Abstract
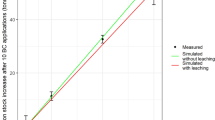
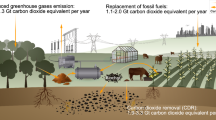
Paddy fields are major contributors to agricultural greenhouse gas emissions. Applying ~1% biochar by topsoil weight (high single, HS) effectively reduces greenhouse gas emissions from paddy fields, but long-term impacts are unclear. Here we present 8-year field experiments showing HS reduces CO2 equivalent per hectare by 59% and yields a net benefit of US$1,810 per hectare. However, its effectiveness declines over time due to the decreased soil carbon content and methanotrophic activity triggered by higher soil ammonium concentrations. To counteract this, the annual-low method, involving yearly biochar recycling, surpasses the HS approach with a 52% CO2 reduction and yields a net benefit of US$2,801 (35%) per hectare—highlighting the economic and environmental viability of annual-low biochar use in sustainable paddy field management practices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and the Supplementary Information. Source data are provided with this paper.
References
Vaughan, A. COP26: 105 countries pledge to cut methane emissions by 30 per cent. New Scientist (2 November 2021); https://www.newscientist.com/article/2295810-cop26-105-countries-pledge-to-cut-methane-emissions-by-30-per-cent/
Xu, C. et al. Coupled anaerobic methane oxidation and metal reduction in soil under elevated CO2. Glob. Change Biol. 29, 4670–4685 (2023).
Ritchie, H., Roser, M. & Rosado, P. CO2 and greenhouse gas emissions. Our World in Data (2020). https://ourworldindata.org/co2-and-greenhouse-gas-emissions
Jeffery, S., Verheijen, F. G., Kammann, C. & Abalos, D. Biochar effects on methane emissions from soils: a meta-analysis. Soil Biol. Biochem. 101, 251–258 (2016).
Nan, Q., Xin, L., Qin, Y., Waqas, M. & Wu, W. Exploring long-term effects of biochar on mitigating methane emissions from paddy soil: a review. Biochar 3, 125–134 (2021).
Nan, Q., Fang, C., Cheng, L., Hao, W. & Wu, W. Elevation of NO3−-N from biochar amendment facilitates mitigating paddy CH4 emission stably over seven years. Environ. Pollut. 295, 118707 (2022).
Tan, G. & Yu, H.-Q. Rethinking biochar: black gold or not? Nat. Rev. Mater. 9, 4–5 (2024).
Roberts, K. G., Gloy, B. A., Joseph, S., Scott, N. R. & Lehmann, J. Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 44, 827–833 (2010).
Patel, M. R. & Panwar, N. L. Biochar from agricultural crop residues: environmental, production, and life cycle assessment overview. Resour. Conserv. Recycl. Adv. 19, 200173 (2023).
Jiang, Y. et al. Acclimation of methane emissions from rice paddy fields to straw addition. Sci. Adv. 5, eaau9038 (2019).
Xia, L. et al. Integrated biochar solutions can achieve carbon-neutral staple crop production. Nat. Food 4, 236–246 (2023).
Hayes, M. Biochar and biofuels for a brighter future. Nature 443, 144–144 (2006).
Das, S. K., Ghosh, G. K. & Avasthe, R. Application of biochar in agriculture and environment, and its safety issues. Biomass Convers. Biorefin. 13, 1359–1369 (2023).
Yi, Q. et al. Temporal physicochemical changes and transformation of biochar in a rice paddy: insights from a 9-year field experiment. Sci. Total Environ. 721, 137670 (2020).
Han, X. G. et al. Mitigating methane emission from paddy soil with rice-straw biochar amendment under projected climate change. Sci. Rep. 6, 24731 (2016).
Nan, Q., Hu, S., Qin, Y. & Wu, W. Methane oxidation activity inhibition via high amount aged biochar application in paddy soil. Sci. Total Environ. 796, 149050 (2021).
Chen, Z., Han, S., Dong, Z., Li, H. & Zhang, A. Trade-off between soil carbon sequestration and net ecosystem economic benefits for paddy fields under long-term application of biochar. GCB Bioenergy 16, e13116 (2024).
Lili, H. et al. Biochar mitigated more N-related global warming potential in rice season than that in wheat season: an investigation from ten-year biochar-amended rice-wheat cropping system of China. Sci. Total Environ. 821, 153344 (2022).
Nan, Q., Tang, L., Chi, W., Waqas, M. & Wu, W. The implication from six years of field experiment: the aging process induced lower rice production even with a high amount of biochar application. Biochar 5, 27 (2023).
Wang, C., Sun, H. F., Zhang, X. X., Zhang, J. N. & Zhou, S. Optimal straw retention strategies for low-carbon rice production: 5 year results of an in situ trial in eastern China. Agronomy 13, 1038–1045 (2023).
Nan, Q., Wang, C., Wang, H., Yi, Q. & Wu, W. Mitigating methane emission via annual biochar amendment pyrolyzed with rice straw from the same paddy field. Sci. Total Environ. 746, 141351 (2020).
Dong, D. et al. Responses of methane emissions and rice yield to applications of biochar and straw in a paddy field. J. Soils Sediments 13, 1450–1460 (2013).
Mia, S., Dijkstra, F. A. & Singh, B. Long-term aging of biochar: a molecular understanding with agricultural and environmental implications. Adv. Agron. 141, 1–51 (2017).
Zhang, A. et al. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 139, 469–475 (2010).
Zhang, A. et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: a field study of 2 consecutive rice growing cycles. Field Crops Res. 127, 153–160 (2012).
Sun, X., Ding, J., Jiang, Z. & Xu, J. Biochar improved rice yield and mitigated CH4 and N2O emissions from paddy field under controlled irrigation in the Taihu Lake Region of China. Atmos. Environ. 200, 69–77 (2019).
Zhu, J. et al. Microbiology and potential applications of aerobic methane oxidation coupled to denitrification (AME-D) process: a review. Water Res. 90, 203–215 (2016).
Wu, Z. et al. Biochar can mitigate methane emissions by improving methanotrophs for prolonged period in fertilized paddy soils. Environ. Pollut. 253, 1038–1046 (2019).
Mishra, V. K., Shukla, R. & Shukla, P. N. Inhibition of soil methane oxidation by fertilizer application: an intriguing but persistent paradigm. Environ. Pollut. Prot. 3, 57–69 (2018).
Nyerges, G. R., Han, S.-K. & Stein, L. Y. Effects of ammonium and nitrite on growth and competitive fitness of cultivated methanotrophic bacteria. Appl. Environ. Microbiol. 76, 5648–5651 (2010).
Stein, L. Y. The double life of Methanoperedens. Nat. Microbiol. 8, 189–190 (2023).
McIlroy, S. J. et al. Anaerobic methanotroph ‘Candidatus Methanoperedens nitroreducens’ has a pleomorphic life cycle. Nat. Microbiol. 8, 321–331 (2023).
Yu, Q. & Li, H. Moderate separation of household kitchen waste towards global optimization of municipal solid waste management. J. Cleaner Prod. 277, 123330 (2020).
FAO Voluntary Guidelines for Sustainable Soil Management (FAO, 2024).
Xunhua, Z. et al. Comparison of manual and automatic methods for measurement of methane emission from rice paddy fields. Adv. Atmos. Sci. 15, 569–579 (1998).
Tamames, J. & Puente-Sánchez, F. SqueezeMeta, a highly portable, fully automatic metagenomic analysis pipeline. Front. Microbiol. 9, 3349 (2019).
Li, D., Liu, C., Luo, R., Sadakane, K. & Lam, T. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 11, 119 (2010).
Aramaki, T. et al. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252 (2020).
Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794 (2022).
Nayfach, S. et al. A genomic catalog of Earth’s microbiomes. Nat. Biotechnol. 39, 499–509 (2021).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinf. 5, 113 (2004).
Kolb, S., Knief, C., Stubner, S. & Conrad, R. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 69, 2423–2429 (2003).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., Von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (42407006, 42077032, 42325707 and 41571241), Postdoctoral Talent Funding Program (2023C03G2072956) and the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2015BAC02B01). We gratefully acknowledge the financial support from the China Scholarship Council (202106320251) and the Doctoral Rising Star Program of Zhejiang University. We thank X. Fan for the constructive suggestions.
Author information
Authors and Affiliations
Contributions
W.W. designed the study. Q.N. and B.G. prepared and analysed the data. Q.N. and B.G. wrote the first draft of the paper. Q.N. and W.C. conducted the long-term field experiment. Y.Q. reviewed this article. Q.N., D.R.S. and J.M. conducted metagenomics analysis. Q.N. provided visualization support. All authors contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Food thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cumulative CH4 emission over 8 years with different amendment strategies.
Different letters above the bar present the significant differences among treatment for each year. There is no interaction between the year and the treatment. Data are presented as mean values ± SEM to account for variability within the replicates (n = 3). The letters above bars denote significant differences (p < 0.05) between treatments for each year in one-way ANOVA. Post-hoc comparisons were conducted using the LSD test to determine specific group differences.
Extended Data Fig. 2 Urea application on CH4 emissions.
The effect of urea application on CH4 emissions in paddy field (a) and its decisive factors (b).The data collected from the long-term field experiment and Banger et al. The data collection method is in Supplementary Method 5. The reference was listed in the supplementary information.
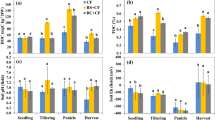
Extended Data Fig. 3 Rice yield with rice straw and biochar amendments over 8 years.
CK, control treatment, no rice straw, and biochar incorporation. RS, rice straw application at 8 Mg ha-1 annually; HS, rice straw biochar application at 22.5 Mg ha-1 only in the first year; AL, rice straw biochar application at 2.8 Mg ha-1 annually. The letters above bars denote significant differences (p ≤ 0.05) between different treatments for each year in a one-sided comparison. Data are presented as mean values ± SEM to account for variability within the replicates (n = 3). The bars show standard error. The letters above bars denote significant differences (p < 0.05) between treatments for each year in one-way ANOVA. Post-hoc comparisons were conducted using the LSD test to determine specific group differences. There is no interaction between the year and the treatment.
Extended Data Fig. 4 Methanogens and methanotrophs community structure.
Community structure variation of methanogens (a-c) and methanotrophs (d-f) between AL and HS, observed in 2016 (a,d), 2018 (b,e) and 2021 (c,f). Methanogen and methanotroph abundances were derived from 16S rRNA sequencing (V34 for methanogens, V45 for methanotrophs) in 2016 and 2018, and from metagenome sequencing in 2021. Sampling methods are detailed in Supplementary Method 1.
Extended Data Fig. 5 Methanotrophs structure within biochar amendments.
The relative methanotrophs abundance of between AL and HS, observed from years of 2016 (a), 2018 (b), and 2021 (c).
Extended Data Fig. 6 CO2-eq reduction and the net benefit over 8 experimental years in RS.
a, GHG emissions reduction after rice straw incorporation at 8 Mg ha-1. b, the GHG emissions reduction over 8 years in RS. c, the corresponding net benefits after rice straw incorporation at 8 Mg ha-1. d, the total benefits over 8 years in RS.
Extended Data Fig. 7 The effect of fresh and aged biochar on CH4 mitigation.
This schematic figure illustrates the adverse effects of biochar aging on CH₄ emission reduction, highlighting the degradation of its physical structure, loss of nutrient retention, decline in root oxygen exudation capacity, and the resulting increase in soil ammonium levels. As biochar ages, its pore structure breaks down, gradually diminishing its ability to supplement nutrients. Compared to fresh biochar, aged biochar in HS is less effective at supporting root growth than in AL, leading to weakened root systems with reduced oxygen exudation capacity. This decline in root activity is directly linked to decreased aerobic methanotrophic activity. In HS, repeated agricultural practices further accelerate the breakdown of biochar’s pores, exposing more surface area and speeding up its oxidation. This process increases the concentration of oxygen-containing functional groups, such as carboxyl groups, which in turn raises soil ammonium ion levels. The elevated ammonium concentration is detrimental to methanotrophs unless they can mitigate ammonium toxicity, leading to decreased methanotroph diversity. In the long term, this inhibition may not only reduce CH₄ mitigation but could potentially promote CH₄ emissions.
Supplementary information
Supplementary Information
Supplementary Methods 1–5, Discussions 1–5, Figs. 1–3 and Tables 1 and 2.
Supplementary Data 1
All the datasets for the supplementary information.
Supplementary Code 1
The R code for linear regression.
Source data
Source Data Figs. 2–6 and Extended Data Figs. 1–6
One Excel workbook with multiple sheets containing all the data from the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nan, Q., Speth, D.R., Qin, Y. et al. Biochar application using recycled annual self straw reduces long-term greenhouse gas emissions from paddy fields with economic benefits. Nat Food 6, 456–465 (2025). https://doi.org/10.1038/s43016-025-01124-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43016-025-01124-z