Abstract
Trimetallic nickel–iron–molybdenum oxides are excellent electrocatalysts for alkaline water electrolysis despite experiencing severe molybdenum dissolution. While the impact of molybdenum on fresh samples is well-understood, its substantial loss during operation without compromising performance presents a unique puzzle. Here, we show that the initial presence of molybdenum induces the formation of nickel vacancies and distorts octahedral nickel sites. This structural distortion induces charge transfer between lattice oxygen and nickel, inducing an early formation and stabilization of active nickel oxyhydroxides. Even after complete molybdenum leaching and transitioning into a bimetallic nickel-iron oxide, the catalyst retains its exceptional performance due to the persistence of distorted octahedral nickel sites. Understanding this process enables the exploration of alternative metals that could induce similar structural distortions, as well as inspire similar strategies in other electrocatalysts.

Similar content being viewed by others
Introduction
Trimetallic Ni–Fe–Mo oxides stand out as leading noble-metal-free electrocatalysts for the oxygen evolution reaction (OER) in alkaline environments1,2,3,4,5. These materials exhibit outstanding stability, maintaining consistent catalytic activity over extended periods. Their excellent OER performance is attributed to the vast formation of the highly active NiOOH. The incorporation of iron modulates the redox properties of nickel and adsorption energies of OER intermediates6,7, induces structural disorder8, and improves electrical conductivity7,9. Simultaneously, molybdenum incorporation facilitates phase control, promoting the transformation of α-Ni(OH)2 to β-Ni(OH)2, and subsequent formation of β-NiOOH10,11,12. It is also reported that the presence of Mo favours the lattice oxygen-mediated mechanism with high rate of oxygen exchange10. This mechanism offers a lower energy barrier when compared to the adsorbate evolution mechanism, leading to improved catalytic activity13.
Despite the promising OER activity of Ni–Fe–Mo oxides, the precise role of molybdenum remains an area of active research. While the effects of Mo in freshly prepared samples are well-understood, its significant dissolution of ~40–50% within the initial 12 h of operation11,14,15 is often overlooked and rarely discussed10,11,16,17,18. The exceptional operational stability of these trimetallic oxides, coupled with the lack of recognition of Mo dissolution, can lead to misleading interpretations. For instance, minimal changes in polarisation curves before and after prolonged stability testing might erroneously suggest the absence of surface reconstruction on the electrode17.
In this work, we produced a trimetallic NiFe0.3MoOx oxide in the form of a coating using solution precursor plasma spraying (SPPS) as a versatile and scalable production method of mixed oxides. The trimetallic oxide was deposited on fluorine-doped tin oxide (FTO) coated glass. We examined all the changes in morphology, crystal structure, surface oxidation states, and catalytic performance caused by the incorporation of Mo. Subsequently, we subjected the trimetallic oxide to electrochemical stress tests to accelerate the dissolution of molybdenum. We monitored the transformation of the trimetallic oxide to a NiO-like structure that was further transformed with ease to the highly active γ-NiOOH. The characteristics of the material after molybdenum dissolution and the underlying mechanism responsible for the high catalytic activity were explored.
Results
Effect on the electrocatalytic activity
SPPS is an ideal technique to produce multi-metal nanostructured coatings from solutions containing a desired metal composition4,12,19,20. The coatings are formed by a successive deposition of particles until the desired loading is achieved, and they exhibit a great variety of nanostructured features, resulting in a large surface area. We used this technique to produce a highly active trimetallic Ni–Fe–Mo oxide (NiFe0.3MoOx). We also produced two other oxide coatings, NiOx and NiFe0.3Ox, to observe and evaluate the effects of molybdenum.
We begin with the electrocatalytic activity and then continue with electronic and structural changes. From the polarisation curves in Fig. 1a (using a standard three-electrode cell, pH = 14), it is clear that the presence of Mo makes a vast improvement in the catalytic activity by reducing the overpotential to reach a current density of 20 mA cm−2 (η20) to only 300 mV, when compared to 430 mV for NiFe0.3Ox, and 500 mV for NiOx (depicted in Fig. S1). It is also evident that NiFe0.3MoOx exhibits a large contribution from the Ni2+/Ni3+ redox couple (1.40−1.55 V vs. reversible hydrogen electrode (RHE))21. This is indicative of the large accessibility of superficial Ni atoms. The Tafel slope (Fig. 1b), obtained from the cathodic sweep to minimise the contribution from the redox couple, is smaller in NiFe0.3MoOx (38 mV dec−1) than the one seen in NiFe0.3Ox (47 mV dec−1), where both metal oxides exhibit the same rate-determining step, being the adsorption of water molecules22. The values of η20 and Tafel slope are well in line with other state-of-the-art Ni-based electrocatalysts (Table S1)16,21,23,24. Electrochemical impedance spectroscopy (EIS) studies indicate that the presence of Mo considerably reduces the charge transfer resistance (R3) by nearly a factor of 10, as observed from the Nyquist plots (Fig. 1c, equivalent circuit model shown in the inset). Values for R3 of 1.2 and 10.8 Ω are observed for NiFe0.3MoOx and NiFe0.3Ox, respectively. Meanwhile, the values of R1 (ohmic resistance) and R2 (associated with the substrate and the substrate–film interface)21,25 exhibit only minor changes. In this case, R1 was similar (~12.0 Ω) for both cases, while R2 increased from 0.5 Ω for NiFe0.3Ox to 0.8 Ω for NiFe0.3MoOx. A summary is presented in Table S2.
a iR-corrected polarisation curves. b Tafel slope. c Nyquist plot evaluated at 1.6 V vs. RHE, and its equivalent circuit diagram. iR-corrected polarisation curves of (d) NiFe0.3MoOx, and e NiFe0.3Ox after 15 CVs, after 1000 CVs (1.2–2.0 V vs. RHE, 100 mV s−1), and after 24 h CA test. f Current response of NiFe0.3MoOx (NiFe0.3Ox) when applying a constant potential needed to reach a current density of 20 (10) mA cm−2 for 24 h.
The incorporation of Mo does not cause any appreciable change in the operational stability (Fig. 1d, e) of the trimetallic oxide coating after being subjected to 1000 cyclic voltammetry (CV) cycles (1.2–2.0 V vs. RHE, 100 mV s−1) followed by a chronoamperometric (CA) test for 24 h (Fig. 1f). The polarisation curves (5 mV s−1, obtained after each step) revealed a slight increase in OER activity typically observed due to a better penetration of the electrolyte into the nanostructured network and subsequent accessibility of active sites. The η20 decreases 10 mV and 90 mV for NiFe0.3MoOx and NiFe0.3Ox, respectively. Note that due to the differences in activity, the CA test was carried out at a current density of 20 and 10 mA cm−2 for NiFe0.3MoOx and NiFe0.3Ox, respectively.
Molybdenum dissolution under OER conditions
Prior to any electrochemical stress, the as-produced trimetallic oxide NiFe0.3MoOx exhibits a compacted sponge-like configuration and a highly porous structure (Fig. 2a, d). These characteristics are unique for NiFe0.3MoOx and are not seen in the bimetallic NiFe0.3Ox (Fig. S2a).The observed fissures are a common characteristic of coatings produced via SPPS that are subjected to a subsequent thermal annealing4,12,19. Electron microscopy studies reveal an enlargement of the fissures after only 15 CVs (Fig. 2b), and a clear loss of material after the stability test (1000 CVs + 24 h CA, Figs. 2c and S3). Material dissolution is also observed for the bimetallic oxide NiFe0.3Ox (see Fig. S2b), likely caused by the poor adhesion to the FTO glass substrate and the dissolution of metals due to the anodic conditions.
Despite the material loss in NiFe0.3MoOx, the electrochemical activity remained stable with slight improvements in the overpotential as described before. Furthermore, EDX (Energy dispersive X-ray) elemental mapping reveals a significant decrease in Mo content from 14.8 At% to 4.4 At%, and later to 0.3 At% for the as-produced, after 15 CVs, and after stability test (1000 CVs + 24 h CA), respectively. This represents a loss of 99.8% of the Mo in the trimetallic oxide, effectively making it similar to the bimetallic oxide when considering only the elemental composition. Note that the accumulation of Mo in the electrolyte had no observable effect on the catalytic activity (see Fig. S4). The remaining Ni, Fe, and Mo on the catalysts still exhibit a homogeneous distribution (EDX mapping, Fig. S3c), with the Mo map closely matching that of oxygen. A summary of the elemental composition obtained from both X-ray fluorescence (XRF) spectroscopy and EDX elemental mapping is reported in Table S3 and Table S4, respectively. Overall, the remaining NiFe0.3MoOx is highly defective in nature with a porous and fractured surface providing a high degree of electrolyte diffusion and more exposed active sites. These results agree with previous reports of trimetallic Ni–Fe–Mo oxides21,26.
X-ray diffraction (XRD) studies (Figs. 3 and S5) revealed that as-produced NiFe0.3MoOx exhibits a crystal structure similar to α-NiMoO4 and β-NiMoO4, where the main difference is the coordination number of the Mo6+ ion (octahedral for α-NiMoO4, and tetrahedral for β-NiMoO4)27,28. Note that no signs of segregation of Ni or Fe (in the form of oxides) were observed. In addition, both high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) studies (Fig. S6a) reveal characteristic lattice fringes and diffraction features that are also consistent with NiMoO4. To better investigate this issue, we produced an additional coating containing only Ni in the precursor solution (all other synthesis parameters were kept similar to the mixed oxides), resulting in the formation of pure NiO with a face-centred cubic structure (Fig. 3)29. The major difference between the single-metal oxide (NiOx sample) and the bimetallic NiFe0.3Ox is that the addition of Fe decreases the crystallite size due to disruption in the lattice periodicity that limits the crystal growth8,30. As already mentioned, the addition of Mo leads to a crystal structure similar to that of NiMoO4. After the stability test (1000 CVs + 24 h CA), the trimetallic oxide changed its crystal structure to that of NiO but with broad and low intensity peaks (Fig. 3). HRTEM and SAED studies also support the transformation towards NiO with less defined lattice fringes (Fig. S6b), but still crystalline characteristics. Note that there are still no signs of phase segregation, and thus the remanent Fe (seen during EDX studies) must be part of the NiO crystal structure, as corroborated by near-edge X-ray absorption fine structure (NEXAFS) studies discussed below.
X-ray absorption spectroscopy studies
NEXAFS was used to evaluate the L-edges of nickel and iron, the K-edge of oxygen, and the M-edge of molybdenum. The results for the as-produced samples are depicted in Fig. 4. The Ni L-edge (Fig. 4a) features are the result of dipole transitions from Ni 2p to 3d empty states. The first two features correspond to the L3-edge (2p3/2 → 3d) with two peaks at 852.7 and 854.7 eV (labelled A and B). The other two features at higher energies correspond to the L2-edge (2p1/2 → 3d) with two peaks at 869.9 and 871.0 eV (C and D). These transitions are sensitive to electronic and oxidation state and local geometry. The spectral shape is consistent with high-spin Ni2+. The formation of NiFe0.3Ox and/or NiFe0.3MoOx does not alter the Ni L-edge spectra significantly when compared to the NiOx coating. The only observable change was a slight increase in the B/A ratio (from 0.26 to 0.28) after the addition of Fe for both NiFe0.3Ox and/or NiFe0.3MoOx. An increase in the B peak intensity has been associated with Ni in a higher oxidation state (e.g. Ni2+δ)31.
To better analyse the Fe L-edge, we produced a coating comprising only α-Fe2O3 as a reference for the NEXAFS studies. In this case, the Fe L-edge (Fig. 4b) is characterised by the L3-edge peaks at 708.0 (A’) and 709.5 eV (B’), and the L2-edge peaks at 721.0 (C’) and 722.6 eV (D’). The spectral shape agrees with Fe3+ in high-spin octahedral configuration, as expected from α-Fe2O3. In the octahedral symmetry, the t2g levels are lower in energy than the eg levels. The Fe L-edge in NiFe0.3Ox and NiFe0.3MoOx exhibits a similar spectra shape with minimal changes. The only noticeable change is a decrease in intensity of the A’ peak. This has been associated with a tetragonally-distorted octahedral configuration32, likely caused by the reduced crystallite size observed in XRD (Fig. 3) and the presence of defects.
The O K-edge spectra are the result of transition from O 1s to O 2p orbitals mixed with 3d orbitals of the transition metal. Therefore, it provides information regarding the type of metal oxide, as seen by the O K-edge spectra in Fig. 4c. It exhibits significant differences between NiOx, NiFe0.3Ox, and NiFe0.3MoOx. The O K-edge spectra of NiOx exhibit peaks at 531.6 (B*), 537.1 (C*), 540.2 (D*), and 545.9 (E*) eV. The B* peak is associated with transitions to O 2p states with Ni 3d character, while C*–E* are associated with transitions to O 2p states interacting with Ni 4s and 4p bands. The addition of Fe introduces a new feature at 529.9 eV (A*). This pre-edge feature has been associated with an increase in hybridisation between O 2p and 3d states, leading to a charge injection from oxygen to the metal site31,33. Consequently, the emergence of this new feature reduces the intensity of the B* peak. This feature has also been associated with impurity states in the band gap created by Ni vacancies, resulting in transitions to nickel vacancies induced O 2p states right above the Fermi level34,35.
The addition of Mo drastically alters the O K-edge spectra (Fig. 4c, top panel) due to the formation of NiMoO4. Two new prominent features appear at 530.6 (F*) and 532.7 eV (G*), while the spectra above 535 eV is broader, but still with clear contribution from peaks characteristic of NiO. The F* and G* features are originated from transitions to O 2p states hybridised with Mo 4d states, where Mo6+ exhibits an octahedral coordination. For the case of Mo6+, the six t2g and the four eg orbitals are empty, and thus several transitions from O 1s to O 2p–Mo 4d states can occur. The broader spectra covering the NiOx peaks are a result of distorted MoO6 octahedra, which leads to multiple splitting of the t2g and eg states36. Mo M2,3-edge (Fig. S7) is only detectable for pristine NiFe0.3MoOx; in this case, the M3-edge exhibits two peaks due to a crystal-field splitting caused by symmetry-breaking distortions of MoO6 octahedra36.
Overall, this information indicates that the addition of Fe promotes the formation of Ni vacancies, the presence of Fe and Mo leads to a reduced structural order, impurity states in the band gap, and better covalency in Ni–O bonds. And all transition metals exhibit a distorted coordination environment.
Surface changes and molybdenum corrosion
Ex-situ Raman spectroscopy studies also indicate that the trimetallic oxide NiFe0.3MoOx exhibits a structure similar to α-NiMoO4 and β-NiMoO421,28. This is evidenced by four bands at 943, 888, 850, and 817 cm−1 ascribed to Mo–O stretching vibrations27, the Mo–O bending modes in the region 300-500 cm−1, and the peak at 775 cm−1 due to Ni–O–Mo (Fig. 5a)37. None of these features are present in the bimetallic NiFe0.3Ox (Fig. 5b) and single metal NiOx (Fig. 5c) coatings. Note that all coatings underwent thermal annealing at 400 °C, which significantly exceeded the decomposition temperature of Ni(OH)2 (~230 °C). This eliminates the possibility of Ni(OH)2 being present on pristine coatings. With this in mind, the NiOx coating exhibits three main features at 375 and 495 cm−1 related to the one-phonon TO (transverse optical) and LO (longitudinal optical) modes, and 1052 cm−1 associated with the two-phonon 2LO mode of NiO29,38. The large spectral intensity of one-phonon modes (375, 495 cm−1) is due to the presence of nickel vacancies38. While the low intensity of the two-phonon band (1052 cm−1) is caused by composition disorder and off-centre displacement of nickel38. This is well in line with the observations from the NEXAFS studies. This highlights the defective nature of materials produced via SPPS4,12,19. Bimetallic NiFe0.3Ox exhibits a similar spectral shape when compared to NiOx with features at 355, 557, 669, and 1069 cm−1. This indicates that NiFe0.3Ox also exhibits a highly defective NiO-like structure. However, in this case, the Raman bands are shifted towards higher wavenumber when compared to the NiOx coating, likely due to iron incorporation39,40.
After exposure to anodic conditions, the trimetallic oxide depicts significant changes in its Raman spectrum, where most of the Mo–O features fade away just after 15 CVs and disappear completely after the stability test. Two new features at 475 and 555 cm−1 are consistent with the OER active γ-NiOOH8,41,42, but there is also an overlap with bands corresponding to NiO (~550 and 673 cm−1), suggesting a mix of both phases after the OER process. These results are in contrast with NiFe0.3Ox and NiOx, whose Raman spectra remain largely unchanged after the stability test, indicating the poor stability of NiOOH following the electrochemical process. The only discernible change in NiFe0.3Ox is an overall decrease in peak intensity attributed to structural disorder induced during the OER.
We also performed NEXAFS studies of NiFe0.3MoOx and NiFe0.3Ox samples after 15 CVs and after the stability test, see results in Figs. 5d, e and S8. The O K-edge of NiFe0.3MoOx (Fig. 5d) reveals that after 15 CVs, the Mo-related F* and G* features disappear nearly completely, with only a small contribution from the F* peak remaining. After the stability test, the intensity of the F* peak decreases even more, and only features corresponding to NiOx remain (B*–E*), in agreement with XRD studies (Fig. 3). Interestingly, a new peak at 533.3 eV (H*) appears after 15 CVs. This peak has been observed in amorphous NiO, where the symmetry of octahedral Ni2+ has been severely affected by adopting either an elongated tetragonal (Jahn-Teller distortion) or a square-based pyramid coordination, breaking the degeneracy of the Ni eg orbitals seen as the H* feature43. Regarding the Fe L-edge (Fig. S8a), the NiFe0.3MoOx sample exhibits a shift of +0.3 eV in the B’ peak just after 15 CVs. The B’ peak is originated from transitions to the eg orbitals of Fe. The increase in energy shows an increase in the crystal-field splitting, indicating a destabilization of the eg orbitals32. The latter can be caused by distortion in the coordination geometry and/or the nature of the ligands. Similar shift has been observed in amorphous nickel containing heavily distorted octahedral coordination, where some distortions preferentially break the degeneracy of the eg orbitals43. This change seems to be associated to the appearance of the H* peak in the O K-edge. Finally, the Ni L-edge (Fig. S8b) reveals no changes in the spectra after the electrochemical tests, and the Mo M-edge is no longer detectable on samples exposed to any electrochemical test, indicating that Mo dissolution at the surface (~1 nm for total electron yield (TEY) mode) occurs fast.
NEXAFS studies reveal that the remanent material in NiFe0.3MoOx after the stability test exhibits distinct differences from the bimetallic NiFe0.3Ox. These differences include the presence of metal vacancies, distorted octahedral sites, impurity states in the bandgap, and enhanced TM 3d-O 2p hybridisation, leading to an enhanced charge transfer to metal sites and lower OER activation energies.
Surface changes and Mo corrosion via in situ Raman
In-situ Raman spectroscopy (details provided in the experimental section) was carried out under applied bias with a staircase constant potential in the range of 1.2–2.0 V (vs. RHE) with 100 mV intervals (Fig. 6). Two additional Raman spectra were acquired at open-circuit potential (OCP) before and after the oxidative test. At OCP before the anodic polarisation, the trimetallic oxide NiFe0.3MoOx exhibits peaks associated with Mo-O stretching vibrations (700–950 cm−1) as observed in the dry sample in Fig. 5a. There is only a slight increase in wavenumber when compared to the dry sample; similar behaviour has been observed in hydrated NiMoO4•xH2O27. Once the step-like polarisation curve was initiated, the Mo-O features remained relatively stable until a potential of 1.5 V (vs. RHE) was achieved (Figs. 6a and S9). As the applied potential increased to 1.3–1.5 V (vs. RHE), a minor feature was observed at 525 cm−1, indicating the formation of α-Ni(OH)2, where its subsequent transformation to NiOOH is expected at 1.4–1.55 V (vs. RHE)21. The Raman features characteristic of γ-NiOOH at 475 cm−1 (Eg bending mode, δ(Ni–O)) and 550 cm−1 (A1g stretching mode, ν(Ni–O))8,41,42 only became visible at 1.6 V (vs. RHE), once the amount of NiOOH was significant. At this potential, the Mo–O features diminished significantly. Note that Raman intensities for Ni(OH)2 are lower than those for NiOOH due to differences in scattering cross-section44. γ-NiOOH features became more prominent at higher potentials, this indicates an enrichment of the electroactive species Ni3+ at the surface of NiFe0.3MoOx. Therefore, Mo incorporation, or more precisely, the presence of structural distortions and enhanced metal-oxygen hybridisation, facilitates the oxidation of Ni to form the conductive and active γ-NiOOH. Similar behaviour has been observed for Co as dopant7. Finally, the Raman spectrum at OCP after polarisation remains very similar to that of 1.9 and 2.0 V (vs. RHE) with clear γ-NiOOH features. These spectra also exhibit new features at 315 and 895 cm−1 corresponding to β-Ni(OH)2, commonly observed after electrochemical cycling between Ni(OH)2 and NiOOH45,46. From these experiments, it is evident that the trimetallic oxide undergoes significant changes at potentials around 1.5 V (vs. RHE), in which Mo-O features start to fade away, and the appearance of the highly active γ-NiOOH begins. This is consistent with the polarisation curve in Fig. 1a, where a large contribution of the Ni2+/Ni3+ at 1.40–1.55 was observed, indicating that Mo aids the formation of γ-NiOOH.
In contrast, the stabilisation and formation of γ-NiOOH was not observed in the bimetallic NiFe0.3Ox oxide nor in the single metal oxide NiOx. In-situ Raman spectroscopy for NiFe0.3Ox (Fig. 6b) reveals features at 567 cm−1 corresponding to vibrations of Ni2+–O bonds in Ni2+(OH)247,48, and 694 cm−1 (likely contribution of α-FeOOH). In this case, β-NiOOH was formed instead of the active γ-NiOOH. β-NiOOH features, seen as a broadening of the region 490–560 cm−1 (marked by a red rectangle)42,45, appear at 1.6 V (vs. RHE). These features are even less evident for NiOx (Fig. 6c), which initially exhibits two sharp features at 462 and 497 cm−1, but at potentials above 1.5 V (vs. RHE) these features get broader, indicating the formation of Ni(OH)2 and β-NiOOH (region marked with a red rectangle)42,45,46. The changes observed after the step-like polarisation in NiFe0.3Ox and NiOx can be due to the lattice distortions, chemical changes, and OH− accumulation while exposed to the anodic potential49.
Chemical states of the trimetallic oxide
X-ray photoelectron spectroscopy (XPS) studies of the trimetallic oxide NiFe0.3MoOx are consistent with the previous characterisation techniques. The spin-orbit splitting between 2p3/2 and 2p1/2 in Ni 2p region of 17.7 eV (Fig. 7a) indicates the presence of NiOOH at the top surface50,51. The peak fitting in the Ni 2p region reveals predominant peaks at 854.6 and 857.2 eV, which are associated with Ni(OH)2 and NiOOH, respectively52. Meanwhile, a slight contribution of NiO appears at 852.6 eV along with three shakeup satellites at 860.7, 863.4, and 866.0 eV. Upon the electrochemical stress, the Ni 2p region evidence similar peaks under the envelope, although the peak associated with NiOOH experienced an increase with respect to the Ni(OH)2 peak. The area ratio of NiOOH/Ni(OH)2 increased from 2.8 to 4.7 after the stability test, respectively. This information correlates well with the Raman spectra of NiFe0.3MoOx in Fig. 5a, where the predominant bands of γ-NiOOH were observed.
The O 1s region (Fig. 7b) indicates the preferred formation and stabilisation of hydroxyl groups (OH−, 531.2 eV) as evidenced by the reduced contribution of oxide anions (O2−, 529.4 eV) after the electrochemical stress tests. The O2− features are due to metal (Ni, Fe, or Mo) bonded oxygen within a regular oxide crystal53, whereas the hydroxyl groups are related to Ni bonded to OH−. In addition, there is an energy shift of +0.3 eV in both peaks and a new peak proportion which closely resembles that of NiOOH54. The Fe 2p region, accompanied by the Ni LMM Auger electrons (see fitting details in the experimental section)55,56,57, indicate the presence of two peaks located at 710.3 and 713.6 eV ascribed to Fe2+ and Fe3+, respectively (Fig. 7c). In addition, the satellite line at 718.2 eV arises from Fe3+ cations interacting with octahedral Ni2+ (NiOOH)58. The deconvolution of Mo 3d from NiFe0.3MoOx (Fig. S10) confirms the existence of Mo6+ prior to any applied bias19. As seen in the Raman spectrum of Fig. 5a, Mo leaches out from NiFe0.3MoOx after the stability test, and XPS also corroborates this information due to the reduced signal arising from Mo6+ (Fig. S10).
The bimetallic oxide NiFe0.3Ox (Fig. 7d–f) reveals a major contribution of NiO, rather than NiOOH, as seen by the 2p3/2 and 2p1/2 spin orbit splitting in Ni 2p of 18.3 eV50. There is also clear contribution of Ni2+ (853.8 eV) and Ni3+ (855.8 eV). The shakeup satellites appear at high energies (861.1, 864.2, and 866.3 eV), likely due to oxygen deficiency or due to interaction with neighbouring NiO6 octahedra55. After the stability test, the NiFe0.3Ox catalyst exhibits similar peak positions to its pristine counterpart, and the Ni2+/Ni3+ ratio increases only slightly from 0.5 to 0.52 after the stability test. These results are in line with the Raman spectra of Fig. 5b where no changes were observed in the NiFe0.3Ox crystal structure. The O 1 s peak fitting for NiFe0.3Ox catalysts, Fig. 7e, exhibits predominantly two peaks at 529.4 eV and 531.1 eV assigned to O2− and Odef, respectively. The first peak is characteristic of lattice oxides, whereas the second feature arises due to oxygen adjacent to Ni vacancies59. Before the electrochemical stress, a third peak is found at 532.8 eV due to adsorbed water. The Fe 2p region shows two peaks at 708.7 and 711.5 eV associated with Fe2+ and Fe3+. The absence of a shakeup satellite confirms that no Fe3+ is present prior to any applied bias in NiFe0.3Ox. However, after the stability test, the peak fitting reveals a new peak around 718.1 eV, suggesting the formation Fe3+. Besides, a new satellite peak is also found at 715.8 eV arising from Fe2+60. It should be noticed that the shift seen in Fig. 7f could also be associated with the presence of Ni LMM lines.
Discussion
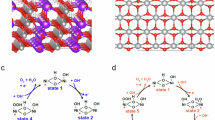
Molybdenum oxides are inherently unstable in alkaline solutions under anodic potentials, readily forming soluble molybdate ions \({{{\rm{MoO}}}}_{4}^{2-}\) as expected from its Pourbaix diagram. This likely occurs in the trimetallic Ni–Fe–Mo oxide after being exposed to OER conditions, leading to Mo leaching as seen by the increase of Mo in the electrolyte (Table S5). This results in the formation of a structurally distorted Ni–Fe oxide with a rock salt structure. Both Ni and Fe adopt octahedral configurations, but Raman and XAS spectroscopy reveal a picture of nickel and iron in distorted octahedral configurations, such as tetragonal elongation (Jahn-Teller distortion) and pyramid coordination (one absent ligand), see Fig. 8. Distorted octahedral sites lift the degeneracy of the metal 3d orbitals, affecting both t2g and eg orbitals. However, in Ni2+ the eg orbitals are unoccupied, and transition from O 1s to hybridised O 2p - TM 3d states correspond to the first peak in the XAS spectrum of O K-edge at 531.6 eV (B*, Fig. 5e). The broken octahedral symmetry differentiates the eg orbitals into the \({d}_{{z}^{2}}\) and \({d}_{{x}^{2}}-{d}_{{y}^{2}}\) orbitals as represented in Fig. 8. This is evident in the XAS spectrum as two peaks, one at the original position (B*), and a second one at 533.3 eV (H*) as seen in Fig. 5d, e. These structural changes result in the appearance of impurity states within the band gap and enhanced metal-oxygen covalency. Jahn-Teller and other lattice distortions can result in charge redistribution due to variations in bond length and changes in crystalline orbitals significantly varying the local charge distribution around the defective site61,62.
a Ideal NiO6 centre with well-defined eg and t2g orbitals and its corresponding transition from the O 1s states to hybridised O 2p-TM 3d orbitals. Occupied oxygen (metal) states are shown in red (blue). Empty states are given with striped colours. b Elongated tetragonal distortion, and square-based pyramid coordination of nickel sites. Splitting of eg and t2g gives rise to an additional feature in the O K-edge spectrum. Experimental NEXAFS spectra of O K-edge for NiFe0.3Ox and NiFe0.3MoOx are shown.
The latter, combined with smaller crystallite size, a defect-rich structure, and charge redistribution, favours the formation of α-Ni(OH)2. Lattice defects are known to enhance chemical reactivity2,10, promoting the interaction with hydroxide (OH−) ions, as well as improving the lattice oxygen exchange with the electrolyte10. On the other hand, charge redistribution can stabilise Ni2+–O complexes31, facilitating the formation of nickel hydroxides and their subsequent transformation to γ-NiOOH during the OER process. The formation of γ-NiOOH and its presence after the OER process was a unique characteristic of the trimetallic oxide, while both NiFe0.3Ox and NiOx exclusively formed β-NiOOH. Although the relative OER activity between β-NiOOH and γ-NiOOH remains a subject of ongoing research, the ability of the trimetallic oxide to generate substantial amounts of NiOOH at lower overpotentials correlates well with its enhanced OER activity. Similar improvements in catalytic performance have been reported in Mo-intercalated Co(OH)263, Mo-doped Mn2O364,65, Mo-doped Fe3O46, and Fe–Co–Mo oxides20,66. However, these studies often omit discussion of Mo dissolution or the underlying mechanisms responsible for the improved performance. Our study elucidates the activation process of these oxides, enabling the search for alternative metals that can induce similar electronic and structural changes, not only in Ni-Mo oxides, but also in other 3d transition metal oxides.
Conclusions
Our study revealed that the presence of iron and molybdenum in NiFe0.3MoOx resulted in a reduced structural order, including reduced crystalline size, nickel vacancies, and distorted octahedral coordination. Additionally, changes in the electronic properties were observed, such as the emergence of impurity states in the band gap and enhanced Ni-O covalency. The exposure to anodic conditions led to molybdenum dissolution, transforming the NiFe0.3MoOx into a NiFeO-like structure. Despite the molybdenum dissolution, the electrochemical activity remained stable. In-situ Raman spectroscopy revealed that the trimetallic oxide undergoes structural changes at potentials around 1.5 V (vs. RHE), with Mo-O features fading and γ-NiOOH bands appearing. The γ-NiOOH features continued after the OER process and even under dry conditions. The enhanced OER activity, seen as a reduced onset potential, Tafel slope, and charge transfer resistance, was attributed to an earlier oxidation of nickel facilitated by persistent structural defects, impurity states, and enhanced metal-oxygen hybridisation. By understanding this mechanism, we can explore alternative metals capable of inducing similar structural distortions and apply similar strategies to other electrocatalysts.
Methods
Materials
Nickel (II) nitrate hexahydrate (Ni (NO3)2·6H2O, 98%), iron (III) nitrate nonahydrate (Fe (NO3)3·9H2O, >98%), and ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, ≥99.0%) were purchased from Merck (Sigma-Aldrich). FTO-coated glass slides (7 Ω sq−1) were acquired from Merck (Sigma-Aldrich). Deionized water (Milli-Q, 18.25 MΩ) was obtained through an EMD Millipore water purification system.
Synthesis method
NiOx, NiFe0.3Ox, and NiFe0.3MoOx coatings were deposited onto FTO-coated glass slides (1 × 2 cm) via SPPS. FTO substrates were placed in the spraying area with a mask to limit the coating area to 1 × 1 cm2 in each substrate. A total of six substrates were coated simultaneously. Different aqueous solutions with a constant precursor concentration of 0.34 M, but different metal ratios (Ni:Fe (5:1), and Ni:Fe:Mo (5:1:3.5)) were prepared. FTO substrates were first cleaned in a sonication bath containing EtOH/H2O (1:1) for 1 h. An atmospheric plasma spraying system (Metallisation Met-PCC (PLAS)) equipped with a PL50 pistol (6 mm nozzle) was used to create the coatings directly onto the FTO substrates. Ar and N2 were used as primary and secondary gases with flow rates of 50.0 NL min−1 and 2.0 NL min−1, respectively. A current of 500 A was used to generate the plasma, resulting in a power of 25.5 kW. The precursor solution was injected (15 ml min−1) into the plasma plume using a high-pressure syringe pump. The solution was premixed with N2 gas (3 NL min−1) to create a mist just before the injection. A robotic arm (ABB 2600) was used to manipulate the plasma torch. A raster scan spraying pattern with a lateral velocity of 250 mm s−1, a vertical displacement of 4 mm per turn, and eight steps was used to fully cover the area of the FTO substrates. The final coating was achieved by applying a total of 5 layers. After the spraying process, samples were annealed in air at 400 °C for 2 h (heating rate of 7 °C min−1). The samples were later stored at ambient conditions until use.
Material characterisation
XRD was carried out in a PANalytical X’pert powder diffractometer equipped with Cu Kα radiation (λ = 1.5406 Å) in the range of 5–95° with a step size and time per step of 0.01395° and 0.5 s, respectively. Scanning electron microscopy (SEM) studies were carried out on a Carl Zeiss Merlin equipped with an energy dispersive X-ray spectrometer. HRTEM studies and SAED were performed on a Glacios cryo-TEM (Thermo Scientific) with an acceleration voltage of 200 kV. The samples were prepared by collecting the coating from the FTO. The powder was dispersed in ethanol and drop-casted onto TEM grids. XPS was performed on a Kratos Axis Ultra DLD electron spectrometer equipped with a monochromatic X-ray source (Al Kα line of 1486.6 eV). The spectra were calibrated using the C 1s set to 284.3 eV (C–C sp2). The peak fitting for Fe was performed by first evaluating the 2p1/2 region and later adding their counterparts in the 2p3/2, while keeping an area ratio of 1:2. The separation distance between the 2p1/2 and 2p3/2 was kept to ~13.6 eV, and the ratio of the full width half maximum between them was set to ~167,68. Raman spectra were recorded in a Renishaw Qontor Raman spectrometer using a 532 nm laser diode calibrated with a Si crystal at 521 cm−1, the laser was operated at 5% of power for 2 s with a total of five accumulations.
X-ray absorption spectroscopy experiments were performed under ultra-high vacuum and room temperature conditions. The L-edge of Ni and Fe, the K-edge of O, and the M-edge of Mo were evaluated. The experiments were performed at the Flexible PhotoElectron Spectroscopy beamline at MAX IV synchrotron source in Lund, Sweden69. An exit slit of 10 µm was used with a photon flux 1012 photons s−1 and a photon energy resolution of 26 meV. The beam size at the sample was 1 × 0.5 mm2 (defocused mode). The data were recorded in the TEY detection mode, and all spectra were normalised after background subtraction.
Elemental analysis using XRF spectroscopy was conducted using an ARL Quant’X Energy-Dispersive Analyzer (Thermo Scientific). The instrument is equipped with a rhodium anode as the X-ray source, operated at 30 kV with an automatically controlled tube current with a maximum output of 50 W. A collimator of 8 mm in diameter is used to control the target area. The X-ray beam is transmitted to the sample through a 50 μm air-cooled beryllium window. Peltier-cooled Silicon Drift Detector was used as the detector with <140 eV resolution at 200 kcps (Mn Kα). The measurements were conducted under ambient air conditions. For liquid samples (5 mL), 32 mm double open-ended XRF sample cells sealed with a 4 µm thick polypropylene film were used. For quantitative analysis, the UniQuant (Thermo Scientific) software was employed. Before each measurement, the system was calibrated using nine certified references (Al, Ti, Cr, Fe, Ni, Mo, Sn, W, and Pb). The analysis involved scanning 79 chemical elements by using diverse filters to optimise detection across different element groups.
Electrochemical measurements
The polarisation curves were recorded in a three-electrode cell with an Ag/AgCl (3.0 M KCl) reference electrode and a graphite rod as a counter electrode, using CV in Ar-saturated 1.0 M KOH electrolyte (pH = 14) at room temperature. The data was collected in a potentiostat (Ivium Technologies). The working electrode consisted of the activated NiFe0.3Ox or NiFe0.3MoOx coatings with an area of 1 cm2, and the electrical contact was made through the FTO with crocodile clips. The catalytic activity was measured by CVs at a scan rate of 5 mV s−1 in a range of 0.164 to 0.964 V vs. Ag/AgCl. A total of 15 CV cycles were used to achieve a stable polarisation curve. The last anodic scan was used in this work. The applied voltage was converted to the RHE using the Nernst equation, where ERHE = EAg/AgCl + 0.059 × pH + E0Ag/AgCl. EAg/AgCl is the potential measured vs. Ag/AgCl, E0Ag/AgCl = 0.210 V for Ag/AgCl. EIS measurements were performed at an overpotential of 1.6 V vs RHE within the frequency range of 10 kHz to 0.1 Hz with an amplitude of 10 mA. The durability test was performed using CV in the range of 1.2–2.0 V vs. RHE for 1000 cycles with a scan rate of 100 mV s−1. A subsequent chronoamperometric study was carried out at a constant potential for 24 h to achieve a current density of 10 mA cm−2 and 20 mA cm−2 for table, respectively. The iR compensation was performed by subtracting 90% of the voltage drop caused by the ohmic resistance (RΩ) obtained from EIS studies using the expression EiR-free = Emeasured (vs. RHE)−0.90 × iRΩ.
In situ Raman spectroscopy studies were performed in a conventional three-electrode cell with a horizontal quartz window where the working electrode is accessible to the laser. A Pt wire and Ag/AgCl (3.0 M KCl) electrode were used as a counter and a reference electrode, respectively. The spectra were acquired using a 532 nm laser at 10% of power, 5 s exposure time, and 10 accumulations. The sample was polarised by applying a potential from 1.2 to 2.0 V vs. RHE in steps of 100 mV. Each step was held for 120 s, and the Raman spectra were recorded in the last 60 s of each step. All samples were exposed to anodic conditions only once, so the Raman spectra would capture changes caused by the first anodic polarisation. For the trimetallic oxide, the applied potential was held for 180 s, and three Raman spectra were recorded (2 s exposure time, and 10 accumulations), and each spectrum was taken after waiting 30 s. The average spectra were used in this study.
Statistics and reproducibility
Data obtained from HRTEM, SEM, and EDX are presented without alterations in contrasts or brightness. XRD data is plotted without alterations and indexed using the HighScore software version 4.5. The XPS data was fitted using CasaXPS version 2.3.24PR1.0. The EIS analysis was performed using the software Ivium Technologies version 4.1. The quantitative analysis from XRF was performed using the UniQuant software from Thermo Scientific.
Data availability
All data generated during and/or analysed during this study are included in this published article and its supplementary information files.
References
Corbin, J. et al. Challenges and progress in oxygen evolution reaction catalyst development for seawater electrolysis for hydrogen production. RSC Adv. 14, 6416 (2024).
Perivoliotis, D. K. et al. Recent progress on defect-rich electrocatalysts for hydrogen and oxygen evolution reactions. Nano Today 50, 101883 (2023).
Kang, Q., Lai, D., Tang, W., Lu, Q. & Gao, F. Intrinsic activity modulation and structural design of NiFe alloy catalysts for an efficient oxygen evolution reaction. Chem. Sci. 12, 3818 (2021).
Rafei, M. et al. Non-stoichiometric NiFeMo solid solutions; tuning the hydrogen adsorption energy via molybdenum incorporation. Adv. Mater. Interfaces 9, 2201214 (2022).
Ekspong, J. et al. Solar-driven water splitting at 13.8% solar-to-hydrogen efficiency by an Earth-abundant electrolyzer. ACS Sustain. Chem. Eng. 9, 14070 (2021).
Wei, L. et al. High-valence Mo doping for highly promoted water oxidation of NiFe (oxy)hydroxide. J. Mater. Chem. A 10, 23790 (2022).
Bates, M. K., Jia, Q., Doan, H., Liang, W. & Mukerjee, S. Charge-transfer effects in Ni–Fe and Ni–Fe–Co mixed-metal oxides for the alkaline oxygen evolution reaction. ACS Catal. 6, 155 (2016).
Klaus, S., Cai, Y., Louie, M. W., Trotochaud, L. & Bell, A. T. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity. J. Phys. Chem. C119, 7243 (2015).
Burke, M. S., Enman, L. J., Batchellor, A. S., Zou, S. & Boettcher, S. W. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chem. Mater. 27, 7549 (2015).
He, Z. et al. Activating lattice oxygen in NiFe-based (oxy)hydroxide for water electrolysis. Nat. Commun. 13, 2191 (2022).
Huang, Y., Wu, Y., Zhang, Z., Yang, L. & Zang, Q. Rapid electrodeposited of self-supporting Ni-Fe-Mo film on Ni foam as affordable electrocatalysts for oxygen evolution reaction. Electrochim. Acta 390, 138754 (2021).
Pineiro-Garcia, A. et al. Quaternary mixed oxides of non-noble metals with enhanced stability during the oxygen evolution reaction. ACS Appl. Mater. Interfaces 16, 70429 (2024).
Wang, F. et al. Activating lattice oxygen in high-entropy LDH for robust and durable water oxidation. Nat. Commun. 14, 6019 (2023).
Li, C. et al. Enhancing hydrogen evolution reaction by synergistically coupling NiMo alloy with Ni(OH)2 nanosheet on carbon cloth. ChemistrySelect 5, 6774 (2020).
Du, W., Shi, Y., Zhou, W., Yu, Y. & Zhang, B. Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction. Angew. Chem. Int. Ed. 60, 7051 (2021).
Qin, F. et al. Trimetallic NiFeMo for overall electrochemical water splitting with a low cell voltage. ACS Energy Lett. 3, 546 (2018).
Gao, W. et al. Magnetic-field-regulated Ni-Fe-Mo ternary alloy electrocatalysts with enduring spin polarization enhanced oxygen evolution reaction. Chem. Eng. J. 455, 140821 (2023).
Chen, Y., Dong, C., Zhang, J., Zhang, C. & Zhang, Z. Hierarchically porous Mo-doped Ni–Fe oxide nanowires efficiently catalyzing oxygen/hydrogen evolution reactions. J. Mater. Chem. A 6, 8430 (2018).
Piñeiro-García, A., Wu, X., Rafei, M., Mörk, P. J. & Gracia-Espino, E. A Quaternary mixed oxide protective scaffold for ruthenium during oxygen evolution reaction in acidic media. Commun. Eng. 2, 28 (2023).
Wu, X. et al. Scalable production of foam-like nickel–molybdenum coatings via plasma spraying as bifunctional electrocatalysts for water splitting. Phys. Chem. Chem. Phys. 25, 20794 (2023).
Rajput, A., Adak, M. K. & Chakraborty, B. Intrinsic Lability of NiMoO4 to Excel the Oxygen Evolution Reaction. Inorg. Chem. 61, 11189 (2022).
Ekspong, J., Gracia-Espino, E. & Wågberg, T. Hydrogen evolution reaction activity of heterogeneous materials: a theoretical model. J. Phys. Chem. C124, 20911 (2020).
Huang, J. et al. Identification of key reversible intermediates in self-reconstructed nickel-based hybrid electrocatalysts for oxygen evolution. Angew. Chem. Int. Ed. 58, 17458 (2019).
Xiao, J., Cai, D., Zhang, Y. & Luo, X. Ni4Mo alloy nanosheets coating on carbon tube arrays as high-performance electrocatalyst toward overall water splitting. J. Alloy. Compd. 886, 161180 (2021).
Pehlivan, İB., Arvizu, M. A., Qiu, Z., Niklasson, G. A. & Edvinsson, T. Impedance spectroscopy modeling of nickel–molybdenum alloys on porous and flat substrates for applications in water splitting. J. Phys. Chem. C123, 23890 (2019).
Li, Y.-K., Zhang, G., Lu, W.-T. & Cao, F.-F. Amorphous Ni–Fe–Mo suboxides coupled with Ni network as porous nanoplate array on nickel foam: a highly efficient and durable bifunctional electrode for overall water splitting. Adv. Sci. 7, 1902034 (2020).
Sharma, P., Minakshi, M., Whale, J., Jean-Fulcrand, A. & Garnweitner, G. Effect of the anionic counterpart: molybdate vs. tungstate in energy storage for pseudo-capacitor applications. Nanomaterials 11, 580 (2021).
Sanyal, G., Mondal, B., Rout, C. S. & Chakraborty, B. Recent developments and future perspectives on energy storage and conversion applications of nickel molybdates. Energy Storage 5, e432 (2023).
Terlemezoglu, M., Surucu, O., Isik, M., Gasanly, N. M. & Parlak, M. Temperature-dependent optical characteristics of sputtered NiO thin films. Appl. Phys. A 128, 50 (2021).
Shtukenberg, A. G., Ward, M. D. & Kahr, B. Crystal growth inhibition by impurity stoppers, now. J. Cryst. Growth 597, 126839 (2022).
Drevon, D. et al. Uncovering the role of oxygen in Ni-Fe(OxHy) electrocatalysts using in situ soft X-ray absorption spectroscopy during the oxygen evolution reaction. Sci. Rep. 9, 1532 (2019).
Banday, A., Shahid, R., Gupta, M. & Murugavel, S. Tunable electronic structure of heterosite FePO4: an in-depth structural study and polaron transport. RSC Adv. 13, 18332 (2023).
Suntivich, J. et al. Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C118, 1856 (2014).
Kuiper, P., Kruizinga, G., Ghijsen, J., Sawatzky, G. A. & Verweij, H. Character of holes in LixNi1-xO and their magnetic behavior. Phys. Rev. Lett. 62, 221 (1989).
Peng, H. Y. et al. Deterministic conversion between memory and threshold resistive switching via tuning the strong electron correlation. Sci. Rep. 2, 442 (2012).
Thakur, P. et al. X-ray absorption and magnetic circular dichroism characterization of Mo1–xFexO2 (x = 0–0.05) thin films grown by pulsed laser ablation. Hyperfine Interact. 197, 95 (2010).
Flores-Aguilar, J. F., Ibarra, I. S., Guevara-Lara, A. & Vázquez-Garrido, I. Raman spectroscopy of NiMo catalysts supported on solid oxides. A review. Appl. Spectrosc. Rev. 59, 601–631 (2023).
Cazzanelli, E., Kuzmin, A., Mariotto, G. & Mironova-Ulmane, N. Study of vibrational and magnetic excitations in NicMg1−cO solid solutions by Raman spectroscopy. J. Phys. Condens. Matter 15, 2045 (2003).
Sahu, B., Panigrahi, U. K., Chakravarty, S., Hussain, S. & Mallick, P. Structural, optical, and magnetic properties of NiO/NiFe2O4 nanocomposites. Appl. Phys. A 129, 584 (2023).
Gong, M. & Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 8, 23 (2015).
Mavrič, A., Fanetti, M., Lin, Y., Valant, M. & Cui, C. Spectroelectrochemical tracking of nickel hydroxide reveals its irreversible redox states upon operation at high current density. ACS Catal. 10, 9451 (2020).
Lai, W. et al. In situ Raman spectroscopic study towards the growth and excellent HER catalysis of Ni/Ni(OH)2 heterostructure. Int. J. Hydrog. Energy 46, 26861 (2021).
Li, R. et al. Short-range order in amorphous nickel oxide nanosheets enables selective and efficient electrochemical hydrogen peroxide production. Cell Rep. Phys. Sci. 3, 100788 (2022).
Diaz-Morales, O., Ferrus-Suspedra, D. & Koper, M. T. M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation. Chem. Sci. 7, 2639 (2016).
Hall, D. S., Lockwood, D. J., Bock, C. & MacDougall, B. R. Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proc. R. Soc. A Math., Phys. Eng. Sci. 471, 20140792 (2015).
Hall, D. S., Lockwood, D. J., Poirier, S., Bock, C. & MacDougall, B. R. Raman and infrared spectroscopy of α and β phases of thin nickel hydroxide films electrochemically formed on nickel. J. Phys. Chem. A 116, 6771 (2012).
Bai, Y. et al. Promoting nickel oxidation state transitions in single-layer NiFeB hydroxide nanosheets for efficient oxygen evolution. Nat. Commun. 13, 6094 (2022).
Ciambriello, L., Alessandri, I., Ferroni, M., Gavioli, L. & Vassalini, I. Unexpected resilience of NiFe catalysts for the alkaline oxygen evolution reaction. ACS Appl. Energy Mater. 7, 3462 (2024).
Dong, J. et al. In situ Raman spectroscopy reveals the structure evolution and lattice oxygen reaction pathway induced by the crystalline–amorphous heterojunction for water oxidation. Chem. Sci. 13, 5639 (2022).
Kitchamsetti, N. et al. DFT and experimental investigations on the photocatalytic activities of NiO nanobelts for removal of organic pollutants. J. Alloy. Compd. 855, 157337 (2021).
Weidler, N. et al. X-ray photoelectron spectroscopic investigation of plasma-enhanced chemical vapor deposited NiOx, NiOx(OH)y, and CoNiOx(OH)y: influence of the chemical composition on the catalytic activity for the oxygen evolution reaction. J. Phys. Chem. C121, 6455 (2017).
Shih, Y.-J., Huang, Y.-H. & Huang, C. P. Electrocatalytic ammonia oxidation over a nickel foam electrode: role of Ni(OH)2(s)-NiOOH(s) nanocatalysts. Electrochim. Acta 263, 261 (2018).
Wang, P. et al. Synchronous defect and interface engineering of NiMoO4 nanowire arrays for high-performance supercapacitors. Nanomaterials 12, 1094 (2022).
Payne, B. P., Biesinger, M. C. & McIntyre, N. S. The study of polycrystalline nickel metal oxidation by water vapour. J. Electron Spectrosc. Relat. Phenom. 175, 55 (2009).
Salunkhe, P., AV, M. A. & Kekuda, D. Investigation on tailoring physical properties of nickel oxide thin films grown by dc magnetron sputtering. Mater. Res. Express 7, 016427 (2020).
Elsener, B., Atzei, D., Krolikowski, A. & Rossi, A. Effect of phosphorus concentration on the electronic structure of nanocrystalline electrodeposited Ni–P alloys: an XPS and XAES investigation. Surf. Interface Anal. 40, 919 (2008).
Acharya, S. S. et al. Temperature dependence of L3M45M45 Auger transition in Fe1−xNix alloys. J. Electron Spectrosc. Relat. Phenom. 212, 1 (2016).
Dong, C., Kou, T., Gao, H., Peng, Z. & Zhang, Z. Eutectic-derived mesoporous Ni-Fe-O nanowire network catalyzing oxygen evolution and overall water splitting. Adv. Energy Mater. 8, 1701347 (2018).
Payne, B. P., Biesinger, M. C. & McIntyre, N. S. Use of oxygen/nickel ratios in the XPS characterisation of oxide phases on nickel metal and nickel alloy surfaces. J. Electron Spectrosc. Relat. Phenom. 185, 159 (2012).
Kong, H., Song, J. & Jang, J. One-step fabrication of magnetic γ-Fe2O3/polyrhodanine nanoparticles using in situ chemical oxidation polymerization and their antibacterial properties. Chem. Commun. 46, 6735 (2010).
Zvejnieks, G., Mastrikov, Y. & Gryaznov, D. Jahn–Teller distortion in Sr2FeO4: group-theoretical analysis and hybrid DFT calculations. Sci. Rep. 13, 16446 (2023).
Fischer, J. & Aidhy, D. S. Local charge distortion due to Cr in Ni-based concentrated alloys. Acta Mater. 279, 120285 (2024).
Meng, Y.-L. et al. Hierarchical MoO42–intercalating α-Co(OH)2 nanosheet assemblies: green synthesis and ultrafast reconstruction for boosting electrochemical oxygen evolution. Energy Fuels 35, 2775 (2021).
Zhao, L. et al. The Jahn–Teller distortion-induced electronic structure regulation of Mn-doped Co3O4 for enhanced acetone detection. InfoMat. 7, e12634 (2025).
Balaghi, S. E., Triana, C. A. & Patzke, G. R. Molybdenum-doped manganese oxide as a highly efficient and economical water oxidation catalyst. ACS Catal. 10, 2074 (2020).
Zhang, Y. et al. Cobalt-nanoparticle-decorated cobalt–molybdenum bimetal oxides embedded in flower-like N-doped carbon as a durable and efficient electrocatalyst for oxygen evolution reaction. ACS Sustain. Chem. Eng. 9, 11052 (2021).
Yamashita, T. & Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 254, 2441 (2008).
Elmi, C., Guggenheim, S. & Gieré, R. Surface crystal chemistry of phyllosilicates using X-ray photoelectron spectroscopy: a review. Clays Clay Miner. 64, 537 (2016).
Preobrajenski, A. et al. FlexPES: a versatile soft X-ray beamline at MAX IV Laboratory. J. Synchrotron Radiat. 30, 831 (2023).
Acknowledgements
The authors acknowledge support from the Kempe Foundation (JCK-2132), the Carl Tryggers Foundation (CTS 21-1581), the Olle Engkvist Foundation (219-0116), Vetenskapsrådet (2021-04629), and the Swedish Foundation for Strategic Research (SSF-Agenda 2030—PUSH, and ID22-0062). We acknowledge the MAX IV Laboratory for beamtime on the Flexible PhotoElectron Spectroscopy (FlexPES) beamline under proposal 20240163. Research conducted at MAX IV, a Swedish national user facility, is supported by Vetenskapsrådet (Swedish Research Council, VR) under contract 2018-07152, Vinnova (Swedish Governmental Agency for Innovation Systems) under contract 2018-04969, and Formas under contract 2019-02496. We also thank the Umeå Core Facility for Electron Microscopy (UCEM), the Vibrational Spectroscopy Core Facility (ViSp), and the XPS platform at Umeå University.
Funding
Open access funding provided by Umea University.
Author information
Authors and Affiliations
Contributions
M.R. performed experiments and contributed to data analysis and writing. A.P.-G. contributed to data analysis and writing. X.W. contributed to data analysis. E.P.-P. performed experiments. S.A. performed experiments. T.U. performed experiments. T.W. contributed to writing. E.G.-E. conceived and supervised the project, contributed to data analysis and writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Pan Liu and Peng Peng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jet-Sing Lee. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rafei, M., Piñeiro-García, A., Wu, X. et al. Distorted octahedral sites drive early formation and stabilisation of nickel oxyhydroxides in trimetallic nickel–iron–molybdenum oxides. Commun Mater 6, 115 (2025). https://doi.org/10.1038/s43246-025-00842-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-025-00842-y