Abstract
Balancing the high photocurrent production and the nonradiative recombination suppression is of practical importance to design high-performance organic bulk heterojunction (BHJ) solar cells. Most efforts in this field have been directed towards the understanding of the electronic charge and photogenerated exciton related optoelectronic and transient processes. Little is known about the spin polaron pairs (PPs) dependent dissociation and nonradiative decay at charge transfer states (CTS), where the coherent spin mixing plays a key role. In this work, we combine magneto-photocurrent (MPC) and coherent spin mixing analyses for the IT- and Y-series, i.e., prototypical nonfullerene acceptors (NFA), based organic BHJ systems. We find that increasing polaron pair dissociation rates at singlet charge transfer states (CTS)S give rise to the photocurrent generation. Within the same systems, strong internal fields that include the hyperfine (HF) fields, spin-orbit coupling (SOC), and Δg value may promote the intersystem crossing (ISC) and the polaron back transfer (PBT), leading to the energy loss. By exploring the impact of the coherent spin mixing on the molecular functionality, our study opens new directions for improving photovoltaic performance of BHJ systems.
Similar content being viewed by others
Introduction
The utilization of nonfullerene acceptors (NFAs) in bulk heterojunction (BHJ) organic solar cells (OSCs) has boosted photovoltaic power conversion efficiencies (PCE) to a completely new height1,2,3,4,5,6. In a view to keep chasing championed PCE, a great deal of efforts has been devoted to nonfullerene molecular syntheses7,8,9,10,11. They are made to be practically useful for efficient12, flexible13,14, and large-scale printable15 OSCs. All these make them a hot topic in the field of green energy research and applications. In the photovoltaic process of the donor-acceptor (D-A) based organic BHJ solar cell, it involves the exciton generation, migration, dissociation, carrier transport and collection16,17,18. All these rely on the electronic charge property. Nevertheless, the electron is endowed with the spin attribute19,20,21. The photovoltaic process is indeed inseparable with the organic spin-optoelectronics.
This newly raised multidisciplinary research for probing the spin behavior requires a joint of organic optoelectronics and spintronics. While both technical and scientific supports are highly demanded. Although the transient absorption spectroscopy (TAS)21,22 and transient electron paramagnetic resonance (t-EPR)23,24 are of exceptional importance for understanding kinetics of photogenerated spin species, it remains critical for us to consider exploring the interior photophysics from the photovoltaic device basis. Furthermore, spin-related physical quantities need to be precisely quantified in order to connect them with photovoltaic performance. We think that all these can be realized through the nondestructive in-situ magneto-photocurrent (MPC) technique and the coherent spin mixing theory20. They act as a complementary method for understanding spin-related phenomena along with TAS and t-EPR. The MPC is one of the organic magnetic field effects25,26. The effects also include the magneto-resistance (MR)27, magneto-photoluminescence (MPL)28, and magneto-electroluminescence (MEL)29. In all the cases, externally applied magnetic fields play an effective tool for probing and disturbing internal fields, as well as generating spin precession.
In the organic BHJ photovoltaic system, the D-A based intermolecular charge transfer states (CTS) act as the core unit for the transformation of firmly bound excitons to weakly bound polaron pairs (PPs)21. They are also known as the charge transfer excitons (CTEs)21,22,30. From the angle of spin-optoelectronics, hybridized interfacial states due to overlaps of molecular wavefunctions are highly spin-decided31. At photoexcited states, the spin PPs possess the spin parallel \((\uparrow \uparrow )\) and the antiparallel \((\uparrow \downarrow )\) configurations, yielding distinct dissociation rates. This is analogous to thermally activated delay fluorescence (TADF) molecules in organic light emitting diodes32. Furthermore, the interconversion of the spin singlet and triplet at CTS is governed by the coherent spin mixing26. The spin mixing that is determined by the hyperfine interaction (HFI), spin-orbit coupling (SOC) and \(\Delta g\) plays a dominant role for the short-circuit photocurrent (Jsc) production and the open-circuit voltage (Voc) loss. All these have been less considered in the organic BHJ solar cells.
In this work, we focus on MPC responses and analyses for various NFAs based organic BHJ solar cells. We adopted some prototypical Y-series (PM6:L8-BO, PM6:BTP-ec9) and IT-series (PBDB-T:ITIC, PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl) molecules for fabricating high performance OSCs. A joint of experimental and theoretical study was performed for unraveling the interior coherent spin mixing mechanism at CTS. HFI, SOC, and \(\triangle g\) were quantified and analyzed in order to understand their contributions on the mixing mechanism. We also compared them with typical fullerene-derivatives based OSCs. It should be noted that all the designed organic photovoltaic materials as well as their blends cannot yield any efficient luminescence. Since there exist some large differences of NFAs and TADF molecules, we do not intend to discuss MEL and MPL mechanisms in this work. All the results are limited to the spin PPs dependent dissociation and the nonradiative decay at CTS.
Results and discussion
Theoretical models
First, we discuss the fundamental photophysics for depicting the principle of the coherent spin mixing for the molecular D-A based CTS in OSCs (Fig. 1a). In the photovoltaic process, intramolecular singlet excitons (SEs) are created by the photoexcitation (hv). It complies with the optical selection rule and the spin conservation. Owing to large binding energies of SEs (0.1–0.5 eV)33,34 and small dielectric constants of organic molecules (3–5)35, the dissociation of SEs is inefficient. However, the internal quantum efficiency (IQE) of this system is close to 100%36,37, and SEs diffuse likely to the intermolecular CTS forming singlet PPs. We use (CTS)S to denote it. In most prototypical NFAs based organic BHJs, CTS have energies inbetween the first excited singlet and triplet energy states (Supplementary Fig. 1 and Supplementary Table 1). In this case, (CTS)S undergoes two possible decay pathways38. It dissociates into free polarons directly, or it turns into a triplet PP at CTS ((CTS)T) through the intersystem crossing (ISC). Thus, CTS are spin-decided. Analogous to intramolecular triplet excitons (TEs), the long-lived (CTS)T may not get an efficient dissociation and it may fuse into TE. This polaron back transfer (PBT) process that gives rise to the nonradiative recombination is known as the geminate recombination21. On the other hand, the nongeminate recombination can be realized by free polarons. The free polarons can decay back to (CTS)T and end up to TE. Thus, there are two possible routes for the energy loss39. Based on these, we need carefully considering two critical aspects in order to well elucidate the photophysical process and to construct its relationship with the solar cell performance. To quantify the decay rates for (CTS)S and (CTS)T; and, to find out the photophysical quantities that govern ISC.
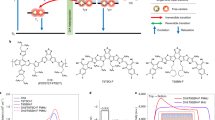
a Schematic illustration for the photophysics of an organic donor-acceptor bulk heterojunction. b–g The pictorial illustration for the coherent spin mixing. h Schematic diagram for the magneto-photocurrent setup. i The expeimental result of the room temperature magneto-photocurrent for the organic solar cell comprising ITO(glass)/ZnO/PBDB-T:ITIC/MoO3/A (black circles). We used three different models for testings (red/green/blue solid lines). The photoexcitation wavelength and power density was 635 nm and 240 mW/cm2 respectively. The symbolic notations are defined by: the singlet exciton recombination rate \(({r}_{S})\), the triplet exciton recombination rate \(({r}_{T})\), the singlet polaron pair dissociation rate \(({k}_{S})\) and fusion rate \(({f}_{S})\) at single charge transfer states \(({\left({\rm{CTS}}\right)}^{{\rm{S}}})\), the triplet polaron pair dissociation rate \(({k}_{T})\) and fusion rate \(({f}_{T})\) at triplet charge transfer states \(({\left({\rm{CTS}}\right)}^{{\rm{T}}})\), \({\omega }_{S-T}\) is the spin mixing rate or the interconversion rate of \({({\rm{CTS}})}^{{\rm{S}}}\) and \({({\rm{CTS}})}^{{\rm{T}}}\). The singlet and triplet polaron pair decay rates are defined by \({\gamma }_{S}\) \(({\rm{i}}.{\rm{e}}.,\,{\gamma }_{S}={k}_{S}+{f}_{S})\) and \({\gamma }_{T}\) \(({\rm{i}}.{\rm{e}}.,\,{\gamma }_{T}={k}_{T}+{f}_{T})\) respectively. \({\vec{S}}_{1}\) and \({\vec{S}}_{2}\) denote two spin vectors. The hyperfine field 1 and 2 are \({\vec{B}}_{{hf}1}\) and \({\vec{B}}_{{hf}2}\) respectively. The spin-orbit coupling field 1 and 2 are \({\vec{B}}_{{SOC}1}\) and \({\vec{B}}_{{SOC}2}\) respectively.
In the semiclassical three-state system of Fig.1a, we define a few critical parameters as per the above discussion. They include the SE recombination rate \(({r}_{S})\), the TE recombination rate \(({r}_{T})\), the singlet PP dissociation rate \(({k}_{S})\) and fusion rate \((\,{f}_{S})\) at \({({\rm{CTS}})}^{{\rm{S}}}\), the triplet PP dissociation rate \(({k}_{T})\) and fusion rate \((\,{f}_{T})\) at \({({\rm{CTS}})}^{{\rm{T}}}\), and the spin mixing rate (\({\omega }_{S-T}\)). The singlet and triplet PP decay rates are defined by \({\gamma }_{S}\) \(({\rm{i}}.{\rm{e}}.,\,{\gamma }_{S}={k}_{S}+{f}_{S})\) and \({\gamma }_{T}\) \(({\rm{i}}.{\rm{e}}.,\,{\gamma }_{T}={k}_{T}+{f}_{T})\) respectively. \({\omega }_{S-T}\) decides the interconversion of \({({\rm{CTS}})}^{{\rm{S}}}\) and \({({\rm{CTS}})}^{{\rm{T}}}\).
An applied magnetic field acts as a probe so that the interior fields get disturbed with it. Now, we discuss the detection of the spin PP at CTS using MPC40. We use \({m}_{s}=\pm \frac{1}{2}\) to denote the spin quantum number. Without externally applied magnetic fields (i.e., B = 0), the spin-dependent eigenstates for instance |Ψ1〉, |Ψ2〉, |Ψ3〉 and |Ψ4〉 are formed by the spin-related basis states, known as the singlet (S, \({E}_{S}=\frac{J}{2}\)) and the three-fold degenerated triplets (T1, T0, T-1, \({E}_{S}=-\frac{J}{2}\)). \({E}_{S}\) represents the exchange energy for the eigenstates. \(J\) denotes the exchange interaction strength for two spins. The exchange interaction for PPs at CTS is weak, of the order of \(J/2{\mu }_{B} < 10{\rm{mT}}\). Under a certain temperature, spin statistics are thermally stabilized by the internal fields in the organic BHJ system. With applied magnetic fields, the spin statistics are disturbed leading to a change of photocurrent. Indeed, MPC is magnetic field strength dependent. It is the summation over the low-field magneto-photocurrent (LFMPC, <100 mT) and the high-field magneto-photocurrent (HFMPC, ≥100 mT)41, for example42:
where, the PC(B) and PC(0) represent the photocurrent measured with and without a magnetic field. The term \({\rm{PC}}\left(B\right)\) can be expanded by ref. 43:
in which, \({tr}[\ldots ]\) is the trace operation for the multiplication of the spin state projection operator \(({P}_{S,T})\) and the time variant spin density \((\rho \left(t\right))\). \(\rho (t)\) can be further written by ref. 43:
where, \({\rho }_{0}\) is the spin density at CTS for t = 0 s. The Hamiltonian \({\mathcal{H}}\) is defined by \({\mathcal{H}}={{\mathcal{H}}}_{0}-i\hslash \varGamma /2\). \({{\mathcal{H}}}_{0}\) contains the exchange interaction \(({{\mathcal{H}}}_{{ex}})\), the hyperfine energy \(({{\mathcal{H}}}_{{HF}})\), the spin-orbit coupling energy \(({{\mathcal{H}}}_{{SOC}})\) and the Zeeman splitting energy \(({{\mathcal{H}}}_{z})\)40. Besides, \(\varGamma \) denotes the summation for the spin dependent decay rate for either singlet and triplet states \((\varGamma =\sum _{S,T}{\gamma }_{S,T}{P}_{S,T})\). \({{\hslash }}\) is the Dirac constant. At the steady state for an input photoexcitation and an output photocurrent in equilibrium, \({\rm{PC}}\left(B\right)\) is the field-associated integral over the complete time interval in Eq. (2). Essence, MPC is decided by PPs’ densities at CTS, while the coherent spin mixing has a certain impact on ISC. In the following, we discuss the coherent spin mixing at different field strengths separately.
Low-field magneto-photocurrent (LFMPC)
The spin-1/2 positive and negative polaron are indicated by \({\vec{S}}_{1}\) and \({\vec{S}}_{2}\) respectively. Without externally magnetic fields (Fig. 1b, c), both of them undergo the spin precession due to the hyperfine field (e.g., \({\vec{B}}_{{hf}1}\) and \({\vec{B}}_{{hf}2}\)) and the SOC fields (e.g., \({\vec{B}}_{{SOC}1}\) and \({\vec{B}}_{{SOC}2}\))44. The precession leads to a periodic change for the spin parallel (↑↑) and antiparallel (↑↓) configurations, which results in the mixing event between them. This model can be thought equivalently for spin PPs at the intermolecular (CTS)S and (CTS)T. If a low magnetic field (i.e., \(B \, < \, 100{\rm{mT}}\)) is applied from zero and it has comparable strengths with the hyperfine field, they will be added up and turned into an effective field. If \(B\, \gg \, {B}_{{hf}\mathrm{1,2}}\), the hyperfine field is overruled and the spin mixing is entirely determined by \(B\) (Fig. 1d). The similar scenario is applied for \({B}_{{SOC}\mathrm{1,2}}\) (Fig. 1e). The spin PPs are preferably in the parallel configuration at large fields. The analytical formular for evaluating the lineshape of LFMPC is refs. 44,45,46:
in which, \({{\rm{A}}}_{{\rm{HF}}}\) and \({{\rm{A}}}_{{\rm{SOC}}}\) are the dimensionless constants. \({a}_{{HF}}/{\mu }_{B}\) and \({a}_{{SOC}}/{\mu }_{B}\) represent the HFI and SOC parameters respectively. The fitting values of \({a}_{{HF}}/{\mu }_{B}\) and \({a}_{{SOC}}/{\mu }_{B}\) scale with the strengths of \({B}_{{hf}\mathrm{1,2}}\) and \({B}_{{SOC}\mathrm{1,2}}\) respectively. It is known that \({B}_{{SOC}\mathrm{1,2}}\) arises due to chemical constitutes with larger atomic numbers such as sulfur (S), chlorine (Cl), iridium (Ir), etc47. It may be considerable for orbital hybridizations at interfaces and structural deformations as well48,49.
High-field magneto-photocurrent (HFMPC)
When an applied magnetic field becomes exceedingly large, the significant Zeeman splitting can be triggered. It is worthwhile to mention that the hyperfine field and SOC field should be disregarded in this case since they are overruled. Consequently, the Hamiltonian (H) for the spin PPs contains the Zeeman and the exchange interaction terms only41:
in which, \({g}_{i}\) represents the \(g\)-factor of the \({i}^{{\rm{th}}}\) polaron, \({\mu }_{B}\) is the Bohr magneton, \({\vec{S}}_{i}\) denotes the \({i}^{{\rm{th}}}\) spin angular momentum. By considering the spin singlet and the projection of the triplet along the z-direction (|S, T0, T1, T−1〉), the matrix form of the Hamiltonian for the Zeeman term \(({H}_{{\rm{zeeman}}})\) is41:
The energy element \(\omega \) is expressed by \(\omega ={\mu }_{B}B({g}_{1}+{g}_{2})/\hslash \), in which, \({g}_{1}\) and \({g}_{2}\) are the Laudé g-factors for the two spin PPs. \(\Delta {\omega }_{p}\) denotes the difference of their precession frequencies.
In fact, the activation of HFMPC is due to the Larmor precession42. The precession frequency is proportional to \(B\) and \(g\) factor difference \((\Delta g)\). The nonzero \(\Delta g\) indicates the positive and negative polarons precess with distinct frequencies. Prior to the dissociation, they undergo the oscillation in-between the singlet and triplet configurations. The rate change from the singlet to triplet is proportional to \(\Delta g{B}_{0}\) (i.e., \({\omega }_{S-T}\propto \Delta {gB}\)). In the organic BHJ, it causes the magnetic field dependent spin mixing between (CTS)S and (CTS)T26. The analytical formular for HFMPC is42:
In the expression, \(c\) and \({b}^{2}\) can be further written as:
where, \({\gamma }_{S}\), \({\gamma }_{{T}_{0}}\), \({k}_{S}\) and \({k}_{{T}_{0}}\) have been defined previously.
In order to verify the models, we applied the MPC characterization for the organic BHJ solar cell comprising ITO(glass)/ZnO/PBDB-T:ITIC/MoO3/Ag. During the measurement, the solar cell was placed in between a pair of electromagnets. The photocurrent was continueously recorded by a source-meter unit as the solar cell was photoexcited in a sweeping field (Fig. 1h). The experimental result of MPC (the black circles) exhibits the symmetric lineshape with repect to the zero field. We tried the three different models. It seems that the three factors (i.e., HFI, SOC, \(\Delta g\)) need to be all included so as to achieve the relatively neat fitting (Fig. 1i, the red solid line).
Experimental analyses
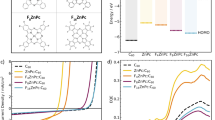
In the experiment, all the organic BHJ solar cells were fabricated with the same device structure ITO(glass)/ZnO/organic BHJ/MoO3/Ag (Fig. 2a). The ZnO and MoO3 films act as the electron and hole transport layers respectively. The organic BHJ was made by various D-A blends, for instance PM6:L8-BO, PM6:BTP-ec9, PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl, PBDB-T:ITIC, P3HT:PC71BM, and P3HT:PC61BM. Based on the molecular structures, they can be grouped into the Y-series, the IT-series, and the fullerene-derivatives. The material energy levels together with the work functions of the metallic electrodes are pictorially displayed in Fig. 2b. From the photovoltaic J-V characteristic curves (Fig. 2c, d), the photovoaltic parameters were summarized in Tab. 1. All OSCs reached the highest records for the performance. There were no needs to further optimize the performance50,51,52,53,54,55. As an example, the PM6:L8-BO based organic BHJ solar cell produced the PCE of approximately 17.66% with Jsc = 25.83 mA/cm2, Voc = 0.880 V and FF = 77.66%. Judging from the EQE and the integrated Jsc (Fig. 2e, f), they are close in agreement with the calculated Jsc in Table 1. The similar conclusions can be drawn from other devices.
a The drawing for the inverted organic bulk heterojunction solar cell structure consisting of ITO(glass)/ZnO/BHJ/MoO3/Ag, the organic bulk heterojunctions were choosen to be PM6:L8-BO, PM6:BTP-ec9, PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl, PBDB-T:ITIC, P3HT:PC61BM, and P3HT:PC71BM. b The pictorial illustration for energy levels of molecular donors, molecular acceptors, transport materials and metallic electrodes. The black dash lines are used to separate them into donor-acceptor bianry groups. c Experimental results of current density-voltage (J-V) curves for the Y-series organic solar cells (OSCs). d Experimental results of J-V curves for the IT-series organic solar cells. e Experimental results of external quantum efficiencies (EQE) spectra (left y-axis) and integrated short-circuit current density (Jsc) (right y-axis) for the Y-series OSCs. f Experimental results of EQE spectra (left y-axis) and integrated Jsc (right y-axis) for the IT-series OSCs. g Ultraviolet-visible (UV-vis) absorption spectra for the Y-series OSCs. h UV-vis absorption spectra for the IT-series OSCs.
The statistical summary in Table 1 tells us the Voc and Jsc differences for the Y- and IT- series OSCs. Conventionally, we know that Voc is associated with the molecular energetic disorder56. The nonradiative recombination is one of the major routes for the energy loss and the Voc loss57. Jsc is related to the light harvesting property (Fig. 2g, h). In addition, the surface morphology5, phase separation58, and carrier mobility59 are decisive to Jsc and Voc. On the other hand, the full understanding of the interior photophysics needs to include the photoexcited spin states60,61. Figure 3a–c show the experimental results of MPC (dotted lines) in percentage for the PM6:L8-BO and PM6:BTP-ec9 based Y-series organic BHJ solar cells. The original data for the measurements are given in the Supplementary Fig. 2. In our case, three different photoexcitation wavelengths were selected for instance λ = 532 nm, 635 nm and 721 nm. The MPC measurements work nicely for all the organic BHJ systems. All the lineshapes are not identical. The effect is strongly material and photoexcitation wavelength dependent. All the red solid lines are the fitting curves (Fig. 3a–c). Judging from the overlap of the tracing and retracing curves (Supplementary Fig. 3), the MPC measurements are highly reproducible.
Experimental results of magneto-photocurrent (MPC, dotted lines) for the organic blends using the three different photoexcitation wavelengths, such as a λ = 532 nm, b λ = 635 nm, and c λ = 721 nm. The photoactive layers are made of PM6:L8-BO and PM6:BTP-ec9. All the red solid lines are the fitting curves. Extracted numerical values through theoretical fittings of (a–c) for the laser wavelength-dependent d hyperfine field parameter \(({a}_{{HF}}/{\mu }_{B})\), e spin-orbit coupling field parameter \(({a}_{{SOC}}/{\mu }_{B})\), f polaron pair \(g\)-facor difference \((\Delta g)\), g singlet polaron pair decay rate \(({\gamma }_{S})\), h triplet polaron pair decay rate \(({\gamma }_{T})\), and i ratio of the singlet to triplet decay rate \(({\gamma }_{S}/{\gamma }_{T})\).
For the PM6:L8-BO and PM6:BTP-ec9 based organic BHJ systems, the extracted numerical values of \({a}_{{HF}}/{\mu }_{B}\), \({a}_{{SOC}}/{\mu }_{B}\), and \(\Delta g\) are shown in Fig. 3d–f respectively. The PM6:BTP-ec9 based system produces relatively larger \({a}_{{HF}}/{\mu }_{B}\), \({a}_{{SOC}}/{\mu }_{B}\), and \(\Delta g\) by comparing with those of the PM6:L8-BO system. The results indicate that ISC can be easily activated by the coherent spin mixing for the PM6:BTP-ec9 system. This may lead to an increasing number of TE through PBT, giving rise to the nonradiative recombination. Consequently, Voc may be suppressed57,62. We found that the PM6:BTP-ec9 based solar cell has the Voc of approximately 0.839 V (Table 1). It is indeed smaller than the one produced by the PM6:L8-BO based solar cell (~0.880 V). We also performed the light power density dependent Jsc experiment (Supplementary Fig. 4a) for the two systems. Both produce the nearly same exponential values (~0.94 and 0.95). Since the values are close to the unity, we thought the TE formation from free polarons may play a minute role. Its formation mainly comes from BPT. This conclusion is close in agreement with the MPC measurements. Despite this, the numerical values of the decay rates were extracted by the HFMPC measurements. As suggested in Fig. 3g, \({\gamma }_{{\rm{S}}}\) is relatively larger for the PM6:BTP-ec9 based solar cell than the one for the PM6:L8-BO based solar cell. By taking the ratio of \({\gamma }_{{\rm{S}}}/{\gamma }_{{\rm{T}}}\) (Fig. 3i), it manifests that the organic BHJ system PM6:BTP-ec9 can dissociate more efficiently at (CTS)S. It thus generates higher Jsc (Table 1).
At the same measuring condition, we performed the MPC characterization and analysis for the PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl and PBDB-T:ITIC based organic BHJ solar cells (Fig. 4a–c). In the IT-series, \({a}_{{HF}}/{\mu }_{B}\), \({a}_{{SOC}}/{\mu }_{B}\), and \(\Delta g\) are relatively smaller for the PBDB-T:ITIC system than those for the fluorine (F, PBDB-T-SF:IT-4F) and chlorine (Cl, PBDB-T-2Cl:IT-4Cl) decorated molecular systems (Fig. 4d–f). This leads to the suppression of the coherent spin mixing. Thus, PBT may not be easily triggered via ISC for the PBDB-T:ITIC system. Nonetheless, as we noticed from the light power density dependent Jsc measurements (Supplementary Fig. 4b), the nongeminate recombination from free polarons may be the major route for the energy loss. Figure 4g–i provides the extracted numerical values for the photoexcitation wavelength dependent decay rates. As suggested, the PBDB-T-2Cl:IT-4Cl and PBDB-T-SF:IT-4F based solar cells have the nearly same \({\gamma }_{{\rm{S}}}\), higher than the one of the PBDB-T:ITIC system. Furthermore, \({\gamma }_{{\rm{T}}}\) are the nearly same for the PBDB-T-2Cl:IT-4Cl and PBDB-T-SF:IT-4F systems. By taking the ratio \({\gamma }_{{\rm{S}}}/{\gamma }_{{\rm{T}}}\) (Fig. 4i), it can be seen that \({\gamma }_{S}/{\gamma }_{T}\approx 1\) for the PBDB-T-2Cl:IT-4Cl and PBDB-T-SF:IT-4F systems, indicating the equal decay rates for (CTS)S and (CTS)T. With the stark contrast, the PBDB-T:ITIC system has \({\gamma }_{S}/{\gamma }_{T} < 1\). The results indicate the relatively higher Jsc for the F and Cl decorated nonfullerene OSCs in comparison with the PBDB-T:ITIC based OSC. From the material point of view, to implement the F or Cl atoms into molecular donors and acceptors are the strategies for improving photovoltaic performance63,64. Both have relatively larger electronegativities so that higher intramolecular electrostatic polarizations are acquired65. The pronounced intramolecular push-pull effect gives rise to the intermolecular dipole-dipole interaction66,67. This usually promotes the electron-hole dissociation68,69. Nevertheless, we have looked at it from the spin-dependent dissociation in this work.
Experimental results of magneto-photocurrent (MPC, dotted lines) for the organic blends using the three different photoexcitation wavelengths, such as a λ = 532 nm, b λ = 635 nm, and c λ = 721 nm. The photoactive layers are made of PBDB-T:ITIC, PBDB-T-SF:IT-4F, and PBDB-T-2Cl:IT-4Cl. All the red solid lines are the fitting curves. Extracted numerical values through theoretical fittings of a-c for the laser wavelength-dependent d hyperfine field parameter \(({a}_{{HF}}/{\mu }_{B})\), e spin-orbit coupling field parameter \(({a}_{{SOC}}/{\mu }_{B})\), f polaron pair \(g\)-facor difference \((\Delta g)\), g singlet polaron pair decay rate \(({\gamma }_{S})\), h triplet polaron pair decay rate \(({\gamma }_{T})\), and i ratio of the singlet to triplet decay rate \(({\gamma }_{S}/{\gamma }_{T})\).
By comparing the Y- and IT-series based organic BHJ solar cells, the relatively greater Jsc for the Y-series OSCs can be ascribed to the efficient dissociation of singlet PPs at CTS. For example, the optimal Jsc of the PM6:BTP-ec9 system is 26.44 mA/cm2, and the optimal Jsc of the PBDB-T:ITIC system is 17.50 mA/cm2. Their corresponding \({\gamma }_{S}\) are approximately 7.58 × 109 s-1 and 2.50 × 109 s-1. We also compared their spin-dependent singlet PP dissociation rates with the prototypical fullerene-derivatives based organic BHJ systems, such as P3HT:PC61BM and P3HT:PC71BM (Supplimentary Fig. 5a, b). The almost half reduced Jsc can be attributed to the nearly one order magnitude decreased \({\gamma }_{S}\).
In order to further validate the above conclusion from the MPC measurements, we selected the IT-series and examined the PPs’ kinetics at CTS using the transient absorption spectroscopy (TAS) (Fig. 5a–c). The insets of Fig. 5a–c show the transient spectra for the organic BHJ systems PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl, and PBDB-T:ITIC respectively. With the tri-exponential fittings for the transient spectra, the organic BHJ system, PBDB-T:ITIC, was found to have the longest average lifetime \(({\tau }_{{\rm{ave}}})\) in the IT-series (Table 2). Since the long lifetime corresponds to the short decay rate, the result consists with our measurement in Fig. 4g. Apart from TAS, we adopted the continuous wave-EPR (cw-EPR) spectroscopy for detecting the g-factor and the number of spin polarons at the dark and illumination conditions70. We selected two photoactive layers, such as PBDB-T-SF:IT-4F and PBDB-T:ITIC. Both samples were prepared at the same condition and have the same dimensionality. The film thicknesses were the same as those for the organic BHJ solar cells. The details about the fabrication method and the characterization condition are given in the method part. Prior to the measurement, the EPR spectral responses were checked to be distinct due to the light off and on for PBDB-T:ITIC. Moreover, the different photoexcitation wavelengths had influence on the spectral intensity due to the distinct absorption strengths (Supplementary Fig. 6). Figure 5d–f provide the room temperature cw-EPR spectra for the two systems under the photoexcitation wavelengths λ = 532 nm, 635 nm, and 721 nm, respectively. The two EPR bands that stem from the positive and negative polarons can be well distinguished for the PBDB-T:ITIC system. The results indicate the existence of the long lived PPs, which is close in agreement with the MPC and TAS measurements. In order to validate the light-induced EPR signals, we also did the EPR characterization in the dark condition (Supplementary Fig. 6 and the inset). After fitting, the Lauda g-factor was found not only includes the spin contribution (~2.0023), but also contains the orbital contribution. This leads to the development of SOC effect (Table 3). This consists with the inclusion of the SOC term in the model (Eq. 4). In all the cases, we did the spectral integration (Fig. 5g–i) in order to estimate the number of photogenerated spin polarons (Table 4). As we can see from Fig. 5g–i for the three different photoexcitation wavelengths, the numbers of the spin polarons for PBDB-T:ITIC are significantly larger than those for PBDB-T-SF:IT-4F. It seems that the spin polarons likely appear in the PBDB-T:ITIC system with less annihilation. The results are close in agreement with the MPC measurements and analyses. Based on this work, we finally provide our suggestion on the molecular design. One of the critical ways to have an outstanding performance of the organic BHJ solar cell is to acquire an efficient dissociation rate for (CTS)S. At the same time, ISC and PBT should be largely inhibited, which can be realized by suppressing the internal fields, such as HFI and SOC.
a–c Transient absorption spectra for the PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl and PBDB-T:ITIC organic bulk heterojunction systems respectively. The insets of a-c are the transient spectra for the organic BHJ systems PBDB-T-SF:IT-4F, PBDB-T-2Cl:IT-4Cl, and PBDB-T:ITIC respectively. d–f Photoexcitation wavelength depdent electron paramagnetic resonance spectra (first-order) for the PBDB-T-SF:IT-4F, and PBDB-T:ITIC organic BHJ systems respectively. g–i Wavelength dependent EPR absorption strengths for the PBDB-T-SF:IT-4F, and PBDB-T:ITIC organic bulk heterojunction systems respectively.
Conclusions
In this work, we introduced the theoretical basis for the coherent spin mixing in the organic BHJ system. We performed the MPC experiment for the prototypical Y-, IT-series and fullerene-derivatives based organic BHJ solar cells. With a joint of experimental and theoretical analyses, the interior photophysics, and its connection to the photocurrent generation and the energy loss were systematically studied. The TAS and light-induced EPR were adopted to further validate MPC. Our results elucidate the photocurrent generation and the energy loss from the spin aspect. We suggest that to maximize the singlet PP dissociation rate and to minimize the triplet decay rate, particularly to reduce the PBT rate in the molecular design are beneficial for the enhancement of the photovoltaic performance. It is merit to design molecules with TE energy states above energies of CTS so that PBT can be greatly avoided.
Methods
Materials
In the experiment, the poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophen-2-yl)-benzo[1,2-b:4,5-b’]dithiophene))-alt-(5,5-(1’,3’-di-2-thienyl-5’,7’-bis(2-ethylhexyl)benzo[1’,2’-c:4’,5’-c’]dithiophene-4,8-dione)] (PM6), poly[(2,6-(4,8-bis(5-(2-ethylhexylthio)-4-fluorothiophen-2-yl)-benzo[1,2-b:4,5-b’]dithiophene))-alt-(5,5-(1’,3’-di-2-thienyl-5’,7’-bis(2-ethylhexyl)benzo[1’,2’-c:4’,5’-c’]dithiophene-4,8-dione)] (PBDB-T-SF), poly[(2,6-(4,8-bis(5-(2-ethylhexylthio)-4-fluorothiophen-2-yl)-benzo[1,2-b:4,5-b’]dithiophene))-alt-(5,5-(1’,3’-di-2-thienyl-5’,7’-bis(2-ethylhexyl)benzo[1’,2’-c:4’,5’-c’]dithiophene-4,8-dione)] (PBDB-T-2Cl), poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b’]-dithiophene))-alt-(5,5-(1’,3’-di-2-thienyl-5’,7’-bis(2-ethylhexyl)benzo[1’,2’-c:4’,5’-c’]dithiophene-4,8-dione))] (PBDB-T) and poly(3-hexylthiophene-2,5-diyl) (P3HT) were chosen as the molecular donors. The 2,2’-((2Z,2’Z)-((12,13-bis(2-ethylhexyl)-3,9-(2-butyloctyl)-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2”,3’‘:4’,5’]thieno[2’,3’: 4,5]pyrrolo[3,2-g]thieno[2’,3’:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (L8-BO), 2,2’-[[12,13-Bis(2-butyloctyl)-12,13-dihydro-3,9-dinonylbisthieno [2”,3”:4’,5’]thieno[2’,3’:4,5]pyrrolo[3,2-e:2’,3’-g][2,1,3]benzothiadiazole-2,10-diyl]bis[methylidyne(5,6-chloro-3-oxo-1H-indene-2,1(3H)-diylidene)]]bis[propanedinitrile] (BTP-ec9), 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-difluoro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2’,3’-d’]-s-indaceno[1,2-b:5,6-b’]dithiophene (IT-4F), 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-dichloro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2’,3’-d’]-s-indaceno[1,2-b:5,6-b’]dithiophene (IT-4Cl), 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2’,3’-d’]-s-indaceno[1,2-b:5,6-b’] dithiophene (ITIC), [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) and [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) were singled out as the molecular acceptors. All of them were commercially available and could be directly used without further purifications. Other commercially available products also include zinc acetate dehydrate (Zn(CH3COO)2·2H2O), ethanolamine (NH2CH2CH2OH), 2-methoxyethanol (CH3OCH2CH2OH), chlorobenzene (CB), chloroform (CF), and chloronaphthalene (CN), 1, 8-Diiodooctane (DIO).
Device fabrication
Before the OSC fabrication, indium tin oxide coated glass substrates (ITO(glass)) were rinsed in an ultrasonic bath by standard chemical means. The substrates were fully dried by blowing with a pure nitrogen gas. After this, they experienced a plasma oxidation in an enclosed chamber for approximately 1 min. N-type zinc oxide (ZnO) layers were spin-coated onto the ITO(glass) substrates. They were annealed at 150 °C for approximately 40 min. Subsequently, they were immediately transferred into an ultra-high purity nitrogen-filled glove-box. The system contains an integrated thermal evaporation system. Then PM6:L8-BO (1:1.2), PM6:BTP-ec9 (1:1.2), PBDB-T:ITIC (1:1), PBDB-T-SF:IT-4F (1:1), PBDB-T-2Cl:IT-4Cl (1:1), P3HT:PC61BM (1:1), P3HT:PC71BM (1:1) heterojunction solutions were respectively dissolved in CF:CN (volume ratio is 99.5:0.5), CF:CN (99.5:0.5), CB:DIO (99.5:0.5), CB:DIO (99.5:0.5), CB:DIO (99.5:0.5), CB:DIO (99.5:0.5) and CB:DIO (99.5:0.5). The concentration of the solution was estimated to be 15.4 mg/mL, 15.4 mg/mL, 16 mg/mL, 16 mg/mL, 16 mg/mL, 10 mg/mL, and 10 mg/mL respectively. The photoactive layers were spin-coated onto ITO(glass)/ZnO substrates with a spin-speed of 3000 rpm for 60 s. After annealing, the thickness of the photoactive layers was estimated to be approximately 100 nm. Finally, all the samples were transferred into the vacuum chamber. Approximately 10 nm thick MoO3 films and 100 nm thick Ag electrodes were evaporated onto ITO(glass)/ZnO/ photoactive layers forming cross-bar structures. The electronic transport areas were patterned by self-designed stainless steel made shadow mask. The areas are 3.8 mm2.
Electronic and magneto- transport measurements
All electronic and magneto- transport measurements were carried out in the ambient condition. Current density-voltage (J-V) characteristic curves were measured by a source-meter unit (Keysight B2912A). Prior to the MPC measurement, an OSC was fixed on a sample holder and all its patterned electrodes were wired bonded onto metallic contact pads. The sample holder was inserted between a pair of electromagnets with maximum field strengths of ±1 T. MPC was measured by continuously recording a short-circuit current density \(({J}_{{\rm{sc}}})\) of the OSC in a sweeping field. Three semiconductor laser diodes with different emissive wavelengths such as 532 nm, 635, 721 nm were used as the photoexcitation sources. The distance of the solar cell and the laser source was approximately 1 m and all laser power densities were set to be about 240 mW/cm2. The magnitude of MPC in percentage can be calculated by \({\rm{MPC}}( \% )=\frac{I\left(B\right)-I(0)}{I(0)}\), in which \(I(B)\) and \(I(0)\) are the photocurrent measured with and without the B-field respectively.
Material Characterizations
Photo-absorption spectra of organic thin films were characterized by an UV-visible spectrophotometer (Shimadzu UV-2600 PC) in the ambient condition. Femtosecond transient photo-absorption spectroscopy (f-TAS) measurements were performed in the ambient condition. The instrument model is Vitara T-Legend Elite-TOPAS-Helios-EOS-Omni. In the measurements, the pumping wavelength, the pulse width and the pump fluence were set to be 550 nm, 25 fs, and 5 μJ/cm2 respectively. A supercontinuum white light was generated by the femtosecond pump beam passing through CaF2, sapphire, or YAG crystals. It was used as a probe light ranging from 400 nm to 1600 nm, with a maximum time resolution of 100 fs. The pump and probe beams were spatially overlapped at a target sample, and a transmitted probe light was collected by a charge-coupled device (CCD).
Electron paramagnetic resonance (EPR) measurements
EPR measurements were performed by a Bruker system (Model: A300 spectrometer, X-band). The system was initially calibrated by a standard stable and long lifetime radical sample such as a chromium (Cr) metallic ball. Prior to the EPR measurement, the organic films were spin-coated on top of high-quality quartz substrates in an ultrahigh purity nitrogen-filled glove box. The concentrations of the solution for the organic blends and the spin-coating speed were as the same as those for the solar cell fabrications. After this, the samples were diced into small strips with the same dimensionality (3.8 mm × 3.8 mm). At the same environment, they were well fitted into 4 mm nitrogen-filled suprasil tubes for background free EPR spectra. The sample tube was inserted into a resonator while both locate inbetween a pair of electromagnets. The sample film front faced perpendicularly to a light source. All spectra were measured by setting the instrument such as 0.1 mW microwave power and 2 G modulation amplitude at 100 kHz field. Samples were measured with the light off and on in a dark room. The photoexcitation was produced by three individual continuous wave semiconductor laser diodes with different wavelengths such as 532 nm, 635 nm, and 721 nm. The output power density and the distance between the sample and the laser diode were all set to be 240 mW/cm2 and 1 m, the same as the MPC measuring condition. All spectra were well fitted and analyzed by the routines of EasySpin (a MatlabTM Package 6.0.0). The EPR measurement does not involve any pulsed and transient measurements.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions of the paper are present in the paper or supplementary materials. The raw data that support the plots within this study are available from the corresponding author upon reasonable request.
References
Fu, J. et al. Rational molecular and device design enables organic solar cells approaching 20% efficiency. Nat. Commun. 15, 1830 (2024).
Liu, K. et al. 19.7% efficiency binary organic solar cells achieved by selective core fluorination of nonfullerene electron acceptors. Joule 8, 835–851 (2024).
Wang, L. et al. Donor–acceptor mutually diluted heterojunctions for layer-by-layer fabrication of high-performance organic solar cells. Nat. Energy 9, 208–218 (2024).
Ding, G. et al. Solid additive-assisted layer-by-layer processing for 19% efficiency binary organic solar cells. Nano-Micro Lett 15, 92 (2023).
Zhu, L. et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 21, 656–663 (2022).
Sun, F. et al. 1,5-Diiodocycloctane: a cyclane solvent additive that can extend the exciton diffusion length in thick film organic solar cells. Energy Environ. Sci. 17, 1916–1930 (2024).
Lin, Y. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Yuan, J. et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 3, 1140–1151 (2019).
Sun, R. et al. Single-junction organic solar cells with 19.17% efficiency enabled by introducing one asymmetric guest acceptor. Adv. Mater. 34, e2110147 (2022).
Yi, J., Zhang, G., Yu, H. & Yan, H. Advantages, challenges and molecular design of different material types used in organic solar cells. Nat. Rev. Mater. 9, 46–62 (2023).
Fan, B., Gao, H. & Jen, A. K. Y. Biaxially conjugated materials for organic solar cells. ACS Nano 18, 136–154 (2024).
Xie, Y. et al. High-reproducibility layer-by-layer non-fullerene organic photovoltaics with 19.18% efficiency enabled by vacuum-assisted molecular drift treatment. Adv. Energy Mater. 14, 2400013 (2024).
Yu, H. et al. Improved photovoltaic performance and robustness of all-polymer solar cells enabled by a polyfullerene guest acceptor. Nat. Commun. 14, 2323 (2023).
Ma, L. et al. High-efficiency and mechanically robust all-polymer organic photovoltaic cells enabled by optimized fibril network morphology. Adv. Mater. 35, e2208926 (2023).
Zhang, H. et al. Concretized structural evolution supported assembly-controlled film-forming kinetics in slot-die coated organic photovoltaics. Nat. Commun. 14, 6312 (2023).
Wang, Y. et al. Origins of the open-circuit voltage in ternary organic solar cells and design rules for minimized voltage losses. Nat. Energy 8, 978–988 (2023).
Zhou, G. et al. Spontaneous carrier generation and low recombination in high-efficiency non-fullerene solar cells. Energy Environ. Sci. 15, 3483–3493 (2022).
Khan, S. U. Z. et al. Nonradiative recombination via charge‐transfer‐exciton to polaron energy transfer limits photocurrent in organic solar cells. Adv. Energy Mater. 12, 2200551 (2022).
Hu, J. et al. Anisotropic organic magnetoresistance in fused-ring electron-acceptor-based bulk heterojunction systems. ACS Mater. Lett. 5, 2058–2064 (2023).
Kan, L. et al. Insights into magneto-photocurrent and coherent spin mixing for binary and ternary nonfullerene bulk heterojunction organic solar cells. Chem. Mater. 34, 10113–10122 (2022).
Gillett, A. J. et al. The role of charge recombination to triplet excitons in organic solar cells. Nature 597, 666–671 (2021).
Zhang, K.-N. & Hao, X.-T. Multiple-time scale exciton dynamics in organic photovoltaic devices. J. Phys. Chem. Lett. 14, 6051–6060 (2023).
Kotova, M. S. et al. On the absence of triplet exciton loss pathways in non-fullerene acceptor based organic solar cells. Mater. Horiz. 7, 1641–1649 (2020).
Grüne, J. et al. Triplet excitons and associated efficiency‐limiting pathways in organic solar cell blends based on (Non‐) Halogenated PBDB‐T and Y‐Series. Adv. Funct. Mater. 33, 2212640 (2023).
Zhang, C. et al. Spin dependent polaron pair dissociation at charge transfer states enables low driving force for nonfullerene organic photovoltaic system. Appl. Phys. Lett. 118, 233302 (2021).
Zhang, C. et al. Essential relation of spin states, trap states and photo-induced polarization for efficient charge dissociation in a polymer-nonfullerene based organic photovoltaic system. Nano Energy 78, 105324 (2020).
Li, J. et al. Investigating underlying mechanisms for nonfullerene hybrid bulk heterojunctions‐based organic magnetoresistance. Adv. Electron. Mater. 9, 2200983 (2022).
Tamarat, P. et al. Universal scaling laws for charge-carrier interactions with quantum confinement in lead-halide perovskites. Nat. Commun. 14, 229 (2023).
Nguyen, T. D. et al. Isotope effect in spin response of π-conjugated polymer films and devices. Nat. Mater. 9, 345–352 (2010).
Coropceanu, V., Chen, X.-K., Wang, T., Zheng, Z. & Brédas, J.-L. Charge-transfer electronic states in organic solar cells. Nat. Rev. Mater. 4, 689–707 (2019).
Noda, H. et al. Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors. Nat. Mater. 18, 1084–1090 (2019).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 8, 326–332 (2014).
Wang, J., Xue, P., Jiang, Y., Huo, Y. & Zhan, X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 6, 614–634 (2022).
Zhu, L. et al. Small exciton binding energies enabling direct charge photogeneration towards low-driving-force organic solar cells. Angew. Chem. Int. Ed. Engl 60, 15348–15353 (2021).
Zhu, L., Yi, Y. & Wei, Z. Exciton binding energies of nonfullerene small molecule acceptors: implication for exciton dissociation driving forces in organic solar cells. J. Phys. Chem. C 122, 22309–22316 (2018).
Karuthedath, S. et al. Intrinsic efficiency limits in low-bandgap non-fullerene acceptor organic solar cells. Nat. Mater. 20, 378–384 (2021).
Liu, J. et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 1, 16089 (2016).
Wang, R. et al. Nonradiative triplet loss suppressed in organic photovoltaic blends with fluoridated nonfullerene acceptors. J. Am. Chem. Soc. 143, 4359–4366 (2021).
Han, G., Hu, T. & Yi, Y. Reducing the singlet-triplet energy gap by end-group pi-pi stacking toward high-efficiency organic photovoltaics. Adv. Mater. 32, e2000975 (2020).
Oviedo-Casado, S., Urbina, A. & Prior, J. Magnetic field enhancement of organic photovoltaic cells performance. Sci. Rep. 7, 4297 (2017).
Devir-Wolfman, A. H. et al. Short-lived charge-transfer excitons in organic photovoltaic cells studied by high-field magneto-photocurrent. Nat. Commun. 5, 4529 (2014).
Nikiforov, D. & Ehrenfreund, E. Magnetic field effects of charge transfer excitons in organic semiconductor devices. Isr. J. Chem. 62, e202100091 (2021).
Khachatryan, B. et al. Magnetophotocurrent in organic bulk heterojunction photovoltaic cells at low temperatures and high magnetic fields. Phys. Rev. Appl. 5, 044001 (2016).
Sheng, Y., Nguyen, T. D., Veeraraghavan, G., Mermer, Ö. & Wohlgenannt, M. Effect of spin-orbit coupling on magnetoresistance in organic semiconductors. Phys. Rev. B 75, 035202 (2007).
Niu, L. B. et al. Hyperfine interaction vs. spin–orbit coupling in organic semiconductors. RSC Adv. 6, 111421–111426 (2016).
Duan, J., Feng, K. & Xu, L. Photoinduced spin-orbital coupling effect at donor: acceptor interface in non-fullerene organic solar cells. Org. Electron. 109, 106613 (2022).
Yang, X. et al. A Heavy-atom-free Molecular Motif Based on Centrosymmetric Bird-like Structured Tetraphenylenes with Room-Temperature Phosphorescence (RTP) Afterglow over 8s. Angew. Chem. Int. Ed. Engl. 62, e202306475 (2023).
Cui, L.-S. et al. Fast spin-flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photonics 14, 636–642 (2020).
Kim, J. U. et al. Nanosecond-time-scale delayed fluorescence molecule for deep-blue OLEDs with small efficiency rolloff. Nat. Commun. 11, 1765 (2020).
Xie, J. et al. Sequentially N-Doped acceptor primer layer facilitates electron collection of inverted non-fullerene organic solar cells. Adv. Funct. Mater. 34, 2309511 (2024).
Wu, J. et al. Interface modification of Tin oxide electron‐transport layer for the efficiency and stability enhancement of organic solar cells. Adv. Energy Mater. 14, 2302932 (2023).
Cai, P. et al. A robust and thickness-insensitive hybrid cathode interlayer for high-efficiency and stable inverted organic solar cells. J. Mater. Chem. A 11, 18723–18732 (2023).
Zhang, Y. et al. Fluorination vs. chlorination: a case study on high performance organic photovoltaic materials. Sci. China Chem. 61, 1328–1337 (2018).
Fan, Q. et al. Synergistic effect of fluorination on both donor and acceptor materials for high performance non-fullerene polymer solar cells with 13.5% efficiency. Sci. China Chem. 61, 531–537 (2018).
Lee, J. U., Jung, J. W., Emrick, T., Russell, T. P. & Jo, W. H. Synthesis of C60-end capped P3HT and its application for high performance of P3HT/PCBM bulk heterojunction solar cells. J. Mater. Chem. 20, 3287 (2010).
Zhu, X. et al. Exploring deep and shallow trap states in a non-fullerene acceptor ITIC-based organic bulk heterojunction photovoltaic system. J. Phys. Chem. C 123, 20691–20697 (2019).
Pang, B. et al. B⎕N-Bond-Embedded triplet terpolymers with small singlet-triplet energy gaps for suppressing non-radiative recombination and improving blend morphology in organic solar cells. Adv. Mater. 35, e2211871 (2023).
Bao, S. et al. Volatilizable solid additive-assisted treatment enables organic solar cells with efficiency over 18.8% and fill factor exceeding 80%. Adv. Mater. 33, 2105301 (2021).
Luke, J., Yang, E. J., Labanti, C., Park, S. Y. & Kim, J.-S. Key molecular perspectives for high stability in organic photovoltaics. Nat. Rev. Mater. 8, 839–852 (2023).
Zhao, F. et al. Spin‐dependent electron–hole recombination and dissociation in nonfullerene acceptor ITIC‐based organic photovoltaic systems. Sol. RRL 3, 1900063 (2019).
Tamai, Y., Murata, Y., Si, Natsuda & Sakamoto, Y. How to interpret transient absorption data?: An overview of case studies for application to organic solar cells. Adv. Energy Mater. 14, 2301890 (2023).
Marin‐Beloqui, J. M. et al. Triplet‐charge annihilation in a small molecule donor: acceptor blend as a major loss mechanism in organic photovoltaics. Adv. Energy Mater. 11, 2100539 (2021).
Hu, H. et al. The role of demixing and crystallization kinetics on the stability of non‐fullerene organic solar cells. Adv. Mater. 32, 2005348 (2020).
Zhou, D. et al. Binary blend all‐polymer solar cells with a record efficiency of 17.41% enabled by programmed fluorination both on donor and acceptor blocks. Adv. Sci. 9, 2202022 (2022).
Li, G. et al. Non-fullerene acceptors with direct and indirect hexa-fluorination afford >17% efficiency in polymer solar cells. Energy Environ. Sci. 15, 645–659 (2022).
Hsiao, Y.-C. et al. Revealing optically induced dipole-dipole interaction effects on charge dissociation at donor:acceptor interfaces in organic solar cells under device-operating condition. Nano Energy 26, 595–602 (2016).
Li, W. et al. A high‐efficiency organic solar cell enabled by the strong intramolecular electron push–pull effect of the nonfullerene acceptor. Adv. Mater. 30, 1707170 (2018).
Aplan, M. P., Lee, Y., Wilkie, C. A., Wang, Q. & Gomez, E. D. Push–pull architecture eliminates chain length effects on exciton dissociation. J. Mater. Chem. A 6, 22758–22767 (2018).
Liu, Z. et al. Intersystem crossing in acceptor–donor–acceptor type organic photovoltaic molecules promoted by symmetry breaking in polar environments. J. Phys. Chem. Lett. 13, 10305–10311 (2022).
Qin, L. et al. Triplet acceptors with a D-A structure and twisted conformation for efficient organic solar cells. Angew. Chem. Int. Ed. Engl 59, 15043–15049 (2020).
Acknowledgements
K.W. acknowledges the financial supports from the National Key R&D Program of China (2024YFA1209603), the Fundamental Research Funds for the Central Universities, Key Project (2023JBZY002), the Tangshan Science and Technology Bureau (23130226E), and the Natural Science Foundation of Hebei Province (F2024105019).
Author information
Authors and Affiliations
Contributions
Kai Wang conceived the research concept and the experimental designs. Kai Wang supervised and guided the whole project. Kai Wang and Lixuan Kan analyzed the data and wrote the manuscript. Lixuan Kan performed the fabrication, optimization, and characterization of the organic solar cells. Lixuan Kan and Fenggui Zhao did the MPC measurements. Fenggui Zhao, Jiaji Hu, and Yongchao Xie assisted with the device fabrication and optimization. Jing Li helped with the transient absorption spectroscopic measurements. Fenggui Zhao and Jiashuo Zhang helped with the EPR characterization and analysis. Xixiang Zhu, Xiaoling Ma, and Haomiao Yu assisted with the MPC measurements. Jinpeng Li and Fujun Zhang provided fruitful discussions on the manuscript. All the authors discussed the results and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kan, L., Zhao, F., Zhang, J. et al. Coherent spin mixing at charge transfer states for spin polaron pair dissociation and energy loss in organic bulk heterojunction solar cells. Commun Phys 8, 47 (2025). https://doi.org/10.1038/s42005-025-01967-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42005-025-01967-9