Abstract
Primary mitochondrial diseases (PMDs) are associated with pediatric neurological disorders and are traditionally related to oxidative phosphorylation system (OXPHOS) defects in neurons. Interestingly, both PMD mouse models and patients with PMD show gliosis, and pharmacological depletion of microglia, the innate immune cells of the brain, ameliorates multiple symptoms in a mouse model. Given that microglia activation correlates with the expression of OXPHOS genes, we studied whether OXPHOS deficits in microglia may contribute to PMDs. We first observed that the metabolic rewiring associated with microglia stimulation in vitro (via IL-33 or TAU treatment) was partially changed by complex I (CI) inhibition (via rotenone treatment). In vivo, we generated a mouse model deficient for CI activity in microglia (MGcCI). MGcCI microglia showed metabolic rewiring and gradual transcriptional activation, which led to hypertrophy and dysfunction in juvenile (1-month-old) and adult (3-month-old) stages, respectively. MGcCI mice presented widespread reactive astrocytes, a decrease of synaptic markers accompanied by an increased number of parvalbumin neurons, a behavioral deficit characterized by prolonged periods of immobility, loss of weight and premature death that was partially rescued by pharmacologic depletion of microglia. Our data demonstrate that microglia development depends on mitochondrial CI and suggest a direct microglial contribution to PMDs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Original data are provided as Source Data files, indicating the correspondence with each main and extended figure. Transcriptomics data are available from the Gene Expression Omnibus Dataset GSE254585. Source data are provided with the paper.
References
Beccari, S. et al. Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy. Autophagy 19, 1952–1981 (2023).
March-Diaz, R. et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging 1, 385–399 (2021).
Oosterhof, N. et al. Homozygous mutations in CSF1R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am. J. Hum. Genet. 104, 936–947 (2019).
Guo, L. et al. Bi-allelic CSF1R mutations cause skeletal dysplasia of dysosteosclerosis–Pyle disease spectrum and degenerative encephalopathy with brain malformation. Am. J. Hum. Genet. 104, 925–935 (2019).
Matuleviciute, R. et al. Microglial contribution to the pathology of neurodevelopmental disorders in humans. Acta Neuropathol. 146, 663–683 (2023).
He, D. et al. Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function. Immunity 55, 159–173.e9 (2022).
McFarland, R., Taylor, R. W. & Turnbull, D. M. A neurological perspective on mitochondrial disease. Lancet Neurol. 9, 829–840 (2010).
Fernández-Agüera, M. C. et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015).
González-Rodríguez, P. et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2, 133–137 (2021).
Altea-Manzano, P. et al. Reversal of mitochondrial malate dehydrogenase 2 enables anaplerosis via redox rescue in respiration-deficient cells. Mol. Cell 82, 4537–4547.e7 (2022).
Kashani-Poor, N., Zwicker, K., Kerscher, S. & Brandt, U. A central functional role for the 49-kDa subunit within the catalytic core of mitochondrial complex I. J. Biol. Chem. 276, 24082–24087 (2001).
Baradaran, R., Berrisford, J. M., Minhas, G. S. & Sazanov, L. A. Crystal structure of the entire respiratory complex I. Nature 494, 443–448 (2013).
Haimon, Z. et al. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 19, 636–644 (2018).
Fornasiero, E. F. et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 9, 4230 (2018).
Yu, W. et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell 75, 1147–1160.e5 (2019).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Ulland, T. K. et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e13 (2017).
Villadiego, J. et al. Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate. Nat. Neurosci. 26, 226–238 (2023).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Sierra, A. et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 (2010).
Lee, J., Lim, E., Kim, Y., Li, E. & Park, S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J. Endocrinol. 205, 263–270 (2010).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Molnar, M. J. & Kovacs, G. G. Mitochondrial diseases. Handb. Clin. Neurol. 145, 147–155 (2018).
Orre, M. et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol. Aging 35, 2746–2760 (2014).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Wculek, S. K. et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity 56, 516–530.e9 (2023).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Arnold, T. D. et al. Impaired αVβ8 and TGFβ signaling lead to microglial dysmaturation and neuromotor dysfunction. J. Exp. Med. 216, 900–915 (2019).
Ramos, B. et al. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer’s disease. Neurobiol. Aging 27, 1658–1672 (2006).
Xiang, X. et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 13, eabe5640 (2021).
Li, Y. et al. Hexokinase 2-dependent hyperglycolysis driving microglial activation contributes to ischemic brain injury. J. Neurochem. 144, 186–200 (2018).
Hu, Y. et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat. Metab. 4, 1756–1774 (2022).
Bernier, L. P. et al. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 11, 1559 (2020).
Baik, S. H. et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 30, 493–507.e6 (2019).
Fairley, L. H. et al. Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 120, e2209177120 (2023).
Peruzzotti-Jametti, L. et al. Mitochondrial complex I activity in microglia sustains neuroinflammation. Nature 628, 195–203 (2024).
Pan, X. et al. A genetically encoded tool to increase cellular NADH/NAD+ ratio in living cells. Nat. Chem. Biol. 20, 594–604 (2024).
Chandel, N. S. Mitochondria. Cold Spring Harb. Perspect. Biol. 13, a040543 (2021).
Seo, B. B. et al. Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc. Natl Acad. Sci. USA 95, 9167–9171 (1998).
Goodman, R. P. et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature 583, 122–126 (2020).
Rojo, R. et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat. Commun. 10, 3215 (2019).
Valdearcos, M., Myers, M. G. & Koliwad, S. K. Hypothalamic microglia as potential regulators of metabolic physiology. Nat. Metab. 1, 314–320 (2019).
Stoolman, J. S. et al. Mitochondrial respiration in microglia is essential for response to demyelinating injury but not proliferation. Nat. Metab. https://doi.org/10.1038/s42255-024-01080-1 (2024).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Saura, J., Tusell, J. M. & Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189 (2003).
Mailliot, C. et al. Pathological tau phenotypes. The weight of mutations, polymorphisms, and differential neuronal vulnerabilities. Ann. N. Y. Acad. Sci. 920, 107–114 (2000).
Orre, M. et al. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol. Aging 35, 1–14 (2014).
Herzenberg, L. A., Tung, J., Moore, W. A., Herzenberg, L. A. & Parks, D. R. Interpreting flow cytometry data: a guide for the perplexed. Nat. Immunol. 7, 681–685 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Alvarez-Vergara, M. I. et al. Non-productive angiogenesis disassembles Aß plaque-associated blood vessels. Nat. Commun. 12, 3098 (2021).
Acknowledgements
We thank D. Carnero and M. Bustos for helping with the optimization of the metabolomic methods used here and A. Ciulkinyte, from Blanca Diaz-Castro’s laboratory (UK DRI, University of Edinburgh), for providing us with a linear mixed-effects model script. B.M.-R. was the recipient of a ‘Junta de Andalucía’ predoctoral fellowship (2021), N.C.-C., M.I.A.-V. and E.M.M. were each the recipients of an FPU/FPI fellowship from the Spanish Ministry of Education (FPU20/03320, FPU15/02898 and PRE2019-087729) and J.J.P.-M. and A.E.R.-N. were the recipients of JdlC-I and JdlC-F fellowships from MCIN/AEI/ 10.13039/501100011033 (IJC2019-038819-I and FJCI-2015-23708). A.E.R.N. is funded by VII PPIT-US. This work was supported by grants to A.P., J.V., A.G. and D.M. by MCIN/AEI/ 10.13039/501100011033, ISCIII (FORT23/00008 (to A.P.); PI21/00915 (to J.V.); and PI21/00915 (to A.G.), and FEDER (RTI2018-096629-B-100, PID2021-126894OB-I00 ‘y por FEDER Una manera de hacer Europa’, SAF2017-90794-REDT, and PIE13/0004), by the regional Government of Andalusia (‘Proyectos de Excelencia’ P12‐CTS‐2138, P20_01312; BIOT22_00018_1, and ProyExcel_00845) co-funded by CEC, REC_EU and FEDER funds, and by the ‘Ayuda de Biomedicina 2018’, Fundación Domingo Martínez. We also thank K. Levitsky and J. Pearson (microscopy), M. J. Castro and C. Henderson (flow cytometry), F. J. Moron and R. March-Diaz (genomics) and R. Duran (histology) for advice and technical assistance in experiments at the IBiS core facilities; as well as C. A. Parejo-Perez at ‘Instituto de Bioquímica Vegetal y Fotosíntesis’ (IBVF, Seville) metabolomic facility.
Author information
Authors and Affiliations
Contributions
A.P., J.J.P.-M., B.M.-R. and N.C.-C. conceived of and designed the research. B.M.-R., N.C.-C., J.J.P.-M., M.I.A.-V., L.T.-E., C.R.-M., E.M.-M., N.M.-C., M.V., J.L.N.-G., P.G.-J.-C., D.M., A.E.R.-N. and A.P. performed the experiments. B.M.-R., N.C.-C., J.J.P.-M., L.T.-E., C.R.-M., E.M.-M., N.M.-C., M.V., J.L.N.-G., P.G.-J.-C., J.V., A.G., A.E.R.-N. and A.P. analyzed the data. J.L.-B. and J.V. contributed mouse models and samples. A.P. and J.J.P.-M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Gimenez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
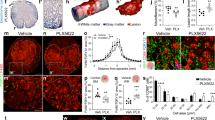
Extended Data Fig. 1 CI inhibition modifies stimulated microglial metabolism in vitro.
a,b, Mouse primary microglial cell cultures were treated with vehicle -C-; stimulus (IL-33: a or TAU: b); rotenone -R-, to inhibit CI; or both R and stimulus for 24 h. The levels of the metabolites were estimated by targeted metabolomics. (a) n = 8, C and R; n = 6, IL-33 and R + IL-33 and (b) n = 6 independent cultures.and MGcCI mice was analyzed by P values from two-sided ANOVA with post-hoc Tukey’s test. All data are presented as means ± s.e.m; a.u.: arbitrary units.
Extended Data Fig. 2 NDUFS2 levels in astrocytes or neurons in MGcCI.
a–e, Gate identification was performed according to guidelines and previous reports in contour density plots. a, Debris, and dead cells were discarded by forward (FSC) and side (SSC) scatters dispersion of events. b,c, Singlets of events were selected according to FSC wide (FSC-W) versus area (FSC-A) (b) and SCC wide (SCC-W) and area (SSC-A) (c). d,e, Microglial cells, reactive for CD45 and CD11b markers (d) or astrocytes, reactive for ACSA2 marker (e) were selected. f, Ndufs2 mRNA expression in FACS-isolated astrocytes from 3-month-old (mo) control (C) and MGcCI mice was analyzed by qRT-PCR. n = 4 mice. g,h, Ndufs2 mRNA (g) and NDUFS2 protein (h) expression in cortical, hippocampal, and striatal samples were analyzed by qRT-PCR and western blot (using GAPDH levels to normalize load). n = 4 mice. i, Ndufs2 mRNA and NDUFS2 protein expression in primary astrocytic cell cultures was analyzed by qRT-PCR (n = 6 C and n = 4 MGcCI mice) and western blot (n = 4 mice). All data are presented as means ± s.e.m; a.u.: arbitrary units. P values from two-sided Student t-test.
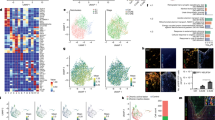
Extended Data Fig. 3 Microglial mitochondria and transcriptomic in MGcCI.
a, Top, electron microscopy images of cortical brain sections of Control –C– and MGcCI mice. Microglia (blue) can be recognized by its darker cytoplasm and mitochondria (brown) by their morphology. Bottom, graphs of the quantified parameters. Scale bar is 1 µm. Mitochondria or cells are shown as datapoint from n = 3 mice. P values from two-sided type III ANOVA with Satterthwaite approximation on linear mixed effects models (LMM). b, GSEA of FACS-isolated microglia showing MGcCI versus C from 1mo and 3mo mice. Left, enplot graphs and right, heat maps show the top-ranking genes of different gene sets. Red symbolizes upregulation and blue represents downregulation. FC: fold change. All data are presented as means ± s.e.m. n values represent the number of biologically independent experiments.
Extended Data Fig. 4 Schematic representation of the main metabolic pathways studied.
1mo (a) and 3mo (b) MGcCI, phagocytic (c), MAPTP301S/+ (TAU; d), or APP751SL/+ (Aß; e) microglia. Red color indicates upregulation and green downregulation of the gene/metabolic pathway. GLY: glycolysis; SSP: serine synthesis pathway; PPP: pentose phosphate pathway; c1CP: cytosolic 1-carbon by folate pathway; m1CP: mitochondrial 1CP; MC: methionine cycle; TCA: tricarboxylic acid cycle. Statistic values and fold change of the enzymes are shown in Extended Data Table 4. f, GSEA of FACS-isolated microglia showing MGcCI versus C from 3mo mice. g, GSEA of FACS-isolated phagocytic versus non-phagocytic microglia. (f,g) Left, enplot graphs and right, heat maps show the top-ranking genes of different gene sets. Red symbolizes upregulation and blue represents downregulation. FC: fold change.
Extended Data Fig. 5 Morphology, proliferation, and phagocytosis in MGcCI microglia.
a,b, Morphologic analysis of control -C- and MGcCI striatal microglia from 1-month-old (1mo) (a) and 3mo mice (b). Left images show the microglia marker (IBA1); left-middle images show the surface of the reconstructed microglia; middle-right and right images show, respectively, the surface and the filament reconstruction of a microglia cell. Graphs show the morphologic parameters analyzed. Scale bars, 30 µm and 10 µm in low and high magnification images, respectively. Cells are shown as datapoints, from n = 3 mice per genotype and condition. P values from two-sided type III ANOVA with Satterthwaite approximation on linear mixed effects models (LMM). c, GSEA of FACS-isolated microglia showing MGcCI versus C from 1mo mice. d, Brain coronal sections of 3mo MGcCI mice immunostained for microglia (IBA1), proliferation (Ki67), and nuclei (DAPI) markers. Scale bar, 10 µm. Graphs show the number of Ki67+-IBA1+ (yellow arrowheads) cells/total number of IBA1+ cells and the microglial density. Yellow arrows indicate non-microglia Ki67+ cells. n = 4 C and n = 6 MGcCI mice. P values from two-sided Student’s t-test. e, GSEA of FACS-isolated microglia showing MGcCI versus C 3mo mice. (c,e) Left, enplot graphs and right, heat maps show the top-ranking genes of different gene sets. Red symbolizes upregulation and blue represents downregulation. FC: fold change. f, Quantification of phagocytic index in the cortex, hippocampus, and striatum. n = 4 C (cortex: 48 and hippocampus: 49 dying cells analyzed) and MGcCI (cortex: 121 and hippocampus: 51 dying cells analyzed) mice in cortex and hippocampus; n = 3, C (14 dying cells analyzed) and MGcCI (10 dying cells analyzed) mice in striatum. P values from two-sided Student t-test. All data are presented as means ± s.e.m. n values represent the number of biologically independent experiments.
Extended Data Fig. 6 GFAP levels in MGcCI and glial analysis in LYcCI.
a Quantification of the cortical density of GFAP+ astrocytes and the percentage of cortex occupied by GFAP signal (load). n = 4 1-month-old (1mo) mice. b,c, Gfap mRNA (b) and GFAP protein (c) expression from C and MGcCI mice, analyzed by qRT-PCR or western blot (cortical samples) using GAPDH or ß-ACTIN levels to normalize load. n = 4 mice. d, Quantification of microglial and astrocytic related-parameters in the Lyz2-Cre/+; Ndufs2Flox/Flox (LYcCI) mouse model. n = 6 mice. All data are presented as means ± s.e.m. n values represent the number of biologically independent experiments. P values from two-sided Student t-test.
Extended Data Fig. 7 MGcCI mice develop neuronal changes, behavioral dysfunction, and premature death.
a, Pre (VGAT and VGLUT) and postsynaptic (PSD95) markers were analyzed by western blot in 1-month-old mice. GAPDH was used to normalize loads. n = 3, control (C) cortex and n = 4 in other samples. b, Pvalb and Sst mRNA expressions in 1mo and 3mo mice were analyzed by qRT-PCR. n = 4 mice except in 3mo C cortex samples: n = 3 mice. c, Top panels, cortical sections of 1mo mice were stained with a parvalbumin marker (PV). Scale bar, 100 µm. Bottom graphs, quantification of the cortical density of parvalbumin cells in 1mo mice. n = 4 mice. d,e, C and MGcCI mice were recorded in an open field arena for 15 min. Quantification of the number of ambulation and freezing events (d), and track length and average speed (e). n = 14 C and n = 8 MGcCI mice. f, Weight of the mice used to perform open field analysis. n = 14 C and n = 8 MGcCI mice. g, Weight of female and male C and MGcCI mice. n is indicated between brackets. h–j, Comparison between adult C and Lyz2-Cre/+; Ndufs2Flox/Flox (LYcCI) mice. h, Weight. n = 5 mice except for male LYcCI n = 6 mice. i, Freezing time. n = 7 C and n = 5 LYcCI mice. j, Survival curve of C and LYcCI mice. n = 11 mice. All data are presented as means ± s.e.m. n values represent the number of biologically independent experiments. a.u.: arbitrary units. P values from two-sided Student t-test (a–c,f,h,i), two-sided Mann-Whitney test (d,e,g), or long rank Mantel-Cox test (j).
Supplementary information
Supplementary Table 1
Microglial metabolomics.
Supplementary Table 2
Microglial transcriptomics.
Supplementary Table 3
Microglial GSEA.
Supplementary Table 4
Astrocytic transcriptomics.
Supplementary Video 1
Representative open field recording of a wild-type mouse.
Supplementary Video 2
Representative open field recording of a MGcCI mouse.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Fig./Table 2
Statistical source data.
Source Data Extended Data Fig./Table 3
Statistical source data.
Source Data Extended Data Fig./Table 5
Statistical source data.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 2
Unprocessed western blots.
Source Data Extended Data Fig./Table 6
Unprocessed western blots.
Source Data Extended Data Fig./Table 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mora-Romero, B., Capelo-Carrasco, N., Pérez-Moreno, J.J. et al. Microglia mitochondrial complex I deficiency during development induces glial dysfunction and early lethality. Nat Metab 6, 1479–1491 (2024). https://doi.org/10.1038/s42255-024-01081-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01081-0
This article is cited by
-
Microglia Mitochondrial Metabolism in Neurological Diseases
Molecular Neurobiology (2026)
-
Dynamic changes in mitochondria support phenotypic flexibility of microglia
Nature Communications (2025)
-
Mitochondria are absent from microglial processes performing surveillance, chemotaxis, and phagocytic engulfment
Nature Communications (2025)
-
Targeting Microglial Connexin43 Hemichannels: A Novel Therapeutic Avenue for Alzheimer’s Disease
Neuroscience Bulletin (2025)
-
Mitochondrial respiration in microglia is essential for response to demyelinating injury but not proliferation
Nature Metabolism (2024)