Abstract
Memory loss is a manifestation of type 1 diabetes mellitus (T1DM)-induced brain damage resulting from hyperglycemia. However, the mechanism underlying T1DM-induced memory deficit remains largely unknown. In diabetes, ketogenesis occurs upon insulin deficiency, and β-hydroxybutyrate (β-OHB) is synthesized and plays a dominant role in diabetic ketoacidosis. In the present study, we investigate the effect of β-OHB-mediated lysine β-hydroxybutyrylation (kbhb) of hippocampal calcium/calmodulin-dependent kinase II-α (CaMKII-α) on memory deficits in male T1DM mice. We find that streptozotocin (STZ) induced a significant increase in the concentration of hippocampal β-OHB in T1DM mice. High β-OHB levels promote CaMKII-α kbhb at the K42 and K267 residues and further inhibit CaMKII activity. The suppression of CaMKII-α kbhb in the hippocampus via the inhibition of P300, a kbhb transferase, reverse the decrease in CaMKII activity and alleviate memory deficits in T1DM mice. Molecular dynamics (MD) simulations further reveale that the enhanced flexibility caused by CaMKII-α kbhb on the critical, conserved residue K42, which alters its side chain, in the catalytic ATP-binding site of this enzyme may be one of the factors responsible for the observed reduction enzymatic activity. Collectively, our results show that a high β-OHB concentration dysregulates hippocampal CaMKII-α kbhb, which may contribute to memory deficits in T1DM mice.

Similar content being viewed by others
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by hyperglycemia due to the destruction of pancreatic β-cells1,2. The brain is especially vulnerable to hyperglycemia, which may lead to diabetic neuropathy by increasing reactive oxygen species (ROS) production and cytokine release and further triggering brain inflammation3,4. In addition, hyperglycemia can cause long-term neurological complications, such as cognitive and memory deficits and dementia5. Patients with diabetes are more likely than individuals without diabetes to experience cognitive dysfunction6. Preclinical studies have also shown that rodents administered streptozotocin (STZ) to induce a model of T1DM exhibit poor performance in memory-related tests7. Therefore, T1DM is considered a strong risk factor for cognitive decline. To date, several potential mechanisms of hyperglycemia-induced cognitive dysfunction have been proposed, including the excessive production of free radicals and ROS8 and the dysregulation of several extracellular and intracellular signaling cascades in the brain; these mechanisms are also thought to be involved in impaired neuronal synaptic function9. We also found that during the recurrent hypoglycemic episodes that occur after insulin injection in T1DM patients, cognitive performance can be seriously impaired, which has been attributed to repeated severe hypoglycemia causing both significant neuronal death and cognitive impairment10,11.
Currently, alterations in memory are associated with changes in brain metabolite levels in T1DM patients12. In individuals with diabetes, ketogenesis results from absolute or relative insulin deficiency, which is accompanied by increased concentrations of glucagon and other counterregulatory hormones during periods of hyperglycemia. This results in increased lipolysis and induces the liver to produce large quantities of ketone bodies via fatty acid oxidation13. β-Hydroxybutyrate (β-OHB), the most abundant ketone body, is synthesized in the liver and plays a dominant role in diabetic ketoacidosis; additionally, its concentration is strongly correlated with the degree of ketoacidosis14,15. The high levels of β-OHB found in the hippocampi of T1DM patients can alter the functional connectivity between cortical and hippocampal regions and affect memory12. However, the mechanism by which high levels of β-OHB affect memory in individuals with T1DM remains unclear.
β-OHB circulates among extrahepatic tissues during starvation, prolonged exercise, or adherence to a ketogenic diet (KD)16. This ketone body acts as the major fuel for energy production and replaces glucose as an important source of energy for the brain during KD consumption17. Interestingly, β-OHB-mediated lysine β-hydroxybutyrylation (kbhb) has been identified as a novel posttranslational modification (PTM) in recent years18. During kbhb, β-OHB serves as the β-hydroxybutyryl donor; the intermediate metabolite β-hydroxybutyryl-CoA is synthesized first and is subsequently be used by P300 as a cofactor for kbhb19,20.
The α isoform of calcium/calmodulin-dependent kinase II (CaMKII-α) is an important synaptic kinase for memory formation21. Upon N-methyl-D-aspartate (NMDA) receptor activation, CaMKII-α undergoes autophosphorylation, activating CaMKII22. Because CaMKII activity is essential for NMDA receptor-dependent long-term potentiation (LTP) and contextual long-term memory formation23, the inhibition of CaMKII activity impairs the retention of spatial memory and the formation of contextual fear memory24,25. We previously reported that when β-OHB is present at high levels, it acts as a β-hydroxybutyryl donor and affects hippocampal CaMKII-α kbhb, thus attenuating cocaine-associated memory26. However, whether a high concentration of β-OHB in the hippocampus contributes to T1DM-induced memory deficit by altering CaMKII-α kbhb is unclear.
In this study, we found that the level of hippocampal β-OHB was significantly increased in T1DM mice with impaired memory. β-OHB significantly promoted kbhb of hippocampal CaMKII-α at the K42 and K267 residues and thus reduced CaMKII activity. Our findings reveal a new link between metabolite-mediated PTMs and memory deficits in T1DM mice and identify a potential therapeutic target for diabetes-induced memory deficits.
Results
High levels of β-OHB promote CaMKII-α kbhb in the hippocampi of T1DM mice
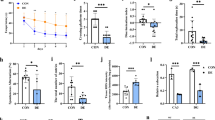
Previous studies have shown that a single dose of 200 mg/kg STZ induces T1DM27 and is accompanied by reduced insulin levels, hyperglycemia, and elevated blood levels of β-OHB in mice12,28. We injected STZ into mice to construct a T1DM mouse model in the present study. Most of the β-OHB present in the brain is produced by the liver and enters the brain via the bloodstream17. We collected liver tissue, blood plasma and hippocampal tissue from T1DM mice to measure the levels of β-OHB. As expected, 4 days after STZ injection, the blood glucose levels were significantly greater in the STZ-treated mice than in the control mice, indicating that the T1DM mouse model was successfully established (Fig. 1A; t(12) = 21.14, p < 0.0001). Furthermore, the levels of β-OHB were significantly increased in the liver, blood and hippocampus of STZ-treated mice 4 days after injection (Fig. 1B–D; liver β-OHB: t(10) = 7.449, p < 0.0001; blood β-OHB: t(10) = 46.79, p < 0.0001; hippocampal β-OHB: t(10) = 9.128, p < 0.0001). To explore whether hippocampal β-OHB production increased in a time-dependent manner after STZ injection, we detected the levels of β-OHB in the hippocampus at 4, 7, 14 and 28 days after STZ injection and found that it was increased at 14 and 28 days (Fig. 1E; F(3, 20) = 36.36, p < 0.0001).
A–D Levels of blood glucose, liver β-OHB, blood β-OHB, and hippocampal β-OHB in mice treated with STZ or vehicle 4 days after injection (blood glucose: n = 7 per group; liver β-OHB: n = 6 per group; blood β-OHB: n = 6 per group; and hippocampal β-OHB: n = 6 per group). E Increase in the hippocampal β-OHB concentration in mice 4 to 28 days after injection of STZ (n = 6 per group). F Degree of exogenous CaMKII-α kbhb at the K42 and K267 sites in HT22 cells treated with LV expressing CaMKII-α or mutant CaMKII-α in the presence of β-OHB (CaMKII-α kbhb at the K42 and K267 sites: n = 3 per group). G Degree of kbhb at the K42 and K267 sites in the recombinant CaMKII-α protein after β-OHB-CoA treatment. H Degree of hippocampal CaMKII-α kbhb at the K42 and K267 sites in mice 4 to 28 days after injection of STZ (n = 6 per group). The data are presented as the means ± SEMs; unpaired t test or one-way ANOVA; **p < 0.01 and ****p < 0.0001. Veh: vehicle; STZ: streptozotocin; bhb: β-hydroxybutyrylation.
Because β-OHB acts as a β-hydroxybutyryl donor to induce CaMKII-α kbhb at K42 and K26726, we continued to examine whether the CaMKII-α kbhb level in the hippocampus increased in a time-dependent manner at 4, 7, 14 and 28 days after STZ injection. First, to validate the specific antibodies targeting the K42 and K267 kbhb sites of CaMKII-α, we transfected HT22 cells (a mouse hippocampal neuronal cell line) with a lentivirus (LV) expressing Flag-tagged CaMKII-α or mutated CaMKII-α (K42M and K267A). We purified and enriched the wild-type and mutated CaMKII-α proteins from β-OHB-treated or untreated HT22 cells by immunoprecipitation (IP), followed by detection of protein modification using two specific antibodies. The results revealed that the degrees of K42 and K267 bhb were significantly increased in β-OHB-treated cells, whereas K42 and K267 bhb were completely abolished in cells expressing mutant CaMKII-α regardless of whether they were treated with β-OHB (Fig. 1F). Second, the recombinant CaMKII-α protein was incubated with β-hydroxybutyryl-CoA at 37 °C for 2 h, and the antibodies were able to detect the K42 and K267 residues of CaMKII-α after β-hydroxybutyryl-CoA treatment. However, these kbhb sites were not detected in the absence of β-hydroxybutyryl-CoA (Fig. 1G). These findings suggest that these two specific antibodies could target the K42 and K267 residues of CaMKII-α after kbhb. Finally, we detected the degree of CaMKII-α kbhb using these two specific antibodies and found that the degree of CaMKII-α kbhb (K42 and K267) increased in the hippocampus 14 and 28 days after STZ injection (Fig. 1H; K42 bhb: F(3, 20) = 11.63, p < 0.001; K267 bhb: F(3, 20) = 12.4, p < 0.0001), indicating that STZ injection increased the levels of hippocampal β-OHB and CaMKII-α kbhb over time.
To evaluate whether hippocampus-dependent memory is affected in T1DM mice, we performed four well-established behavioral tests, the Y maze test, a novel object location test, a novel object recognition test, and a contextual fear conditioning test29, 4 weeks after STZ injection30. In the Y maze test, which assesses working memory, STZ-treated mice spent a significantly reduced percentage of time in the novel arm, suggesting working memory deficit (Fig. 2A; t(12) = 2.563, p < 0.05). In the novel object location test, which is a spatial memory test, T1DM mice displayed less preference for the location of the novel object (Fig. 2B; t(12) = 2.316, p < 0.05), indicating a deficit in spatial memory. Similarly, in the novel object recognition test, which analyzes long-term recognition memory, T1DM mice showed a reduced preference to explore the novel object (Fig. 2C; t(12) = 5.174, p < 0.001), indicating a deficit in long-term memory. We also conducted a contextual fear conditioning test to assess context-associated memory and pattern separation. The mice were conditioned to a context with 3 footshocks (context A) and exposed to an unconditioned but somewhat similar context (context B) the next morning. We then quantified their ability to discriminate between the two contexts after training. The results revealed that T1DM mice exhibited significant impairments in context-associated memory and pattern separation, as evidenced by a reduction in freezing in response to context A and a decreased contextual freezing ability one day after training (Fig. 2D–F; context A: t(12) = 5.066, p < 0.001; discrimination index: t(12) = 2.186, p < 0.05). Collectively, our data revealed a link between high hippocampal β-OHB concentrations and memory deficits in T1DM mice.
A–C Mice were treated with STZ or vehicle, and the Y-maze test, novel object location test and novel object recognition test were subsequently performed (n = 7 per group). D–F Freezing behavior in context A (conditioned) and context B (unconditioned) and the discrimination index of mice treated with STZ or vehicle (n = 7 per group). G Hippocampal HDAC activity in mice treated with STZ or vehicle (n = 5 per group). H, I Degree of hippocampal CaMKII-α kbhb at the K42 and K267 sites and CaMKII activity in mice treated with STZ or vehicle (n = 6 per group). The data are presented as the means ± SEMs; unpaired t test; *p < 0.05 and ***p < 0.001. Veh vehicle, STZ streptozotocin, bhb β-hydroxybutyrylation.
A recent study revealed that β-OHB, which acts as an endogenous blocker of histone deacetylase (HDAC), increases histone acetylation at the Foxo3a and Mt2 promoters31 and modulates hippocampus-dependent memory deficits in mice with Kabuki syndrome32. We thus investigated whether hippocampal HDAC activity was altered in T1DM mice after the behavioral tests. Interestingly, we found that STZ injection did not affect hippocampal HDAC activity (Fig. 2G), suggesting that endogenous HDAC may not be involved in the induction of memory impairment by high levels of β-OHB in T1DM mice. We further detected the degree of CaMKII-α kbhb and found that STZ injection increased the degree of CaMKII-α kbhb at K42 and K267 in the hippocampus (Fig. 2H; K42 bhb: t(10) = 2.925, p < 0.05; K267 bhb: t(10) = 3.016, p < 0.05). To further explore the potential link between CaMKII-α kbhb and CaMKII activity in the hippocampus of T1DM mice, we measured hippocampal CaMKII activity. Notably, we observed a significant decrease in hippocampal CaMKII activity in T1DM mice (Fig. 2I; t(10) = 2.301, p < 0.05), suggesting that high levels of β-OHB may promote CaMKII-α kbhb and attenuate CaMKII activity in T1DM mice.
High levels of β-OHB promote CaMKII-α kbhb in the hippocampus and impair memory
To investigate whether a high concentration of β-OHB directly affects memory in mice through CaMKII-α kbhb, mice were injected (i.p.) with β-OHB (600 mg/kg) once daily for 10 consecutive days. We found that β-OHB-treated mice exhibited high levels of β-OHB in both the blood and hippocampus (Fig. 3A, B; blood β-OHB: t(10) = 38.2, p < 0.0001; hippocampal β-OHB: t(10) = 7.273, p < 0.0001). We also measured the weights of the mice before and after β-OHB treatment, and there were no differences in body weight after β-OHB treatment (Fig. S1A and B), suggesting that β-OHB injection did not affect the mouse weight. In the memory-related behavior tests, β-OHB-treated mice displayed memory impairment, as evidenced by the reduction in the percentage of time spent in the novel arm in the Y maze test (Fig. 3C; t(12) = 2.898, p < 0.05), a decreased preference for the novel object in both the novel object location and novel object recognition tests (Fig. 3D, E; novel object position: t(12) = 4.735, p < 0.001; novel object recognition: t(14) = 7.307, p < 0.0001), and less freezing behavior in context A and contextual freezing (Fig. 3F–H; context A: t(12) = 5.596, p < 0.001; discrimination index: t(12) = 2.827, p < 0.05). To further explore the difference between unlearned freezing and memory after β-OHB treatment in the conditioning test, β-OHB-treated mice were kept in the conditioning context A chamber to receive footshock or not. We found an increase in the freezing behavior in context A in the mice that received footshock (Fig. S2A–C), indicating that contextual fear memory expression could be assessed by manually evaluating learned freezing behavior after β-OHB treatment. We continued to explore whether footshock affects the pain threshold of mice. In the hot plate test, there was no difference in the pain thresholds of the mice that did and did not receive footshock (Fig. S2D). Similarly, we also found that pain latency was not different between the STZ- or β-OHB-treated mice and the control mice (Fig. S3A).
A, B Blood and hippocampal levels of β-OHB in mice injected with β-OHB or saline for 10 days (blood β-OHB: n = 6 per group; hippocampal β-OHB: n = 6 per group). C–E Mice were injected with β-OHB or saline, and the Y-maze test, novel object location test and novel object recognition test were subsequently performed (Y-maze test: n = 7 per group; novel object position test: n = 7 per group; novel object recognition test: n = 8 per group). F–H Freezing behavior in context A (conditioned) and context B (unconditioned) and the discrimination index of mice injected with β-OHB or saline for 10 days (n = 7 per group). I Hippocampal HDAC activity in mice injected with β-OHB or saline for 10 days (n = 6 per group). J Hippocampal H2Aac, H2A, H3ac, H3, H4ac and H4 levels in mice injected with β-OHB or saline for 10 days (n = 6 per group). K, L Degree of hippocampal CaMKII-α kbhb at the K42 and K267 sites and CaMKII activity in mice injected with β-OHB or saline for 10 days (n = 6 per group). The data are presented as the means ± SEMs; unpaired t test; *p < 0.05, ***p < 0.001 and ****p < 0.0001. bhb: β-hydroxybutyrylation.
In addition, previous studies have shown that β-OHB can act as an endogenous HDAC inhibitor31, and suppression of HDAC activity increases histone acetylation to affect cognition and memory in diabetic mice33. Interestingly, the mice that received a high concentration of β-OHB exhibited no change in HDAC activity or histone (2A, 3 and 4) acetylation in the hippocampus (Fig. 3I, J) but did show a significant increase in the degrees of CaMKII-α kbhb at K42 and K267 and a decrease in hippocampal CaMKII activity (Fig. 3K, L; K42 bhb: t(10) = 2.981, p < 0.05; K267 bhb: t(10) = 3.035, p < 0.05; CaMKII activity: t(10) = 2.41, p < 0.05). In addition, we detected hippocampal CaMKII activity before or after the behavior test in β-OHB-treated mice and found that CaMKII activity was not altered (Fig. S4A, B), indicating that hippocampal CaMKII activity was regulated mainly by β-OHB. Collectively, these data showed high concentrations of β-OHB may impair memory in mice by increasing CaMKII-α kbhb rather than modulating HDAC activity or histone acetylation in the hippocampus.
Finally, to confirm that insulin could alleviate the memory effects induced by high concentrations of β-OHB, mice were pretreated with insulin (10 I.U./kg/day) daily for 5 days during the administration of β-OHB. We found that insulin decreased mouse blood glucose levels (Fig. S5B; F(2, 21) = 15.00, p < 0.0001) but did not reverse the β-OHB-induced memory deficits detected in the memory-related behavior tests (Fig. S5C–K; Y maze test: F(2, 18) = 5.622, p < 0.05; novel object position: F(2, 18) = 12.25, p < 0.001; novel object recognition: F(2, 18) = 20.2, p < 0.0001; context A: F(2, 18) = 8.133, p < 0.01; discrimination index: F(2, 18) = 3.075, p < 0.05).
High levels of β-OHB impair hippocampal synaptic function
Because CaMKII activity plays an important role in long-term potentiation (LTP), we hypothesized that the decrease in CaMKII activity induced by β-OHB may decrease the plasticity of hippocampal excitatory synapses and thus cause memory deficits. To this end, we explored synaptic short-term plasticity using a paired-pulse facilitation (PPF) protocol in hippocampal slices. Typical PPF traces with a 50-ms interpulse interval were observed in the two groups of mice (Fig. 4A). However, we detected a significant reduction in the P2/P1 ratio at all tested P1 and P2 intervals in the β-OHB-treated mice at 50 and 100 ms (Fig. 4B; 50 ms: t(15) = 2.371, p < 0.05; 100 ms: t(15) = 2.571, p < 0.05), suggesting decreased hippocampal short-term synaptic plasticity. We further measured theta burst stimuli-induced LTP in the hippocampal Schaffer collateral-CA1 pathway. The time to a normalized field excitatory postsynaptic potential (fEPSP) slope (baseline was 1) was plotted, and the results revealed impairments in LTP induction (0–10 min after theta burst stimulation) and maintenance (50–60 min after theta burst stimulation) in the β-OHB-treated mice (Fig. 4C, D). Moreover, statistical analyses revealed that the extents to which LTP was induced and maintained decreased in the β-OHB-treated mice (Fig. 4E, F; induction: t(38) = 3.74, p < 0.01; maintenance: t(38) = 94.74, p < 0.0001), suggesting that LTP was reduced in the β-OHB-treated mice during the induction and maintenance phases.
A Typical fEPSP responses evoked by two stimuli at an interval of 50 ms. B P2/P1 ratios evoked by different paired-pulse stimulation intervals (25, 50, 100, and 400 ms) (n = 8 for the saline group; n = 9 for the β-OHB group). C Typical fEPSPs recorded before and TBS. D Summary of hippocampal CA1 LTP data from mice injected with β-OHB or saline. E, F LTP induction (0–10 min after tetanic stimulation) and maintenance (50–60 min after tetanic stimulation) in mice injected with β-OHB or saline (n = 20 slices from 8 mice in the saline group; n = 20 slices from 9 mice in the β-OHB group). The data are presented as the means ± SEMs; unpaired t test; *p < 0.05, ***p < 0.001 and ****p < 0.0001. TBS theta burst stimulation.
CaMKII-α kbhb in the hippocampal CA1 region is involved in memory deficits in T1DM mice
To explore the involvement of CaMKII-α kbhb at the K42 and K267 residues in the hippocampal CA1 region in T1DM mice with memory impairment, an adeno-associated virus (AAV)-expressing CaMKII-α or mutant CaMKII-α (K42M and K267A) was infused into the hippocampal CA1 region of the mice 20 days before STZ injection (Fig. 5A, B; Fig. S6A). Western blot analysis revealed that STZ injection increased K42 and K267 kbhb in the hippocampal CA1 region of AAV-CaMKII-α-treated mice; however, the degree of kbhb did not change in the mice treated with AAV or AAV-mutant CaMKII-α alone (Fig. 5C; K42 bhb: F(2,15) = 6.832, p < 0.01; K267 bhb: F(2.15) = 7.34, p < 0.01; CaMKII-α: F(2,15) = 7.263, p < 0.01). Behavioral tests revealed that T1DM mice treated with AAV-CaMKII-α presented more impaired memory in the Y-maze test (Fig. 5D; F(2, 25) = 10.19, p < 0.001) and reduced exploration and recognition of the object in the novel location and novel object tests (Fig. 5E, F; object in the novel location: F(2, 25) = 6.106, p < 0.01; novel object: F(2, 25) = 5.214, p < 0.05), indicating further deficits in spatial memory and long-term memory. In the contextual fear conditioning test, T1DM mice overexpressing CaMKII-α in the hippocampus also exhibited further impairments in fear-associated memory and contextual freezing ability (Fig. 5G–I; context A: F(2, 22) = 7.504, p < 0.01; discrimination index: F(2, 22) = 7.045, p < 0.01). However, compared with AAV-CaMKII-α-treated mice, AAV-mutant CaMKII-α-treated mice presented increased memory in these tests (Fig. 5D–I). Together, our results showed that increasing CaMKII-α kbhb at K42 and K267 in the hippocampal CA1 region may lead to further memory deficits in T1DM mice.
A Schematic of the AAV injection procedure. All the mice were treated with STZ to establish a T1DM model before being subjected to memory-related behavior tests. B Representative images of exogenous CaMKII-α expression in the hippocampal CA1 region (green) and DAPI (blue) staining; scale bar, 100 μm. C The degree of CaMKII-α kbhb at the K42 and K267 sites in the hippocampal CA1 region of mice injected with AAVs expressing CaMKII-α or mutant CaMKII-α (n = 6 per group). D–F Mice were injected with AAV-expressing CaMKII-α or mutant CaMKII-α, and the Y-maze test, novel object location test and novel object recognition test were subsequently performed (n = 9 for the AAV group; n = 10 for the AAV-CaMKII-α group; n = 9 for the AAV-mutant CaMKII-α group). G–I Freezing behavior in context A (conditioned) and context B (unconditioned) and the discrimination index of mice injected with an AAV expressing CaMKII-α or mutant CaMKII-α were measured (n = 8 for the AAV group; n = 9 for the AAV-CaMKII-α group; n = 8 for the AAV-mutant CaMKII-α group). The data are presented as the means ± SEMs; one-way ANOVA; *p < 0.05 and **p < 0.01. Mutant CaMKII-α: K42M and K267A mutations; AAV refers to AAV-EGFP mice; AAV-CaMKII-α refers to CaMKII-α-overexpressing mice; and AAV-mutant CaMKII-α refers to mutant CaMKII-α-overexpressing mice.
To confirm the involvement of CaMKII-α kbhb at the K42 and K267 residues in the CA1 region in synaptic short-term plasticity, AAV-expressing CaMKII-α or mutant CaMKII-α was infused into the hippocampal CA1 region of mice 20 days before β-OHB injection (Fig. 6A). Typical PPF traces with a 50-ms interpulse interval were observed in each group (Fig. 6B). However, we observed a significant further decrease in the P2/P1 ratio at all tested P1 and P2 intervals in AAV-CaMKII-α-treated mice at 50 and 100 ms after β-OHB injection (Fig. 6C; 50 ms: F(2, 15) = 9.469, p < 0.05; 100 ms: F(2, 15) = 11.39, p < 0.05; 400 ms: F(2, 15) = 9.005, p < 0.05). In the LTP test, the degrees of LTP induction and maintenance further decreased in the AAV-CaMKII-α-treated mice after β-OHB injection (Fig. 6D–G; induction: Kruskal‒Wallis statistic=7.52, p < 0.05; maintenance: Kruskal‒Wallis statistic=10.15, p < 0.01). Interestingly, the P2/P1 ratio and LTP maintenance in AAV- and AAV-mutant CaMKII-α-treated mice were greater than those of AAV-CaMKII-α-treated mice after β-OHB injection (Fig. 6C–G).
A Schematic of the AAV injection procedure. All the mice were injected with β-OHB to achieve a high concentration of β-OHB, after which electrophysiological analysis was performed. B Typical fEPSP responses evoked by two stimuli at an interval of 50 ms. C P2/P1 ratios evoked by different paired-pulse stimulation intervals (25, 50, 100, and 400 ms) (n = 6 per group). D Typical fEPSPs in each group. E Summary of hippocampal CA1 LTP data from mice injected with an AAV expressing CaMKII-α or mutant CaMKII-α. F, G LTP induction and maintenance in mice injected with an AAV expressing CaMKII-α or mutant CaMKII-α (n = 6 slices from 3 mice in the AAV group; n = 6 slices from 4 mice in the AAV-CaMKII-α group; n = 6 slices from 4 mice in the AAV-mutant CaMKII-α group). The data are presented as the means ± SEMs; one-way ANOVA or the Kruskal‒Wallis test; *p < 0.05 and **p < 0.01. Mutant CaMKII-α: K42M and K267A mutations; AAV refers to AAV-EGFP mice; AAV-CaMKII-α refers to CaMKII-α-overexpressing mice; and AAV-mutant CaMKII-α refers to mutant CaMKII-α-overexpressing mice.
β-OHB suppresses hippocampal CaMKII activity via kbhb at K42 and K67
We next investigated whether CaMKII-α kbhb directly regulates CaMKII activity in vitro by treating HT22 cells with β-OHB for 24 h. As expected, 24 h of treatment with 40 mM β-OHB markedly increased the degree of CaMKII-α kbhb at K42 and K267 but decreased CaMKII activity (Fig. S7A, B; K42 bhb: t(6) = 3.042, p < 0.05; K267 bhb: t(6) = 2.838, p < 0.05; CaMKII activity: t(6) = 3.101, p < 0.05). To determine whether CaMKII-α kbhb at K42 and K267 regulates CaMKII activity, HT22 cells were transfected with a LV expressing CaMKII-α or mutated CaMKII-α (K42M and K267A). As expected, β-OHB (40 mM) treatment significantly increased CaMKII-α in LV-CaMKII-α-transfected cells (Fig. S7C; K42 bhb: F(2, 9) = 11.08, p < 0.05; K267 bhb: F(2, 9) = 10.91, p < 0.05; CaMKII-α: F(2, 9) = 27.44, p < 0.01), but the degree of CaMKII-α kbhb at K42 and K267 was not altered in the LV-mutated CaMKII-α-transfected cells (Fig. S7C). In addition, β-OHB (40 mM) treatment further decreased CaMKII activity in the LV-CaMKII-α-transfected cells compared with that in the LV- and LV-mutated CaMKII-α-transfected cells (Fig. S7D; F(2, 9) = 17.82; p < 0.01).
P300 is a transcriptional coactivator that regulates gene transcription through histone acylation34 and can catalyze multiple types of lysine acylation, such as acetylation, crotonylation35 and 2-hydroxyisobutyrylation36. We thus hypothesized that P300 catalyzes CaMKII-α kbhb. To test this hypothesis, HT22 cells were treated with the selective P300 inhibitor A485 (3 μM) and then with β-OHB (40 mM). We found that β-OHB significantly increased CaMKII-α kbhb at K42 and K267; however, this increase was reversed by the addition of A485 (Fig. S7E; K42 bhb: F(2, 9) = 16.3, p < 0.05; K267 bhb: F(2, 9) = 32.25, p < 0.0001), indicating that inhibiting P300 directly suppressed CaMKII-α kbhb. Similarly, the β-OHB-mediated decrease in CaMKII activity was reversed by A485 treatment (Fig. S7F; F(2, 9) = 5.367, p < 0.05). To confirm that P300 indeed directly catalyzed CaMKII-α kbhb, siRNA oligo pairs targeting P300 were transfected into HT22 cells, and P300 expression was efficiently knocked down (Fig. S7G; t(6) = 7.306, p < 0.01). Western blot analysis revealed decreased CaMKII-α kbhb at K42 and K267 in P300-deficient cells (Fig. S7G; K42 bhb: t(6) = 3.367, p < 0.05; K267 bhb: t(6) = 4.335, p < 0.01), indicating that P300 is a β-hydroxybutyryltransferase for CaMKII-α kbhb at the K42 and K267 residues after β-OHB (40 mM) treatment. Similarly, CaMKII activity was significantly increased in P300-knockdown cells after β-OHB (40 mM) treatment (Fig. S7H; t(8) = 2.77, p < 0.05). Taken together, these findings indicate that β-OHB can induce CaMKII-α kbhb at K42 and K267 and thus inhibit CaMKII activity and that this effect may be mediated directly by P300 in vitro.
We further assessed whether inhibiting CaMKII-α kbhb in the hippocampal CA1 region of T1DM mice could alleviate memory deficits by increasing CaMKII activity. AAV-sh-P300 was stereotactically infused into the hippocampal CA1 region of the mice 20 days before STZ injection (Fig. 7A). Not surprisingly, inhibition of P300 markedly reduced kbhb at K42 and K267 but increased CaMKII activity in the hippocampal CA1 region of T1DM mice (Fig. 7B, C; P300: t(10) = 5.462, p < 0.001; K42 bhb: t(10) = 3.116, p < 0.05; K267 bhb: t(10) = 2.781, p < 0.05; CaMKII activity: t(6) = 3.336, p < 0.05). In addition, AAV-sh-P300 treatment increased the performance of T1DM mice in the memory-related Y-maze, novel object position test, novel object recognition test and contextual fear conditioning test compared with that of the AAV-treated mice (Fig. 7D–I; Y-maze: t(12) = 2.755, p < 0.05; novel object position: t(12) = 2.483, p < 0.05; novel object recognition: t(12) = 2.294, p < 0.05; context A: t(12) = 2.315, p < 0.05; discrimination index: t(12) = 2.355, p < 0.05). Collectively, these findings indicate that P300-mediated CaMKII-α kbhb in the hippocampal CA1 region is involved in memory deficits in T1DM mice.
A Schematic of the AAV injection procedure and memory-related behavior tests. B, C Level of P300 and degree of CaMKII-α kbhb in the hippocampal CA1 region and CaMKII activity in T1DM mice injected with AAV expressing sh-P300 (P300, CaMKII-α kbhb at K42 and K267: n = 6 per group; CaMKII activity: n = 4 per group). D–F T1DM mice were injected with AAV expressing sh-P300, and the Y-maze test, novel object location test and novel object recognition test were subsequently performed (n = 7 per group). G–I Freezing behavior in context A (conditioned) and context B (unconditioned) and the discrimination index of T1DM mice injected with AAV expressing sh-P300 (n = 7 per group). The data are presented as the means ± SEMs; unpaired t test; *p < 0.05 and ***p < 0.001.
Molecular dynamics (MD) simulations revealed the potential mechanism by which kbhb at K42 and K267 affects CaMKII-α activity
Through comparative MD simulations, we further examined the potential mechanism by which kbhb at K42 and K267 affects CaMKII activity. First, we constructed wild-type CaMKII-α, CaMKII-α kbhb and mutant CaMKII-α systems through side chain modification and virtual site-directed mutagenesis on the basis of the CaMKII-α holoenzyme structure (Protein Data Bank [PDB] code: 5U6Y). Because CaMKII-α autophosphorylation at the critical residue threonine 286 (T286) is involved in CaMKII activation37, we next explored whether kbhb at K42 and K267 affects the autophosphorylation of CaMKII-α at T286 to ultimately reduce CaMKII activity. As indicated by the full-length holoenzyme structure, the linker is a flexible loop composed of 32 residues, and the connected hub is separated from the critical K42, K267 and T286 sites (Fig. S8A). The energies of the wild-type CaMKII-α, CaMKII-α kbhb and mutant CaMKII-α systems reached equilibrium after 5 ns, with average potential energies/standard deviations of –1.58 × 105/430, –1.52 × 105/460 and –1.58 × 105/430 kcal/mol, respectively (Fig. S8B). Because the sequences contained more than 300 residues, the fluctuation rate of ~0.3% was essentially negligible, indicating that these comparative MD simulations were stable and reliable. As expected, the root mean square deviation (RMSD) of CaMKII-α kbhb was clearly greater than that of normal and mutant CaMKII-α, indicating that residues that undergo kbhb directly affect the flexibility of the whole system and increase its overall motion (Fig. S8C). Moreover, as an important complement to the global convergence of the system, the last 80 ns of the MD trajectories (120–200 ns) of each of the three systems were relatively stable; these segments were defined as the equilibrium portions and were supported by the distributions of the radii of gyration (Fig. S8D).
At the structural level, the key residue T286 is buried at the interface between the regulatory segment and the kinase domain (Fig. S8A); thus, for T286 to be autophosphorylated, it must be exposed to the solvent37. To investigate the effects of CaMKII-α kbhb and mutant CaMKII-α on the degree of solvation of T286, solvent-accessible surface area (SASA) analysis, a reliable method for residual solvation measurement, equilibrium MD trajectories were employed. Unsurprisingly, the average SASAs of the T286 site in the three systems were negative because T286 remained in a hydrophobic cavity due to the lack of Ca2+/CaM binding (Fig. S8E). Specifically, the SASA of T286 in the CaMKII-α kbhb system was slightly lower than that in the other two systems, which indicated that kbhb at K42 and K267 could prevent the exposure of T286 to the solvent and was not conducive to T286 autophosphorylation. To further investigate protein flexibility at the residue level, root mean square fluctuation (RMSF) values were calculated for the three CaMKII-α systems, and their distribution trends appeared to be similar. The RMSF results revealed that the molecular flexibilities of the systems followed the order of CaMKII-α kbhb>mutant CaMKII-α > CaMKII-α (Fig. S8F). These findings were basically consistent with the results from the SASA analysis. Notably, residue K42 is conserved across all kinases that bind ATP and catalyze phosphotransferase reactions. Catalytic residues typically display structural rigidity to maintain a defined conformation and spatial arrangement so that the enzyme can interact with the substrate efficiently, thereby accelerating the catalytic reaction. In contrast, the enhanced flexibility caused by the PTM on this critical Lys residue in the ATP-binding pocket is anticipated to perturb ATP binding by altering its side chain, consequently leading to no autophosphorylation and activity loss.
To further analyze the motion of and conformational changes in the three CaMKII-α systems, principal component analysis (PCA), a standard method of effectively extracting functional motion information from complex MD trajectories through dimensionality reduction, was carried out for the three systems. We obtained the free energy landscapes (FELs) and corresponding principal component fluctuations for the three CaMKII-α systems on the basis of their MD trajectories (Fig. S9A–F). The results suggested that the wild-type (Fig. S9A, D) and mutant CaMKII-α systems (Fig. S9C, F) had FELs. In the CaMKII-α kbhb system, the FEL could be divided into four independent regions of low free energy in which the conformation of the system expanded significantly (Fig. S9B, E). These data indicated that the CaMKII-α kbhb system exhibited the largest conformational changes among the three systems, which was consistent with the previous RMSD and RMSF data.
According to the representative snapshots of the low free energy regions in the three systems, there were clear differences in the conformations of the N-lobe (Y13-L91) and CaM-binding element (F293-T310) on the regulatory segment. In the representative conformations of the CaMKII-α kbhb system, the initially disordered CaM-binding region tended to form an α-helix instead of a flexible loop (Fig. S9H). However, similar conformations were not observed with the wild-type or mutant CaMKII-α systems (Fig. S9G, I). Therefore, we quantitatively examined the secondary structures of the whole regulatory segment (R274-S314) throughout each MD simulation in the three CaMKII-α systems (Fig. S8G). The results showed that the residues T305-N312 formed an additional stable α-helix in the CaMKII-α kbhb system, and nearly 60% of the regulatory segment was composed of helices. However, the percentages of helices in the wild-type CaMKII-α and mutant CaMKII-α systems were 45.7% and 32.9%, respectively (Fig. S8G). These results also suggested that CaMKII-α kbhb induces the distal regulatory segment to adopt a highly α-helical conformation, indicating the long-range allosteric regulation of kbhb.
Discussion
Cognitive dysfunction and memory deficit are the most common symptoms of advanced diabetes38. Previous studies on memory loss in diabetes patients have focused mainly on blood-brain barrier disruption or neuroinflammation, which are accompanied by microglial activation and cytokine release, and little attention has been given to the effects of β-OHB. Thus, elucidating the neurobiological basis of T1DM-induced memory deficits is critical for developing treatment strategies. In the present study, we revealed that high levels of β-OHB increased the degree of CaMKII-α kbhb in the hippocampus and thus inhibited CaMKII activity, contributing to memory deficits in T1DM mice. Therefore, inhibiting abnormal CaMKII-α kbhb in the hippocampus is a potential strategy for the treatment of diabetes-induced memory deficits.
Previous studies have shown that a single β-OHB infusion can improve the working memory performance of patients with type 2 diabetes39, indicating that β-OHB can improve memory in these individuals. However, our results showed that high levels of hippocampal β-OHB may contribute to memory deficits in T1DM mice. To date, studies on the beneficial effects of ketones on memory impairment have focused mainly on the use of glucose as the primary energy source for the body40,41. For example, a moderate increase in ketone levels can reduce cerebral glucose metabolism and improve brain function42. Moreover, long-term ketogenic treatment can alleviate mild cognitive deficits in patients by inducing only slight ketosis to reduce cerebral glucose metabolism43. In addition, β-OHB sustains synaptic activity in adult rats only when blood glucose levels are lower44. These studies showed that a low concentration of ketones may effectively alleviate cognitive deficits by reducing cerebral glucose metabolism.
In patients with diabetes, the absence of insulin coupled with glucagon release drives gluconeogenesis in the liver, lipolysis in adipose tissue, and hepatic ketogenesis45. Increased ketogenesis is the major factor responsible for the development of ketoacidosis, which is known to elicit oxidative stress and inflammatory responses in patients with diabetes. The buildup of ketones in the blood, which can decrease the blood pH, can lead to ketoacidosis, which may induce cognitive deficits and memory loss in T1DM patients5. Considering these previous results together with our findings, we speculate that the role of β-OHB in T1DM-induced memory deficit may depend on the concentration of β-OHB in the brain.
Under disease conditions, β-OHB serves as an epigenetic regulator in addition to a metabolite and participates in cellular signaling, epigenetic control and histone lysine PTM18,46,47. At high levels, β-OHB preferentially serves as a substrate for lysine kbhb26. Intriguingly, we found that the HDAC activity and histone acetylation levels in the hippocampus were not altered by β-OHB, suggesting that at high levels, β-OHB may preferentially serve as a substrate for CaMKII-α kbhb in mice with T1DM. Our findings revealed a link between β-OHB-mediated CaMKII-α kbhb and memory deficits, indicating the mechanism underlying how high levels of β-OHB affect memory in T1DM mice.
In the present study, we found that P300 selectively catalyzed kbhb of CaMKII-α, a nonhistone protein, which is in line with the findings of previous studies showing that P300 can selectively catalyze lysine PTMs on distinct protein substrates36. However, the differences in the specificity of lysine acetylation and other types of lysine acylation remain unknown. For example, P300, CREBBP-binding protein (CBP), and lysine acetyltransferase 8 (KAT8) can use a broad range of acyl-CoAs as substrates to catalyze acetylation and other types of acylation34,48,49, suggesting that these enzymes exhibit little selectivity. However, the difference between enzymatically catalyzed acetylation and other types of acylation mainly depends on the concentration of the catalytic substrate36. In the present study, P300 may have been the preferred substrate of β-OHB for kbhb rather than for acylation. This hypothesis was supported by the finding that CaMKII-α is β-hydroxybutyrylated at the K42 and K267 sites in HT22 cells treated with β-OHB. We speculate that an internal regulatory mechanism by which P300 selects substrates for either lysine acetylation or other lysine acylation reactions in the presence of different extracellular catalytic substrates or treatments exists.
CaMKII-α, the major synaptic protein in the forebrain, is autophosphorylated at T28650. However, the subunit being phosphorylated must be bound to Ca2+/CaM for T286 autophosphorylation51. The autophosphorylation of T286 markedly increases the affinity of the kinase for Ca2+/CaM52, which results in the frequency dependence of CaMKII activation53. Diabetes accompanied by long-term hyperglycemia provokes several neurovascular and cellular changes and disturbs neuronal Ca2+ homeostasis, which in turn alters synaptic plasticity54. Previous studies have shown that the inhibition of Ca2+ influx in neuronal cells and the maintenance of cellular Ca2+ homeostasis by tranilast increase the activity of CaMKII in the brain, which further ameliorates cognitive decline in diabetic rats55. In the present study, T1DM mice presented a significant reduction in hippocampal CaMKII activity, which is consistent with the findings of previous studies that have shown the inhibition of CaMKII autophosphorylation and activity in the hippocampi of diabetic animals55,56.
The mechanism of CaMKII autoinhibition is currently poorly understood. The SASA analysis based on our MD simulations suggested that CaMKII-α kbhb slightly prevented the solvent exposure of T286 and was not conducive to T286 autophosphorylation. Notably, K42 is conserved across all kinases that bind ATP and catalyze phosphotransferase reactions. Thus, it is plausible that the enhanced flexibility caused by PTM of this critical Lys residue in the catalytic ATP-binding site could abolish ATP binding and enzymatic activity by altering its side chain.
Recent studies have reported that LTP induction by CaMKII requires CaMKII to bind to the NMDAR subunit GluN2B and does not involve CaMKII catalysis57. In the present study, we found that CaMKII-α kbhb at the K42 and K267 sites suppressed CaMKII-α activity as well as LTP in the hippocampus. One possible reason for this is that CaMKII-α kbhb may also interfere with GluN2B binding to CaMKII by altering the structure of CaMKII. Thus, future studies are needed to explore whether CaMKII-α kbhb affects LTP by altering the structure of CaMKII to inhibit GluN2B binding.
In conclusion, the present study revealed that at high levels, β-OHB acts as a β-hydroxybutyryl donor to promote CaMKII-α kbhb and thus suppresses CaMKII activity in the hippocampus, which may contribute to memory deficits in T1DM mice. Preventing the dysregulation of CaMKII-α kbhb could be a potential therapeutic approach for diabetes-induced memory deficits. Thus, our findings help elucidate the pathogenesis of metabolite-induced brain damage.
Materials and methods
Animals and ethical statement
Male C57BL/6J wild-type mice (8–10 weeks of age) were obtained from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China) for use in this study. All the mice were housed in the animal room on a regular 12-h light/12-h dark cycle at a constant temperature with free access to food and water. All experimental and animal care procedures were carried out in compliance with the guidelines established by the Association for Assessment and Accreditation of Laboratory Animal Care and the Institutional Animal Care and Use Committee of Sichuan University (Approval no. 20240428001). All the efforts were made to minimize the suffering of the mice.
The mice were given 200 mg/kg (i.p.) STZ to induce T1DM. Blood was collected from the mice four days after STZ injection, at which time T1DM induction was confirmed27. In the present study, T1DM mice were characterized by a blood glucose level greater than 16.0 mmol/L. The mice in the β-OHB-treated group were given β-OHB (600 mg/kg) once a day (i.p.) for 10 consecutive days. After the behavioral tests were performed, blood and tissue samples were collected from each group of mice.
Drugs
(R)-(-)-3-β-Hydroxybutyrate sodium was purchased from Sigma‒Aldrich (54965, USA). STZ (13104, USA) and A485 (24119, USA) were obtained from Cayman Chemical. Puromycin was purchased from Selleck (S7417, USA). Insulin was obtained from Wanbang Pharmaceutical Company (40 IU/ml, China).
A single dose of STZ (200 mg/kg) was injected (i.p.) to construct the T1DM model, and a single dose of β-OHB (600 mg/kg) was injected (i.p.) once daily for 10 consecutive days to achieve a high concentration of β-OHB in the hippocampi of mice. Insulin (10 I.U./kg/day) was injected (i.p.) once daily for 5 consecutive days.
Cell culture and transfection
The mouse hippocampal neuronal cell line HT22 (CL-0697, Pricella) was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 μ/ml), and streptomycin (100 mg/ml) in a 5% CO2 environment at 37 °C. Mutant CaMKII-α was generated on the basis of the murine full-length CaMKII-α amino acid sequence with point mutations of lysine 42 to methionine (K42M) and lysine 267 to alanine (K267A). The LVs pLenti-EF1a-CMV-CaMKII-α-Flag-GFP-2A-Puro and pLenti-EF1a-CMV-mutant CaMKII-α-Flag-GFP-2A-Puro (Vigene Biotechnology Co. Ltd, China) were constructed for the cell studies, and recombinant lentiviruses were produced by transient transfection of HEK 293T cells. Furthermore, the P300 shRNA was cloned and inserted into pLKD-CMV-2A-Puro-U6-shRNA and sequenced. The sequences of the scrambled control shRNA and mouse P300 shRNA were 5′-TTCTCCGAACGTGTCACGT-3′ and 5′-GCAATGGACAAGGGATAATTT-3′, respectively (Vigene Biotechnology Co. Ltd, China).
To achieve stable expression of CaMKII-α-Flag or mutant CaMKII-α-Flag in HT22 cells, the media (DMEM) was replaced with serum-free DMEM 2 h before transfection, and the cells were then incubated with a LV expressing mouse CaMKII-α or mutant CaMKII-α. Antibiotic-resistant clones were selected with 2.5 g/ml puromycin and grown in DMEM supplemented with puromycin. To promote CaMKII-α kbhb in vitro, HT22 cells were treated for 24 h with (R)-(-)-3-hydroxybutyric acid sodium salt (40 mM).
Behavior tests
Y-maze test: The experiment was carried out with a Y-maze composed of a starting arm and two target arms. The mice were acclimated to the room for 10 min prior to testing. On the test day, the mice were placed at the end of one arm (identified as the starting arm) and trained for 10 min to leave the starting arm and enter the right arm (familiar arm) rather than the left arm (novel arm). The novel arm was opened after a 120-min intertrial interval, and the mice were allowed to explore all three arms. During a 5-min period, the time spent in the novel and familiar arms was automatically recorded using ANY-maze software (USA). The correct alternation was determined using the following formula: time (novel arm)/(time (novel arm)+time (familiar arm)).
Novel object position experiments: On the first day, the animals were placed in a test chamber (48 cm × 48 cm) for 10 min without objects to acclimate to the chamber. The mice were then placed in the test chamber with two identical objects for 10 min on the second day. On the third day, the familiar object was moved to a diagonal position (a different position, but was the same size and color as the familiar object). The animals were returned to the area and allowed to explore the object in its new location for 10 min. The time spent exploring the two objects was recorded with EthoVision 7.0 software (Noldus Information Technology, Leesburg, VA, USA). The formula for calculating the exploratory preference was as follows: time (novel)/(time (novel)+time (familiar)).
Novel object recognition experiments: On the first day, the mice were placed in a test chamber (48 cm × 48 cm) for 10 min without objects to acclimate to the chamber. The mice were placed in the test chamber with two identical objects for 10 min on the second day. On the third day, one of the familiar items was exchanged for a new object (different size and color from the familiar object). The animals were placed in the area again and given 10 min to explore the novel object. EthoVision 7.0 software (Noldus Information Technology, Leesburg, VA, USA) was used to document the time spent exploring the objects. The formula for calculating the exploratory preference was as follows: time (novel)/(time (novel)+time (familiar)).
Contextual fear conditioning: We used two alternative settings for contextual fear conditioning (context A and context B). The context A chamber had light cues and a grid floor composed of 24 stainless steel rods, and the context B chamber had a different set of context cues than the context A chamber, including darkness and a white plastic floor. The mice were kept in the conditioning context A chamber for a total of 300 s during the training period, and footshocks (2 s, 0.75 mA) were applied at 120, 180, and 240 s. To assess fear discrimination, the mice were placed in the context B chamber the next morning and permitted to explore for 180 s to observe their freezing behavior, and the freezing time in the context A chamber was evaluated after 2 h. Contextual fear memory expression was assessed by manually examining freezing behavior, and freezing was defined as at least 2 s of no movement. The context-associated fear memory discrimination index was determined using the following formula: (freezing time (A)-freezing time (B))/(freezing time (A)+freezing time (B)).
Hot plate Test: When a mouse is on an object whose surface temperature increases, the mouse will lick or lift its paw. Our study determined the time that elapsed after the mouse licked their paw or jumped after lying down on the surface of the hot plate (55 °C), which was considered the pain latency period. To avoid damaging the tissues of the mouse paw, the maximum period was 40 s. The hot plate test was cleaned after each mouse.
Measurement of the β-OHB and glucose levels
A β-OHB assay kit (MAK041, Sigma) was used to measure the levels of β-OHB in the blood and hippocampal tissues in accordance with the manufacturer’s recommendations. A contour blood glucose monitor (Bayer) was used to measure the mouse blood glucose levels.
Tissue isolation
The mice were sacrificed by rapid decapitation after the memory-related behavioral tests were complete. Hippocampal or CA1 tissues were removed from the brain, snap frozen on dry ice and stored at −80 °C until use.
HDAC activity assay
A nuclear extraction kit (40028, ACTIVE MOTIF) was used to extract the nuclear proteins from the hippocampal tissue. HDAC enzymatic activity was assessed using an HDAC activity assay kit (K330, BioVision) according to the manufacturer’s instructions. Briefly, 15 µl of test sample and 85 µl of ddH2O was added to each well, followed by 5 µl of HDAC colorimetric substrate, and the mixture was incubated at 37°C for 1 h. The reaction was stopped by adding 10 µl of stop buffer and mixing well with incubation at 37 °C for 30 min. Finally, the HDAC activity in each group was determined by measuring the optical density (OD) at 405 nm.
CaMKII activity assay
Hippocampal tissues and HT22 cells were harvested for CaMKII activity assays using a simplified CaMKII activity assay kit (JM-11610M1, Jingmei Biotechnology) according to the manufacturer’s instructions. Briefly, tissues or cells were homogenized by using a homogenizer and centrifuged for 10 min at 4 °C and 3000 rpm in a cold microcentrifuge to remove any insoluble material. The supernatant was collected and transferred to a new tube for analysis. A total of 10 µl of test sample and 40 µl of sample diluent was added to each well, followed by the addition of 100 µl of colorimetric substrate for incubation at 37 °C for 1 h. The reaction was stopped by adding 50 µl of stop buffer to each well and mixing well. The CaMKII activity in each group was determined by measuring the OD at 450 nm.
In vitro CaMKII-α bhb
CaMKII-α bhb was measured by mixing reaction buffer (25 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM DTT, 100 mM EDTA, 10% glycerol, protease inhibitor cocktail, 10 mM nicotinamide and 100 ng/ml TSA), 2 mg of recombinant CaMKII-α (ab60899, Abcam) and 10 mM DL-β-hydroxybutyryl-CoA lithium salt (H0261, Sigma) for 2 h in a 37 °C water bath. The reaction was stopped by the addition of SDS loading buffer, after which western blot analysis was performed.
Western blotting
Proteins were extracted from brain tissues and cells using a mammalian cell and tissue extraction kit (K269-500, BioVision; each milliliter of lysis buffer contained 2 μl of 1 M DTT and 2 μl of protease inhibitor cocktail) with phosphatase inhibitors (4906845001, Roche). A Bradford assay kit (P0006, Beyotime) was used to determine the total protein concentration. The proteins were separated via 10 or 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes after loading. The membranes were incubated with primary antibodies at 4 °C overnight. The next day, the cells were incubated with the secondary antibody at room temperature for 2 h, and the immunoreactivity of the proteins was observed using a chemiluminescence substrate and a chemiluminescence imaging system (CLINX, Shanghai, China). Sequence-specific anti-kbhb K42 and anti-kbhb K267 antibodies were constructed by PTM Biolabs, Inc. Anti-H2Aac (39643), anti-H3ac (61638) and anti-H4ac (39926) antibodies were purchased from Active Motif. Anti-H2A (16441-1-AP), anti-H3 (17168-1-AP) and anti-H4 (16047-1-AP) antibodies were purchased from Proteintech. Anti-GAPDH (ab8245) and anti-P300 (ab275378) antibodies were purchased from Abcam. Anti-CaMKII-α (13-7300) and anti-Flag (F1084) antibodies were purchased from Invitrogen and Sigma, respectively.
Protein purification
CaMKII-α- and mutant CaMKII-α-overexpressing HT22 cells were collected, and proteins were extracted by using a mammalian cell and tissue extraction kit (K269-500, BioVision; each milliliter of lysis buffer contained 2 μl of 1 M DTT and 2 μl of protease inhibitor cocktail). The protein supernatant from each cell type was incubated with anti-Flag beads (B26101, Bimake) overnight at 4 °C with rotation. The next day, the beads were washed two times with TBS, and the supernatants were collected. The purified CaMKII-α proteins were competitively eluted with the Flag peptide (B23111, Bimake) at room temperature with constant vortexing. The eluted proteins were then detected via western blotting after boiling in 1× SDS buffer.
Adeno-associated virus (AAVs) injection
In the present study, the AAVs 2/9-CaMKII-EGFP-Flag and AAV2/9-mutant CaMKII-EGFP-Flag were used for the animal experiments (Vigene Biotechnology Co., Ltd, China). Mice (aged 8 weeks) were fixed on a stereotaxic apparatus after being anesthetized with sodium pentobarbital (60 mg/kg). To inject the virus into the hippocampal CA1 region, very small craniotomy holes were drilled using a skull rotor (RWD Life Science). Then, AAV2/9-CaMKII-EGFP-Flag (1 μl, 0.1 μl/min) or AAV2/9-mutant CaMKII-EGFP-Flag (1 μl, 0.1 μl/min) was injected into the bilateral CA1 region of the hippocampus (AP, −1.7 mm; ML, ±1.2 mm; DV, −1.3 mm). To allow the virus to spread, a syringe was inserted after each injection, maintained in place for another 5 min, and then slowly removed. The placement of the AAV injections was confirmed in 10-μm-thick coronal sections stained with 4’,6-diamidino-2-phenylindole (DAPI), and images were acquired using a fluorescence microscope. Mice with misplaced injections were excluded from the statistical analysis.
Hippocampal slice preparation and electrophysiology recordings
For hippocampal slice preparation, the mice were anesthetized with sodium pentobarbital (60 mg/kg), and the brain was rapidly removed and immersed in cold (4 °C) artificial cerebrospinal fluid (ACSF). Subsequently, 95% O2 and 5% CO2 were constantly bubbled into the ACSF. A vibratome was used to cut several 400-μm-thick coronal sections containing the dorsal hippocampus. The sections were subsequently incubated at room temperature (21 ± 1 °C) for at least 60 min before testing. One slice was removed from a bath with continually dripping oxygenated ACSF (2.0 to 2.5 ml/min) and placed in a liquid air interface chamber and suspended on a nylon net. A feedback circuit at 31 (accurate to ±0.5 °C) was used to control the bath temperature, and humidified carbogen was administered along the upper surface of the slice.
Electrophysiological recordings: The electrophysiological recording procedure was adapted from a previously described method58 with slight modifications. A recording electrode was positioned at the CA1 apical dendrite area to capture synaptic potentials. Input‒output connections were used to determine the stimulus intensity, which was set to 50% of the maximum response. Two stimuli at 50% maximum strength were induced at intervals of 25, 50, 100, and 400 ms to investigate PPF. After 20 min of stable baseline synaptic potential recordings at 50% maximum intensity, theta burst tetanic stimulation with 15 burst trains of 5 Hz (each train consisted of five pulses of 100 Hz) was applied to measure LTP. Subsequent theta burst stimulation consisted of five trains with duration of 10 ms (100 Hz) applied 5 times at 200-ms intervals. The initial fEPSPs (50% of maximum stimulation with 0.33 Hz) were captured for 20 to 30 min. Theta burst tetanic stimuli were administered if the variation in the baseline was within ±10%. The baseline intensity-evoked fEPSPs were recorded for 60 min at 0.33 Hz. Axoclamp-2B amplifiers recorded all the evoked responses, and pClamp 10.2 software (Molecular Devices, Sunnyvale, California, USA) was used for data collection.
For the CaMKII-α- or mutant CaMKII-α-overexpressing mouse hippocampus CA1 LTP test, slices were transferred to a recording chamber, and carbogen-saturated ACSF was perfused constantly at room temperature. To record synaptic potentials, a recording electrode was placed at the CA1 apical dendrite region. A glass microelectrode filled with ACSF was positioned in the CA1 stratum radiatum to record fEPSPs. fEPSPs were evoked with an intensity (1 ms) that elicited ~50% of the maximum amplitude at 0.05 Hz. To record LTP, stable baseline synaptic potentials (50% of the maximal intensity) were recorded for 20 min, and then high-frequency stimulation (HFS; two consecutive 1 s trains of a 100 Hz stimulus with a 20 s interval between trains) was delivered. Potentiation was calculated as the percent increase in the average fEPSP slope during the first and last 10 min normalized to the average of the baseline slopes. The signals were acquired with a MultiClamp 700B amplifier, filtered at 1 kHz and sampled at 20 kHz with a Digidata 1440A interface. Data were obtained with Clampex 10.2 software and analyzed with Clampfit 10.2.
MD stimulations
The cryo-electron microscopy (EM) structure of the CaMKII-α holoenzyme (PDB code: 5U6Y) was used to prepare the initial computational model59. To reduce simulation complexity, the flexible loop and the connected hub (residues S314-V345) were removed manually, and only the kinase domain and regulatory segment, including the CaM-binding element in a single subunit, were retained to examine T286 autophosphorylation (Fig. S8A). On the basis of this wild-type CaMKII-α model, the CaMKII-α kbhb and mutant CaMKII-α systems were constructed by side chain modification and virtual site-directed mutagenesis, respectively. The RESP charges of the β-OHB-modified lysine residues were calculated using the RESP scheme provided by Multiwfn60, which were needed for subsequent MD simulations (Table S1).
Three MD simulations were then performed for the CaMKII-α, CaMKII-α kbhb and mutant CaMKII-α systems at 300 K with the AMBER 18 package, and the ff14SB force field61 was used for protein atoms with the TIP3P water model. After minimization, the MD simulations were performed in two stages. First, we restrained the solutes (restraining force constant of 100 kcal/mol Å2) and slowly heated the systems from 0 to 300 K over 5 ns. Three nonrestraint MD simulations at 300 K were then performed for 195 ns using the SHAKE algorithm to constrain the hydrogen-containing atoms with a nonbonded interaction radius of 12 Å. The integration step was 2 fs, conformational snapshots were collected every 20 ps, and 10,000 total conformations were obtained during each MD simulation. The MD trajectories were analyzed with CPPTRAJ62.
Statistics and reproducibility
The data were analyzed with GraphPad Prism 7 software, are presented as the means ± SEMs, and were subjected to the Kolmogorov-Smirnov test to assess the normality of the distribution. For simple comparisons, an unpaired two-tailed Student’s t test was used. For multiple comparisons, one-way repeated-measures ANOVA was used when necessary, followed by the Bonferroni post hoc correction for each experiment. The data that did not pass the normality test were analyzed using the Kruskal-Wallis test with a post hoc test. In all cases, n refers to the number of animals. For all the results, p < 0.05 was considered to indicate statistical significance.
Reporting summary
Further information on the research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and Supplementary Information (Supplementary material and Supplementary Data 1). The data presented in this study can be obtained upon reasonable request from the corresponding author.
References
DiMeglio, L. A., Evans-Molina, C. & Oram, R. A. Type 1 diabetes. Lancet 391, 2449–2462 (2018).
Ingelfinger, J. R. & Jarcho, J. A. Increase in the incidence of diabetes and its implications. N. Engl. J. Med. 376, 1473–1474 (2017).
Yagihashi, S., Mizukami, H. & Sugimoto, K. Mechanism of diabetic neuropathy: where are we now and where to go?. J. Diabetes Investig. 2, 18–32 (2011).
Feldman, E. L., Nave, K. A., Jensen, T. S. & Bennett, D. L. H. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93, 1296–1313 (2017).
Shalimova, A. et al. Cognitive dysfunction in type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 104, 2239–2249 (2019).
Jacobson, A. M. et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 54, 245–255 (2011).
Gao, H. et al. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 165541 (2019).
Mastrocola, R. et al. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J. Endocrinol. 187, 37–44 (2005).
Gupta, M., Pandey, S., Rumman, M., Singh, B. & Mahdi, A. A. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neurosci. Rep. 14, 57–63 (2023).
Suh, S. W., Gum, E. T., Hamby, A. M., Chan, P. H. & Swanson, R. A. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J. Clin. Invest. 117, 910–918 (2007).
Bree, A. J., Puente, E. C., Daphna-Iken, D. & Fisher, S. J. Diabetes increases brain damage caused by severe hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 297, E194–E201 (2009).
Creo, A. L. et al. Brain functions and cognition on transient insulin deprivation in type 1 diabetes. JCI Insight 6, e144014 (2021).
Laffel, L. M. et al. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet. Med. 23, 278–284 (2006).
Sheikh-Ali, M. et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis?. Diabetes Care 31, 643–647 (2008).
Turan, S., Omar, A. & Bereket, A. Comparison of capillary blood ketone measurement by electrochemical method and urinary ketone in treatment of diabetic ketosis and ketoacidosis in children. Acta Diabetol. 45, 83–85 (2008).
D’Andrea Meira, I. et al. Ketogenic diet and epilepsy: what we know so far. Front. Neurosci. 13, 5 (2019).
Achanta, L. B. & Rae, C. D. beta-hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem. Res. 42, 35–49 (2017).
Xie, Z. et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206 (2016).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 7, 9 (2021).
Kaczmarska, Z. et al. Structure of p300 in complex with acyl-CoA variants. Nat. Chem. Biol. 13, 21–29 (2017).
Buard, I. et al. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 30, 8214–8220 (2010).
Lisman, J., Schulman, H. & Cline, H. J. N. R. N. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3, 175–190 (2002).
Lisman, J., Yasuda, R. & Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012).
Babcock, A. M. et al. In vivo inhibition of hippocampal Ca2+/calmodulin-dependent protein kinase II by RNA interference. Mol. Ther. 11, 899–905 (2005).
Irvine, E. et al. Properties of contextual memory formed in the absence of αCaMKII autophosphorylation. Mol. Brain 4, 8 (2011).
Li, H. et al. beta-hydroxybutyrate reduces reinstatement of cocaine conditioned place preference through hippocampal CaMKII-alpha beta-hydroxybutyrylation. Cell Rep. 41, 111724 (2022).
Koronowski, K. B. et al. Ketogenesis impact on liver metabolism revealed by proteomics of lysine β-hydroxybutyrylation. Cell Rep. 36, 109487 (2021).
Gruber, N. et al. Fatty acid-binding protein 4: a key regulator of ketoacidosis in new-onset type 1 diabetes. Diabetologia 65, 366–374 (2022).
Wang, J. et al. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25, 754–767.e759 (2019).
Farajpour, R. et al. Chronic administration of Rosa canina hydro-alcoholic extract attenuates depressive-like behavior and recognition memory impairment in diabetic mice: a possible role of oxidative stress. Med. Princ. Pract. 26, 245–250 (2017).
Shimazu, T. et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Benjamin, J. S. et al. A ketogenic diet rescues hippocampal memory defects in a mouse model of Kabuki syndrome. Proc. Natl Acad. Sci. USA 114, 125–130 (2017).
Aggarwal, A., Yadav, B., Sharma, N., Kaur, R. & Rishi, V. Disruption of histone acetylation homeostasis triggers cognitive dysfunction in experimental diabetes. Neurochem. Int. 170, 105592 (2023).
Dancy, B. M. & Cole, P. A. Protein lysine acetylation by p300/CBP. Chem. Rev. 115, 2419–2452 (2015).
Sabari, B. R. et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 58, 203–215 (2015).
Huang, H. et al. p300-mediated lysine 2-hydroxyisobutyrylation regulates glycolysis. Mol. Cell 70, 663–678 e666 (2018).
Bhattacharyya, M., Karandur, D. & Kuriyan, J. Structural insights into the regulation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII). Cold Spring Harb. Perspect. Biol. 12, 6 (2020).
Biessels, G. J. & Despa, F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604 (2018).
Jensen, N. J. et al. Effects of beta-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur. J. Endocrinol. 182, 233–242 (2020).
Gasior, M., Rogawski, M. A. & Hartman, A. L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharm. 17, 431–439 (2006).
Haces, M. L. et al. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 211, 85–96 (2008).
Svart, M. et al. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 13, e0190556 (2018).
Fortier, M. et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 15, 625–634 (2019).
Page, K. A. et al. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 58, 1237–1244 (2009).
Wolfsdorf, J., Glaser, N. & Sperling, M. A. Diabetic ketoacidosis in infants, children, and adolescents. Diabetes Care 29, 1150–1159 (2006).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284 (2017).
Liu, K. et al. p53 beta-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 10, 243 (2019).
Chen, Y. et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell Proteom. 6, 812–819 (2007).
Liu, X. et al. MOF as an evolutionarily conserved histone crotonyltransferase and transcriptional activation by histone acetyltransferase-deficient and crotonyltransferase-competent CBP/p300. Cell Discov. 3, 17016 (2017).
Easton, A. C. et al. alphaCaMKII autophosphorylation controls the establishment of alcohol-induced conditioned place preference in mice. Behav. Brain Res. 252, 72–76 (2013).
Rich, R. C. & Schulman, H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 273, 28424–28429 (1998).
Meyer, T., Hanson, P. I., Stryer, L. & Schulman, H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science 256, 1199–1202 (1992).
Chang, J. Y. et al. CaMKII autophosphorylation is necessary for optimal integration of Ca2+ signals during LTP induction, but not maintenance. Neuron 94, 800–808.e804 (2017).
Hardigan, T., Ward, R. & Ergul, A. Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clin. Sci.130, 1807–1822 (2016).
Thapak, P., Bishnoi, M. & Sharma, S. S. Amelioration of diabetes-induced cognitive impairment by Transient Receptor Potential Vanilloid 2 (TRPV2) channel inhibitor: behavioral and mechanistic study. Neurochem. Int. 139, 104783 (2020).
Liao, M. H. et al. The disturbance of hippocampal CaMKII/PKA/PKC phosphorylation in early experimental diabetes mellitus. CNS Neurosci. Ther. 19, 329–336 (2013).
Tullis, J. E. et al. LTP induction by structural rather than enzymatic functions of CaMKII. Nature 621, 146–153 (2023).
Sun, G. Z. et al. Hippocampal synaptic and neural network deficits in young mice carrying the human APOE4 gene. CNS Neurosci. Ther. 23, 748–758 (2017).
Myers, J. B. et al. The CaMKII holoenzyme structure in activation-competent conformations. Nat. Commun. 8, 15742 (2017).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Roe, D. R. & Cheatham, T. E. 3rd. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
We are grateful to Jianping Hu (College of Pharmacy and Biological Engineering, Sichuan Industrial Institute of Antibiotics, Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, Chengdu University) and Zhixiang Wu (Faculty of Environmental and Life Science, Beijing University of Technology) for performing the MD simulation analyses. This work was partially supported by the National Natural Science Foundation of China (grant numbers 82071494, 81871043, 82371498, 81272459 and 82404598) and the 1·3·5 Project for Disciplines of Excellence of West China Hospital Sichuan University (ZYGD23011).
Author information
Authors and Affiliations
Contributions
Hongchun Li: Funding acquisition, project administration, methodology, statistical analysis and writing of the manuscript. Rong Chen: Software, analysis and interpretation of the data. Hongbo Wang: Supervision. Jingwei Tian: Supervision. Yinglan Zhao: Supervision and conceptualization. Xiaobo Cen: Funding acquisition, project conception, experimental design, research supervision and manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Christoph Anacker and Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Chen, R., Wang, H. et al. Hippocampal CaMKII-α β-hydroxybutyrylation induces memory deficits in mice with type 1 diabetes mellitus. Commun Biol 8, 1435 (2025). https://doi.org/10.1038/s42003-025-08832-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08832-z