival curves of the ΔeseJ and ΔeseJΔfimA groups remained statistically indistinguishable (Fig. 1). This indicates that the deletion of fimA in the absence of EseJ did not have an additional effect. These results demonstrate that EseJ significantly increases the virulence of E. piscicida, particularly during the initial stages of infection. This suggests that EseJ may play a role in modulating the innate immune response.
Twenty naive blue gouramis per group were infected intramuscularly with E. piscicida wild-type (WT) strain, ΔeseJ strain or ΔeseJΔfimA strain at ~2.48 × 105 CFU per fish per strain. Survival of blue gourami were monitored for 7 days. Means ± SEM of three independent experiments are shown. *P < 0.05; **P < 0.01; ***P < 0.001. ΔeseJ VS WT, red asterisks; ΔeseJΔfimA VS WT, blue asterisks.
The T3SS-translocated protein EseJ suppresses pyroptosis in murine macrophages
In order to investigate the phenotype of EseJ, J774A.1 murine macrophage cells were infected with E. piscicida strains expressing GFP from the plasmid pFPV25.1 at a multiplicity of infection (MOI) of 10. At 3 h post infection (hpi), J774A.1 cells infected with the ΔeseJ strain exhibited significant cell swelling and membrane rupture (Fig. 2a). Propidium iodide (PI) staining, which indicates loss of membrane integrity, revealed a significantly higher percentage of PI-positive cells in ΔeseJ-infected cells (29.8 ± 6.1%) than in the WT strain (15.8 ± 4.0%) or the complemented strain ΔeseJ[eseJ] (8.9 ± 3.0%) (Fig. 2a, b). The release of lactate dehydrogenase (LDH), a marker of cytolysis, was consistently higher in ΔeseJ-infected cells (26.9 ± 2.3%) than in WT-infected cells (14.3 ± 1.6%), and returned to normal levels in ΔeseJ[eseJ]-infected cells (21.2 ± 0.6%) (Fig. 2c). These data suggest that EseJ inhibits cytolytic cell death in J774A.1 cells.
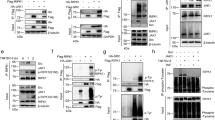
a Confocal images of J774A.1 cells infected with E. piscicida strains. J774A.1 cells were infected with WT/GFP, ΔeseJ/GFP, and ΔeseJ[eseJ]/GFP strains (green signal), and live stained with PI (red signal) in DMEM. b Percentage of PI-positive in positively infected J774A.1 cells at 3 h post-infection (%). Twenty images per infection were quantified. Means ± SD for a representative experiment are shown. ***P < 0.001. c LDH released by J774A.1 cells infected with E. piscicida strains. Murine macrophage J774A.1 cells were infected with WT, ΔeseJ, and ΔeseJ[eseJ] strains at an MOI of 10; 3 h post infection, culture supernatants were collected and the amount of LDH released into the supernatant was measured. Means ± SD from six repeats for a representative experiment are shown. ***P < .001. Immunoblotting of cleaved caspase-1 (p20) (d) and cleaved GSDMD (f) from J774A.1 cells infected with E. piscicida WT, ΔeseJ, and ΔeseJ[eseJ] strains at 3 h post infection. Actin was used to confirm that similar amounts of protein were loaded per lane. Quantitative analysis of caspase-1 (e) and GSDMD (g) cleavage from J774A.1 cells infected with the E. piscicida strains shown in Fig. 2d, f, respectively. The amount of cleaved caspase-1 or cleaved GSDMD was quantified by densitometry and normalized to the amount of actin. The graph shows the relative ratio (means ± SEM) of cleaved caspase-1 or cleaved GSDMD to actin from three independent experiments. **P < 0.01; ***P < 0.001. Immunoblotting of cleaved caspase-1 (p20) (h) and cleaved GSDMD (j) from J774A.1 cells infected with E. piscicida WT, ΔeseJ, ΔesaN, and ΔeseJΔesaN strains. Actin was used to confirm that similar amounts of protein were loaded per lane. Quantitative analysis of cleaved caspase-1 (i) or GSDMD (k) from J774A.1 cells infected with the E. piscicida strains shown in (h, j), respectively. The amount of cleaved caspase-1 or cleaved GSDMD was quantified by densitometry and normalized to that of actin. The graph shows the relative ratios (means ± SEM) of cleaved caspase-1 or cleaved GSDMD from three independent experiments. NS not significant; **p < 0.01; ***p < 0.001. Immunoblotting on cleaved caspase-1 (p20) from J774A.1 cells infected with E. piscicida ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains (l) or WT, ΔeseJ, ΔfimH, and ΔfimH[eseJ] strains (n). Actin was used to confirm that similar amounts of protein were loaded per lane. m,o Quantitative analysis of cleaved caspase-1 from J774A.1 cells infected with the E. piscicida strains shown in (l, n) respectively. The amount of cleaved caspase-1 was quantified by densitometry and normalized to that of actin. The graph shows the relative ratio (means ± SEM) of cleaved caspase-1 from three independent experiments. **P < 0.01; ***P < 0.001.
Rupture of the cell membrane is one of the hallmarks of pyroptosis, as is the activation of caspase-1. The level of cleaved caspase-1 protein detected in J774A.1 cells infected with the ΔeseJ strain was significantly higher than in cells infected with the WT or complemented ΔeseJ[eseJ] strain at 3 hpi (Fig. 2d, e). Similarly, increased cleavage of the pyroptosis executioner Gasdermin D (GSDMD) was observed in ΔeseJ-infected J774A.1 cells (Fig. 2f, g). This was associated with the formation of pores and pyroptotic cell death. However, the further deletion of esaN (the ΔeseJΔesaN strain) significantly decreased the cleavage of caspase-1 and GSDMD to the same level as that observed in the ΔesaN strain (Fig. 2h, k). As the EseN gene encodes the ATPase that energizes the delivery of T3SS effectors16, these results demonstrate that inhibition of pyroptosis by EseJ depends on an active T3SS.
In addition to acting as a T3SS effector, EseJ negatively regulates the expression of type 1 fimbriae and inhibits apoptosis11,17. This raises the question of whether EseJ continues to play a role in pyroptosis when type 1 fimbriae are absent. To investigate this, J774A.1 cells were infected with the following strains at an MOI of 20 for 3 h: ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH. The adhesion and invasion ratios of these four strains are similar11. Similarly low levels of cleaved caspase-1 were found in J774A.1 cells infected with the ΔfimA or ΔfimH strains. However, further deletion of eseJ (in the ΔeseJΔfimA strain or ΔeseJΔfimH strain) stimulated a significant increase in cleaved caspase-1 (Fig. 2l, m). This suggests that EseJ likely functions as a T3SS effector, suppressing pyroptosis in the absence of type 1 fimbriae. For further clarification, pACYC-eseJ-HA was introduced into the ΔfimH strain. Overexpression of EseJ dramatically and significantly reduced the level of cleaved caspase-1 in the ΔfimH strain compared to the wild type (Fig. 2n, o). This further demonstrates that, as a T3SS effector, EseJ suppresses pyroptosis in J774A.1 cells.
Taken together, these data show that the translocation of EseJ by the T3SS inhibits pyroptosis in macrophages by preventing the activation of caspase-1 and the processing of GSDMD.
EseJ inhibits pyroptosis by deactivating the caspase-8 and NLRP3 inflammasome
In our previous study, we demonstrated that EseJ inhibits host cell apoptosis by specifically suppressing the type 1 fimbria-dependent activation of caspase-8 and following cleavage of caspase-323. Caspase-8 plays a crucial role in pyroptosis by activating both caspase-1 and GSDMD37,47. To determine whether the activation of caspase-1 and GSDMD was the result of caspase-8 activation, J774A.1 cells were pretreated for 1 h with 50 μM Z-IETD-FMK (a caspase-8 inhibitor), followed by infection with the ΔeseJ strain at an MOI of 10 for 3 h. It was found that cleaved caspase-8, cleaved caspase-1 and cleaved GSDMD were not present in J774A.1 cells that had been pre-treated with a caspase-8 inhibitor and then infected (Fig. 3a). This suggests that caspase-8 may play a role in activating caspase-1 and GSDMD in J774A.1 cells infected with the ΔeseJ strain.
a Immunoblotting of cleaved caspase-1 (p20), cleaved GSDMD and cleaved caspase-8 from J774A.1 cells infected with E. piscicida strains at 3 hpi. J774A.1 cells were pre-treated with the caspase-8 inhibitor Z-IETD-fmk for 1 h prior to infection with the ΔeseJ strain. J774A.1 cells infected with WT strain and ΔeseJ strain were used as negative and positive controls, respectively. Actin was used to confirm that similar amounts of protein were loaded per lane. b Confocal images of J774A.1 cells infected with E. piscicida strains. J774A.1 cells were pretreated with 50 μM caspase-8 inhibitor Z-IETD-fmk for 1 h prior to infection with the ΔeseJ strain. The morphology of cell rounding in J774A.1 cells infected with ΔeseJ and WT strains was observed at 1 hpi. Infected J774A.1 cells were live stained with PI at 3 hpi. Black arrows and white arrows indicate cell rounding and cell rupture, respectively. c Percentage of PI-positive cells at 3 h post infection. Twelve images per infection were quantified. Means ± SD for a representative experiment are shown. ***P < 0.001. d Immunoblotting of cleaved caspase-1 (p20), cleaved GSDMD, cleaved caspase-3 and cleaved caspase-8 from J774A.1 cells infected with E. piscicida ΔfimH, ΔeseJΔfimH, and ΔeseJΔfimH + Z-IETD-fmk. J774A.1 cells were pretreated with the caspase-8 inhibitor Z-IETD-fmk for 1 h prior to infection with the ΔeseJΔfimH strain. Actin was used to confirm that similar amounts of protein were loaded per lane. e Immunoblotting of cleaved caspase-1 (p20), cleaved GSDMD and cleaved caspase-8 from J774A.1 cells infected with E. piscicida strains. J774A.1 cells were pretreated with 650 μM NLRP3 inhibitor MCC950 for 1 h prior to infection with the ΔeseJΔfimH strain. J774A.1 cells infected with ΔfimH and ΔeseJΔfimH strains were used as negative and positive controls, respectively. Actin was used to confirm that similar amounts of protein were loaded per lane. f Confocal images of PI-positive J774A.1 cells infected with E. piscicida strains. J774A.1 cells were pretreated with 650 μM NLRP3 inhibitor MCC950 for 1 h prior to infection with the ΔeseJΔfimH strain. J774A.1 cells infected with ΔfimH and ΔeseJΔfimH strains were used as negative and positive controls, respectively. J774A.1 cells infected with the different E. piscicida strains were stained with PI at 3 hpi. g Quantification of cells that were PI-positive at 3 hpi. Twelve views per infection were quantified from (f). Means ± SD for one representative experiment are shown. ***p < 0.001. Quantification of infected cells that were PI positive at 3 hpi. HEK293T cells were co-transfected with pLVX-caspase-8 siRNA (h), pLVX-NLRP3 siRNA (i) or pLVX-control siRNA, as well as pMD2.g (envelope plasmid) and pSPAX2 (packing plasmid). 48 h later, the cell culture supernatant was collected to harvest the lentiviral particles. J774A.1 cells were infected with the concentrated lentivirus for 48 h before being infected with ΔfimH and ΔeseJΔfimH strains. Three hours post-infection, both cells and cell culture media were harvested and precipitated, followed by immunoblotting for caspase-8 (h), NLRP3 (i), and cleaved caspase-1 (p20). Actin was used as a loading control. j Leukocytes were isolated from the head kidney of mandarin fish, treated with either the caspase-8 inhibitor z-IETD-fmk or the NLRP3 inhibitor MCC950 for one hour, and then infected with the ΔeseJΔfimH strain at an MOI of 2.5, 5 or 10. Three hours post-infection, the culture supernatant was collected and the amount of LDH released was measured. Means ± SD from four samples for a representative experiment are shown. *P < 0.05; **P < 0.01; ***P < 0.001.
For corroboration, the morphology of J774A.1 cells infected with E. piscicida strains was examined at 1 and 3 hpi. One hour post-infection (hpi), J774A.1 cells infected with the ΔeseJ strain exhibited rapid and dramatic cell rounding (black arrows in the annotated zoomed area), which is a hallmark of apoptosis. This was compared to that infected with the wild-type (WT) strain (Fig. 3b). As the infection progressed, the ΔeseJ strain caused significant cell rupture (indicated by white arrows in the zoomed-in area) and an increase in the number of PI-positive cells. Pretreatment with Z-IETD-FMK significantly reduced PI uptake (Fig. 3b, c). This indicates that the uptake of PI by J774A.1 cells infected with ΔeseJ was inhibited by the caspase-8 inhibitor Z-IETD-FMK. This confirms that EseJ inhibits pyroptosis via the caspase-8-related pathway.
To further investigate the role of EseJ in the absence of type 1 fimbriae, J774A.1 cells were pre-treated with the 50 μM caspase-8 inhibitor Z-IETD-FMK for 1 h, after which they were infected with the ΔeseJΔfimH strain at an MOI of 20 for 3 h. It was observed that the caspase-8 inhibitor Z-IETD-fmk reduced the steady-state protein levels of cleaved caspase-3, cleaved caspase-1 and cleaved GSDMD (Fig. 3d). This suggests that, even in the absence of type 1 fimbriae, EseJ can inhibit pyroptosis by deactivating caspase-8.
Caspase-8, which is activated during apoptosis, can directly or indirectly trigger the assembly of the NLRP3 inflammasome, thereby inducing pyroptosis48,49. To determine whether the observed pyroptosis stimulated by the ΔeseJΔfimH strain was dependent on NLRP3 activation, J774A.1 cells were pre-treated with 650 μM MCC950, an NLRP3 inhibitor, for 1 h prior to infection with the ΔeseJΔfimH strain at an MOI of 20 for 3 h. A significant reduction in cleaved caspase-1, cleaved caspase-8 and cleaved GSDMD levels was observed in J774A.1 cells pretreated with MCC950 prior to infection with the ΔeseJΔfimH strain (Fig. 3e). Consistently, MCC950 suppressed the increased ratio of PI-positive cells caused by infection with the ΔeseJΔfimH strain (Fig. 3f, g). These results demonstrate that the activation of caspase-1 and GSDMD by ΔeseJΔfimH infection is mediated by the activation of the NLRP3 inflammasome. Furthermore, the maturation of the NLRP3 inflammasome promotes the activation of caspase-8. Moreover, the activation of caspase-1 triggered by the ΔeseJ strain was notably reduced by either the knockdown of caspase-8 or NLRP3 (Fig. 3h, i). Taken together, these data suggest that suppression of pyroptosis by EseJ depends on both the caspase-8 and NLRP3 pathways. Pretreatment with caspase-8 (Z-IETD-FMK) or NLRP3 (MCC950) inhibitors in fish leukocytes prior to infection with the ΔeseJΔfimH strain (MOI: 2.5, 5, or 10) reduced the release of lactate dehydrogenase (LDH) (Fig. 3j). This suggests that EseJ suppresses pyroptosis-like cell death via caspase-8- and NLRP3-dependent pathways in fish leukocytes infected. This is consistent with its role in inhibiting pyroptosis in murine macrophages.
Taken together, these data demonstrate that the translocation of E. piscicida EseJ via the T3SS inhibits pyroptosis in macrophages by suppressing the activation of caspase-8 and the NLRP3 inflammasome.
EseJ inhibits the NLRP3-caspase-8-RIPK1 PANoptosome, which is responsible for PANoptosis
PANoptosis is a unique form of PCD involving the activation of pyroptosis, apoptosis and necroptosis31,35. Compared to cells infected with the WT strain or the ΔeseJ[eseJ] strain, infection with the ΔeseJ strain induced significantly elevated levels of p-MLKL (a key necroptosis executioner) in J774A.1 cells (Fig. 4a–d), indicating that EseJ suppresses necroptosis. Necroptosis is initiated by the phosphorylation of RIPK1 and RIPK3. These proteins then assemble into the necroptosome, which propagates death signals via MLKL29.
Immunoblotting on p-MLKL from J774A.1 cells infected with E. piscicida WT, ΔeseJ, and ΔeseJ[eseJ] strains (a) or ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains (c). Actin was used to confirm that similar amounts of protein were loaded per lane. b, d Quantitative analysis of p-MLKL from J774A.1 cells infected with the E. piscicida strains shown in (a, c). The amount of p-MLKL was quantified by densitometry and normalized to that of actin. The graph shows the relative ratios (means ± SEM) of p-MLKL from three independent experiments. *P < 0.05; **P < 0.01. e Immunoblotting of cleaved caspase-1 (p20), cleaved caspase-3 and p-MLKL from J774A.1 cells infected with E. piscicida strains. J774A.1 cells were pretreated with RIPK1 inhibitor (Nec-1s) and RIPK3 inhibitor (GSK'872) for 1 h before infection with the ΔeseJ strain. J774A.1 cells infected with WT and ΔeseJ strains were used as negative and positive controls, respectively. Actin was used to confirm that similar amounts of protein were loaded per lane. Quantitative analysis of cleaved caspase-1 (p20) (f), cleaved caspase-3 (g), p-MLKL (h) shown in (e). The amount of cleaved caspase-1 (p20), cleaved caspase-3, p-MLKL was quantified by densitometry and normalized to that of actin. The graph shows the relative ratios (Means ± SEM) of cleaved caspase-1 (p20), cleaved caspase-3, p-MLKL from three independent experiments. NS, no significance; **P < 0.01; ***P < 0.001. Immunoblotting on p-TAK1 from J774A.1 cells infected with E. piscicida WT, ΔeseJ, and ΔeseJ[eseJ] strains (i) or ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains (k). Actin was used to confirm that similar amounts of protein were loaded per lane. j, l Quantitative analysis of p-TAK1 from J774A.1 cells infected with the E. piscicida strains shown in (i, k). The level of p-TAK1 was quantified by densitometry and normalized to that of actin. The graph shows the relative ratios (Means ± SEM) of p-TAK1 from three independent experiments. *P < 0.05; ***P < 0.001. m Immunoprecipitation of endogenous NLRP3 from lysates of J774A.1 cells pretreated with the NLRP3 inhibitor MCC950 prior to infection with the WT or ΔeseJ strain, followed by immunoblotting analysis of the PANoptosome components RIPK1 and caspase-8. n Quantitative analysis of caspase-8 and RIPK1 from immunoprecipitated samples shown in (m). The amount of caspase-8 or RIPK1 was quantified by densitometry and normalized to that of actin. The graph shows the relative ratios (Means ± SEM) of caspase-8 or RIPK1 from three independent experiments. *P < 0.05; **P < 0.01. J774A.1 cells were infected with WT, ΔeseJ, and ΔeseJ[eseJ] strains (o) or ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains (p) for 5 h, after which culture supernatants were collected and levels of secreted IL-1β were assessed by ELISA. Supernatant from an uninfected cell culture was used as a control. Means ± SD from five repeats for a representative experiment are shown. ***P < 0.001.
Pre-treatment with the RIPK3 inhibitor GSK’872 stimulated a substantial decrease in cleaved caspase-1 and abolished p-MLKL. It also produced a dramatic increase in cleaved caspase-3. However, no difference was detected in RIPK1 inhibitor (Nec-1) pre-treatment. This demonstrates that RIPK3 plays a pivotal role in regulating apoptosis, pyroptosis, and necroptosis stimulated by ΔeseJ infection.
As PANoptosis activation requires molecular interactions downstream of TAK1, the level of phosphorylated TAK1 (p-TAK1) in J774A.1 cells infected with E. piscicida strains was examined. A dramatically decreased level of p-TAK1 was detected in J774A.1 cells infected with the ΔeseJ strain compared to the WT strain or the ΔeseJ[eseJ] strain (see Fig. 4i–l), indicating that EseJ enhances TAK1 phosphorylation.
The PANoptosome provides a molecular scaffold that allows the interaction and activation of the components required for PANoptosis, including those involved in pyroptosis (NLRP3 and ASC), apoptosis (caspase-8) and necroptosis (RIPK3 and RIPK1)35. In order to investigate the role of EseJ in PANoptosome formation, J774A.1 cells were pre-treated with the NLRP3 inhibitor MCC950 before being infected with either the wild-type (WT) strain or the ΔeseJ strain. We then immunoprecipitated endogenous NLRP3 to assess its interactions with RIPK1 and caspase-8. As shown in Fig. 4m, n, a minimal level of interaction was observed between NLRP3 and caspase-8 or RIPK1 in uninfected J774A.1 cells. Following pre-treatment with MCC950 and WT infection, these interactions increased moderately. However, pre-treatment with MCC950 prior to infection with the ΔeseJ strain stimulated significantly stronger NLRP3-RIPK1/caspase-8 binding. This result demonstrates that EseJ inhibits the assembly of the NLRP3-caspase-8-RIPK1 PANoptosome. In conclusion, EseJ inhibits PANoptosis by suppressing the formation of the PANoptosome in a TAK1-dependent manner.
PANoptosis is a distinct form of PCD accompanied by inflammation, typically involving the release of inflammatory cytokines (e.g., IL-1β)31. Interestingly, the secretion of IL-1β from J774A.1 cells stimulated by the EseJ (wt vs ΔeseJ, ΔfimA vs ΔeseJΔfimA, ΔfimH vs ΔeseJΔfimH strain) was significantly and substantially decreased (Fig. 4o, p), indicating that T3SS-translocated EseJ promotes IL-1β secretion.
Discussion
Innate immune signaling and inflammatory cell death (pyroptosis, apoptosis, necroptosis and PANoptosis) form the primary defence mechanism against pathogens. However, pathogens have evolved multiple strategies, including T3SS effectors, to evade or inhibit PANoptosis31,32. Previous studies have shown that the T3SS protein EseJ in E. piscicida suppresses caspase-8-dependent apoptosis by down-regulating type 1 fimbriae23. In this study, we demonstrate that T3SS-translocated EseJ inhibits NLRP3-dependent pyroptosis by deactivating caspase-8. Additionally, EseJ activates TAK1, which suppresses the assembly of the NLRP3-caspase-8-RIPK1 PANoptosome and thereby inhibits PANoptosis.
The coordinated activation of caspases and gasdermins across cell death modalities reveals a dynamic regulatory network that balances apoptosis and pyroptosis during bacterial infection. This study observed apoptotic cell rounding in J774A.1 cells infected with the ΔeseJ strain as early as 1 hpi, followed by pyroptotic cell rupture. This sequential, caspase-8-dependent transition was demonstrated by using the caspase-8 inhibitor Z-IETD-fmk to block this process. These findings align with the well-established role of caspase-8 as a molecular hub that mediates the conversion from apoptosis to pyroptosis when upstream signaling pathways (e.g., TAK1/IκK) are disrupted37,47,50. The hypothesis that the functional inhibition of TAK1 redirects the activity of caspase-8 away from apoptotic effectors (e.g., caspase-3) towards pyroptotic substrates (e.g., caspase-1 and GSDMD), thereby promoting lytic cell death, is further supported by the significant activation of caspase-8 and the concomitant reduction in the phosphorylation of TAK1 in cells infected with the ΔeseJ strain. However, the investigation of the caspase-8- or NLRP3-dependent mechanisms of cell death used pharmacological inhibitors, which limits the strength of the conclusions that can be drawn. To further clarification, the caspase-8 and NLRP3 were knocked down in J774A.1 macrophages, and caspase-1 activation induced by the ΔeseJ strain was significantly diminished by either caspase-8 or NLRP3 knockdown (Fig. 3h, i). Collectively, the data support that EseJ-mediated suppression of caspase-1 activation depends on caspase-8 and NLRP3. It is hypothesized that infection with the ΔeseJ strain inhibits TAK1 signaling, thereby destabilizing the apoptotic checkpoint and enabling caspase-8 to initiate pyroptosis by cleaving caspase-1 or GSDMD.
A hallmark of PANoptosis under conditions of TAK1 or IκK inhibition is the assembly of multi-protein PANoptosomes involving NLRP3, RIPK1, RIPK3, and caspase-834. In this study, a significant increase in the interaction between NLRP3 and caspase-8 or RIPK1 was observed in J774A.1 cells infected with the ΔeseJ strain (Fig. 4m, n). This suggests that PANoptosome formation is the default pathway in the absence of EseJ-mediated suppression. However, the failure of RIPK1 kinase inhibition to attenuate PANoptosis suggests that RIPK1 acts primarily through a scaffolding role rather than its catalytic activity in this context. This is consistent with the finding that RIPK1 regulates PANoptosis in a kinase-independent manner during TLR priming setting with TAK1 deficiency51. This suggests that RIPK1 may facilitate the assembly of PANoptosome through its scaffolding function. Crucially, the RIPK3 kinase activity turned out to be indispensable for the EseJ-inhibited PANoptosis. Pharmacological blockade of RIPK3 not only abolished necroptosis, but also reduced pyroptosis while reciprocally enhancing apoptosis (Fig. 4e–h). This suggests that the enzymatic function of RIPK3 coordinates multiple death modalities within PANoptosis. Our data converge on a model that EseJ deactivates the NLRP3-caspase-8-RIPK1 PANoptosome in PANoptosis. Whether EseJ binds directly to TAK1 to inhibit PANoptosis by activating the TAK1-IκK pathway awaits further study.
Interesting links between host cell death modes and bacterial virulence are revealed by the survival dynamics of E. piscicida-infected blue gourami (Trichopodus trichopterus). The wild-type (WT) strain induced a sharp decline in host survival between day 2 and day 3, whereas fish infected with the ΔeseJ strain showed a markedly attenuated mortality curve. The inability of the ΔeseJ strain to suppress PANoptosis during early infection is probably responsible for this divergence. Robust PANoptotic activation in ΔeseJ-infected hosts would drive efficient pathogen clearance through the elimination of intracellular replication niches and the release of damage-associated molecular patterns (DAMPs) that amplify immune surveillance, even in the absence of canonical IL-1β-driven inflammation (Fig. 4o, p). Notably, despite reduced IL-1β secretion, widespread lytic cell death may directly limit E. piscicida proliferation by lyzing infected cells, thereby reducing the overall bacterial burden through a non-canonical and inflammation-independent defence mechanism. Surprisingly, over the course of the infection, the ΔeseJ and ΔeseJΔfimA strains showed similar cumulative survival rates at each time point examined. This similarity may be due to the fact that the blue gourami were infected by intramuscular injection, which eliminates significant differences in adhesion and invasion between strains shown by bath immersion. However, it is necessary to verify whether these findings are applicable to murine or turbot (Scophthalmus maximus) through further investigation.
To accurately assess the contribution of EseJ to pyroptosis, the ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains of E. piscicida were used for infection, where adhesion and invasion of each strain were at similarly low levels. An MOI of 20 was used to stimulate pyroptosis because cleaved caspase-1 was barely detectable in J774A.1 cells infected with the ΔeseJΔfimA strain or the ΔeseJΔfimH strain at an MOI of 5 or 10. However, when infected with wild-type and ΔeseJ strains, an MOI of 5 or 10 was used to monitor cell morphological changes, as a lower MOI helps to avoid rapid and overwhelming cell detachment.
The E. piscicida T3SS mutant induces a slight decrease in cleaved caspase-1 and cleaved GSDMD compared to the WT strain. This finding is in agreement with the conclusion of Chen et al.8 and Xie et al.46. Disruption of the T3SS ATPase EsaN or its rod protein EsaI has little effect on levels of cleaved caspase-1 or IL-1β8. In contrast, infection with the ΔeseJ strain results in a significant increase in cleaved caspase-1 and GSDMD. Further deletion of esaN (the ΔeseJΔesaN strain) reduces these elevated levels to those of the ΔesaN strain (Fig. 2h–k). The flagellin protein FliC could also be translocated into host cells by T3SS and trigger pyroptosis46,52. It is therefore speculated that the weak role of T3SS in pyroptosis stimulation by E. piscicida WT strain could be the coordinated result of translocated EseJ, which inhibits pyroptosis, and some other T3SS proteins, such as EsaI and FliC, which stimulate pyroptosis. At present, we have not been able to provide direct evidence of pyroptosis stimulation or inhibition by EseJ in murine macrophages due to the low efficiency of macrophage transfection.
In summary, the E. piscicida T3SS protein EseJ inhibits PANoptosis (pyroptosis, apoptosis and necroptosis) in murine macrophages (Fig. 5). On contact with host cells, EseJ suppresses apoptotic cell rounding by down-regulating the type 1 fimbriae, which activates the cleavage of caspase-8 and caspase-3, leading to cell apoptosis23. Once internalized, the T3SS-translocated EseJ stimulates the phosphorylation of TAK1, inhibits NLRP3-caspase-8-RIPK1-mediated PANoptosome formation and thereby inhibits PANoptosis. EseJ inhibits pyroptosis by deactivating NLRP3 and caspase-8 and preventing caspase-1 and GSDMD cleavage. By deactivating MLKL and MLKL pore formation, EseJ also inhibits necroptosis. These findings reveal a novel mechanism by which the T3SS protein EseJ manipulates macrophage fate for E. piscicida survival and replication.
The E. piscicida T3SS protein EseJ controls the conversion from apoptotic cell rounding to PANoptotic cell lysis through activating TAK1. The EseJ protein suppresses apoptosis by down regulating type 1 fimbriae, which would otherwise interact with host cell receptors to activate caspase-8 mediated extrinsic apoptosis21. Upon being internalized, EseJ translocated by T3SS stimulates TAK1 phosphorylation and inhibits the assembly of NLRP3-caspase-8-RIPK1 PANoptosome, thereby (1) suppressing GSDMD cleavage, GSDMD pore formation and pyroptosis; (2) inhibiting caspase-3 activation and apoptosis; (3) deactivating MLKL phosphorylation, MLKL pore formation and necroptosis. The E. piscicida T3SS protein EseJ controls the transition from apoptotic cell rounding to PANoptotic cell lysis by activating TAK1.
Materials and methods
Bacterial strains, cell and cultivation
Bacterial strains, cell and cultivation E. piscicida PPD130/91, formerly known as E. tarda PPD130/912, was used in this study. The bacterial strains and plasmids used in this study are described in Table 1. E. piscicida strains were grown in tryptic soy broth (TSB) at 28 °C. Antibiotics were used when necessary at the following concentrations 12.5 μg/mL colistin (Col; Sigma), 50 μg/mL gentamicin (Gem; Sigma) and 15 μg/mL tetracycline (Tet; Amresco).
Murine macrophages J774A.1 (China Center for Type Culture Collection, Cat# GDC0176) were cultured in Dulbecco’s minimal essential medium (DMEM) (HyClone, Cat# SH30022.01) supplemented with 10% FBS (Gibco) in a 5% CO2 atmosphere.
Isolating mandarin fish leukocytes
Head kidney from healthy mandarin fish (Siniperca chuatsi, approximately 500 g) were minced in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin and 20 U/mL sodium heparin using a 100 μm sterile cell strainer to produce a single-cell suspension. The separated cell suspension was carefully layered on a discontinuous 34%/51% Percoll density gradient and centrifuged at 400 × g for 40 min at 4 °C. leukocytes located at the interface between the Percoll layers were collected, washed three times in DMEM supplemented with 2% fetal bovine serum (FBS) for subsequent experiments.
Cytotoxicity assay based on the uptake of propidium iodide
The J774A.1 macrophage monolayers were infected with different E. piscicida strains as described previously46 with slight modifications. Briefly, E. piscicida strains expressing GFP were opsonized with naive mouse serum prior to infection at an MOI of 10. Cultures were then centrifuged at 170 × g for 5 min at room temperature and incubated at 35 °C. After 30 min, the macrophages were washed three times and fresh culture medium containing 50.0 μg/mL gentamicin was added for 3 h. The macrophages were then stained live with 2.0 μg/mL propidium iodide in DMEM for 15 min in the dark. Cytotoxic cells were identified and scored for PI uptake. PI uptake cells were then counted from more than 12 fields of three coverslips per infection. Images were captured using a confocal laser scanning microscope (NOL-LSM 710; Carl Zeiss, Germany).
To evaluate the effect of caspase-8 inhibitor or NLRP3 inhibitor on infection-induced pyroptosis, J774A.1 cells were pretreated with 50 μM Z-IETD-fmk (BioVision) or 650 μM MCC950 (Selleck) for 1 h before infection in 10% FBS-DMEM. PI staining was performed at 3 h after infection.
Cytotoxicity assays using lactate dehydrogenase (LDH) release
The release of lactate dehydrogenase (LDH) from macrophage J774A.1 monolayers or fish leukocytes infected with different E. piscicida strains was determined using a previously described method with slight modifications46. The percentage of LDH release per infection was calculated as follows: percentage of LDH release = [(infected cell LDH release - spontaneous LDH release)/(total LDH release - spontaneous LDH release)] × 100%.
Immunoblotting assay
J774A.1 cells were seeded and infected as previously described with slight modifications23. Briefly, J774A.1 cells were seeded at a density of 5 × 105 cells per well and infected at an MOI of 10 or 20. At time zero post infection, the cell monolayers were rinsed twice and incubated in 250 µl DMEM supplemented with gentamicin (50 µg/mL). At 3 h post infection, cell culture supernatants were collected and cells were lyzed with 60 µl IP lysis buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol) (Pierce) on ice for 10 min. The supernatants and cell lysates from each infection were precipitated with methanol and chloroform, respectively, as described by Wang et al.53. The resulting pellets were dried at room temperature and dissolved in 1.5 × SDS loading buffer.
Protein samples were separated by electrophoresis on NuPAGE 10% bis-Tris gel (Novex) and then transferred to polyvinylidene difluoride (PVDF) membranes (pore size, 0.2 μm). After transfer, the membranes were blocked in 5% skimmed milk and probed overnight at 4 °C with a monoclonal mouse anti-cleaved caspase-1 (p20) antibody (Adipogen, Cat# AG20B0042C100) at a 1:2000 dilution, rabbit anti-phosphorylated TAK1 (CST, Cat# 9339 s), rabbit anti-phosphorylated MLKL (CST, Cat# 37333 s) monoclonal antibody, rabbit anti-cleaved caspase-3 (CST, Cat# 9661 s), rabbit cleaved caspase-8 (CST, Cat# 8592 s), rabbit anti caspase-3 monoclonal antibody (CST, Cat# 9662), rabbit anti caspase-8 monoclonal antibody (CST, Cat# 4790) at 1:1000 dilution, rabbit anti-NLRP3 antibody (Cat# ab270449, Abcam), mouse anti-caspase-8 antibody (Cat# SC-81656, Santa Cruz), rabbit anti-RIPK1 antibody (Cat# 3493 s, CST), and mouse anti-GSDMD (EPR19828) monoclonal antibody (Abcam, Cat# ab209845) at a 1:1000 dilution, and rabbit anti-actin polyclonal antibody (ABclonal, Cat# AC038) at 1:5000 dilution.
To investigate the effect of different inhibitors on infection-induced pyroptosis, cells were pretreated with specific inhibitors, including 50 μM caspase-8 inhibitor Z-IETD-fmk (BioVision), 650 μM NLRP3 inhibitor MCC950, 250 μM RIPK1 inhibitor Nec-1s, and 100 μM RIPK3 inhibitor GSK’872 (Selleck) for 1 h before infection in 10% FBS-DMEM. Supernatants and cell lysates were collected at 3 h post infection for analysis.
Immunoprecipitation
For immunoprecipitation, J774A.1 cells were seeded 24 h before infection. J774A.1 cells were pretreated with 650 μM NLRP3 inhibitor MCC950 for 1 h prior to infection with WT and ΔeseJ strains. After 3 h post infection, J774A.1 were lyzed in IP lysis buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol, and protease inhibitor cocktail) for 30 min. Whole cell lysates were harvested and incubated with 2.0 μg NLRP3 antibody for 4 h at 4 °C. Protein G agarose beads were added to these samples and incubated overnight at 4 °C. The agarose beads were centrifuged and washed five times in IP lysis buffer, and the immunoprecipitates were eluted by adding sample buffer and subjected to immunoblotting analysis.
Single strain infection in blue gourami
The blue gourami (Trichopodus trichopterus) is a tropical fish susceptible to Edwardsiella piscicida, and is therefore widely used in studies of its pathogenesis17. For this study, healthy, wild-type blue gourami (approximately eight months old) were purchased from an aquaculture company and kept in a recirculating aquaculture system for two weeks. The fish, which were of a similar size (5.25 ± 0.94 g), were then randomly divided into four tanks (20 fish per tank) for the infection process. E. piscicida wild-type strain, ΔeseJ strain and ΔeseJΔfimA strain were subcultured at 1:20 into TSB for 2 h, where OD540 reaches ~0.5. Fish was infected by injection intramuscularly near the dorsal fin with (2.48 ± 0.12) × 105 CFU of E. piscicida wild-type strain, (2.47 ± 0.16) × 105 CFU of ΔeseJ strain, or (2.48 ± 0.07) × 105 CFU of ΔeseJΔfimA strain. The PBS injection group was used as the negative control. The fish were kept at 28 °C after infection and mortality was recorded over a period of seven days.The tropical fish that survived the experiment were euthanised by an ice bath. This experimental procedure was repeated 3 times independently.
IL-1β measurement
Levels of IL-1β secreted into culture media during infection by WT, ΔeseJ, and ΔeseJ[eseJ] strains or ΔfimA, ΔeseJΔfimA, ΔfimH, and ΔeseJΔfimH strains were measured as described in Xie et al. with slight modification46. At 5 hpi, the supernatants from three replicate wells per infection condition were collected, secreted level of IL-1β was analyzed with the Quantikine mouse IL-1β immunoassay kit following the manufacturer’s instructions (R&D Systems). The replicate values for each infection condition were averaged.
Lentiviral production and RNA interference
The RNAi target sequences were “GGCCTTACTTCAATCTGTT” for NLRP3 and “AACCTCGGGGATACTGTCT” for Caspase-8, and the scrambled sequence “AATCGCATAGCGTATGCCG” for the control. These were amplified by PCR from mouse genomic DNA and inserted into the pLVX-shRNA2-Puro to form the recombinant plasmids pLVX-NLRP3 siRNA, pLVX-caspase-8 siRNA, and pLVX-control siRNA. The oligonucleotides used in this study are described in Table 2. HEK293T cells were co-transfected with the recombinant plasmids and the envelope and packaging plasmids (pMD2.g and pSPAX2, respectively) using a standard transfection protocol. 48 h later, the cell culture supernatant was collected, filtered through a 0.45 μm filter and centrifuged to harvest the lentiviral particles. J774A.1 cells were infected with the concentrated lentivirus for 48 h before being infected with ΔfimH and ΔeseJΔfimH strains at an MOI of 20. Three hours post-infection, both cells and cell culture supernatants were harvested and precipitated, followed by immunoblotting for caspase-8 (i), NLRP3 (j) and cleaved caspase-1 (p20). Actin was used to indicate similar amounts of protein loading per lane.
Statistical and reproducibility
All experiments were repeated at least three times independently and are presented as the mean ± standard deviationm (SD) or the mean ± standard error of the mean (SEM) of triplicate samples per experimental condition, unless otherwise noted. Data were analyzed using a Student’s t test in Graphpad Prism (version 8.4.3), and a P value of less than 0.05 was considered to be statistically significant.
Ethics
We have complied with all relevant ethical regulations for animals use. All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Uncropped immunoblotting images are provided in the Supplementary Information. The numerical source data for the graphs in this paper can be found in the Supplementary Data. All other original data supporting this study are available upon request to the corresponding author. The complete sequences of nlrp3 and caspase-8 knockdown-related plasmids have been deposited in NCBI under the accession number PP098725-PP098733.
References
Padros, F., Zarza, C., Dopazo, L., Cuadrado, M. & Crespo, S. Pathology of Edwardsiella tarda infection in turbot, Scophthalmus maximus (L.). J. Fish. Dis. 29, 87–94 (2006).
Shao, S. et al. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813(T) encoding two distinct T3SS and three T6SS gene clusters: propose a novel species as Edwardsiella anguillarum sp. nov. Syst. Appl. Microbiol. 38, 36–47 (2015).
Ashford, R. U., Sargeant, P. D. & Lum, G. D. Septic arthritis of the knee caused by Edwardsiella tarda after a catfish puncture wound. Med. J. Aust. 168, 443–444 (1998).
Gilani, A., Sarmadian, R., Kahbazi, M. & Yousefichaijan, P. Urinary tract infection caused by Edwardsiella tarda: a report of the first case in Iran. BMC Infect. Dis. 22, 962 (2022).
Sakai, T., Kanai, K., Osatomi, K. & Yoshikoshi, K. Identification and characterization of a fimbrial gene cluster of Edwardsiella tarda expressing mannose-resistant hemagglutination. Fish. Pathol. 39, 87–93 (2004).
Tan, Y. P., Zheng, J., Tung, S. L., Rosenshine, I. & Leung, K. Y. Role of type III secretion in Edwardsiella tarda virulence. Microbiology 151, 2301–2313 (2005).
Zheng, J. & Leung, K. Y. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66, 1192–1206 (2007).
Chen, H. et al. The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca(2+)-dependent MAPK-Jnk pathway. Cell Host Microbe 21, 47–58 (2017).
McLay, R. B. et al. Level of fimbriation alters the adhesion of Escherichia coli bacteria to interfaces. Langmuir 34, 1133–1142 (2018).
Thumbikat, P. et al. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 5, e1000415 (2009).
Zhang, Q. et al. The Edwardsiella piscicida type III effector EseJ suppresses expression of type 1 fimbriae, leading to decreased bacterial adherence to host cells. Infect. Immun. 87, e00187-19 (2019).
Sakai, T., Kanai, K., Osatomi, K. & Yoshikoshi, K. Identification of a 19.3-kDa protein in MRHA-positive Edwardsiella tarda: putative fimbrial major subunit. FEMS Microbiol. Lett. 226, 127–133 (2003).
Galan, J. E. & Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573 (2006).
Zeng, Z. X. et al. Secreted in a type III secretion system-dependent manner, EsaH and EscE are the cochaperones of the T3SS needle protein EsaG of Edwardsiella piscicida. mBio 13, e0125022 (2022).
Chatterjee, S., Chaudhury, S., McShan, A. C., Kaur, K. & De Guzman, R. N. Structure and biophysics of type III secretion in bacteria. Biochemistry 52, 2508–2517 (2013).
Xie, H. X. et al. EseG, an effector of the type III secretion system of Edwardsiella tarda, triggers microtubule destabilization. Infect. Immun. 78, 5011–5021 (2010).
Xie, H. X. et al. Identification and functional characterization of the novel Edwardsiella tarda effector EseJ. Infect. Immun. 83, 1650–1660 (2015).
Hou, M. et al. Identification and functional characterization of EseH, a new effector of the type III secretion system of Edwardsiella piscicida. Cell Microbiol. 19, e12638 (2017).
Cao, H. et al. Novel T3SS effector EseK in Edwardsiella piscicida is chaperoned by EscH and EscS to express virulence. Cell Microbiol. 20, e12790 (2018).
Liao, X. J. et al. Unraveling and characterization of novel T3SS effectors in Edwardsiella piscicida. mSphere 8, e0034623 (2023).
Zhou, M. et al. Type III secretion system effector YfiD inhibits the activation of host poly(ADP-ribose) polymerase-1 to promote bacterial infection. Commun. Biol. 7, 162 (2024).
Zhang, L. et al. Edwardsiella piscicida interferes with classical endocytic trafficking and replicates in a specialized replication-permissive niche in nonphagocytic cells. J. Bacteriol. 203, e0050520 (2021).
He, T. T. et al. Edwardsiella piscicida type III protein EseJ suppresses apoptosis through down regulating type 1 fimbriae, which stimulate the cleavage of caspase-8. Cell Microbiol. 22, e13193 (2020).
Chai, Q., Lei, Z. & Liu, C. H. Pyroptosis modulation by bacterial effector proteins. Semin Immunol. 69, 101804 (2023).
Jorgensen, I., Rayamajhi, M. & Miao, E. A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 17, 151–164 (2017).
Taylor, R. C., Cullen, S. P. & Martin, S. J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9, 231–241 (2008).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Xue, Y., Enosi Tuipulotu, D., Tan, W. H., Kay, C. & Man, S. M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 40, 1035–1052 (2019).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Wang, H. et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014).
Malireddi, R. K. S. et al. RIPK1 distinctly regulates Yersinia-induced inflammatory cell death, PANoptosis. Immunohorizons 4, 789–796 (2020).
Yuan, H. et al. Salmonella effector SopF regulates PANoptosis of intestinal epithelial cells to aggravate systemic infection. Gut Microbes 15, 2180315 (2023).
Zheng, M. & Kanneganti, T. D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 297, 26–38 (2020).
Christgen, S. et al. Identification of the PANoptosome: a molecular platform triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 10, 237 (2020).
Samir, P., Malireddi, R. K. S. & Kanneganti, T. D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 10, 238 (2020).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Newton, K. et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431 (2019).
Malireddi, R. K. S. et al. Inflammatory cell death, PANoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons 5, 568–580 (2021).
Karki, R. et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight 5, e136720 (2020).
Malireddi, R. K. S. et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 215, 1023–1034 (2018).
Sweet, C. R., Conlon, J., Golenbock, D. T., Goguen, J. & Silverman, N. YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell Microbiol. 9, 2700–2715 (2007).
Paquette, N. et al. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl. Acad. Sci. USA 109, 12710–12715 (2012).
Okuda, J. et al. Intracellular replication of Edwardsiella tarda in murine macrophage is dependent on the type III secretion system and induces an up-regulation of anti-apoptotic NF-kappaB target genes protecting the macrophage from staurosporine-induced apoptosis. Micro. Pathog. 41, 226–240 (2006).
Zhou, Z. J. & Sun, L. Edwardsiella tarda-induced inhibition of apoptosis: a strategy for intracellular survival. Front. Cell Infect. Microbiol. 6, 76 (2016).
Xie, H. X. et al. Edwardsiella tarda-induced cytotoxicity depends on its type III secretion system and flagellin. Infect. Immun. 82, 3436–3445 (2014).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 115, E10888–E10897 (2018).
Vince, J. E. et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1beta activation. Cell Rep. 25, 2339–2353.e2334 (2018).
Chen, K. W. et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 38, e101638 (2019).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Malireddi, R. K. S. et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med. 217, jem.20191644 (2020).
Sun Y. H, Rolan H. G & Tsolis R. M. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem 282, 33897–33901 (2007).
Wang, X. et al. NLRP3 inflammasome activation in murine macrophages caused by Neospora caninum infection. Parasites Vectors 10, 266 (2017).
Ling, S. H. M., Wang, X. H., Xie, L., Lim, T. M. & Leung, K. Y. Use of green fluorescent protein (GFP) to study the invasion pathways of Edwardsiella tarda in in vivo and in vitro fish models. Microbiology 146, 7–19 (2000).
Valdivia, R. H. & Falkow, S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277, 2007–2011 (1997).
Wang, Z. et al. Direct lysine dimethylation of IRF3 by the methyltransferase SMYD3 attenuates antiviral innate immunity. Proc. Natl. Acad. Sci. USA 122, e2320644122 (2025).
Acknowledgements
We would like to thank Dr. Hong Bing Yu (Department of Microbiology, Molecular Genetics and Immunology, University of Kansas Medical Center) for his helpful discussions. This research was supported by the National Natural Science Foundation of China (Grants 32202992 and 32073018).
Author information
Authors and Affiliations
Contributions
HX.X and P.N. initiated and supervised the project. HX.X and TT.H. designed the experiments, which were executed by TT.H. with help from PY.T., XL.J. and SS.S. PY.T. contributed to the blue gourami infection and reagent preparation. XL.J. and SS.S. helped perform some of the immunofluorescence experiments. TT.H. prepared the figures and wrote the manuscript with HX.X. All authors participated in the discussion, revision, and approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Dipshikha Chakravortty and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Karthika Rajeeve and Johannes Stortz. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, T.T., Tang, P.Y., Jiang, X.L. et al. T3SS effector EseJ in Edwardsiella piscicida inhibits PANoptosis in macrophages. Commun Biol 8, 1420 (2025). https://doi.org/10.1038/s42003-025-08823-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08823-0