Abstract
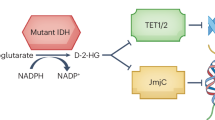
Isocitrate dehydrogenase (IDH) mutants define a class of gliomas that are initially slow-growing but inevitably progress to fatal disease. To characterize their malignant cell hierarchy, we profiled chromatin accessibility and gene expression across single cells from low-grade and high-grade IDH-mutant gliomas and ascertained their developmental states through a comparison to normal brain cells. We provide evidence that these tumors are initially fueled by slow-cycling oligodendrocyte progenitor cell-like cells. During progression, a more proliferative neural progenitor cell-like population expands, potentially through partial reprogramming of ‘permissive’ chromatin in progenitors. This transition is accompanied by a switch from methylation-based drivers to genetic ones. In low-grade IDH-mutant tumors or organoids, DNA hypermethylation appears to suppress interferon (IFN) signaling, which is induced by IDH or DNA methyltransferase 1 inhibitors. High-grade tumors frequently lose this hypermethylation and instead acquire genetic alterations that disrupt IFN and other tumor-suppressive programs. Our findings explain how these slow-growing tumors may progress to lethal malignancies and have implications for therapies that target their epigenetic underpinnings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Processed scATAC-seq and scRNA-seq data are available through the Gene Expression Omnibus under accession number GSE241745. Raw data are available through the dbGaP under accession number phs003697. The human glioma bulk RNA-seq and methylation data were obtained from TCGA Research Network (http://cancergenome.nih.gov/). All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Original code for the analyses performed in this study was deposited on GitHub (https://github.com/BernsteinLab/IDH_mutant_gliomas_progression_2024).
References
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Miller, J. J. et al. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 25, 4–25 (2023).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465, 966 (2010).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17, 510–522 (2010).
Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–483 (2012).
Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114 (2016).
Rahme, G. J. et al. Modeling epigenetic lesions that cause gliomas. Cell 186, 3674–3685 (2023).
Fueyo, J. et al. Hypermethylation of the CpG island of p16/CDKN2 correlates with gene inactivation in gliomas. Oncogene 13, 1615–1619 (1996).
Costello, J. F., Berger, M. S., Huang, H. S. & Cavenee, W. K. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res. 56, 2405–2410 (1996).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Notarangelo, G. et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377, 1519–1529 (2022).
Bunse, L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 24, 1192–1203 (2018).
Ceccarelli, M. et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164, 550–563 (2016).
Tirosh, I. et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539, 309–313 (2016).
Venteicher, A. S. et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, eaai8478 (2017).
Wei, Y. et al. Stalled oligodendrocyte differentiation in IDH-mutant gliomas. Genome Med. 15, 24 (2023).
Babikir, H. et al. ATRX regulates glial identity and the tumor microenvironment in IDH-mutant glioma. Genome Biol. 22, 311 (2021).
Wang, L. et al. The phenotypes of proliferating glioblastoma cells reside on a single axis of variation. Cancer Discov. 9, 1708–1719 (2019).
Yu, K. et al. Surveying brain tumor heterogeneity by single-cell RNA-sequencing of multi-sector biopsies. Natl Sci Rev 7, 1306–1318 (2020).
Johnson, K. C. et al. Single-cell multimodal glioma analyses identify epigenetic regulators of cellular plasticity and environmental stress response. Nat. Genet. 53, 1456–1468 (2021).
Chaligne, R. et al. Epigenetic encoding, heritability and plasticity of glioma transcriptional cell states. Nat. Genet. 53, 1469–1479 (2021).
Trevino, A. E. et al. Chromatin and gene-regulatory dynamics of the developing human cerebral cortex at single-cell resolution. Cell 184, 5053–5069 (2021).
Morabito, S. et al. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet. 53, 1143–1155 (2021).
Couturier, C. P. et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 11, 3406 (2020).
Burdziak, C. et al. Epigenetic plasticity cooperates with cell–cell interactions to direct pancreatic tumorigenesis. Science 380, eadd5327 (2023).
Yanchus, C. et al. A noncoding single-nucleotide polymorphism at 8q24 drives IDH1-mutant glioma formation. Science 378, 68–78 (2022).
Cancer Genome Atlas Research Network et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498 (2015).
Varn, F. S. et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 185, 2184–2199 (2022).
Blanco-Carmona, E. et al. Tumor heterogeneity and tumor–microglia interactions in primary and recurrent IDH1-mutant gliomas. Cell Rep. Med. 4, 101249 (2023).
Miller, T. E. et al. Programs, origins, and niches of immunomodulatory myeloid cells in gliomas. Preprint at bioRxiv https://doi.org/10.1101/2023.10.24.563466 (2023).
Barriga, F. M. et al. MACHETE identifies interferon-encompassing chromosome 9p21.3 deletions as mediators of immune evasion and metastasis. Nat. Cancer 3, 1367–1385 (2022).
Chen, K. et al. Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell 170, 492–506 (2017).
Jacob, F. et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 180, 188–2042 (2020).
Abdullah, K. G. et al. Establishment of patient-derived organoid models of lower-grade glioma. Neuro Oncol. 24, 612–623 (2021).
Gorbunova, V. et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature 596, 43–53 (2021).
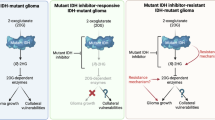
Mellinghoff, I. K. et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 389, 589–601 (2023).
Mellinghoff, I. K. et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat. Med. 29, 615–622 (2023).
Spitzer, A. et al. Mutant IDH inhibitors induce lineage differentiation in IDH-mutant oligodendroglioma. Cancer Cell 42, 904–914.e9 (2024).
Couturier, C. P. et al. Glioblastoma scRNA-seq shows treatment-induced, immune-dependent increase in mesenchymal cancer cells and structural variants in distal neural stem cells. Neuro Oncol. 24, 1494–1508 (2022).
Giovannoni, F. & Quintana, F. J. The role of astrocytes in CNS inflammation. Trends Immunol. 41, 805–819 (2020).
Rahme, G. J., Luikart, B. W., Cheng, C. & Israel, M. A. A recombinant lentiviral PDGF-driven mouse model of proneural glioblastoma. Neuro Oncol. 20, 332–342 (2018).
Jun, H. J. et al. A PDGFRα-driven mouse model of glioblastoma reveals a stathmin1-mediated mechanism of sensitivity to vinblastine. Nat. Commun. 9, 3116 (2018).
Goenka, A. et al. Oncogenic long noncoding RNA LINC02283 enhances PDGF receptor A-mediated signaling and drives glioblastoma tumorigenesis. Neuro Oncol. 25, 1592–1604 (2023).
Ganz, J. et al. Rates and patterns of clonal oncogenic mutations in the normal human brain. Cancer Discov. 12, 172–185 (2022).
Herman, J. G. et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 55, 4525–4530 (1995).
Gonzalez-Zulueta, M. et al. Methylation of the 5′ CpG Island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 55, 4531–4535 (1995).
Kohanbash, G. et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J. Clin. Invest. 127, 1425–1437 (2017).
Amankulor, N. M. et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786 (2017).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Hu, J. et al. STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature 616, 806–813 (2023).
Volkman, H. E., Cambier, S., Gray, E. E. & Stetson, D. B. Tight nuclear tethering of cGAS is essential for preventing autoreactivity. eLife 8, e47491 (2019).
Fang, L. et al. Methionine restriction promotes cGAS activation and chromatin untethering through demethylation to enhance antitumor immunity. Cancer Cell 41, 1118–1133 (2023).
Turcan, S. et al. Mutant-IDH1-dependent chromatin state reprogramming, reversibility, and persistence. Nat. Genet. 50, 62–72 (2018).
Gierahn, T. M. et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398 (2017).
Satpathy, A. T. et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 37, 925–936 (2019).
Azevedo Portilho, N. et al. The DNMT1 inhibitor GSK-3484862 mediates global demethylation in murine embryonic stem cells. Epigenetics Chromatin 14, 56 (2021).
Kaminow, B., Yunusov, D. & Dobin, A. STARsolo: accurate, fast and versatile mapping/quantification of single-cell and single-nucleus RNA-seq data. Preprint at bioRxiv https://doi.org/10.1101/2021.05.05.442755 (2021).
Bravo González-Blas, C. et al. cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. Nat. Methods 16, 397–400 (2019).
Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with Signac. Nat. Methods 18, 1333–1341 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Kotliar, D. et al. Identifying gene expression programs of cell-type identity and cellular activity with single-cell RNA-Seq. eLife 8, e43803 (2019).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Vierstra, J. et al. Global reference mapping of human transcription factor footprints. Nature 583, 729–736 (2020).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020).
Turcan, S. et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT inhibitor decitabine. Oncotarget 4, 1729–1736 (2013).
Guilhamon, P. et al. Single-cell chromatin accessibility profiling of glioblastoma identifies an invasive cancer stem cell population associated with lower survival. eLife 10, e64090 (2021).
Barthel, F. P. et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature 576, 112–120 (2019).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 (2019).
Acknowledgements
We thank G. Rahme, C. Couturier, J. Verga, M. Thompsom and all members of the Bernstein laboratory for their discussions. We thank S. Ma, V. Kartha, C. Lareau and J. Buenrostro for guiding the scATAC-seq experiment and analysis. We thank S. Gritsch and D. Silverbush for providing help with the preparation and analysis of glioma samples. This work was supported by funds from the National Cancr Institute (NCI) and National Institutes of Health (NIH) Director’s Fund (DP1CA216873 to B.E.B.) and the Ludwig Center at Harvard. J.W. was supported by a Damon Runyon postdoctoral fellowship award and King Trust fellowship award. L.N.G.C was supported by NIH award K12CA090354 and a Harold Amos faculty development award from the Robert Wood Johnson Foundation. B.E.B. is the Richard and Nancy Lubin Family Endowed Chair at the Dana-Farber Cancer Institute and an American Cancer Society research professor. This work was supported by grants to M.L.S. from the Mark Foundation (emerging leader award), the Sontag Foundation (distinguished scientist award), the MGH Research Scholars and NCI R37CA245523 and R01CA258763.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design, J.W., L.N.G.C., M.L.S. and B.E.B. Methodology and data acquisition, J.W., L.N.G.C., S.B., J.D.A. and T.E.M. Analysis and interpretation of data, J.W., L.N.G.C., C.A.E.F., M.L.S. and B.E.B. Manuscript writing and revision, J.W., L.N.G.C., M.L.S. and B.E.B.
Corresponding author
Ethics declarations
Competing interests
L.N.G.C. has received research funding from Merck & Co. (to the Dana-Farber Cancer Institute) and has received consulting fees from Elsevier, Servier Laboratories, BMJ Best Practice, Prime Education and Oakstone Publishing. B.E.B. discloses financial interests in HiFiBio, Arsenal Biosciences, Chroma Medicine, Cell Signaling Technologies and Design Pharmaceuticals. M.L.S. discloses financial interests in Immunitas Therapeutics. T.E.M. discloses financial interest in Reify Health, Care Access Research and Telomere Diagnostics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Shi-Yuan Cheng, Roel Verhaak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of malignant cells and their developmental states from scRNA-seq and scATAC-seq data.
a. Genome tracks show aggregated (pseudo-bulk) scATAC-seq data for IDH-mutant gliomas (n=10) over a representative neural locus (ASCL1). b. UMAP visualization of IDH-mutant glioma cells profiled by scRNA-seq and scATAC-seq. The leftmost plots indicate annotated malignant cells, while the others depict expression or promoter and gene body accessibility (red) of cell type-specific genes. c. Genome tracks show aggregated scRNA-seq and scATAC-seq data over representative cell type-specific genes. d. CNAs inferred from scATAC-seq data for malignant cells from IDH-mutant cohorts used in this study (see Methods). CNAs inferred directly from the scATAC-seq data (right) were consistent with CNAs derived by applying inferCNV to imputed gene activity scores (left). e. UMAP visualizations of integrated scRNA-seq and scATAC-seq data from normal fetal and adult brain cells. Cells are colored by annotated cell types (left) and donor types (right). f. Pie charts depict the distributions of cell state annotations nominated by scRNA-seq (left) or scATAC-seq (right). g. Gene programs enriched in OPC-like cells from IDH-mutant oligodendrogliomas (IDH-O) or astrocytomas (IDH-A) by NMF analysis. h. Genome tracks show aggregated scATAC-seq data for each cell state over oligodendrocyte- (APOD) and astrocyte-specific (APOE) genes.
Extended Data Fig. 2 Marker genes and TF motifs associated with glioma cell states.
a. Heatmaps show the expression of variable genes (rows) across individual brain or malignant cells (columns). Cells are grouped by their assigned states. b. Heatmaps depict TF motif enrichments (rows) in scATAC-seq profiles for individual brain or malignant cells (columns). c. Genome tracks show aggregate accessibility over portions of the MYC and PDGFRA loci in normal brain and malignant glioma cell types. Shaded intervals correspond to an OPC-specific enhancer in the MYC locus that harbors a genetic variant associated with glioma risk (left) and an OPC-specific enhancer implicated in PDGFRA induction. d. Heatmaps show pairwise correlations of scRNA-seq (top) or scATAC-seq (bottom) data for normal brain cells or malignant glioma cells grouped by their cell type classifications, as in Fig. 2c, but with data from three high-grade IDH-mutant gliomas with matched scRNA-seq and scATAC-seq data.
Extended Data Fig. 3 Glioma progression associated with increasing proportions of NPC-like cells.
a. Box plots depict proportions of OPC-like and NPC-like cells in IDH-mutant gliomas, stratified by grade and subtype (n=7 for IDHO grade 2, n=2 for IDHO grade 3, n=5 for IDHA grade 2, n=11 for IDHA grade 3, n=5 for IDHA grade 4). Boxes depict 25th, 50th and 75th percentiles, and whiskers depict extreme values. One-tailed t-test P-value: 0.013 for NPC-like proportions. b. Box plots depict relative proportions of NPC-like versus OPC-like cells, inferred from bulk expression data for IDH-mutant gliomas (TCGA), stratified by grade and subtype (n=81 for IDHO grade 2, n=70 for IDHO grade 3, n=114 for IDHA grade 2, n=104 for IDHA grade 3, n=7 for IDHA grade 4). One-tailed t-test P-value: 0.039; *** defines p<0.001. c. Barplot depicts the ratio of NPC-like to OPC-like cells in scRNA-seq data for six matched pairs of primary and recurrent tumors. Data are presented as mean values +/- SEM. d. UMAP visualization of malignant cells projected onto normal brain cells, as in Figs. 1b and 3a, with heat depicting the proliferation scores of individual malignant cells. e. Trajectory analysis was performed on combined scRNA-seq data for malignant cells and normal OPCs, using the Monocle package. The pseudotime coloring and best-fit trajectory are consistent with progression from normal OPC to OPC-like and then NPC-like malignant cells. f. Plot depicts CNAs for loci subject to CNAs (rows) across single cells (columns) from a second IDH-mutant glioma (OPK438), as in Fig. 3g. Malignant cells are grouped into subclones based on CNAs, and compared to normal cells from the same resection (left). Malignant cell state assignments indicated.
Extended Data Fig. 4 Validation of IFN signature in malignant cells and glioma organoids.
a. Boxplot depicts IFN signature scores (RNA-seq) for grade 2 IDH mutant and WT gliomas (n=35 for IDH mutant and n=5 for IDH WT) with high purity estimates (>80%). Boxes depict 25th, 50th and 75th percentiles, and whiskers depict extreme values. One-tailed t-test P-values: *** defines p<0.001. b. Heatmap shows DNA methylation levels, proliferation scores, and IFN scores (rows) in grade 2 IDH-mutant and grade 2 IDH WT TCGA gliomas (columns) based on the 2016 WHO classification. c. Heatmap depicts the expression of IFN pathway genes (columns) clustered by their expression across malignant cells, immune cell lineages, and oligodendrocytes (rows). This analysis distinguished malignant cell-specific IFN-related genes. d. UMAP visualization of scRNA-seq data compares cells from a glioma organoid (red) and the primary tumor from which it was derived (blue). e. Heatmaps depict the expression of variable genes associated with different cell states (rows) in single cells (columns) grouped by nominal cell identity. The glioma organoids recapitulate cell types and transcriptional programs as seen in the primary tumors.
Extended Data Fig. 5 IFN genes are upregulated by DNMT1 and IDH inhibitors.
a. Box plots depict module scores (Seurat) for gene correlates of DNA methylation loss in IDH-mutant glioma organoids treated with DNMT1 inhibitor or control (n=3 technical replicates). Boxes depict 25th, 50th and 75th percentiles, and whiskers depict extreme values. b. Running Enrichment Score (GSEA) for IFNa genes regulated by DNMT1 inhibitor. c. Box plots depict module scores (Seurat) for gene correlates of methylation loss in IDH-mutant gliomas resected from patients treated with IDH inhibitors. Left: paired pre- and post-treatment samples from a single patient. Right: unpaired samples from 6 untreated and 2 treated patients. Two-tailed t-test P values: ***p<0.001. d. Running Enrichment Score (GSEA) for IFNa genes in IDH-mutant gliomas resected from patients treated with IDH inhibitor (as in c). e. Multivariate survival analysis for high-grade IDH-mutant gliomas (grade 3 and 4) stratified by NPC enrichment score and tumor grade. Hazard ratios (HR) and P-value associated with NPC-enriched tumors are indicated.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table
Supplementary Table 1: The quality control information for ATAC-seq samples. Supplementary Table 2: The P value between NPC ratio and proliferation scores and between genetic lesions and DNA methylation. Supplementary Table 3: The percentage of codeletion. Supplementary Table 4: The subtype and grade information of TCGA cohorts. Supplementary Table 5: Genes upregulated upon DNA demethylation in glioma cell line.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Gonzalez Castro, L.N., Battaglia, S. et al. Evolving cell states and oncogenic drivers during the progression of IDH-mutant gliomas. Nat Cancer 6, 145–157 (2025). https://doi.org/10.1038/s43018-024-00865-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-024-00865-3
This article is cited by
-
Immunotherapy and targeted therapy for high grade gliomas: current and future directions
Journal of Neuro-Oncology (2026)
-
From Classical to Emerging Biomarkers of Brain and Central Nervous System Tumors. An Evidence-Based Review with a Focus on Gliomas
Cellular and Molecular Neurobiology (2026)
-
Epigenetic reprogramming as the nexus of cancer stemness and therapy resistance: implications for biomarker discovery
Discover Oncology (2025)
-
Perioperative IDH inhibition in treatment-naive IDH-mutant glioma: a pilot trial
Nature Medicine (2025)