Abstract
Transfer RNA modifications have emerged as critical regulators of translational reprogramming, yet their roles in colorectal cancer (CRC) remain largely elusive. Here, we find that tRNA N1-methyladenosine (m1A) methyltransferase TRMT6 is upregulated in human CRC tissues and high TRMT6 expression correlates with poor survival in patients with CRC. Using orthotopic, metastatic and conditional knockout mouse models, we establish the oncogenic role of TRMT6 in CRC. Mechanistically, TRMT6 increases tRNA m1A levels by maintaining the stability of the TRMT6–TRMT61A complex. Targeting TRMT6-mediated tRNA m1A modification in CRC cells destabilizes tRNA-Lys-TTT-1-1 and impairs histone mRNA translation in a codon-biased manner, thereby restricting histone synthesis and hindering cell cycle progression. Our study provides evidence that TRMT6 functions as a translational checkpoint in the accelerated histone synthesis of CRC cells, highlighting TRMT6 as a promising target for potential anti-CRC therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Ribosome profiling, tRNA sequencing and RNA-seq data can be found in the NCBI’s Gene Expression Omnibus using series accession number GSE233361. The human m1A methyltransferase for molecular docking was obtained from the PDB under accession code 5CCB. All TCGA/CPTAC/GTEx data used in this study are publicly available on cBioPortal (https://www.cbioportal.org/) and GTEx Portal (https://www.gtexportal.org/home/). The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
All relevant package and software information is provided in the Methods. No custom code was generated during the course of this study.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Barbieri, I. & Kouzarides, T. Role of RNA modifications in cancer. Nat. Rev. Cancer 20, 303–322 (2020).
Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 22, 375–392 (2021).
Orellana, E. A., Siegal, E. & Gregory, R. I. tRNA dysregulation and disease. Nat. Rev. Genet. 23, 651–664 (2022).
Dedon, P. C. & Begley, T. J. Dysfunctional tRNA reprogramming and codon-biased translation in cancer. Trends Mol. Med. 28, 964–978 (2022).
Zhang, W., Foo, M., Eren, A. M. & Pan, T. tRNA modification dynamics from individual organisms to metaepitranscriptomics of microbiomes. Mol. Cell 82, 891–906 (2022).
Wang, Y. et al. tRNA modifications: insights into their role in human cancers. Trends Cell Biol. 33, 1035–1048 (2023).
Yuan, W. et al. Dysregulation of tRNA methylation in cancer: mechanisms and targeting therapeutic strategies. Cell Death Discov. 10, 327 (2024).
Wang, Y. et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat. Commun. 12, 6314 (2021).
Blanco, S. et al. Stem cell function and stress response are controlled by protein synthesis. Nature 534, 335–340 (2016).
Luo, Y. et al. The potential role of N7-methylguanosine (m7G) in cancer. J. Hematol. Oncol. 15, 63 (2022).
Boccaletto, P. et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 50, D231–D235 (2022).
Zhang, Z. et al. Global analysis of tRNA and translation factor expression reveals a dynamic landscape of translational regulation in human cancers. Commun. Biol. 1, 234 (2018).
de Crecy-Lagard, V. et al. Matching tRNA modifications in humans to their known and predicted enzymes. Nucleic Acids Res. 47, 2143–2159 (2019).
Chan, C. T. et al. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 6, e1001247 (2010).
Liu, F. et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell 167, 816–828 e816 (2016).
Vasaikar, S. et al. Proteogenomic analysis of human colon cancer reveals new therapeutic opportunities. Cell 177, 1035–1049 e1019 (2019).
Neufert, C., Becker, C. & Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998–2004 (2007).
Finer-Moore, J. et al. Crystal structure of the human tRNA m(1)A58 methyltransferase-tRNA(3)(Lys) complex: refolding of substrate tRNA allows access to the methylation target. J. Mol. Biol. 427, 3862–3876 (2015).
Graille, M. Division of labor in epitranscriptomics: what have we learnt from the structures of eukaryotic and viral multimeric RNA methyltransferases? Wiley Interdiscip. Rev. RNA 13, e1673 (2022).
Schwartz, S. m(1)A within cytoplasmic mRNAs at single nucleotide resolution: a reconciled transcriptome-wide map. RNA 24, 1427–1436 (2018).
Li, X. et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68, 993–1005.e1009 (2017).
Basavappa, R. & Sigler, P. B. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 10, 3105–3111 (1991).
Goodarzi, H. et al. Modulated expression of specific tRNAs drives gene expression and cancer progression. Cell 165, 1416–1427 (2016).
Passarelli, M. C. et al. Leucyl-tRNA synthetase is a tumour suppressor in breast cancer and regulates codon-dependent translation dynamics. Nat. Cell Biol. 24, 307–315 (2022).
Orellana, E. A. et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell 81, 3323–3338.e3314 (2021).
Dai, Z. et al. N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol. Cell 81, 3339–3355 e3338 (2021).
Marzluff, W. F., Wagner, E. J. & Duronio, R. J. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat. Rev. Genet. 9, 843–854 (2008).
Marzluff, W. F. & Koreski, K. P. Birth and death of histone mRNAs. Trends Genet. 33, 745–759 (2017).
Mejlvang, J. et al. New histone supply regulates replication fork speed and PCNA unloading. J. Cell Biol. 204, 29–43 (2014).
Gunesdogan, U., Jackle, H. & Herzig, A. Histone supply regulates S phase timing and cell cycle progression. eLife 3, e02443 (2014).
Armstrong, C. & Spencer, S. L. Replication-dependent histone biosynthesis is coupled to cell-cycle commitment. Proc. Natl Acad. Sci. USA 118, e2100178118 (2021).
Gruber, J. J. et al. HAT1 coordinates histone production and acetylation via H4 promoter binding. Mol. Cell 75, 711–724 e715 (2019).
Hagelskamp, F., Borland, K., Ammann, G. & Kaiser, S. M. Temporal resolution of NAIL-MS of tRNA, rRNA and Poly-A RNA is overcome by actinomycin D. RSC Chem. Biol. 4, 354–362 (2023).
Hu, B. et al. Therapeutic siRNA: state of the art. Signal Transduct. Target. Ther. 5, 101 (2020).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Gao, G. et al. NPAT expression is regulated by E2F and is essential for cell cycle progression. Mol. Cell. Biol. 23, 2821–2833 (2003).
Jung, G., Hernandez-Illan, E., Moreira, L., Balaguer, F. & Goel, A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 17, 111–130 (2020).
Haruehanroengra, P., Zheng, Y. Y., Zhou, Y., Huang, Y. & Sheng, J. RNA modifications and cancer. RNA Biol. 17, 1560–1575 (2020).
Neganova, M. E., Klochkov, S. G., Aleksandrova, Y. R. & Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 83, 452–471 (2022).
Zuo, H., Wu, A., Wang, M., Hong, L. & Wang, H. tRNA m1A modification regulate HSC maintenance and self-renewal via mTORC1 signaling. Nat. Commun. 15, 5706 (2024).
Su, Z. et al. TRMT6/61A-dependent base methylation of tRNA-derived fragments regulates gene-silencing activity and the unfolded protein response in bladder cancer. Nat. Commun. 13, 2165 (2022).
Gingold, H. et al. A dual program for translation regulation in cellular proliferation and differentiation. Cell 158, 1281–1292 (2014).
Torrent, M., Chalancon, G., de Groot, N. S., Wuster, A. & Madan Babu, M. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci. Signal. 11, eaat6409 (2018).
You, J. S. & Jones, P. A. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22, 9–20 (2012).
Gingold, H. & Pilpel, Y. Determinants of translation efficiency and accuracy. Mol. Syst. Biol. 7, 481 (2011).
Liu, Y. et al. tRNA-m(1)A modification promotes T cell expansion via efficient MYC protein synthesis. Nat. Immunol. 23, 1433–1444 (2022).
Wang, H. L. et al. Sirtuin5 protects colorectal cancer from DNA damage by keeping nucleotide availability. Nat. Commun. 13, 6121 (2022).
Pavon-Eternod, M., Gomes, S., Rosner, M. R. & Pan, T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA 19, 461–466 (2013).
Shechter, D., Dormann, H. L., Allis, C. D. & Hake, S. B. Extraction, purification and analysis of histones. Nat. Protoc. 2, 1445–1457 (2007).
Zheng, G. et al. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods 12, 835–837 (2015).
Gogakos, T. et al. Characterizing expression and processing of precursor and mature human tRNAs by hydro-tRNAseq and PAR-CLIP. Cell Rep. 20, 1463–1475 (2017).
Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
Lauria, F. et al. riboWaltz: optimization of ribosome P-site positioning in ribosome profiling data. PLoS Comput. Biol. 14, e1006169 (2018).
Acknowledgements
We thank all patients and individuals for their participation in our study. We thank H.-B. Li (Shanghai Jiao Tong University) for providing the Trmt6flox/flox mice. We thank W. Zou (University of Michigan Medical School), H.-B. Li (Shanghai Jiao Tong University), J. Yang (Shanghai Jiao Tong University) and all members of the Shanghai Institute of Digestive Disease for fruitful discussions and/or technical assistance. We are grateful to Guangzhou Gene Denovo Biotechnology Co. for assisting in sequencing and bioinformatics analysis. This work was supported by the National Natural Science Foundation of China (grant numbers 82273140 (Y.-X.C.), 82002484 (J.T.), 82250005 (J.-Y.F.), 82203269 (H.-L.W.), 82303215 (Q.L.), 82403172 (E.-W.T.)) and the National Key R&D Program of China (grant number 2020YFA0509200 (J.-Y.F.)).
Author information
Authors and Affiliations
Contributions
E.-W.T., Y.W. and J.T. performed the experiments and analyzed the data. Y.C. provided support for IHC staining. T.-Y.S. and Z.-Q.Z. provided support for bioinformatics analysis. Y.H., H.-L.W. and Q.L. were involved in the studies of mice. E.-W.T. drafted the manuscript. Y.-Q.W. and L.F. revised the manuscript critically. H.C. and J.H. provided critical comments and technical support for the project. Q.-Y.G., J.-Y.F. and Y.-X.C. designed or/and supervised this project and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Pierre Close, Rene Jackstadt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Dysregulated tRNA modifiers and TRMT6 upregulation in CRC.
(a) Heatmaps showing differentially expressed tRNA-modifying genes between tumor and normal samples. The mRNA expression level of collected tRNA-modifying genes was analyzed using The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. The Clinical Proteomic Tumor Analysis Consortium (CPTAC) database was also used to analyze the protein levels of these genes (some genes were not found in this database). Each color represents differential gene expression: red represents higher expression in the tumor sample compared with that in the normal sample. The blue box denotes de-modified enzymes, and the others are write-modifying enzymes. (b) TRMT6 mRNA expression and DNA copy number status were evaluated in 590 primary CRC samples from the TCGA CRC database. The analysis revealed that 45.25% (267 of 590) of the samples had TRMT6 copy number gain/amplification as determined by putative copy-number alterations from GISTIC. (c) The correlation between TRMT6 mRNA expression and copy number variation according to two-tailed Pearson correlation analysis (n = 590, r = 0.78, p < 0.0001). The data were collected from the TCGA CRC cohort. (d) Overview and partially enlarged images of TRMT6 protein staining in colorectal normal tissues and CRC tissues using immunohistochemistry (IHC) staining. The staining was performed on two independent tissue microarrays, CRC cohort 1 and CRC cohort 2. The scale bar of enlarged images represents 100 μm.
Extended Data Fig. 2 Knockdown of TRMT6 inhibited proliferation of CRC cells.
(a) Confirmation of TRMT6 knockdown using two independent siRNAs in RKO and SW480 cells, including RNA dot blot (total RNA, 1000 ng) analysis of m1A levels in the indicated cells. (b) Knockdown of TRMT6 inhibited cell viability of RKO and SW480 cells, as assessed using CCK-8 assays (Representative results are shown, n = 6 technical replicates). (c) Knockdown of TRMT6 suppressed cell colony formation of RKO and SW480 cells (mean ± SD, n = 3 biologically independent experiments, p-values as indicated in the figure: 0.0066, 0.0065, and < 0.0001, one-way ANOVA with Tukey’s multiple comparisons test). (d) Knockdown of TRMT6 decreased the percentage of Ki-67 positive cells of RKO, SW480, and HCT116 cells (mean ± SD, n = 3 biologically independent experiments, p-values as indicated in the figure: < 0.0001, 0.0003, and 0.0006, two-tailed unpaired t-test). The representative images were shown, and a scale bar of 50 μm was used for the image. The experiments in a-d were independently performed three times, all with similar results.
Extended Data Fig. 3 TRMT6 depletion showed no apoptosis or intestinal changes.
(a) Left: Representative pictures of the gating strategy for FACS-based quantification of apoptosis cells. Right: Quantified percentage of apoptotic cells in each group (flowjo, mean ± SD, n = 3 biologically independent experiments, ns: not significant (p = 0.3090), two-tailed unpaired t-test). (b) Under light microscopy (scale bar of 100 μm), there was no apparent increase in the presence of cells exhibiting apoptotic morphology upon TRMT6 knockdown. (c) Oxaliplatin (Oxa), known for its apoptotic induction properties, notably elevated the expression levels of the apoptotic marker protein Cleaved-PARP in RKO cells. Conversely, TRMT6 knockdown did not result in the upregulation of Cleaved-PARP expression levels. (d) Confirmation of genotype of conditional knockout (Trmt6flox/flox, Vil1-Cre) mice using reverse transcription PCR of DNA extracted from mouse tail. (e) The mRNA level of Trmt6 was analyzed by real-time PCR in mouse colon tissues from WT and CKO mice (mean ± SD, n = 3 biologically independent animals per group, p < 0.0001, two-tailed unpaired t-test). (f) Confirmation of TRMT6 protein expression in mouse colon tissues from WT and CKO mice by immunoblot. (g) Morphology of the excised colon. Representative images of the colon from 24-week-old WT mice and CKO mice (n = 5 biologically independent animals per group). (h) Representative H&E and Ki-67 IHC of colon tissues from 24-week-old WT and CKO mice, with a scale bar of 1 mm and 100 μm for the images (n = 5 biologically independent animals per group). (i) The mRNA expression levels of TRMT6 were analyzed using qRT–PCR upon knockdown of TRMT6 with siRNA in indicated cell lines (mean ± SD, n = 3 biologically independent experiments, p < 0.0001, two-tailed unpaired t-test). (j) The relative cell viability was assessed using CCK-8 assays after TRMT6 silencing in CRC tumor cells and normal intestinal epithelial cells (NCM460) for 72 h (Representative results are shown, n = 4 technical replicates). The experiments in a, b, c, d, e, f, and i were independently performed three times, the experiments in g, h, and j were independently performed two times, all with similar results.
Extended Data Fig. 4 Correlation between TRMT6 and TRMT61A in CRC.
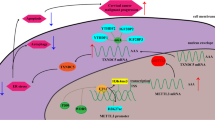
(a) IHC analysis (16 sample pairs, cohort 3) confirmed CRC-specific TRMT6 upregulation (p < 0.0001, two-tailed Mann–Whitney). Box plots show median (center line), 25th–75th percentiles (box edges), and full data range (whiskers). (b) The relative protein and mRNA levels of TRMT6 and TRMT61A upon knockdown of TRMT6 or TRMT61A with ShRNA in RKO cell lines (mean ± SD, n = 3 biologically independent experiments, p < 0.0001, one-way ANOVA with Tukey’s multiple comparisons test). (c) Confocal microscopy was performed to visualize the subcellular localization of HA-tagged TRMT6 and FLAG-tagged TRMT61A in HCT116 cells. Representative images were shown (scale bar of 10 μm). (d) Co-immunoprecipitation (Co-IP) assays were performed to investigate the physical interaction (endogenous + exogenous) between TRMT6 and TRMT61A in HCT116 cells. (e) Left: Based on the TCGA+GTEx databases, we analyzed the differences in relative TRMT6 and TRMT61A mRNA levels between colorectal tumor and normal tissues. Right: Based on the CPTAC database, we analyzed the differences in relative levels of TRMT6 and TRMT6 protein between colorectal tumor and normal tissues. Box plots display median (center line), 25th–75th percentiles (box edges), whiskers at 1.5 × IQR, and individual outliers. Statistical analysis using two-tailed Mann–Whitney test (n is indicated in the figure, p < 0.0001, ns: non-significant (p = 0.3053)). (f, g) A two-tailed Pearson correlation analysis was conducted to assess the correlation between TRMT6 and TRMT61A mRNA expression levels and their protein levels using data from the CPTAC databases and CCLE databases. Red star denotes common CRC cell lines. N, r-value, and p-value were indicated in the figure. (h) This schematic diagram illustrates that in colorectal cancer, overexpression of TRMT6 is driven by copy number gain, stabilizing the TRMT6/TRMT61A complex and ultimately resulting in elevated levels of tRNA m1A modification. Created with BioRender.com. The experiments in b, c, and d were independently performed three times, all with similar results.
Extended Data Fig. 5 The potential key catalytic site of TRMT61A.
(a) Sequence alignment of the MTase domain of TRMT61A across multiple species. Strictly conserved residues are highlighted with red boxes, emphasizing key regions of evolutionary conservation. The sequence alignment was conducted using the Clustal Omega tool available from the EBI website (https://www.ebi.ac.uk/). (b) An overview of the core residues involved in substrate binding within the MTase domain of TRMT61A. The three conserved residues identified from the sequence alignment are highlighted in red text (Ser114, Glu135, Asp181), and all three are substrate-binding residues. The 3D structure of the MTase domain was obtained from the Protein Data Bank (PDB: 5CCB, https://www.rcsb.org/3d-view/5CCB/1?preset=symmetry&sele=0).
Extended Data Fig. 6 TRMT6 knockdown decreased the TE of histone mRNAs.
(a) Puromycin intake assay of TRMT6 or TRMT61A-knockdown RKO cells. The cells were incubated with puromycin (2 μg/ml) for 30 min. Total protein samples were examined by western blotting using an anti-puromycin antibody. (b) The volcano plot of statistical significance and fold changes in mRNA and ribosome-protected mRNA abundance upon knockdown of TRMT6, with a significance threshold of FDR < 0.01 and |Fold Change | > 2 (Red dots indicate upregulated mRNAs and blue dots indicate downregulated mRNAs). RNAs differential expression analysis was performed by DESeq2 software between two different groups. (c) A snapshot depicting the ribosome profiling (RPF) tracks specifically for representative histone genes. This snapshot provides a visual representation of the translation dynamics and ribosome occupancy along these crucial genes involved in histone synthesis. (d) Relative changes in all histone mRNAs after knockdown of TRMT6 in RKO cells. The results were analyzed with data from RNA-seq, Ribo-seq, and qRT–PCR, respectively. As replication-dependent histone mRNAs (without poly(A) tail) cannot be detected using RNA-seq, qRT–PCR experiments were conducted to measure the mRNA levels of these histone mRNAs. The translation efficiency (TE) of these histone mRNA was then calculated using data from Ribo-seq and qRT–PCR. The histone genes with decreased TE were marked in blue font. N.D. means not detected. The data was presented as the mean ± SD (n = 3 biologically independent experiments, two-tailed unpaired t-test, individual p values are shown in the Supplementary Table 8). (e) Differential TE genes (top 500 genes) were subjected to enrichment analysis of GO functions and KEGG pathways. (f) Left: TRMT6 knockdown reduces exogenous HA-tagged histone H3C1 protein levels in SW480 cells (WB); Right: No significant change in H3C1 mRNA levels upon TRMT6 knockdown (qRT–PCR, mean ± SD, n = 3 biologically independent experiments, two-tailed unpaired t-test, ns: not significant (p = 0.5498)). The experiments in a and f were independently performed three times, all with similar results.
Extended Data Fig. 7 TRMT6 knockdown restricted histone synthesis.
(a) The protein levels of newly synthesized cytoplasmic histones (H1, H2B, H3, and H4) in CRC cell lines upon thymidine incubation and removal. Experimental setup (top panel), #1: The cells were incubated with medium for 24 h; #2: The cells were incubated with both medium and thymidine for 24 h respectively; #3: Based on #2, thymidine was removed, and the cells were further cultured in the medium for another 24 h. In the bottom panel, WB analysis showing that cytoplasm-extracted histones were decreased upon thymidine treatment and returned to normal levels after thymidine removal. Thymidine: an inhibitor of DNA synthesis. (b) WB was performed to confirm the efficiency of cytoplasmic and nuclear protein separation, using α-tubulin as a cytoplasmic protein control and Lamin A/C as a nuclear protein control. (c) In TRMT6-depleted and control cells, cytoplasm-extracted and acid-extracted proteins were analyzed by western blot and Coomassie staining (12.5% SDS–PAGE). Upon TRMT6 knockdown, the levels of newly synthesized cytoplasmic histones (cytoplasm-extracted) were decreased, while the levels of chromatin-bound histones (acid-extracted) remained unchanged. (d) The experimental design is as depicted in the diagram: following transfection, cells were incubated with thymidine nucleoside for 20 h, after which the thymidine nucleoside was removed, and the cells were further cultured in complete DMEM medium until the specified time point. The experiments in a, b, and c were independently performed three times, all with similar results.
Extended Data Fig. 8 tRNA-Lys-TTT-1-1 overexpression in CRC tissues.
(a) In RKO cells, different types of histones were overexpressed, and western blot experiments confirmed the expression efficiency of these exogenous histones. (b) Northern blot analysis was used to determine the levels of tRNA-Lys-TTT-1-1 in CRC tissue compared to adjacent normal tissues. Relative expression levels were quantified by ImageJ software, with sample #1 used for normalization. The data shows that the levels of tRNA-Lys-TTT-1-1 were significantly upregulated in CRC tissue compared to adjacent normal tissues (paired dot plots, n = 6 biologically independent samples per group, CRC cohort 3, p = 0.0043, two-tailed paired t-test). (c) The actinomycin D transcription inhibition assay revealed that the degradation of tRNA-Lys-TTT-1-1 was accelerated upon silencing of TRMT6. SW480 cells with TRMT6 silencing and control cells were treated with actinomycin D for different durations and then subjected to northern blot analysis with the indicated probes (left panel); the relative levels of remaining tRNA-Lys-TTT-1-1 were quantified using ImageJ software, with actinomycin D-untreated samples used for normalization (right panel). The experiments in a and c were independently performed two times, all with similar results.
Extended Data Fig. 9 Inhibitory effect of Chol-si-TRMT6 on liver metastasis.
(a) Representative schematic diagram of the chemical modification pattern of Chol-si-NC and Chol-si-TRMT6. The U and G bases in double-stranded siRNA are modified with 2’-O-methyl ribose modification and the double-stranded 3′ ends are modified with cholesterol, enhancing their stability and efficacy in vivo. (b) Schematic diagram of the animal experiments to uncover the inhibitory effect of Chol-si-TRMT6 on liver metastasis. Nude mice were injected in the spleen with luciferase-labeled HCT116 cells (2.0 × 106) to establish a surgical liver metastasis mouse model. Treatments (Chol-si-NC/Chol-si-TRMT6, 5 nmol, tail vein) initiated at Day 8. (c) The growth of experimental liver metastases was measured on days 8, 14 and 20 using an In Vivo Xtreme imaging system. Liver metastases from different groups were dissected and photographed. The luminescence intensities obtained for the Chol-si-NC and Chol-si-TRMT6 groups were further compared (mean ± SD, n = 5 biologically independent animals per group, p = 0.0079, two-tailed unpaired t-test). (d) Schematic representation of the mechanism by which CDK4/6 inhibitor Palbociclib regulates histone mRNA synthesis. The activated Cyclin-CDK4/6 complex initiates phosphorylation of the RB1 protein, leading to RB1 inactivation and dissociation from the transcription factor E2F. Released E2F promotes the transcription of NPAT, which, in turn, acts as a co-transcription factor for histone genes, facilitating histone transcription. Consequently, inhibition of Cyclin-CDK4/6 complex by Palbociclib results in a decrease in histone mRNA levels. (e) Western blot analysis showed that treatment with increasing concentrations of Palbociclib (0.016–20 μM) reduced phosphorylated RB and newly synthesized cytoplasmic H3 in RKO, SW480, and HCT116 cells. Effective concentrations identified as 0.8 μM and 4 μM for RKO, and 4 μM and 20 μM for SW480 and HCT116 cells. (f) The mRNA levels of NPAT and representative histone genes were analyzed using qRT–PCR in various CRC cells (RKO, SW480, and HCT116) treated with Palbociclib at indicated concentrations (mean ± SD, n = 3 biologically independent experiments). The experiments in c were independently performed two times, the experiments in e and f were independently performed three times, all with similar results.
Extended Data Fig. 10 Palbociclib enhances anticancer effect of TRMT6 knockdown.
(a) Left panel: heatmaps of dose-combination responses in RKO cells showing strong anti-proliferative activity observed for combination, and the average percentage inhibition values are indicated in the heatmaps. Right panel: bliss synergy score topography plot recapitulated the broad range of synergistic doses (Bliss score greater than 10 indicates that the interaction between the two drugs is likely synergistic). (b) EdU incorporation assays showed that the combination of si-TRMT6 and Palbociclib significantly reduced EdU-positive cells compared to individual treatments (mean ± SD, n = 3 biologically independent experiments, p-values as indicated in the figure: < 0.0001, 0.0032, 0.0370, and 0.0400, one-way ANOVA with Tukey’s multiple comparisons test). (c) Flow cytometry analysis revealed increased G1-phase and decreased S-phase populations in RKO cells treated with si-TRMT6 and Palbociclib combination, compared to single treatments (FlowJo software, mean ± SD, n = 3 biologically independent experiments, p-values as indicated in the figure: < 0.0001 and 0.0002, one-way ANOVA with Tukey’s multiple comparisons test). (d) WB showed that the combination of si-TRMT6 and Palbociclib (at concentrations of 0.8 μM or 4 μM) significantly decreased the protein levels of newly synthesized cytoplasmic H3 in RKO cells compared to individual treatments. (e) WB analysis showed the protein levels of TRMT6, TRMT61A, phosphorylated Rb, and newly synthesized cytoplasmic H3 in different groups of HCT116 cell-derived xenograft tumors. RNA dot blot analysis showed the m1A levels in different groups of HCT116 cell-derived xenograft tumors (n = 3 biologically independent animals per group). (f) Immunohistochemistry analyses of the proliferation marker (Ki-67) in different groups of HCT116 cell-derived xenograft tumors (Scale bar, 100 μm) (n = 3 biologically independent animals per group). Created with BioRender.com. The experiments in a-e were independently performed three times, and the stainings in f were independently performed twice, all with similar results.
Supplementary information
Supplementary Table
Supplementary Tables 1–8.
Source data
Source Data
Unprocessed western blots and gels.
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, EW., Wang, Y., Tan, J. et al. TRMT6-mediated tRNA m1A modification acts as a translational checkpoint of histone synthesis and facilitates colorectal cancer progression. Nat Cancer 6, 1458–1476 (2025). https://doi.org/10.1038/s43018-025-00977-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00977-4