Abstract
Lithium iron sulfide (Li2FeS2) exhibits unique characteristics, including multielectron redox behavior and abundant valence states, making it a promising candidate for electrode material in lithium-ion batteries. However, the sluggish charge transfer kinetics, low stability, and slow rate performance hamper its practical application. Herein, we propose a strategy to boost the electrochemical performance of Li2FeS2 by substituting F dopants with S sites through a two-step solid-state process. The effects of F dopants on material characteristics and electrochemical behaviors are investigated. Experimental results show that F dopants significantly enhance diffusion kinetics and rate performance, indicating improved interfacial activity in Li2FeS2−xFx. Theoretical calculations confirm that F substitution occurs at the S site, enhancing charge mobility. After 100 cycles, the optimized Li2FeS2-xFx cathode exhibits a specific capacity of 250 mAh g−1, higher than pristine Li2FeS2. The improved electrochemical properties, diffusion kinetics, capacity, and rate performance are attributed to the enhanced structural stability from a stronger metal–fluorine bond compared to metal–sulfur, and increased Li+ ion diffusion due to a greater electronegativity difference.
Similar content being viewed by others
Introduction
The growing energy demand in modern civilization is driving the rapid advancement of energy storage technologies. Among them, electrochemical energy storage devices such as electrochemical capacitors (or supercapacitors) and Li-ion batteries (LIBs) are the most promising systems for electric vehicles and portable electronic devices1,2,3,4. LIBs have become the dominant energy storage technology compared to other electrochemical energy storage systems due to their superior energy density5,6,7. This advantage has prompted scientists to conduct extensive research focused on enhancing the electrochemical kinetics associated with the intercalation and deintercalation of Li+ ions. Despite efforts to increase capacity with new materials or nanostructuring, it still faces challenges related to low safety, environmental toxicity, and higher capital costs8,9.
The cathode is a critical component of battery systems, fundamentally influencing efficiency, energy density, and cycle life10. As a result, significant research has been directed toward optimizing a range of cathode materials to enhance these performance parameters. Among the alternative cathode materials, sulfide-based lithium-rich cathodes such as Li2FeS2 (LFS), have attracted attention due to their high safety, low cost, and high theoretical capacity of about 400 mAh g−111,12. The bulk LFS yet exhibits low specific capacity and unsatisfactory rate performance for practical use due to its low electronic conductivity and sluggish transportation of Li+ ions11,12,13. Therefore, different strategies have been employed to improve its electrochemical properties, including particle size minimization14, substitution of S with Se13, and incorporation of O atoms15. These modifications resulted in a direct impact on the reversible redox processes during charging–discharging cycles, improved the intrinsic conductivity, and prolonged the structural stability. Hence, achieving high-performance LFS-based cathode materials requires tailoring the electronic and crystal structures, such as defect engineering or doping16,17.
Among the potential dopants, F is one of the interesting elements due to its high electronegativity and lightness among the halogen group (group 17). Previous studies have also shown that incorporating F into electrode materials can achieve a stable electrode–electrolyte interface18,19,20,21. It accelerates surface ion diffusion and enhances ion intercalation kinetics on either the cathode or anode sides. This is because of its high electronegativity and its strong attraction to metal ions22. Tamgadge et al. reported that F-doped TiO2 has a three-times larger specific capacitance and superior rate capability compared to the pristine TiO2, attributed to the enhanced electronic charge carrier density and local conductivity23. Liao et al. reported that the F-substituted LiFePO4/C shows a high-rate performance and delivers a discharge capacity of 110 mAh g−1 at 10 C24. On the other hand, it was demonstrated that an appropriate level of F-doping can effectively enhance the structural stability of lithium nickel cobalt aluminum oxides (NCA) cathode materials due to strong M–F bonds, resulting in improved electrochemical performance25. Further, many researchers have reported that it reduces cell impedance for Li-ion diffusion and stabilizes the crystal structure by suppressing the formation of O vacancy during the initial charging process26,27. Despite its promising potential, the strategy of incorporating F atoms into the LFS-based electrode material to enhance the charge storage and facilitate the transportation of Li+ ions has not yet been explored.
In this report, we propose to substitute F into S sites in LFS crystal structure (denoted as Li2FeS2−xFx where \(x\) = 0 to 0.4) using a two-step solid-state method. The effects of F dopant on the material characteristics and electrochemical properties are systemically investigated. The optimized electrode material, where \(x\) = 0.3, shows much better rate performance, diffusion kinetics, and capacity than those of pristine LFS. The enhanced electrochemical properties are attributed to the high electronegativity of F, which promotes the formation of stronger bonds (e.g., Fe–F instead of Fe–S). This strengthens the structural stability of the cathode during charge–discharge cycles and facilitates the formation of a stable cathode electrolyte interphase (CEI), effectively suppressing undesirable side reactions. Additionally, F stabilizes the high oxidation states of transition metals during the cycling process, enabling more efficient utilization of the active material and improving capacity retention. Furthermore, the incorporation of F dopant into the LFS crystal structure enhances Li+ ion diffusion, contributing to improved electrochemical performance. First-principles calculations are further utilized to precisely determine the position of the F atom within the LFS structure and used for Bader charge analysis to explain the enhanced charge mobility.
Results
Synthesis and characterizations of Li2FeS2−xFx
Li2FeS2−xFx powder (with \(x\) = 0, 0.1, 0.2, 0.3, and 0.4) was synthesized through a two-step solid-state process using Li2S, LiF, S, and Fe precursors15. As shown in Fig. 1a, the stoichiometric amounts of the above-mentioned powder precursors were mixed, sealed under a vacuum, and transferred to a box furnace, followed by melting at the desired temperature to get crystallized powder. Inductively coupled plasma optical emission spectroscopy (ICP-OES) was used to confirm elemental composition, where the measured molar ratios align well with the nominal ratios (Supplementary Table 1). Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) images were used to study the surface morphologies and microstructures, respectively. As shown in Fig. 1b, we observed that the pristine and F-substituted samples display a meatball-like morphology with characteristic sizes of tens of micrometers and are tightly packed. Notably, these images show that although the morphology remains almost unchanged, the particle size increases with F doping. Furthermore, the elemental mapping images obtained by SEM equipped with energy-dispersive X-ray spectroscopy (SEM-EDX) show the homogeneous distribution of Fe, S, and F in the solid matrix (Fig. 1c, d and Supplementary Fig. S1). As shown in Fig. 1e, f, the \({d}\)-spacing slightly decreases due to the presence of F which is in agreement with the X-ray diffraction (XRD) patterns and density functional theory (DFT) results, which will be discussed later.
The crystal structure was analyzed by synchrotron powder XRD (Fig. 2a), followed by Rietveld refinement and the corresponding difference curve (Supplementary Fig. 2). Our analysis revealed that the diffraction patterns of the as-prepared cathode materials contain small amounts (~5–6%) of Li2S and LiHFeO as secondary phases. The diffraction patterns of Li2FeS2-xFx are indexed to the trigonal lattice with the \(P3m1\) crystal system which is similar to our previous report15. The enlarged view of (001) planes show a peak shift to higher angle, indicating the crystal structure contraction after F atoms substitution in the S sites. SAED patterns also show that the atomic arrangement is preserved after F substitution with a marginal change in the radius of the fringes (Fig. 2b). To observe the lattice vibrational modes and detect alterations arising from changes in bond angle or bond length, Raman spectroscopy was performed. As shown in Fig. 2c, Raman spectra are fitted with six peaks, labeled with I, II, III, IV, V, and VI. Peaks II and VI show red-shifts and those peaks labeled as IV and V exhibit blue-shifts after F incorporation. Notably, the two other peaks denoted as I and III are remained invariant (Supplementary Table 2). Although the F doping is known to induce shifts in the Raman peaks, further research is needed to elucidate the specific alterations in each vibrational mode, which are contingent on the short-range symmetry.
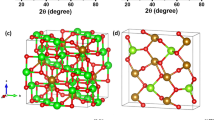
To unveil the possible ways of introducing F atoms into the Li2FeS2 trigonal structure with \(P3m1\) crystal system, DFT calculations were conducted. The structural optimization was performed with the 12 × 12 × 5 gamma centered mesh \(K\)-points. The predicted lattice parameters of pristine LFS, \(a\) = 3.763 Å and \(c\) = 6.019 Å, are in agreement with our previous report15. To model the F-doped material, we considered a Li2FeS2 supercell (2 × 2 × 2) model containing eight Li2FeS2 units with the cell volume of 7.526 Å × 7.526 Å × 12.022 Å. As displayed in Fig. 3a, there are two distinct Li⁺ sites: (i) In the first site, Li⁺ is bonded to four S2- atoms, forming LiS4 tetrahedra. These tetrahedra share corners with six equivalent LiS6 octahedra and six equivalent LiS4 tetrahedra. (ii) In the second site, Li⁺ shares edges with three equivalent LiS6 octahedra and three equivalent FeS4 tetrahedra28. The Li2FeS2 supercell structure with Li and S defects was also investigated and their formation energies were calculated at −0.66 eV and 0.62 eV, respectively. This indicates the Li-defected structure is relatively stable as compared with S-defected one. We considered two possible ways of introducing F atoms into Li2FeS2. The first way is, S atom substituted with F atom (denoted as F sub @S; see Fig. 3b). The formation energy of 1 F and 2 F substituted with S in Li2FeS2 supercell structure is in the range of −5.50 to −8.01 eV. This substitution leads to significantly shorter F–Li bond lengths of 1.881 Å 1.889 Å and F–Fe bond length of 3.33 Å, compared to the original S–Li bond lengths of 2.455 and 2.322 Å. Other bonding such as S–Fe significantly increased from 2.567 to 3.33 Å. The second way of introducing F atoms into the lattices of Li2FeS2 is leaving F atom in an interstitial position (denoted as F@int; see Fig. 3c. For F@int model, we probed the effect of F doping located interstitially at various places in between the Li, Fe, and S layers of Li2FeS2 supercell. We firstly calculated the F atom doping interstitially located in between the six Li atoms, resulted in F–Li bond length and formation energy of ~1.995 Å and −5.42 eV. Among the all-interstitial configurations, the F atom bounds to lattice Li atoms in between two layers (i.e., F @ int-1) has the lowest formation energy of −5.42 eV. The formation energies of F-dopant atoms in different configurations are summarized in Supplementary Table 3. Li migration process barrier results are shown in Supplementary Fig. 3. In pristine Li2FeS2, Li migrates from a subsurface sulfur (S) site to the surface, encountering a migration barrier of 1.5 eV. The distances between the subsurface Li and the top and bottom S layers are 2.453 Å and 2.345 Å, respectively. In Li2FeS2, Li can migrate to the surface, forming a Li@surface(a) configuration, which is exothermic by 0.96 eV. Upon substituting a sulfur atom with fluorine (F), the structure becomes more stable. The F–Li bond shortens to 1.789 Å, while the remaining Li–S bond length increases to 2.445 Å. The presence of F significantly lowers the Li migration barrier to 1.2 eV and 0.3 eV less than in the pristine system. This migration process is also exothermic, with an energy release of –0.68 eV.
a Pristine Li2FeS2, S-defect, Li-defect, and 1F-substituted at S. b 2F-substituted at four different sites, i.e., S-1, S-2, S-3, and S-4. c F-substituted at four different interstitial sites, i.e., F@int-1, F@int-2, F@int-3, and F@int-4. Red, green, yellow, and blue-balls stands for Fe, Li, S, and F atoms, respectively.
The chemical states and stoichiometry of the electrode materials were studied by X-ray photoemission spectroscopy (XPS) analysis. Figure 4 and Supplementary Table 4 show the XPS spectra, peak positions, and the full-width half maximum (FWHMs) of Li 1 s, Fe 2p, S 2p, and F 1 s signals. Figure 4a shows a gradual red-shift that for Li 1 s from 54.68 to 54.39 eV via varying \(x\) from 0 to 0.4. Fe 2p core level spectra exhibit almost a similar profile with an asymmetric behavior due to the presence of multiplets and satellite peaks (Fig. 4b)29. Although the peak positions of the Fe 2p3/2 do not show a trend but it can be assigned to the presence of bivalent Fe²⁺ oxidation state30. S 2p3/2 and S 2p1/2 peaks were fitted by considering the spin-orbit coupling of 1.2 eV with S 2p3/2 peak positions changing from 160.52 to 160.24 eV via \(x\) from 0 to 0.4, standing for S2- oxidation state (Fig. 4c). S 2p spectra further demonstrate the presence of Sx2- oxidation state in larger binding energies due to the presence of S–O bonds31. As displayed in Fig. 4d, the F 1 s peaks are located at 684.29, 684.52, 684.48, and 684.31 eV for \(x\) = 0.1, 0.2, 0.3, and 0.4, respectively. Notably, all the peak positions are within the error bar as depicted in Supplementary Fig. 4, originated from the measurement resolution, binding energy calibration, and fitting process. To probe the concentration of F in LFS, we calculated the F/S, S/Fe, and F/Fe ratios (Fig. 4e). As expected, the F/S and F/Fe ratios increase and the S/Fe ratio decreases with \(x\), which is consistent with the nominal stoichiometric.
Additionally, the Bader charge analysis is performed to quantify the electron density transfer (Fig. 4f). In the pristine sample, Li atom exhibits a partial positive charge of 0.82 e, reflecting its role as a donor within the structure. S atom holds a significant negative charge of −1.34 e, consistent with its function as an electron acceptor due to its high electronegativity and large atomic size. Fe atom carries a moderate positive charge of 1.04 e, suggesting it is partially oxidized, likely near the Fe2+ state. In the F-doped sample, Li atom slightly loses electron density and its charge increasing to 0.85 e (\(\frac{\Delta q}{q}\) = 3.5%). This indicates electron withdrawal by the surrounding environment, driven by the highly electronegative F atom. S atom slightly gains more electron density and its charge becomes more negative of −1.36 e (\(\frac{\Delta q}{q}\) = −1.5%). This reflects a minor redistribution of electron density in response to F substitution, potentially altering the local bonding environment. On the other hand, the positive charge of Fe atom decreases to 0.98 e (\(\frac{\Delta q}{q}\) = −5.8%), indicating electron density transfer towards Fe atoms. This suggests a slight reduction in its oxidation state, likely due to the impact of F in withdrawing electrons from Li and S. Finally, F atom itself, with a charge of −0.9 e, acts as a strong electron acceptor and disrupts the electronic structure of the system by attracting electron density from nearby atoms.
Electrochemical performance
After confirming the successful substitution of electronegative and electrochemically stable F atoms into the LFS structure to enhance interactions with inorganic cations, we assessed the electrochemical properties of Li2FeS2−xFx. This evaluation aims to determine its potential viability as an electrode material for LIB application. Pristine LFS was evaluated under identical measurement conditions for comparison. We specifically assembled a CR2032 coin-type cell, using the Li2FeS2−xFx layer as the working electrode and a Li metal foil as the counter electrode. Since the best performance is achieved for Li2FeS2−xFx with \(x\) = 0.3, it was selected for further in-depth electrochemical study, followed by comparing with the pristine one.
As displayed in Fig. 5a, the redox behaviors of the Li2FeS2 and Li2FeS1.7F0.3 electrodes were evaluated by CV in the potential range of 1.7–3.0 V versus Li+/Li at a scan rate of 0.1 mV s−1. Electrodes show multiple anodic peaks on the first cycle where the intense peak is around 2.73 V, corresponding to the anion oxidation of S2− to (S2)2−12. For the reverse sweep, electrodes exhibit a single cathodic wave at around 1.94 V, albeit with only a slight shift toward higher potentials for the F-substituted electrode. We further operated the Li2FeS2-xFx—Li cell in an increased voltage range (Supplementary Fig. 5). At elevated upper cut-off voltages, electrolyte decomposition, S–S bond cleavage, and structural degradation may occur. GCD measurements were conducted to investigate the impact of anion substitution on the electrochemical energy storage properties. Figure 5b presents the first, second, third, and 35th GCD curves. Similar to previous reported works13,15, there are two distinct regions in the charge profile: (I) Several inflections at a sloping region, and (II) extended flat plateau. The electrochemical behaviors in sloping and extended plateau region are corresponded to Fe2+/3+ and S2−/S22− redox reactions, respectively32. Since the material follows a different mechanistic pathway during reduction, the charge and discharge curves exhibit different behaviors. Initial capacity decay may result from irreversible anion redox reactions and structural stress during the early cycling, which can create microcracks that promote further growth of the CEI. As shown in Fig. 5c, the capacity fading rate after extended cycling in the case of the Li2FeS2 (\(x\) = 0) is higher as compared with Li2FeS1.7F0.3 (\(x\) = 0.3). A specific capacity of about 250 mAh g−1 at C/5 is obtained after 100 cycles for the Li2FeS1.7F0.3, which is much larger than pristine LFS of 160 mAh g−1 (i.e., ~56% enhancement in capacity), showing an improved electrochemical stability compared to other related works (Supplementary Table 5 and Supplementary Table 6). The small ionic radius and high charge density of F⁻ contribute to a more compact and rigid lattice. This increased rigidity reduces volume changes during cycling, thereby enhancing mechanical stability and preventing microcracks or phase transformations. The improvement in electrochemical performance can be attributed to the following reasons. (i) Fluorine doping alters the local electronic environment and narrows the bandgap, thereby enhancing both ionic and electronic conductivity in the material9. (ii) Due to the strong electronegativity of F, it forms stronger bonds (e.g., Fe–F instead of Fe–S), which reinforces the structural stability of the cathode during GCD cycles. (iii) F facilitates the development of a stable CEI, effectively suppressing undesirable side reactions, such as polysulfide dissolution, which is a key challenge for sulfide-based cathodes. (iv) F acts as an electron-withdrawing group, stabilizing high oxidation states of transition metals during cycling. This enables more efficient utilization of the active material and improving capacity retention. (v) The modified electronic structure further accelerates redox reactions, thereby enhancing the overall performance33,34. Li2FeS1.7F0.3 phase also remains after cycling and the reaction mechanism has been proposed (Supplementary Fig. 6)
To probe the impact of F atoms on electrochemical properties, rate performances were examined at varied C-rates from C/5 to 2 C. The rate performance of a battery provides insight into its ability to retain capacity and deliver energy efficiently at varying charge and discharge rates (Fig. 5d). To assess the rate performance of our materials, we added the error bar in the figure. The performance results were averaged from three replicate cells. The Li2FeS1.7F0.3 electrode displays higher reversible capacities over the entire range of 311, 277, 193, and 167 mAh g−1 at C/5, C/2, 1 C, and 2 C than those for the pristine LFS electrode of 266, 214, 82, and 68 mAh g−1 at C/5, C/2, 1 C, and 2 C, mA g−1, respectively. Supplementary Fig. 7 presents the rate performance of other samples (\(x\) = 0.1, 0.2, and 0.4) and the experimental errors of \(x\) = 0 and 0.3. This suggests that incorporating F atoms enhances the accessibility of Li⁺ ions to reaction sites even at high rates, ensuring strong reversibility during rapid charge-discharge processes. It seems that F enhances the bonding within the cathode material owing to its high electronegativity and small ionic radius. This reinforcement stabilizes the lattice structure, reducing degradation during repeated high-rate cycling. This is further supported by the galvanostatic intermittent titration technique GITT experiment, which will be discussed later.
Li+ ion storage kinetics
Besides the storage capacity of Li⁺ ions, the F atoms affect the lithiation–delithiation kinetics. S-based cathodes suffer from sluggish Li+ ion diffusion caused by structural constraints and elevated migration barriers. To assess Li+ ion storage kinetics, we performed the GITT measurements (Fig. 6a, b). Notably, the diffusion coefficient for the Li+ ion (\({D}_{{{Li}}^{+}}\)) was calculated according to Fick’s second law of diffusion (Supplementary Fig. 8)35,36. F-doped electrode demonstrates significantly higher diffusion kinetics than pristine LFS throughout the entire charge profile (Fig. 6c). Introduction of F atoms reduces interfacial resistance by improving surface chemistry and minimizing unwanted side reactions, facilitating faster Li+ ion transfer across the cathode–electrolyte interface. In the discharge profile, the diffusion kinetics of Li2FeS1.7F0.3 is still higher at higher potentials. High electronegativity of F diminishes the electrostatic interaction between Li+ ions and the host lattice, allowing the ions to move more freely towards improving diffusion kinetics.
a Schematic illustration of the half-cell potential during a GITT pulse. b GITT curves of the first cycle of Li2FeS2−xFx for \(x\) = 0 and 0.3. GITT was obtained at C/5 for a 30 min pulse, followed by 5 h relaxation periods. c Variation of \({D}_{{{Li}}^{+}}\) at various charge and discharge states. The diffusion coefficient \({D}_{{{Li}}^{+}}\) was calculated using the GITT curves. d, e Nyquist plots and equivalent circuit model of cathode, respectively.
EIS measurements were also conducted to evaluate the ion transport kinetics (Fig. 6d). The depressed semicircle observed in the high-to-medium frequency range is attributed to the formation of a surface film on the positive electrode and the associated charge-transfer resistance (\({R}_{{ct}}\)), and the straight line at lower frequency represents the Li+ diffusion from the surface into the interior of the crystalline Li2FeS2 or Li2FeS1.7F0.3 (Warburg impedance)37,38,39,40,41. The EIS data were fitted with an equivalent circuit model: \({R}_{s}+{{CPE}}_{1}{||}{R}_{{ct},1}+{{CPE}}_{2}{||}({R}_{{ct},2}+{W}_{O})\) where \({R}_{s}\), \({R}_{{ct},i}\), \({{CPE}}_{i}\), and \({W}_{O}\) stand for the equivalent series resistance, charge transfer resistance, constant phase element, and Warburg elements, respectively (Fig. 6e and Supplementary Table 7). The Nyquist plot revealed that the semicircle of the F-doped electrode is smaller than that of the pristine LFS electrode. This indicates that F-substituted electrode exhibits a lower charge-transfer resistance of \({R}_{{ct},1}\) = 68.2 Ω, which is much smaller than pristine LFS of \({R}_{{ct},1}\) = 112 Ω. The Li2FeS1.7F0.3 material demonstrates higher conductivity compared to Li2FeS2, with values of 2.7 × 10-1 cm2 s⁻1 versus 1.5 × 10-2 cm² s⁻¹, attributed to improved Li⁺ diffusion kinetics. The electronegative properties of F prevent unwanted electrolyte decomposition in Li2FeS2-xFx and create a stable interface between the electrode and electrolyte. This leads to more rapid charge transport and significantly improved Li+ ion storage capacity.
XAS measurements
XANES measurements were conducted for the pristine and optimized electrode material to examine the redox behaviors of the elements (Fig. 7). Electrodes were charged and discharged to various representative points in the galvanostatic profile and measured ex-situ to determine the redox contributions to each observed feature. Both Li2FeS2 and Li2FeS1.7F0.3 were cycled to the following conditions: Open-circuit potential (OCP) state, charged to 3 V, and fully discharged to 1.7 V. The smooth and reversible shift in XANES during cycling indicates the redox couple is both active and kinetically accessible, leading to faster kinetics and improved rate capability. The activity of Fe toward the redox process was investigated using Fe K-edge XANES at three different states of charge (SOCs). The pre-and near-edge data for Li2FeS2 and Li2FeS1.7F0.3 are shown in Fig. 7a, c, respectively. The pre-edge feature, labeled as “a”, corresponds to the weakly allowed Fe 1 s to Fe 3 d transition near 7113.1 eV and is highly sensitive to local symmetry changes. The peak denoted as “c”, is sensitive to changes in oxidation state. Up on charging to 3 V, the rising peak “b” shifts from 7117.2 to 7118.8 eV in both pristine and F-substituted samples, indicating the oxidation of Fe2+, aligning with a previous study42. The presence of a less distorted tetrahedral environment has been supported by the observed increase in the intensity of the pre-edge feature. The pre-edge feature at 7112–7113 eV and the rising edge at 7117.2 eV are associated with Fe2+, while a positive shift to 7118.8 eV indicates the formation of Fe3+. The samples exhibit significant changes during discharge compared to the charged state. The pre-edge intensity decrement closely resembles the OCP state. After discharging, the rising edge shifts back to 7117.2 eV, showing the reversibility of the Fe. The local structure of F-substituted material is not exactly similar to the pristine Li2FeS2, as indicated by the slight difference in intensity of the near-edge feature of Li2FeS1.7F0.3. Li2FeS1.7F0.3 shows reduced Fe redox reversibility compared to Li2FeS2. This is primarily because F⁻ is more electronegative and less polarizable than S2⁻, which leads to stronger Fe–F bonds. This change affects the local structure, bonding, and electronic environment, resulting in less reversible redox processes.
With the same set of samples, S K-edge XANES spectra were also collected to investigate the redox status of sulfur and the covalency extent of the Fe–S bond (Fig. 7b, d)43,44. The S K-edge spectra of pristine and F-doped electrodes display a pre-edge feature, which is associated with the Li2S (charged terminal sulfur) and covalently bonded S to a transition metal (i.e., S–Fe in here)45,46. The pre-edge feature, located at 2470 eV for both materials, corresponds to the S 1 s to Fe 3 d transition, resulting from the mixing of S 3 s and 3p states with the Fe 3 d state. Up on charging to 3 V, the peak intensity decreased which could be related to the S 3 s and 2p states mixing with the unoccupied Fe 3 d states, resulting in a covalent S–Fe bond. Substituting S with F decreases the local S concentration and alters the surrounding S environment by replacing some S–Fe–S linkages with S–Fe–F connections. This substitution can cause a slight shift of the S K-edge to lower energies if the Fe–S bonds weaken, as nearby F⁻ ions pull electron density away, thereby reducing Fe–S covalency. However, some studies indicate a blue shift may occur if S becomes more oxidized due to decreased back-bonding from Fe. Additionally, an increase in S pre-edge intensity may happen if the Fe–S bond becomes more ionic and less covalent, which enhances the S p-character available for X-ray transitions. Moreover, substitution-induced disorder, such as a random distribution of S and F, could lead to broader spectral features.
To study the redox behavior of F in our system, F K-edge XAS has been utilized. The pre-and near-edge data of all SOCs for Li2FeS1.7F0.3 are plotted in Fig. 7e. The F K-edge spectra rising feature is located at 694.2 eV, which is similar to previously reported works47,48. Up on charging to 3 V, there is a difference in the white line intensity denoted (*) than the OCP state. The white line position of F K-edge XAS is at 694.6 eV. This difference in white line intensity could be attributed to the high electronegativity difference between F and S atoms where the electron cloud is distorted. Nonetheless, fully discharging to 1.7 V does not show any reversibility. This could be an indicator that F is not involved in the main redox process. In Li2FeS2₋xFx, fluorine interacts with Fe²⁺, resulting in hybridization between the Fe 3 d and F 2p orbitals. This interaction gives the Fe–F bond a notable covalent character, reflecting significant mixing of the Fe 3 d and F 2p orbitals. The onset of the main absorption edge can shift slightly with variations in the Fe oxidation state. If Fe undergoes minor oxidation due to fluorine substitution, the effective nuclear charge experienced by F increases, leading to a shift of its absorption edge to slightly higher energies.
In summary, we presented an effective strategy to enhance the electrochemical kinetics of lithium-rich iron sulfide (Li2FeS2) cathode by introduction of F dopant towards high-performance electrodes for LIB application. Since F atoms substituted into S sites, it resulted in F–Fe bonds that are responsible for stabilizing the CEI layer, thereby improving the initial capacity fading and enabling reversible electro-transformation for lithiation–delithiation processes at the fast rates. As a result, the optimized Li2FeS2−xFx electrode demonstrated enhanced Li+ ion storage capacity and stable cycling performance over 100 cycles without notable capacity fading, even at high C-rates, such as 2 C, compared to the structurally similar pristine Li2FeS2. The exceptional electrochemical performance of Li2FeS2−xFx was attributed to the favorable ion-binding capability of F atoms, although the F element is not involved in the redox process as confirmed by the XAS, nonetheless the formation of F-bonding enhances the Li+ ion diffusivity for both cathodic and anodic reactions. In particular, stabilizing the electrode–electrolyte interface enhanced the ion transport rate and improved access to internal active storage sites. Hence, our strategy not only enhances the electrochemical properties, which is required for fabrication of practical LIBs, but also provides insight into the design principles of various families of lithium-rich sulfide-based electrodes for use in other electrochemical energy storage systems.
Methods
Chemicals and materials preparation
All precursors were handled inside an Argon-filled glovebox (H2O and O2 < 1 ppm). Li2FeS2 powder was synthesized using the two-step solid-state method, following a procedure similar to our previous report. Stoichiometric quantities of Li2S (Sigma, 99.9%), Fe (Acros Organics, 99.0%), and S (Sigma, 99.8%) powders were ground together and transferred into a boron nitride crucible15. This crucible was then placed inside a quartz ampoule, which was evacuated to pressures below 10 mTorr and sealed with a propane-oxygen torch, avoiding air exposure. The sealed sample was heated in a furnace at a rate of 0.625 °C min−1 to 500 °C, with a dwell time of 24 h to achieve a molten reaction mixture. Afterward, the product was removed from the glass, ground in the glovebox, and re-sealed using the same procedure. It was then heated at a rate of 0.903 °C min−1 to 750 °C, with a dwell time of 15 h to enhance crystallinity. Li2FeS2−xFx powder was prepared similarly, with the addition of LiF (Sigma, 99.0%) powder precursor. After naturally cooling to room temperature, the ampoule was opened inside the glovebox, and the samples were ground into fine powders and stored for further characterization.
Electrochemical characterizations
Electrode preparation is done in an Argon-filled glovebox (H2O and O2 < 1 ppm) using the doctor blade method to coat a slurry onto carbon-coated aluminum current collectors (MTI). The slurry consisted of a mixture of conductive carbon (Super P, Alfa Aesar) and polyvinylidene fluoride (PVDF) binder (Sigma) in a weight ratio of 70:20:10, with N-Methylpyrrolidone (NMP) as solvent (Sigma). After coating, the electrodes were dried overnight (110 °C) and cut into circular shapes with a mass loading of 1.3–2.5 mg per electrode. For the preparation of high mass loading freestanding films, a mixture with a weight ratio of 60:20:20 of active material, conductive carbon, and binder was used. In this case, solvent-free polytetrafluoroethylene (PTFE) binder beads (Sigma) replaced PVDF, allowing for a higher loading of 7–16 mg. Electrochemical analyses were conducted utilizing 2032-coin cells, incorporating Li foil (75 µm, Alfa Aesar) as the anode, polypropylene (Celgard 2400) as the separator, and 35 µl of a carbonate-based electrolyte (1 M LiPF6 in EC/DEC (1:1, v/v)). For galvanostatic charge-discharge (GCD) experiments, either an Arbin cycler (LBT series, Arbin Instruments) or Ubiq BAT 750B battery testers have been utilized. Analyses such as cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were carried out using a Solartron multichannel electrochemical station (1470E). The charge-discharge process was set for 30 minutes at C/5 rate, followed by a 5-h relaxation period, for the galvanostatic intermittent titration technique (GITT) measurement.
Characterizations
Synchrotron-based powder XRD patterns were obtained from samples enclosed in glass capillaries. At a wavelength of \(\lambda\) = 0.61992 Å, the measurements were carried out at the National Synchrotron Radiation Research Center (NSRRC) in Taiwan using the TPS BL 19 A. The Rietveld method with TOPAS V5 software was employed to analyze the diffraction patterns, while crystal structure visualization was facilitated by VESTA (version 3.5.7)49. To estimate the average composition of the samples, inductively coupled plasma optical emission spectroscopy (ICP-OES) was performed using an Agilent 710-ES instrument. Confocal Raman spectra were collected with a Jobin Yvon LabRAM HR800 at excitation wavelength of 532 nm. To prevent air exposure, an airtight Raman cell equipped with a quartz window was utilized. The surface morphologies, elemental distribution, microstructures, and crystal structures were examined using scanning electron microscopy (SEM; JEOL-6700F), SEM-energy-dispersive X-ray spectroscopy (SEM-EDX), high-resolution transmission electron microscopy (HRTEM; JEM-2100F, 200 kV), and selected area electron diffraction (SAED) patterns. Conductivity measurements were performed using a four-point probes Ecopia Hall measurement system. X-ray photoelectron spectroscopy (XPS; ULVAC-PHI Inc./PHI 5000 Versa Probe III) was employed to investigate the chemical states of the samples. Ex situ quick scanning X-ray absorption near edge structure (XANES) experiments were conducted in transmission mode at TPS-44A, electron yield mode at TLS-20A, and fluorescence mode at TLS-16A at NSRRC beamlines for Fe, F, and S. Additionally, spectra from reference Fe foil and HF and S powders were collected.
Calculations
The possible ways of introducing F atom into the Li2FeS2 (\(P3m1\)) structure was analyzed by DFT simulations and calculating the formation energy and electronic properties using the Vienna ab initio simulation package (VASP) and applying DFT + U50. The generalized gradient approximation (GGA) in the formalism of Perdew-Burke-Ernzerhof (PBE) was adopted to describe the electronic exchange-correlation energy. The projector-augmented wave (PAW) pseudo-potentials were chosen to describe ionic cores51,52. Spin-polarized calculations were also carried out throughout the system. The PBE exchange-correlation functional tendency to delocalize unpaired electrons and thus may not give accurate account of magnetic moment of Fe atom. The DFT + U method was utilized to effectively address the strong on-site Coulomb repulsion of Fe 3 d states. The U value of 3.0 eV was adopted from prior research53. We have utilized the standard PBE functional method to improve the van der Waals (vdW) interactions correctly by Becke-Jonson damping potential with the DFT-D3 method54. The convergence criterion for the self-consistent iteration was set at 10-5 eV. The calculations were carried out with plane-wave basis set cutoff energy was set to 520 eV with Gaussian smearing method of 0.05 eV width, to assure well converged total energy and force values. Atomic charges of the optimized structures were calculated by utilizing the Bader method with a program designed by Henkelman and coworkers55. We also calculate the iso-surfaces of charge density difference analysis shows that apparent electron transfers occur from the F to other elements. We considered two possible ways of introducing F-doping atom into the lattices of Li2FeS2. The first was a Li or S atom substituted with an F atom (denoted by F sub @Li and F sub @S); the second was an F doping atom sited in an interstitial position (denoted as F@int). To characterize the stability of the dopant in substitutional and interstitial configurations in Li2FeS2, we calculated the formation energies (\({E}_{f}\)) using the following equations,
where E(Li2FeS2) and E(F˗Li2FeS2) are the total energies of pristine Li2FeS2 and F-doped Li2FeS2 supercells, respectively. The quantities µ(F) and µ(S) represent the chemical potentials of F and S atoms, respectively. The chemical potential for µ(F) and µ(S) is calculated from the α-fluorine and α-solid S8 bulk, respectively. The formation energies of dopant atom in different configurations were calculated by using DFT-D3 method. The Li migration process on the Li2FeS2 (002) surface was studied using the nudged elastic band (NEB) method to determine the minimum energy path and migration energy.
Data availability
All relevant data are either supplied in the Article and Supplementary Information, or available from the corresponding authors upon request. Source data are provided with this paper.
References
Tian, R. et al. Improved interfacial contact and electrochemical performance of highly Ni-rich LiNixCoyMn1-x-yO2 (x>90%) cathode in all-solid-state lithium battery. Energy Storage Mater. 68, 103350 (2024).
Zhang, C. et al. Regulating oxygen covalent electron localization to enhance anionic redox reversibility of lithium-rich layered oxide cathodes. Energy Storage Mater. 46, 512–522 (2022).
Choi, J. H. et al. Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages. Energy Storage Mater. 68, 103368 (2024).
Qorbani, M., Chen, K. H. & Chen, L. C. Hybrid and asymmetric supercapacitors: achieving balanced stored charge across electrode materials. Small 20, 1–28 (2024).
Simon, P. & Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 19, 1151–1163 (2020).
Fahimi, Z. et al. Co3V2O8 hollow spheres with mesoporous walls as high-capacitance electrode for hybrid supercapacitor device. Chem. Eng. J. 436, 135225 (2022).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Yu, Y., Wang, J., Zhang, P. & Zhao, J. A detailed thermal study of usual LiNi0.5Co0.2Mn0.3O2, LiMn2O4 and LiFePO4 cathode materials for lithium ion batteries. J. Energy Storage 12, 37–44 (2017).
Meng, Y. et al. F-doped LiFePO4 @N/B/F-doped carbon as high performance cathode materials for Li-ion batteries. Appl. Surf. Sci. 476, 761–768 (2019).
Qian, H. et al. Surface doping vs. bulk doping of cathode materials for lithium-ion batteries: a review. Electrochem. Energy Rev. 5, 1–32 (2022).
Barker, J. & Kendrick, E. The electrochemical insertion and safety properties of the low-cost Li-ion active material, Li2FeS2. J. Power Sour. 196, 6960–6963 (2011).
Hansen, C. J. et al. Multielectron, cation and anion redox in lithium-rich iron sulfide cathodes. J. Am. Chem. Soc. 142, 6737–6749 (2020).
Martinolich, A. J. et al. Controlling covalency and anion redox potentials through anion substitution in Li-Rich Chalcogenides. Chem. Mater. 33, 378–391 (2021).
Kim, S. S. et al. Promoting reversibility of multielectron redox in alkali-rich sulfide cathodes through cryomilling. Chem. Mater. 34, 3236–3245 (2022).
Hailemariam, A. G. et al. Oxygen-incorporated lithium-rich iron sulfide cathodes for Li-Ion batteries with boosted material stability and electrochemical performance. Chem. Mater. 36, 9370–9379 (2024).
Zhang, Y. et al. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 32, 1–22 (2020).
Kumar, K. & Kundu, R. Doping engineering in electrode material for boosting the performance of sodium ion batteries. ACS Appl. Mater. Interfaces 16, 37346–37362 (2024).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Gong, C. et al. Revealing the role of fluoride-rich battery electrode interphases by operando transmission electron microscopy. Adv. Energy Mater. 11, 2003118 (2021).
Pathak, R. et al. Fluorinated hybrid solid-electrolyte-interphase for dendrite-free lithium deposition. Nat. Commun. 11, 1–10 (2020).
Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Lee, J. et al. Fluorine-rich covalent organic framework to boost electrochemical kinetics and storages of K+ Ions for potassium-ion battery. Adv. Energy Mater. 13, 1–12 (2023).
Tamgadge, R. M. & Shukla, A. Fluorine-doped anatase for improved supercapacitor electrode. Electrochim. Acta 289, 342–353 (2018).
Liao, X. Z., He, Y. S., Ma, Z. F., Zhang, X. M. & Wang, L. Effects of fluorine-substitution on the electrochemical behavior of LiFePO4/C cathode materials. J. Power Sour. 174, 720–725 (2007).
Wang, J. et al. Strengthened the structural stability of in-situ F− doping Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries. Chem. Eng. J. 438, 135537 (2022).
Li, L. et al. Retarded phase transition by fluorine doping in Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. J. Power Sour. 283, 162–170 (2015).
Kang, S. H. & Amine, K. Layered Li(Li0.2Ni0.15 + 0.5zCo0.10Mn 0.55 − 0.5z)O2 - zFz cathode materials for Li-ion secondary batteries. J. Power Sour. 146, 654–657 (2005).
Jain, A. et al. Commentary: the materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 1–12 (2013).
Fathabadi, M. et al. An ultrathin amorphous defective co-doped hematite passivation layer derived via an in situ electrochemical method for durable photoelectrochemical water oxidation. J. Mater. Chem. A 10, 16655–16665 (2022).
Ma, W., Liu, X., Lei, X., Yuan, Z. & Ding, Y. Micro/nano-structured FeS2 for high energy efficiency rechargeable Li-FeS2 battery. Chem. Eng. J. 334, 725–731 (2018).
Mamo, T. T. et al. Enhanced CO2 photoreduction to CH4 via *COOH and *CHO intermediates stabilization by synergistic effect of implanted P and S vacancy in thin-film SnS2. Nano Energy 128, 109863 (2024).
Nagarajan, S., Hwang, S., Balasubramanian, M., Thangavel, N. K. & Arava, L. M. R. Mixed cationic and anionic redox in Ni and Co free chalcogen-based cathode chemistry for Li-Ion batteries. J. Am. Chem. Soc. 143, 15732–15744 (2021).
Zhang, Q. et al. Surface cobaltization for boosted kinetics and excellent stability of nickel-rich layered cathodes. Natl Sci. Open 3, 20240010 (2024).
Wang, Q. et al. Synthesis and electrochemical performance of Ni and F doped LiMn2O4 cathode materials. RSC Adv. 5, 75333–75340 (2015).
Nickol, A. et al. GITT analysis of lithium insertion cathodes for determining the lithium diffusion coefficient at low temperature: challenges and pitfalls. J. Electrochem. Soc. 167, 090546 (2020).
Lee, J., Kim, C., Cheong, J. Y. & Kim, I. D. An angstrom-level d-spacing control of graphite oxide using organofillers for high-rate lithium storage. Chem 8, 2393–2409 (2022).
Zhang, J. et al. Boron and nitrogen codoped carbon layers of LiFePO4 improve the high-rate electrochemical performance for lithium ion batteries. ACS Appl. Mater. Interfaces 7, 20134–20143 (2015).
Syum, Z. et al. Enhancing the lithium-ion storage capability of Cu2ZnSnS4 anodes via a nitrogen-doped conductive support. Chem. Eng. J. 465, 142786 (2023).
Pendashteh, A., Tomey, R. & Vilatela, J. J. Nanotextile 100% Si anodes for the next generation energy-dense Li-ion batteries. Adv. Energy Mater. 14, 1–13 (2024).
Park, S. H. et al. High areal capacity battery electrodes enabled by segregated nanotube networks. Nat. Energy 4, 560–567 (2019).
Sun, Y. et al. One‐Step Calcination Synthesis of Bulk‐Doped Surface‐Modified Ni‐Rich Cathodes with Superlattice for Long‐Cycling Li‐Ion Batteries. Angew. Chemie 135, e202300962 (2023).
Kowalska, J. K. et al. X-ray absorption and emission spectroscopic studies of [L2Fe2S2]n model complexes: implications for the experimental evaluation of redox states in iron-sulfur clusters. Inorg. Chem. 55, 4485–4497 (2016).
Glaser, T., Hedman, B., Hodgson, K. O. & Solomon, E. I. Ligand K-edge X-ray absorption spectroscopy: a direct probe of ligand - Metal covalency. Acc. Chem. Res. 33, 859–868 (2000).
Solomon, E. I., Hedman, B., Hodgson, K. O., Dey, A. & Szilagyi, R. K. Ligand K-edge X-ray absorption spectroscopy: covalency of ligand-metal bonds. Coord. Chem. Rev. 249, 97–129 (2005).
Gorlin, Y. et al. Operando characterization of intermediates produced in a lithium-sulfur battery. J. Electrochem. Soc. 162, A1146–A1155 (2015).
Ko, Y. C. et al. Interconnected microporous and mesoporous carbon derived from pitch for lithium-sulfur batteries. ACS Sustain. Chem. Eng. 10, 4462–4472 (2022).
Roesch, P., Vogel, C., Huthwelker, T., Wittwer, P. & Simon, F. G. Investigation of per- and polyfluoroalkyl substances (PFAS) in soils and sewage sludges by fluorine K-edge XANES spectroscopy and combustion ion chromatography. Environ. Sci. Pollut. Res. 29, 26889–26899 (2022).
Cao, Z. et al. Revealing the unusual mechanism of mixed cationic and anionic redox in oxyfluorosulfide cathode for all-solid-state fluoride-ion batteries. Chem. Mater. 36, 1928–1940 (2024).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 54, 11169–11186 (1996).
Lee, C., Yang, W. & Parr, R. G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Capdevila-Cortada, M., Łodziana, Z. & López, N. Performance of DFT+U approaches in the study of catalytic materials. ACS Catal. 6, 8370–8379 (2016).
Tkatchenko, A., Distasio, R. A., Car, R. & Scheffler, M. Accurate and efficient method for many-body van der Waals interactions. Phys. Rev. Lett. 108, 1–5 (2012).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Acknowledgements
This work was supported by the Ministry of Science and Technology (MOST), Taiwan [grant MOST 110-2124-M-001-001; MOST 110-2636-E-007-020-; MOST 110-2622-8-007-015; MOST 111-2124-M-001-00; MOST 111-2112-M-001-084-MY3]; the Academia Sinica project [grant AS-SS-112-01]; the Academic Summit Project [grant 107-2745-M-002-001-ASP], the Science Vanguard Project [grants 110-2123-M-002-006 and 111-2123-M-002-009]; the iMATE program[grant AS-iMATE-110-34] of Academia Sinica; and the Center of Atomic Initiative for New Materials (AI-Mat), National Taiwan University [112 L9008 and 113 L9008], from the Featured Areas Research Center Program within the frame-work of the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan; National Science and Technology Council (NSTC) of Taiwan [grants NSTC 112-2112-M-002-021-MY2 and NSTC112-2811-M-002-040]. M.Q. acknowledges support from the National Science and Technology Council (NSTC) Taiwan [grant numbers: NSTC 112-2112-M-002-021-MY2, NSTC 112-2811-M-002-040, and NSTC 113-2811-M-002-137].
Author information
Authors and Affiliations
Contributions
A.G.H. conceived the idea of the study, designed the experiments, prepared the samples, conducted all the electrochemical measurements, and material characterizations, analyzed the results, performed XRD data refinement, and wrote the manuscript. M.Q. fitted Raman data, analyzed XPS spectra, and revised the manuscript. A.G.H. and T.T.M. conducted Raman and XRD measurements. P.R. performed DFT calculations. C.-Y.H. recorded SEM images. K.S.B. contributed to conceptualization. H.-L. W. contributed to resources and revising the manuscript. M.-C. L. contributed to performing DFT, ab-initio based MDS, and CPU resources. L.-C.C. and K.-H.C. contributed to directing the research, conceiving the idea of the study, and revising the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Bingan Lu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Guangmin Zhou and Jet-Sing Lee. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hailemariam, A.G., Qorbani, M., Mamo, T.T. et al. Improved electrochemical kinetics and rate performance of lithium-ion batteries by Li2FeS2−xFx cathode materials. Commun Mater 6, 143 (2025). https://doi.org/10.1038/s43246-025-00866-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43246-025-00866-4