Abstract
Human activities and climate change substantially threaten coastal areas, impacting ecosystem functions, services, and human-wellbeing. Trace elements, from both natural and anthropogenic sources, can contaminate coastal regions, and at high concentrations may become toxic to marine biota. Climate change is likely to affect the sources, sinks and cycling of trace elements in coastal systems: for example, riverine runoff is set to increase as precipitation in the Arctic intensifies, and more frequent extreme floods are expected to activate previously deeply buried trace elements. Furthermore, changes in human activity under a warming climate, such as increased Arctic shipping and potential geoengineering projects such as ocean alkalinity enhancement, will likely introduce more trace elements to coastal ecosystems. Advancing our understanding of trace element cycling is at present limited by factors including lack of data coverage in the Global South, challenges in studying multi-stressor effects and ecosystem responses, lack of long-term data, and the difficulty in parametrizing robust models in coastal environments.
Similar content being viewed by others
In the hydrosphere, especially in marine waters, trace elements (TE) play an essential role as micronutrients and tracers of processes of fundamental importance1. Elements such as cadmium (Cd), cobalt (Co), copper (Cu), chromium (Cr), iron (Fe), nickel (Ni), selenium (Se), and zinc (Zn) are considered bio-essential elements since they are required in small quantities for plants and wildlife in natural waters to maintain metabolic processes such as carbon uptake, photosynthesis, and nitrogen fixation2,3,4. On the other hand, while some TE, such as aluminum (Al)5, have no known biological function, others, like arsenic (As) and mercury (Hg) are highly toxic6,7. The principle that „the dose makes the poison“ applies to all bio-essential elements, which can become toxic at high concentrations. This toxicity can result from natural sources, such as hydrothermal vents and groundwater seepage, as well as anthropogenic influences like industrial effluents and emissions7. Additionally, there is often a narrow optimal concentration range for marine biota, as is the case for Cu8.
Isotope ratios of TE, including barium (Ba), Cd, Cr, Cu, Fe, molybdenum (Mo), Ni, lead (Pb), uranium (U), and Zn serve as valuable tracers of human activities9,10,11. They provide insight into various geological, physical, biological, and chemical processes in the ocean, and elucidate the sources and sinks of these elements12,13,14. Furthermore, TE such as silver (Ag), gold (Au), Cd, Co, Cu, Ni, and Mn, alongside technology-critical elements (TCE), like rare earth elements (REE), platinum group elements (PGE), and others, including gallium (Ga), germanium (Ge), indium (In), Niobium (Nb), antimony (Sb), tantalum (Ta), tellurium (Te), and thallium (Tl), are expected to play increasingly significant roles in driving global economic growth15,16.
Notwithstanding their critical role in both environmental and economic contexts as nutrients, contaminants (defined as being present at above their natural background levels), tracers, and resources, our understanding of TE remains incomplete, despite decades of research (e.g., through the GEOTRACES Program17,18). There are notable knowledge gaps in the literature, particularly regarding the complex dynamics and biogeochemical cycles of TE contaminants, including their chemical speciation, physical fractionation, bioavailability, toxicity, and fate under global CC and multiple stressors19. These gaps are especially pronounced for the rapidly increasing TCE, where the sources, sinks, and geochemistry remain largely unknown, requiring in-depth and urgent investigations15,16 to assess their fate and both short- and long-term impact on marine ecosystems.
Today’s anthropogenic pressures are leading to changes happening at unprecedented rates, magnitudes, and variabilities, thereby greatly influencing the dynamics and fate of TE at various temporal and spatial scales, i.e., local, regional, and global20. Major CC drivers involve physical and chemical ocean- and coastal changes, including warming (leading to e.g., ice melt, sea-level rise, altered precipitation patterns, dust deposition, and river runoff rates), deoxygenation, acidification, frequency of extreme events (storms, floods, fires, droughts), (de)salinization, changes in near-shore ocean circulation and mixing, as well as the interaction of co-occurrent multiple stressors. Representative Concentration Pathways (RCP) are climate change scenarios projecting future greenhouse gas concentrations. Even under very stringent RCP 2.6 projection (UN IPCC)21 it is estimated that by the end of the 21st century, atmospheric CO2 concentrations could be as high as 478 μatm, global mean sea surface temperature could increase by 0.73 °C, the mean sea level could rise by 0.55 m, and surface pH and dissolved oxygen could decrease by 0.06 units and 0.6%21, respectively. These perturbations, stemming directly or indirectly from human activities such as fossil fuel combustion, deforestation, fertilizer use, land-use change, and industrial activities22, will inevitably affect the physicochemical nature (e.g., speciation, complexation, redox chemistry, sorption properties, and remobilization) of diverse TE contaminants and thereby impact their reactivity, bioavailability, and ultimate fate. Such changes will have follow-on effects on ecosystem functions and services, coastal and ocean health and sustainability, as well as human health and livelihoods (i.e., seafood safety and security).
Policy frameworks, legislation, and global agreements have been implemented to reduce specific TE contaminants in the marine environment. These include the London Convention/Protocol, and the Oslo Convention (Annex I and II), and, more recently, the Minamata Convention. However, despite these efforts, increasing TE contamination continues to negatively impact ocean health and sustainability, as well as human wellbeing, compromising the achievement of the Sustainable Development Goal Target 14.1 “Reduce Marine Pollution”, and Goal 2 “Zero Hunger” (i.e., seafood safety and security), as well as the outcomes of the UN Decade of Ocean Science for Sustainable Development (2021–2030). Thus, increasing the quality, coverage, and availability of sound data, expertise, and research capabilities to understand marine TE contaminants and their cumulative, long-lasting effects on human health and ecosystem functioning is crucial19.
To guide the scientific community towards future research priorities, this review aims to (i) summarize existing knowledge to provide a broad perspective on the impact of the major CC drivers on sources, sinks, cycling, distribution, transport, toxicity, and fate of TE contaminants, as well as their influence on the marine ecosystem (as shown schematically in Fig. 1), (ii) identify knowledge gaps to help streamline and focus future research efforts, and (iii) make recommendations for future research directions. While CC and human activities will affect the environmental distribution, fate, and toxicity of numerous chemical contaminants in marine ecosystems, this review will focus on TE in coastal ecosystems, for which, contamination is one of the most prominent pressures due to the proximity to sources (Fig. 1). However, where necessary, discussions of ocean waters will be included, given the intrinsic connections between coastal and ocean systems. Our effort will complement the work already undertaken by Working Group 45, titled ‘Climate Change and Greenhouse Gas Related Impacts on Contaminants in the Ocean’ (WG4519), as approved by the Group of Experts on Scientific Aspects of Marine Environmental Protection (GESAMP), and the Vision 2030 White Paper of Challenge 1 ‘Understand and beat marine pollution’23.
Sensitivity of trace element sources and sinks to climate change
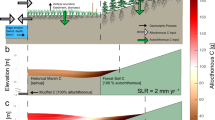
There are numerous sources of TE for the marine environment, stemming from both natural processes and anthropogenic activities. Natural sources include point sources such as rivers, volcanic activity, hydrothermal vents, and forest fires. Diffuse sources include weathering of rocks, atmospheric deposition, sediment resuspension, sea-ice melt/retreat, and submarine groundwater discharge (SGD). Since the industrial revolution, anthropogenic emissions have substantially contributed to TE fluxes, sometimes surpassing natural fluxes along the land-ocean continuum24,25. Anthropogenic inputs can derive from activities like mining (both on land and at sea), smelting, combustion of fossil fuels, aquaculture, agriculture, ports, shipping, industrial and residential activities, waste disposal, as well as medical applications. For most TEs, their final sink in the marine ecosystems is their permanent burial in sediments and/or mineral deposits. Both CC and human activities affect these sources and sink at various spatial and temporal scales, thereby altering the TE partitioning between and across different environmental spheres (atmosphere, hydrosphere, geosphere, and biosphere), their transport pathways, their internal cycling processes, and their physical, chemical, and biological drivers26 as exemplified schematically in Fig. 2.
Natural sources and transport pathways
Hydrological and eolian transport pathways
Observations and climate model simulations indicate significant changes in the hydrological cycle in response to CC, resulting in alterations in the precipitation amount, intensity, and frequency27. Global average precipitation is projected to increase by ca. 2% per °C in response to global warming with the highest increase in annual precipitation expected in high-latitude regions28. Particularly, Arctic precipitation is expected to rise significantly, far exceeding the global average value, with a projected 4.5% increase per °C in this century28. This process is happening along with persistent permafrost thaw and snowmelt, leading to increased river discharge and allowing more and deeper groundwater flow to occur, thus, causing large-scale changes in hydrologic fluxes28,29,30. These changes impact the chemical composition and residence time of chemical exports29,30. Conversely, regions such as Central and South America, Australasia, the Mediterranean, and southern Africa are expected to experience worsening aridity, heightening the risk of droughts and fires31.
Atmospheric dry and wet deposition
Atmospheric deposition has long been recognized as a major source of TE such as Al, Cu, Co, Fe, Mn, Pb titanium (Ti), vanadium (V), and Zn to the surface ocean32,33. While the anthropogenic emissions of TE into the atmosphere emitted through waste incineration, burning of fossil fuels, and traffic-related sources decreased since 1990 due to technological innovation34, there has been a notable increase in global dust mass loading since pre-industrial times. This increase, estimated at 55 ± 30%, is largely attributed to natural and anthropogenic induced CC including desertification, droughts, and wildfire emissions, as well as changes in land use35,36. Climate model simulations, however, have difficulties in reproducing the observed historic increase37 and future changes in dust concentrations diverge widely, that is, it is not clear whether dust concentrations will increase or decrease with CC36,38. Atmospheric dust concentrations over the North Atlantic, for instance, vary in response to drought cycles in Africa and large-scale atmospheric features such as the North Atlantic Oscillation39. The behavior and impact of atmospheric TE deposits, including their solubility and post-deposition processes, are influenced by various environmental factors (Box 1). These complex interactions, combined with diverse sources, sizes, chemical composition, and solubility of atmospheric aerosols, make it challenging to predict future trends in atmospheric dust loading and its impacts on ocean ecosystems40.
Riverine inputs
Simulations with global hydrological and climate models41,42,43 showed a significant increase in mean annual river discharge across the high northern latitudes for the period 2071–2100 relative to 1971–2000, due to large increases in precipitation and snowmelt. Similar trends were also projected for large parts of the tropical regions, including the Amazon, Congo, and Indus catchments, due to increasing monsoon rainfall43. Increased seasonality of river discharge was also projected for ca. 30% of the global area, primarily affecting temperate and continental climate zones43. Conversely, decreases in mean annual river discharge are anticipated for regions in the mid-northern latitudes and southern latitudes, including the southern United States, Central America, Europe, the Middle East, eastern China, southern Africa, and southern Australia42,43. This altered hydrologic cycle resulting from CC is poised to affect weathering, denudation, and riverine runoff, consequently influencing the fluxes of TE contaminants into the coastal ocean across various spatial and temporal scales44. Furthermore, increased runoff is expected to accelerate leaching, resulting in enhanced TE loads45,46 (Box 2).
Submarine groundwater discharge (SGD)
The exchange of groundwater between land and sea, known as submarine groundwater discharge (SGD), is an essential hydrogeological feature that can be a major pathway for delivering contaminants to the coastal zone, influencing coastal ocean chemistry47,48. In addition to the land-to-sea flow via SGD, seawater intrusion into coastal aquifers also occurs. Although the magnitude of fresh SGD to the coastal ocean was reported to be approximately 1% of the annual river discharge49, it represents a globally important source of nutrients, carbon, and TE contaminants to the ocean49,50.
There are early signs indicating that both the quantity and composition of SGD are undergoing changes due to ongoing CC and urbanization. For example, projections for Northern Europe suggest a moderate (0.2 m) increase in average groundwater levels over the next two decades, largely driven by increased precipitation, potentially increasing the mobilization and flux of TE through groundwater by a factor of 2–1051. In addition, seasonal and spatial variations in groundwater, driven by higher evapotranspiration in spring and summer in warmer climates, as well as warmer winters in snow-dominated regions, should also be expected. A decrease in fresh groundwater recharge, coupled with sea-level rise, could lead to significant seawater intrusions into coastal aquifers and prolonged residence times52,53. The associated shift of the freshwater-seawater interface can disturb the SGD chemistry and affect the biogeochemistry of aquifers that have not been in contact with seawater for centuries47,54.
Furthermore, the projected increase in the magnitude and frequency of floods and droughts, attributed to CC, may impact groundwater quality55. During floods, extremely high groundwater levels can increase TE solubility or facilitate their transport by particles from the topsoil51. Contaminated groundwater is already impacting TE concentrations in stream water under both, extremely low and high river flows, potentially diminishing the efficacy of remediation efforts for TE contamination, such as those arising from mining activities56. Consequently, the inland expansion of subterranean estuaries, increasing temperatures, and extreme events becoming more frequent due to CC, could lead to greater mobility of hazardous TE and subsequent contamination of coastal ecosystems (Box 3).
Sediments and coastal erosion under extreme weather events and sea-level rise
Sediments as a potential source of contaminants
Sediments and soils in coastal ecosystems represent an important sink and reservoir for contaminants, linking the atmosphere, geosphere, and hydrosphere through natural and anthropogenic TE fluxes. However, sediments can also act as a secondary source of historically accumulated contaminant loads, which may be released during events that disrupt sediments, leading to the remobilization, transport, and redistribution of contaminants57. Mobilization and abrupt changes in the availability of TE may occur due to hydraulic perturbation, caused by erosion, dredging, trawling, turbulent mixing, tsunamis, biological processes (bioturbation), and modifications of the chemical milieu that disrupt the equilibrium partitioning between water and sediment58,59,60. The mobility of TE in sediments is governed by various environmental factors, such as redox potential, pH changes, and DOM interactions (Box 4). The lack of field observation on the biogeochemical responses of sediments to pressures driven directly or indirectly by CC, particularly in a multi-pressure context, poses considerable challenges for further investigation46.
Extreme floods
Flood and extreme flood events, with extreme floods being high-magnitude events characterized by exceptionally high water levels significantly exceeding the historical flood levels for a given area, are amplified by CC61. Globally, the absolute flooding damage from flooding could increase by a factor of 20 by the end of the century62. By 2100, if no coastal protection or adaptation measures are implemented, 48% of the world’s land area, 52% of the global population, and 46% of global assets will be at risk of flooding63.
Extreme floods are more complex than typical events because of the differences in duration, intensity, and extent (river/estuary/bays versus watershed), and it is predicted that extreme floods will account for 68% of the global coastal area flood events, with regional sea-level rise contributing the remaining 32%63. Flooding alters the physicochemical properties of sediment and soils and influences biological processes64 (Box 5). Extreme floods can also promote the transport and translocation of TE contaminants from deeper anoxic sediments, exposing organisms to high concentrations of TE65,66, and causing ecological adverse effects and health risks through various exposure routes, including bioaccumulation67.
During flooding, rapid depletion of oxygen due to the loss of contact with the oxygenated atmosphere leads to a decrease in redox potential. The associated material transport influences the environmental fate of TE, but the effect on mobility varies among contaminants68. Metal partitioning between particulate and dissolved phases can change during flood events, i.e., the particulate phase may be diluted by coarser, less contaminated particles eroded from bed sediments, while the dissolved phase may become enriched owing to mobilization69,70,71. Further, high-magnitude flood events can wash out bottom sediments, and lead to a decrease in fine-sediment and TE concentrations in labile fractions and, consequently, affect the downstream transfer of contaminants (e.g., Cr, Cu, Fe, Ni, and Zn)72. Moreover, turbulent flow conditions during these events may favor strong TE mobilization due to sediment resuspension, the desorption of TE, and the transformation of TE into more bioavailable forms57,70.
Extreme droughts
Extreme droughts, characterized by prolonged periods of less than average precipitation that significantly exceed the typical or historical drought conditions for a given area, are projected to increase in magnitude and/or frequency in most dry subtropical regions73. Droughts favor water quality deterioration regarding TE contamination, temperature, and eutrophication74 (Box 6). Further, the drying up of contaminated areas may expose TE-enriched sediments to the atmosphere that may be eroded and transported away from their original deposition site75. As global water scarcity increases and the prospect of more frequent or more intense droughts and heatwaves remains, managing drought-driven TE contaminant flux will become increasingly important and requires the understanding of the system’s hydrology and sediment transport dynamics.
Sea-level rise
Predicted sea-level rise will increase the flood risk in coastal zones, exacerbate erosion, and lead to the salinization of groundwater and soils76. This change will have major implications to transport of sediments and associated TE contaminants and their residence times in coastal systems77 (Box 7). For instance, sea-level rise accompanied by warming may produce more anoxic conditions in mangrove environments, not only due to increased flooding but also due to less bioturbation, causing enhanced As mobility and suppressing Sb mobility78.
Coastal plains in many parts of the world contain acidic soils that are highly vulnerable to sea-level rise. These soils are characterized by high concentrations of exchangeable and hydrolyzable acidic metal cations such as Al and Fe, along with other toxic TE released by the weathering of aluminosilicates79. Flooding by seawater may cause rapid, short-term increases in acidity via the desorption and mobilization of acidic metal cations, creating a positive feedback loop that further increases acidity80. This process can lead to declining water quality due to the mobilization of high concentrations of Al, Ni, and Zn80. These examples highlight the importance of understanding the factors that influence the mobility of individual TE at a specific site to predict the impacts of increased flooding duration and magnitude on their environmental fate, bioavailability, toxicity, and risks to affect ecological integrity and human health.
Cryosphere
Polar regions are highly susceptible to a warming climate and, as such, are on the frontline of CC. The main effects of CC in these regions will be visible through changes in TE contaminant transport pathways, including atmospheric, riverine, sea-ice, ocean currents, coastal erosion, groundwater discharge, and biological transport driven by migrating animals (e.g., Hg biomagnified in birds and transported over large distances)81. Further, sea-ice reduction is expected to alter surface albedo, salinity gradients, TE input, and the exchange of heat, moisture, and trace gases between the atmosphere and ocean. These changes affect the stability of the water column, ocean-atmosphere circulation patterns, biological composition, and in-situ biogeochemical conditions, thereby affecting the entire ocean including coastal areas81. Thus, the polar seas and the cryosphere act as precursors for global changes in other ecosystems.
An important aspect of CC in polar regions is the change in the biogeochemical cycling and dynamics of TE since the cryosphere can be rich in nutrients, organic matter, and TE82,83. Consequently, permafrost thawing, glaciers/ice melt, and increased river runoff are expected to enhance the flux of nutrients and TE to the polar seas, potentially affecting the biological productivity in these ecosystems84.
Ice and glaciers melting is a foreseeable CC factor with many concerning implications76. Sea ice serves as a dynamic interface between the atmosphere and the coastal ocean, playing a crucial role in the biogeochemical cycling of TE85. For example, concentrations of TE in the surface water of the cove (Antarctic coastal waters) have increased substantially due to melting ice, with Fe increasing 18-fold, Al and Mn increasing 8–10 fold, and Co, Cr, Ni, and REE increasing up to 4-fold86. Ice melting also allows the input of atmospheric dust, which has been observed in Cu and Cd profiles, showing surface maxima in the Ross Sea87. Glaciers also acquire a significant load of terrigenous material through glacial erosion processes, atmospheric deposition, and direct contact with shelf sediments. Thus, glacier fragments contain elevated levels of land-borne material, including TE, which are released directly into the surrounding water during melting and thaw cycles88,89,90.
Enrichment with particulate TE has been observed in both Arctic and Antarctic Sea ice relative to underlying waters, with Al and Fe showing the highest enrichment91,92. Additionally, the colloidal phase (>80%) was enriched in cryosphere reservoirs for Cd, Fe, Mn, Ni, and Zn, whereas seawater was dominated by the truly dissolved phase83. Given the responses to CC, an important question is how changes in sea-ice coverage, thickness, and melt season length, including the increased dispersal of trapped particulate and colloidal TE and increased direct atmospheric deposition of TE to the surface ocean, will affect TE residence times and bioavailability in polar seas83,93.
Overall, the Arctic is more vulnerable to environmental changes connected to CC than the Antarctic due to greater seasonal warming and freshening, as well as its lower alkalinity and nutrient limitation94. The Arctic Ocean, influenced by higher freshwater input, less biological uptake, and/or less scavenging removal under the sea ice, has exceptionally high concentrations of dissolved bioactive TE such as Co, Cu, Fe, Ni, and Zn82, thereby differing from other oceans. In addition, the release of materials stored in permafrost poses significant risks of increased contaminant levels in Arctic environments (Box 8). Mercury is particularly significant concern (Box 9). Transport of these TE downward along the Transpolar Drift is facilitated by an increased concentration of organic ligands, originating mainly from Arctic rivers82,95,96. This process which will have implications for marine productivity not only in the Arctic region but also in the North Atlantic82,95,96.
Anthropogenic sources
Past and present anthropogenic sources/activities
Rising industrial production, increasing demand for mineral resources, energy, and food, and the continuous development of high-technology products have, among other things, led to an unavoidable increase in TE contaminant inputs into the environment, particularly in coastal marine zones, via atmospheric deposition, surficial runoff, wastewaters, SGD, and riverine input97. It has been estimated that anthropogenic activities have increased the global fluxes of toxic metals such as Pb by more than tenfold98 and of Hg by a factor of 3–799 relative to pre-anthropogenic levels97. A substantial fraction of these TEs eventually end up in the ocean97. Silver (Ag), an element highly toxic to marine biota, is increasingly found in coastal seawater of various regions100 owing to atmospheric deposition from coal burning and riverine input from the increasing use of Ag nanoparticles in antibacterial consumer products20. Additionally, the shipping industry and associated port activities, such as the use of scrubbers in ship stack fumes101, antifouling paints, and biocides, can result in the coastal and oceanic discharge of Cu, V, and Zn102,103,104. Plastics, a major concern in the 21st century, can also act as vectors for the transport of TE contaminants to the marine environment since plastics contain TE, including high concentrations of Cd and Zn, and can also adsorb TE, notably Cu, Pb105,106, and REE107. In addition, decreasing pH has been shown to reduce the net adsorption of Cd, Co, Ni, and Pb to plastic pellets in estuaries108, raising questions about how predicted ocean acidification might affect the interaction between TE and plastics.
Moreover, the decline of sea-ice cover is opening new Arctic shipping routes and allowing access to previously inaccessible areas. These changes lead to secondary follow-on effects, including economic growth, increased maritime activity which increased by 75% from 2013 to 2019109, population growth, changing land use, and increasing land-based activities, all of which pose substantial risks to the sub-Arctic and Arctic regions by introducing new and more TE contaminants into the Arctic environment such as Cd, Cu, Hg, Pb, and Zn109,110,111. A similar situation is occurring in the Southern Hemisphere, where warming, population growth, and industrial development are increasingly introducing TE contaminants to the Antarctic environment, including Cu and Pb, owing to mining and smelting activities in Australia, Chile, Peru, Zaire, and Zambia110. Despite these observations, it remains largely unknown how anthropogenic sources and the biogeochemical behavior and cycling of associated TE (including their interaction) will be affected by CC drivers, making it difficult to predict the environmental impacts of the emerging CC–TE interactions on coastal areas.
Future anthropogenic sources/activities
Anticipated future activities that will affect the TE contaminants input to coastal environments include the exploitation of natural resources (e.g., land and deep-sea mining), the establishment of coastal infrastructure (e.g., wind turbines, seawalls, coastal roads, land reclamation), the development of new and emerging technologies, the expansion of aquaculture, and the implementation of geoengineering activities for climate mitigation and adaptation20. The latter has found particular attention as it is now increasingly seen as a necessary step to achieve the Sustainable Development Goals of the Paris Agreement. Proposed marine geoengineering activities range from the manipulation of the carbon cycle (e.g., carbon removal activities such as Fe fertilization, artificial upwelling, CO2 storage and capture, and alkalinity enhancement), to albedo modification, and hybrid technologies20,112. For example, increasing ocean alkalinity by adding Ca2+ or Mg2+ via minerals (e.g., olivine, calcite) or alkaline industrial residuals, theoretically leads to substantial CO2 uptake from the atmosphere and a decrease in ocean acidification. However, these minerals also contain high amounts of TE (i.e., Cd, Cr, Cu, Fe, Ni, and Pb)113 that could negatively impact the marine environment. Olivine-rich rocks, for example, can contain up to 30–50% Fe and a high abundance of Cr and Ni, with the latter being toxic to marine organisms above a certain threshold114,115. So, while initially beneficial, the knock-on effects of adding large amounts of geological material to marine systems have not yet been systematically studied, especially in the context of a (multi-stressor) changing ocean and CC drivers. The same is true for other anthropogenic activities, such as deep-sea mining. Secondary impacts of CC, such as the corrosion of coastal infrastructure (e.g., wind turbines, dikes, coastal roads, bridges, naval bases, harbors), however, have been fairly well studied116,117.
Investigating the short- and long-term impacts of emerging CC–TE interactions remains critical to environmental risk assessments before any large-scale ocean solutions and new anthropogenic activities (e.g., deep-sea mining) are approved and implemented20. The societal challenge is to develop integrated and coordinated cross-sectoral approaches that balance the sustainable use of the ocean with its protection23.
Sensitivity of trace elements cycling to climate change
In seawater, the reactivity and bioavailability of elements are determined by their chemical and physical speciation. The latter is often also referred to as fractionation, a term used from now onwards. While changes in the speciation of the carbonate system are key to ocean acidification, they also influence the fractionation and speciation of TE. These changes affect the biogeochemical cycles, transport (mobility and solubility), reactivity (residence times, sorption, and desorption), and biological availability (toxicity, bioaccumulation, and trophic transfer) of TE, thereby altering their fate and impact in the marine environment7,118. Figure 3 shows a simplified schematic of the anticipated effects of CC drivers on TE chemistry and the related biological effects in seawater.
Chemical speciation and fractionation
In the marine environment, TE exists in numerous forms (species). Physical species include particulate (>0.2 μm) and dissolved (<0.2 μm) phases, which can be subdivided into colloidal (1 nm–0.2 μm) and ‘truly’ dissolved species (≤1 nm). Along the entire physical size range, TE can also be distinguished by their chemical form, such as oxidation state, and complexation with inorganic (incl. Cl−, CO32−, OH−, SO42−) and organic ligands (incl. siderophores, thiols, phytochelatins, humic(-like) and exopolymeric substances).
Transformations among the various TE species and size fractions occur continuously at different temporal and spatial scales and are controlled by complex and interlinked biogeochemical processes that are dependent on environmental conditions7,119. This control makes TE speciation inherently susceptible to CC-related effects such as ocean acidification, increasing anoxia, and salinity changes7.
In the context of CC, the interplay between TE and ocean acidification is the most studied. Ocean acidification will decrease the concentration of OH− and CO32− ions, which form strong complexes with divalent and trivalent metals impacting their speciation, solubility, adsorption to organic material, redox reactions, and potential toxicity. These anions are expected to decrease in surface waters by 4% and 6%, respectively, if atmospheric pCO2 increases up to 478 μatm by the end of the century (Table 1). The TE most affected by this decrease will be those that form strong complexes with carbonates, such as Co2+, Cu2+, Ni2+, Pb2+, UO22+, or Zn2+ and hydroxides, such as Al3+, Cr3+, or Fe3+.
Most TEs become more soluble at lower pH and increasing temperature (Box 10), with those demonstrating a high degree of carbonate or hydroxide complexation being impacted the most. An exception to this trend is the oxyanion species of Cr (CrO42−), Mo (MoO42−), V (VO43−), and tungsten (W; WO42−) for which solubility decreases (based on own calculations using an ion-pairing model written by CMG van den Berg, Univ. Liverpool)120. It should be noted that these estimates only pertain to the inorganic species and do not consider the effect of pH, temperature, and salinity on their organic complexation, for which little is known, although proton-binding characteristics of marine dissolved organic matter have recently been reported121. This complexation is specifically important for TE that are dominated by their organically complexed species, such as Cd, Co, Cu, Fe, Pb, or Zn122. The binding affinity of organic ligands with acidic functional groups (e.g., carboxylic) could be strongly affected by decreased pH. For Cu, the strength of organic-Cu interactions continuously decreases with the acidification of seawater123. However, the magnitude varies with DOC124, suggesting that variability in DOC may have a greater impact on chemical speciation and bioavailability than the projected changes in inorganic TE speciation. Metals that form strong complexes with Cl−, such as Cd or Hg, will be less affected by ocean acidification since Cl− is insensitive to pH. Also, for TE that is predominantly in the free form (considering only inorganic species), such as Co2+ and Mn2+, the pH decrease should lead to only relatively small changes in their free ion fraction122. Ocean acidification may also impact biological calcification and/or the dissolution of CaCO3, which would increase Ca2+ concentration. The competition of H+ and Ca2+ ions for binding sites with TE on organic ligands would then cause the release of TE from sediments and organic ligands125,126.
Ocean acidification also affects photochemical processes such as the production of O2–, HO2, and H2O2 that can change the oxidation state of TE (e.g., Cr(III)/Cr(VI), Cu(I)/Cu(II), or Fe(II)/Fe(III)122) and oxidizes dissolved organic material. Additionally, altered UV irradiation due to the depletion of ozone can break down the available binding sites for TE on organic ligands, thus increasing the proportion of free TE ions and their bioavailability and/or potential toxicity127,128. Other implications of increased UV irradiation include UV-induced oxidation of Hg0 to Hg2+, which can then be methylated and biomagnified along the food chain129, and the mobilization of dissolved TE (i.e., Al, Cd, Cu, Co, Mn, Mo, Ni, Pb, Ti) from sunscreen products130. While most studies have focused on the effects of ocean acidification in the water column, TE speciation is also affected in contaminated sediments, increasing their toxicity131.
Although empirical data showed that temperature increases have a negligible effect on metal speciation, particularly on free ion activity132, warmer, more stratified, de-oxygenated, and acidified seawater will change microbial community structure and function in general133. This change may impact microbial detoxification abilities, which are closely related to the production of organic ligands134. How CC drivers affect microbial ligand production and associated speciation of TE in the future remains to be determined.
Bioavailability, bioaccumulation, and biological responses
Some TE (e.g., Hg) can be transferred through the marine food chain via bioaccumulation and subsequent biomagnification processes, affecting organisms at higher trophic levels and potentially impacting humans through seafood consumption135. For many TEs, the nature of the cellular transport systems remains unknown, leading to some uncertainty on how climate- and acidification-related changes will affect bioavailability136.
Climate change is expected to affect TE bioavailability and bioaccumulation primarily by altering seawater pH, temperature, oxygen levels, and salinity. Studies examining the combined impacts of CC and TE on marine biota, primarily focus on understanding ocean acidification’s effect126,137,138 (Box 11). Since divalent cations are predominantly transported via ATP-ases that bind to the free inorganic cations, acidification is expected to increase the bioavailability of Cr, Cu, Fe, and Pb but not of Cd, Co, Hg, Mn, Ni, and Zn. It has been shown that the high-affinity Fe uptake system of some phytoplankton cells depends on CO32−, leading to a strong pH sensitivity139. Another point to consider is that the competition between H+ ions and some TE, such as Cr and Cu, at the cell surface could diminish their bioavailability counteracting the increased free ion concentration140,141,142. However, the biotic uptake mechanisms are a bit more complex for TE whose speciation is dominated by complexation with organic ligands, such as Co, Cu, Fe, and Zn143. Traditionally, the strong organic complexes of Cd, Cu, and Zn were assumed to be non-bioavailable144. Contrary to these general assumptions, some Cu complexes with organic ligands have been shown to be available for uptake by phytoplankton and mussels145,146. Zinc organic complexes are relatively weak (e.g., compared to those of Co or Cu)147 and a decrease in phytoplankton Cd and Zn uptake under decreased pH has been observed148. In contrast, in coastal seawater, an increase in bioavailable Zn was found under lower pH149, demonstrating that the role of organic ligands in the interactions between acidification, speciation, and bioavailability is specific to the combination of TE and organic TE-binding ligands and far from being understood.
Another possible effect of acidification on interactions between TE contaminants and biota, independent of changes in the bioavailable species in the medium, is its influence on the efficiency of the uptake and/or elimination systems. Reduced pH and elevated CO2 concentrations can alter the physiological effects of TE contaminants and their associated bioaccumulation in marine organisms, which appears to be an even more important consequence of ocean acidification for marine biota than changes in bioavailability126.
Another important driver of CC, impacting bioavailability and bioaccumulation, is ocean warming (Box 12). Studies have shown that TE uptake and accumulation increase with increasing temperature in numerous aquatic organisms132. In 80% of the cases, ectotherms were more affected. Since temperature changes have a minor effect on TE speciation, this can only be explained by the effects of temperature on biological factors132. Increased temperatures are expected to cause higher TE uptake by decreasing oxygen solubility, thus enhancing gill ventilation, however, it can also lead to higher rates of depuration and detoxification mechanisms125,138.
Furthermore, increased precipitation and temperature due to CC will induce eutrophication and stratification, leading to the expansion of anoxic and hypoxic areas in aquatic ecosystems worldwide125. This additional stressor could exacerbate the effects of TE toxicity, with benthic species being particularly endangered as they are directly exposed to sediment TE contaminants and inhabit areas where hypoxia occurs more frequently than in the water column125,150,151.
Moreover, while it has been found that moderate ocean acidification can mitigate TE toxicity for some marine species, ocean warming can counteract this positive effect, leading to increased TE toxicity under combined ocean warming and acidification conditions. This was shown for As, Cd, or/and Cu in anemones, copepods, corals, and various molluscs138, as well as for Hg in numerous marine organisms150. It was observed that the effect of increasing CO2 alone did not impact the bioaccumulation in mussels M. galloprovincialis but when combined with higher temperatures led to increased bioaccumulation of As, Cd, Cr, Ni, and Pb in mussels adapted to colder waters152. Likewise, the simultaneous exposure to elevated Cu levels, along with ocean acidification and warming exacerbated the detrimental effects on kelp's microscopic early life stages compared to the effects of the single stressors alone153. This synergistic effect was also observed in a study on REE (La and Gd), which revealed increased accumulation patterns in U. rigida154. Therefore, warming appears to pose a greater threat to estuarine and coastal organisms than acidification150,155,156. Given this context, it is crucial to consider the combined effects of acidification and warming to evaluate the risk posed by TE contaminants, especially under global change conditions.
Discussion
Despite the recognized importance and increasing interest in the effects of CC on TE sources, cycling, transport, and fate, research on these topics is still at an early stage. The knowledge gaps are large, and the depth to which they are addressed varies widely around the world and research topic. CC drivers such as ocean acidification and warming receive more attention in the context of TE contamination157 than other equally important primary drivers such as deoxygenation, and secondary drivers such as sea-level rise, ice melting, changes in circulation/mixing, salinization, and extreme events. Additionally, the interactions and feedback mechanisms among multiple stressors are only poorly understood due to their intrinsic complexity and the lack of well-designed experiments that consider the diversity of potential simultaneous TE contaminants and CC stressors. Moreover, TE concentrations are usually determined in contaminant studies, while their speciation, which determines element reactivity and bioavailability, is largely overlooked. This lack of studies limits our understanding of current and future processes in the highly dynamic coastal marine environment. Addressing these knowledge gaps is crucial for making accurate predictions of coastal and ocean system changes, which are essential for effective human health and ecosystem protection measures. To build a comprehensive dataset, we suggest that scientists prioritize nine key research areas in the future. This focus will help bridge current knowledge gaps and address science-policy requirements.
Adoption and development of best practices
We face ever-increasing methodological complexity and diversity owning to the interdependencies of CC drivers, the inherent interaction of multi-stressors in coastal environments, the emergence of new TE contaminants, as well as varying institutional capacities and capabilities. Additionally, the fragmentation of methods and data across regions, nations, and disciplines inhibits effective collaboration, global participation, data sharing, interoperability, and comparability158. A first and foremost requirement for TE contaminant research and monitoring is therefore the need to adopt and follow coherent, well-defined, and reproducible methodologies and quality assurance protocols158,159,160.
Improved geographical and temporal research coverage
Coastal areas experience diverse environmental variations in space and time that affect TE biogeochemistry, organism responses, behavior, and trophic interactions, which are expected to worsen with CC. Comprehensive, long-term monitoring and research are needed in geographical regions that are currently poorly studied but highly affected by CC, such as polar regions, tropical coastal zones, various vulnerable environments such as coral reefs, and estuaries52, and in natural archives of contaminants (e.g., estuarine and mangrove sediments). Ideally, the activities would be scaled to assess contamination impacts in all environmental compartments (i.e., atmosphere, sediments, water, and biota), and at a community and ecosystem level. To understand the global response of TE contaminants to CC, there is a need to better cover our understanding in the much-understudied Global South.
Research on so-far understudied sources and environments
The knowledge of the distribution of TE and their sources and cycling in different marine environments has substantially increased since the implementation of the GEOTRACES Program17. However, some sources, including eolian wet and dry deposition, and coastal shallow hydrothermal vents161, and ecosystems like seagrass beds and tidal flats remain poorly studied162 and should receive more attention given their high significance at regional scales.
Expanding biological research
The effects of key CC drivers on contaminants have been thoroughly explored in recent years, but mostly as single drivers using individual-based organism approaches138,157. Thus, current knowledge is limited to a relatively small set of model organisms. More holistic studies that assess community and ecosystem-level responses using a multi-stressor approach are still scarce157 and limit the comprehension of the CC-contaminant interaction and their associated biological responses. Further, to account for organisms’ ability to adapt to environmental changes, scientists should examine the whole life cycles of organisms. Therefore, to assess the long-term effects of CC and contaminants on marine biota, future studies should include multiple developmental stages, species from different geographic locations, and multiple taxa126,137,138 to better address current management and policy needs (e.g., preservation of biodiversity and food safety157).
Research related to emerging contaminants
A number of new compounds and TE are introduced into the environment each year that have no natural use, termed as emerging contaminants. At present, TEs of emerging concern include TCE, REE, and PGE16,97,163. The introduction of emerging contaminants in the aquatic environment is gaining interest due to limited scientific knowledge about their distribution, cycling, transport, reactivity, toxicity, and ultimate fate, including bioaccumulation processes. Their impact on the coastal environment driven by CC remains unknown and requires urgent attention to avoid potentially irreversible damage.
Research on multiple stressors
While it is evident that CC is causing multiple stressor effects (see Figs. 1 and 2), most studies are concentrated on much simplified scenarios. Although this approach has greatly improved our understanding, it fails to reveal interactive and synergistic effects of multiple contaminants and CC stressors on single organisms, or entire ecosystems19. There is a clear need to increase the knowledge around the interactions and cumulative impacts of the growing mixture of TEs and other chemicals, starting with stressor pairs, to which marine biota are exposed under varying, interacting, and co-occurring CC conditions157,164.
Development of in-situ sensors and low-cost methods and instrumentation
In-situ sensors or continuous samplers should be employed to capture the spatial and temporal variations of TE contaminants in dynamic coastal environments165,166. Although there has been some progress, additional research is needed to develop in-situ sensors and samplers capable of directly measuring TE concentrations and speciation in the aqueous phase166,167,168. However, in-situ sensors and samplers entail substantial costs from development and validation to production. To promote their widespread adoption, supporting the creation and operation of affordable versions is essential.
Development of better models
Gaps in the monitoring and understanding of processes are often filled by marine ecosystem models, which can also be a useful tool. However, our mechanistic understanding of the effects of CC drivers on TE contaminants, particularly their speciation and organic complexation, and their interaction with biology, is currently very limited, making model predictions and calculations non-trivial. Additionally, short-term fluctuations in environmental variables (e.g., salinity, temperature, and pH), common in coastal areas, are generally not accounted for in models. As we gather more high-quality data, with increased temporal resolution, including data on speciation and bioavailability, efforts to enhance modeling capabilities in the near future are underway. This effort is fueled by the current momentum to create a Digital Twin of the ocean (https://www.mercator-ocean.eu/en/digital-twin-ocean/) using machine learning tools.
Inclusion of trace element speciation in legislation and risk assessments
The implementation of appropriate legislation, regulation, and successful management strategies for contaminants is closely linked to the fundamental understanding of the key processes and factors controlling their speciation dynamics in marine ecosystems. However, contaminant speciation remains largely unapplied to hazard assessments, environmental risk assessments, and/or the regulations of contaminants. The biggest challenge to increase the use of speciation-based approaches for contaminants in regulations and assessments is providing practical approaches that are scientifically robust, easy, and fast to implement, and do not come with additional costs compared to the more traditional approaches for determining contaminant concentrations. Ongoing efforts aim to develop tools to meet these needs and thereby increase the ecological relevance of contaminant regulations169. A recent example in this direction is the MONITOOL project (https://www.monitoolproject.eu/), which aims to respond to European Directive demands for the assessment of the chemical status of transitional and coastal waters, by developing speciation-based environmental quality standards (EQS) using passive sampling technique for metals170,171.
Addressing these nine research priorities related to the contaminant-CC nexus over the next years would facilitate the implementation of science-based policies and regulations that support effective mitigation and management strategies in vulnerable coastal areas and in so doing support the achievement of Sustainable Development Goal 14 (“Life Below Water”).
Reporting summary
Further information on research design is available in Nature Portfolio Reporting Summary linked to this article.
Data availability
No new analytical data were generated or presented in this review. All sources of data, including databases where data was extracted from, are stated in-text.
References
Henderson, G. M. Ocean trace element cycles. Philos. Trans. R. Soc. A https://doi.org/10.1098/rsta.2015.0300 (2016).
de Baar, H. J. W., van Heuven, S. M. A. C. & Middag, R. Ocean biochemical cycling and trace elements. Encycl. Earth Sci. Ser. https://doi.org/10.1007/978-3-319-39312-4_356 (2018).
Lane, T. W. et al. A cadmium enzyme from a marine diatom. Nature 435, 42–42 (2005).
Kumudu, R. V. B. & Pathmalal, M. M. Marine Pollution - Recent Developments, Vol. 4 (eds Mancuso, M. & Abbas, M.) (IntechOpen, 2022).
Adams, W. J. & Chapman, P. M. Assessing the Hazard of Metals and Inorganic Metal Substances in Aquatic and Terrestrial Systems 1st edn (CRC Press, 2007).
Hunter, P. A toxic brew we cannot live without - micronutrients give insights into the interplay between geochemistry and evolutionary biology. EMBO Rep. 9, 15–U15 (2008).
Tercier Waeber, M.-L., Stoll, S. & Slaveykova, V. Trace metal behavior in surface waters: emphasis on dynamic speciation, sorption processes and bioavailability. Arch. Sci. 65, 119–142 (2012).
Bruland, K. W., Donat, J. R. & Hutchins, D. A. Interactive influences of bioactive trace-metals on biological production in oceanic waters. Limnol. Oceanogr. 36, 1555–1577 (1991).
De Vera, J. et al. Anthropogenic lead pervasive in Canadian Arctic seawater. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2100023118 (2021).
Zurbrick, C. M. et al. Dissolved Pb and Pb isotopes in the North Atlantic from the GEOVIDE transect (GEOTRACES GA-01) and their decadal evolution. Biogeosciences 15, 4995–5014 (2018).
Packman, H. et al. Tracing natural and anthropogenic sources of aerosols to the Atlantic Ocean using Zn and Cu isotopes. Chem. Geol. https://doi.org/10.1016/j.chemgeo.2022.121091 (2022).
Anderson, R. F. GEOTRACES: accelerating research on the marine biogeochemical cycles of trace elements and their isotopes. Annu. Rev. Mar. Sci. 12, 49–85 (2020).
Conway, T. M., Horner, T. J., Plancherel, Y. & Gonzalez, A. G. A decade of progress in understanding cycles of trace elements and their isotopes in the oceans*. Chem. Geol. https://doi.org/10.1016/j.chemgeo.2021.120381 (2021).
Horner, T. J. et al. Bioactive trace metals and their isotopes as paleoproductivity proxies: an assessment using GEOTRACES-Era Data. Glob. Biogeochem. Cycles https://doi.org/10.1029/2020GB006814 (2021).
Dang, D. H., Filella, M. & Omanovic, D. Technology-critical elements: an emerging and vital resource that requires more in-depth investigation. Arch. Environ. Contam. Toxicol. 81, 517–520 (2021).
Filella, M., Dror, I. & Omanovic, D. Foreword to the special issue on ‘technology critical elements. Environ. Chem. 17, 75–76 (2020).
GEOTRACES Program. https://www.geotraces.org/ 2006).
Robert, F. A. GEOTRACES reflections. Oceanography https://doi.org/10.5670/oceanog.2024.405 (2024).
Hatje, V. et al. Emergent interactive effects of climate change and contaminants in coastal and ocean ecosystems. Front. Mar. Sci. https://doi.org/10.3389/fmars.2022.936109 (2022).
Henderson, G. M., Achterberg, E. P. & Bopp, L. Changing trace element cycles in the 21st century ocean. Elements 14, 409–413 (2018).
Intergovernmental Panel on Climate Change. The Ocean and Cryosphere in a Changing Climate: Special Report of the Intergovernmental Panel on Climate Change. (Cambridge Univ. Press, 2022).
Doney, S. C. The growing human footprint on coastal and open-ocean biogeochemistry. Science 328, 1512–1516 (2010).
UNESCO. Vision 2030 Working Group 1, Challenge 1: Understand and Beat Marine Pollution White Paper (UNESCO, Paris, 2024).
Klee, R. J. & Graedel, T. E. Elemental cycles: a status report on human or natural dominance. Annu. Rev. Environ. Resour. 29, 69–107 (2004).
Sen, I. S. & Peucker-Ehrenbrink, B. Anthropogenic disturbance of element cycles at the Earth’s surface. Environ. Sci. Technol. 46, 8601–8609 (2012).
Noyes, P. D. et al. The toxicology of climate change: environmental contaminants in a warming world. Environ. Int. 35, 971–986 (2009).
Trenberth, K. E. Changes in precipitation with climate change. Clim. Res. 47, 123–138 (2011).
Bintanja, R. & Selten, F. M. Future increases in Arctic precipitation linked to local evaporation and sea-ice retreat. Nature 509, 479 (2014).
Giorgi, F. et al. Chapter 10: Regional climate information - evaluation and projections. In Climate Change 2001: The Scientific Basis (eds Houghton, J. T. et al.) 583–638 (Cambridge Univ. Press, 2001).
Walvoord, M. A. & Striegl, R. G. Increased groundwater to stream discharge from permafrost thawing in the Yukon River basin: potential impacts on lateral export of carbon and nitrogen. Geophys. Res. Lett. https://doi.org/10.1029/2007gl030216 (2007).
IPCC. Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, Geneva, Switzerland, 2023).
Witt, M. L. I., Mather, T. A., Baker, A. R., De Hoog, J. C. M. & Pyle, D. M. Atmospheric trace metals over the south-west Indian Ocean: total gaseous mercury, aerosol trace metal concentrations and lead isotope ratios. Mar. Chem. 121, 2–16 (2010).
Buck, C. S., Aguilar-Islas, A., Marsay, C., Kadko, D. & Landing, W. M. Trace element concentrations, elemental ratios, and enrichment factors observed in aerosol samples collected during the US GEOTRACES eastern Pacific Ocean transect (GP16). Chem. Geol. 511, 212–224 (2019).
Boyle, E. A. et al. Anthropogenic lead emission in the ocean the evolving global experiment. Oceanography 27, 69–75 (2014).
Desboeufs, K. et al. Wet deposition in the remote western and central Mediterranean as a source of trace metals to surface seawater. Atmos. Chem. Phys. 22, 2309–2332 (2022).
Kok, J. F. et al. Mineral dust aerosol impacts on global climate and climate change. Nat. Rev. Earth Environ. 4, 71–86 (2023).
Thornhill, G. et al. Climate-driven chemistry and aerosol feedbacks in CMIP6 Earth system models. Atmos. Chem. Phys. 21, 1105–1126 (2021).
Jickells, T. D., Baker, A. R. & Chance, R. Atmospheric transport of trace elements and nutrients to the oceans. Philos. Trans. R. Soc. A https://doi.org/10.1098/rsta.2015.0286 (2016).
Arimoto, R. Eolian dust and climate: relationships to sources, tropospheric chemistry, transport and deposition. Earth Sci. Rev. 54, 29–42 (2001).
Mahowald, N. M. et al. Aerosol trace metal leaching and impacts on marine microorganisms. Nat. Commun. https://doi.org/10.1038/s41467-018-04970-7 (2018).
Bring, A., Shiklomanov, A. & Lammers, R. B. Pan-Arctic river discharge: prioritizing monitoring of future climate change hot spots. Earths Future 5, 72–92 (2017).
Koirala, S., Hirabayashi, Y., Mahendran, R. & Kanae, S. Global assessment of agreement among streamflow projections using CMIP5 model outputs. Environ. Res. Lett. https://doi.org/10.1088/1748-9326/9/6/064017 (2014).
van Vliet, M. T. H. et al. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 23, 450–464 (2013).
Leon-Munoz, J. et al. Climate and land cover trends affecting freshwater inputs to a Fjord in Northwestern Patagonia. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.628454 (2021).
Wijngaard, R. R., van der Perk, M., van der Grift, B., de Nijs, T. C. M. & Bierkens, M. F. P. The impact of climate change on metal transport in a lowland catchment. Water Air Soil Poll. https://doi.org/10.1007/s11270-017-3261-4 (2017).
de Lacerda, L. D., Ward, R. D., Borges, R. & Ferreira, A. C. Mangrove trace metal biogeochemistry response to global climate change. Front. Glob. Chang. https://doi.org/10.3389/ffgc.2022.817992 (2022).
Moore, W. S. The effect of submarine groundwater discharge on the ocean. Annu Rev. Mar. Sci. 2, 59–88 (2010).
Robinson, C. E. et al. Groundwater dynamics in subterranean estuaries of coastal unconfined aquifers: controls on submarine groundwater discharge and chemical inputs to the ocean. Adv. Water Resour. 115, 315–331 (2018).
Taniguchi, M. et al. Submarine groundwater discharge: updates on its measurement techniques, geophysical drivers, magnitudes, and effects. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2019.00141 (2019).
Tamborski, J. et al. Submarine karstic springs as a source of nutrients and bioactive trace metals for the oligotrophic Northwest Mediterranean Sea. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.139106 (2020).
Jarsjö, J. et al. Projecting impacts of climate change on metal mobilization at contaminated sites: controls by the groundwater level. Sci. Total Environ. 712, 135560–135560 (2020).
Jiang, S., Ibánhez, J. S. P., Wu, Y. & Zhang, J. Geochemical tracers in submarine groundwater discharge research: practice and challenges from a view of climate changes. Environ. Rev. 29, 242–259 (2021).
Werner, A. D. et al. Seawater intrusion processes, investigation and management: recent advances and future challenges. Adv. Water Resour. 51, 3–26 (2013).
Colombani, N., Dinelli, E. & Mastrocicco, M. Trend of heavy metal release according to forecasted climate change in the Po delta. Environ. Process. 3, 553–567 (2016).
Aladejana, J. A., Kalin, R. M., Sentenac, P. & Hassan, I. Assessing the impact of climate change on groundwater quality of the shallow coastal aquifer of eastern Dahomey basin, southwestern Nigeria. Water 12, 224–224 (2020).
Byrne, P. et al. Critical shifts in trace, metal transport and remediation performance under future low river flows. Environ. Sci. Technol. 54, 15742–15750 (2020).
Eggleton, J. & Thomas, K. V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 30, 973–980 (2004).
Bushey, J. T., Driscoll, C. T., Mitchell, M. J., Selvendiran, P. & Montesdeoca, M. R. Mercury transport in response to storm events from a northern forest landscape. Hydrol. Process. 22, 4813–4826 (2008).
Forstner, U. Traceability of sediment analysis. TrAC Trend Anal. Chem. 23, 217–236 (2004).
Vilibic, I., Monserrat, S. & Rabinovich, A. B. Meteorological tsunamis on the US East Coast and in other regions of the World Ocean. Nat. Hazards 74, 1–9 (2014).
Trenberth, K. E., Fasullo, J. T. & Shepherd, T. G. Attribution of climate extreme events. Nat. Clim. Chang. 5, 725–730 (2015).
Winsemius, H. C. et al. Global drivers of future river flood risk. Nat. Clim. Chang. 6, 381–385 (2016).
Kirezci, E. et al. Projections of global-scale extreme sea levels and resulting episodic coastal flooding over the 21st Century. Sci Rep. https://doi.org/10.1038/s41598-020-67736-6 (2020).
Harvey, R. J., Chadwick, D. R., Sanchez-Rodriguez, A. R. & Jones, D. L. Agroecosystem resilience in response to extreme winter flooding. Agr. Ecosyst. Environ. 279, 1–13 (2019).
Barber, L. B. et al. Effects of an extreme flood on trace elements in river water-from urban stream to major river basin. Environ. Sci. Technol. 51, 10344–10356 (2017).
Izaditame, F., Siebecker, M. G. & Sparks, D. L. Sea-level-rise-induced flooding drives arsenic release from coastal sediments. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2021.127161 (2022).
Crawford, S. E. et al. Remobilization of pollutants during extreme flood events poses severe risks to human and environmental health. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2021.126691 (2022).
Kelly, T. J., Hamilton, E., Watts, M. J., Ponting, J. & Sizmur, T. The effect of flooding and drainage duration on the release of trace elements from floodplain soils. Environ. Toxicol. Chem. 39, 2124–2135 (2020).
Shahabi-Ghahfarokhi, S. et al. The response of metal mobilization and redistribution to reoxygenation in Baltic Sea anoxic sediments. Sci. Total Environ. 837, 155809 (2022).
Roussiez, V., Probst, A. & Probst, J. L. Significance of floods in metal dynamics and export in a small agricultural catchment. J. Hydrol. 499, 71–81 (2013).
Layglon, N. et al. Long-term monitoring emphasizes impacts of the dredging on dissolved Cu and Pb contamination along with ultraplankton distribution and structure in Toulon Bay (NW Mediterranean Sea, France). Mar. Pollut. Bull. https://doi.org/10.1016/j.marpolbul.2020.111196 (2020).
Martinez-Santos, M., Probst, A., Garcia-Garcia, J. & Ruiz-Romera, E. Influence of anthropogenic inputs and a high-magnitude flood event on metal contamination pattern in surface bottom sediments from the Deba River urban catchment. Sci. total Environ. 514, 10–25 (2015).
Jimenez Cisneros, B. E. et al. Climate Change 2014: Impacts,Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Field, C. B. et al.) 229–269 (Cambridge Univ. Press, Cambridge, 2014).
van Vliet, M. T. H. & Zwolsman, J. J. G. Impact of summer droughts on the water quality of the Meuse river. J. Hydrol. 353, 1–17 (2008).
Shirani, M., Afzali, K. N., Jahan, S., Strezov, V. & Soleimani-Sardo, M. Pollution and contamination assessment of heavy metals in the sediments of Jazmurian playa in southeast Iran. Sci Rep. https://doi.org/10.1038/s41598-020-61838-x (2020).
Bindoff, N. L. et al. Changing Ocean,Marine Ecosystems, andDependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (eds Pörtner, H. O. et al.) 477–587 (Intergovernmental Panel on Climate Change, 2019).
Robins, P. E. et al. Impact of climate change on UK estuaries: a review of past trends and potential projections. Estuar. Coast. Shelf Sci. 169, 119–135 (2016).
Pan, F. et al. Integrated effects of bioturbation, warming and sea-level rise on mobility of sulfide and metalloids in sediment porewater of mangrove wetlands. Water Res. https://doi.org/10.1016/j.watres.2023.119788 (2023).
Sundstrom, R., Astrom, M. & Osterholm, P. Comparison of the metal content in acid sulfate soil runoff and industrial effluents in Finland. Environ. Sci. Technol. 36, 4269–4272 (2002).
Wong, V. N. L. et al. Seawater causes rapid trace metal mobilisation in coastal lowland acid sulfate soils: Implications of sea level rise for water quality. Geoderma 160, 252–263 (2010).
Pouch, A. & Zaborska, A. Climate change influence on migration of contaminants in the Arctic marine environment. In Impact of Climate Changes on Marine Environments (eds Zielinski, T., Weslawski, M. & Kuliński, K.) 75–90 (Springer, Cham, 2015).
Charette, M. A. et al. The transpolar drift as a source of riverine and shelf‐derived trace elements to the central Arctic Ocean. J. Geophys. Res. Oceans https://doi.org/10.1029/2019JC015920 (2020).
Jensen, L. T. et al. Biogeochemical cycling of colloidal trace metals in the Arctic cryosphere. J. Geophys. Res. Oceans https://doi.org/10.1029/2021JC017394 (2021).
Bopp, L., Aumont, O., Cadule, P., Alvain, S. & Gehlen, M. Response of diatoms distribution to global warming and potential implications: a global model study. Geophys. Res. Lett. https://doi.org/10.1029/2005gl023653 (2005).
Evans, L. K. & Nishioka, J. Accumulation processes of trace metals into Arctic sea ice: distribution of Fe, Mn and Cd associated with ice structure. Mar. Chem. 209, 36–47 (2019).
Kim, I., Kim, G. & Choy, E. J. The significant inputs of trace elements and rare earth elements from melting glaciers in Antarctic coastal waters. Polar Res. 34, 24289–24289 (2015).
Frache, R. et al. Effects of ice melting on Cu, Cd and Pb profiles in Ross Sea waters (Antarctica). Int. J. Environ. Anal. Chem. 79, 301–313 (2001).
Carling, G. T., Rupper, S. B., Fernandez, D. P., Tingey, D. G. & Harrison, C. B. Effect of atmospheric deposition and weathering on trace element concentrations in glacial meltwater at Grand Teton National Park, Wyoming, USA. Arct. Antarct. Alp. Res. 49, 427–440 (2017).
Hopwood, M. J., Cantoni, C., Clarke, J. S., Cozzi, S. & Achterberg, E. P. The heterogeneous nature of Fe delivery from melting icebergs. Geochem. Perspect. Let. 3, 200–208 (2017).
Raiswell, R. Iceberg-hosted nanoparticulate Fe in the Southern Ocean: mineralogy, origin, dissolution kinetics and source of bioavailable Fe. Deep Sea Res. II 58, 1364–1375 (2011).
Bolt, C., Aguilar-Islas, A. & Rember, R. Particulate trace metals in Arctic snow, sea ice, and underlying surface waters during the 2015 US western Arctic GEOTRACES Cruise GN01. ACS Earth Space Chem. 4, 2444–2460 (2020).
Lannuzel, D., Bowie, A. R., van der Merwe, P. C., Townsend, A. T. & Schoemann, V. Distribution of dissolved and particulate metals in Antarctic sea ice. Mar. Chem. 124, 134–146 (2011).
Marsay, C. M. et al. Dissolved and particulate trace elements in late summer Arctic melt ponds. Mar. Chem. 204, 70–85 (2018).
Shadwick, E. H., Trull, T. W., Thomas, H. & Gibson, J. A. E. Vulnerability of polar oceans to anthropogenic acidification: comparison of Arctic and Antarctic seasonal cycles. Sci. Rep. 3, 2339–2339 (2013).
Gerringa, L. J. A. et al. Dissolved Cd, Co, Cu, Fe, Mn, Ni, and Zn in the Arctic Ocean. J. Geophys. Res. Oceans https://doi.org/10.1029/2021JC017323 (2021).
Arnone, V. et al. Natural copper-binding ligands in the Arctic Ocean. The influence of the Transpolar Drift (GEOTRACES GN04). Front. Mar. Sci. https://doi.org/10.3389/fmars.2023.1306278 (2023).
Hatje, V., Lamborg, C. H. & Boyle, E. A. Trace-metal contaminants: human footprint on the ocean. Elements 14, 403–408 (2018).
Nriagu, J. O. Global inventory of natural and anthropogenic emissions of trace-metals to the atmosphere. Nature 279, 409–411 (1979).
Lamborg, C. H. et al. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512, 65–68 (2014).
Lodeiro, P., Achterberg, E. P. & El-Shahawi, M. S. Detection of silver nanoparticles in seawater at ppb levels using UV visible spectrophotometry with long path cells. Talanta 164, 257–260 (2017).
Turner, D. R., Hassellov, I. M., Ytreberg, E. & Rutgersson, A. Shipping and the environment: smokestack emissions, scrubbers and unregulated oceanic consequences. Elementa Sci. Anthrop. https://doi.org/10.1525/elementa.167 (2017).
Andrade, R. L. B. et al. Chronology of anthropogenic impacts reconstructed from sediment records of trace metals and Pb isotopes in Todos os Santos Bay (NE Brazil). Mar. Pollut. Bull. 125, 459–471 (2017).
Caric, H., Cukrov, N. & Omanovic, D. Nautical Tourism in Marine Protected Areas (MPAs): evaluating an impact of copper emission from antifouling coating. Sustainability https://doi.org/10.3390/su132111897 (2021).
Dafforn, K. A., Lewis, J. A. & Johnston, E. L. Antifouling strategies: history and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 62, 453–465 (2011).
Fajkovic, H. et al. Correlation of metals and degraded marine (micro)plastic litter in geologically similar coastal areas with different anthropogenic characteristics. Mar. Pollut. Bull. https://doi.org/10.1016/j.marpolbul.2022.114041 (2022).
Munier, B. & Bendell, L. I. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE https://doi.org/10.1371/journal.pone.0191759 (2018).
Souza, L. A., Santos, A. C. S. S., Leao, J. M., Schaeppi, O. C. & Hatje, V. Occurrence and contents of trace metals and rare earth elements on plastic pellets. Mar. Pollut. Bull. https://doi.org/10.1016/j.marpolbul.2022.114261 (2022).
Holmes, L. A., Turner, A. & Thompson, R. C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 167, 25–32 (2014).
Svavarsson, J., Guls, H. D., Sham, R. C., Leung, K. M. Y. & Halldórsson, H. P. Pollutants from shipping - new environmental challenges in the subarctic and the Arctic Ocean. Mar. Pollut. Bull. 164, 112004 (2021).
Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 400, 212–226 (2008).
Macdonald, R. W., Harner, T. & Fyfe, J. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci. Total Environ. 342, 5–86 (2005).
Boyd, P. W. & Vivian, C. M. G. (eds) High level review of a wide range of proposed marine geoengineering techniques, Rep. Stud. GESAMP No. 98. 144 (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UN Environment/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, 2019).
Bach, L. T., Gill, S. J., Rickaby, R. E. M., Gore, S. & Renforth, P. CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and co-benefits for marine pelagic ecosystems. Front. Clim. https://doi.org/10.3389/fclim.2019.00007 (2019).
Flipkens, G., Blust, R. & Town, R. M. Deriving nickel (Ni(II)) and chromium (Cr(III)) based environmentally safe olivine guidelines for coastal enhanced silicate weathering. Environ. Sci. Technol. 55, 12362–12371 (2021).
Guo, J. Y. A., Strzepek, R., Willis, A., Ferderer, A. & Bach, L. T. Investigating the effect of nickel concentration on phytoplankton growth to assess potential side-effects of ocean alkalinity enhancement. Biogeosciences 19, 3683–3697 (2022).
Peng, L. Z. L., Stewart, M. G. & Melchers, R. E. Corrosion and capacity prediction of marine steel infrastructure under a changing environment. Struct. Infrastruct. Eng. 13, 988–1001 (2017).
Valdez, B. et al. Corrosion assessment of infrastructure assets in coastal seas. J. Mar. Eng. Technol. 15, 124–134 (2016).
Campbell, P. G. C. in Trace Metal Speciation and Bioavailability in Aquatic Systems (eds Tessier, A. & Turner, D. R.) 45–102 (John Wiley & Sons, 1995).
Abdou, M. et al. Estuarine dissolved speciation and partitioning of trace metals: a novel approach to study biogeochemical processes. Environ. Res. https://doi.org/10.1016/j.envres.2021.112596 (2022).
van den Berg, S. https://www.liverpool.ac.uk/~sn35/Documents/Speciation.xls (2014).
Lodeiro, P., Rey-Castro, C., David, C., Humphreys, M. P. & Gledhill, M. Proton binding characteristics of dissolved organic matter extracted from the North Atlantic. Environ. Sci. Technol. 57, 21136–21144 (2023).
Millero, F. J., Woosley, R., Ditrolio, B. & Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22, 72–85 (2009).
Louis, Y., Garnier, C., Lenoble, V., Omanović, D. & Mounier, S. & Pižeta, I. Characterisation and modelling of marine dissolved organic matter interactions with major and trace cations. Mar. Environ. Res. 67, 100–107 (2009).
Gledhill, M., Achterberg, E. P., Li, K. Q., Mohamed, K. N. & Rijkenberg, M. J. A. Influence of ocean acidification on the complexation of iron and copper by organic ligands in estuarine waters. Mar. Chem. 177, 421–433 (2015).
Schiedek, D., Sundelin, B., Readman, J. W. & Macdonald, R. W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 54, 1845–1856 (2007).
Ivanina, A. V. & Sokolova, I. M. Interactive effects of metal pollution and ocean acidification on physiology of marine organisms. Curr. Zool. 61, 653–668 (2015).
Cheloni, G. & Slaveykova, V. I. Combined effects of trace metals and light on photosynthetic microorganisms in aquatic environment. Environments https://doi.org/10.3390/environments5070081 (2018).
Epp, R. G., Erickson, D. J., Paul, N. D. & Sulzberger, B. Interactive effects of solar UV radiation and climate change on biogeochemical cycling. Photoch. Photobio. Sci. 6, 286–300 (2007).
Lalonde, J. D. et al. Photoinduced oxidation of Hg-0 (aq) in the waters from the St. Lawrence estuary. Environ. Sci. Technol. 38, 508–514 (2004).
Rodriguez-Romero, A., Ruiz-Gutierrez, G., Viguri, J. R. & Tovar-Sanchez, A. Sunscreens as a new source of metals and nutrients to coastal waters. Environ. Sci. Technol. 53, 10177–10187 (2019).
Roberts, D. A. et al. Ocean acidification increases the toxicity of contaminated sediments. Glob. Chang. Biol. 19, 340–351 (2013).
Sokolova, I. M. & Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim. Res. 37, 181–201 (2008).
Coelho, F. J. R. C. et al. Interactive effects of global climate change and pollution on marine microbes: the way ahead. Ecol. Evol. 3, 1808–1818 (2013).
Hansen, C. T. et al. Impact of high Fe-concentrations on microbial community structure and dissolved organics in hydrothermal plumes: an experimental study. Sci Rep. https://doi.org/10.1038/s41598-022-25320-0 (2022).
Lu, Y. et al. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ. Pollut. 239, 670–680 (2018).
Hoffmann, L. J., Breitbarth, E., Boyd, P. W. & Hunter, K. A. Influence of ocean warming and acidification on trace metal biogeochemistry. Mar. Ecol. Prog. Ser. 470, 191–205 (2012).
Jin, P. et al. The combined effects of ocean acidification and heavy metals on marine organisms: a meta-analysis. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.801889 (2021).
Rodríguez-Romero, A., Viguri, J. R. & Calosi, P. Acquiring an evolutionary perspective in marine ecotoxicology to tackle emerging concerns in a rapidly changing ocean. Sci. Total Environ. 764, 142816–142816 (2021).
McQuaid, J. B. et al. Carbonate-sensitive phytotransferrin controls high-affinity iron uptake in diatoms. Nature 555, 534 (2018). -+.
Gao, Y. F., Feng, J. F., Wang, C. C. & Zhu, L. Modeling interactions and toxicity of Cu-Zn mixtures to zebrafish larvae. Ecotoxicol. Environ. Saf. 138, 146–153 (2017).
Lacoue-Labarthe, T. et al. Temperature and pCO2 effect on the bioaccumulation of radionuclides and trace elements in the eggs of the common cuttlefish, Sepia officinalis. J. Exp. Mar. Biol. Ecol. 413, 45–49 (2012).
Pascal, P.-Y., Fleeger, J. W., Galvez, F. & Carman, K. R. The toxicological interaction between ocean acidity and metals in coastal meiobenthic copepods. Mar. Pollut. Bull. 60, 2201–2208 (2010).
Stockdale, A., Tipping, E., Lofts, S. & Mortimer, R. J. G. Effect of ocean acidification on organic and inorganic speciation of trace metals. Environ. Sci. Technol. 50, 1906–1913 (2016).
Bruland, K. W. Complexation of zinc by natural organic-ligands in the Central North Pacific. Limnol. Oceanogr. 34, 269–285 (1989).
Lorenzo, J. I., Beiras, R., Mubiana, V. K. & Blust, R. Copper uptake by Mytilus edulis in the presence of humic acids. Environ. Toxicol. Chem. 24, 973–980 (2005).
Semeniuk, D. M., Bundy, R. M., Payne, C. D., Barbeau, K. A. & Maldonado, M. T. Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar. Chem. 173, 222–233 (2015).
Lohan, M. C., Crawford, D. W., Purdie, D. A. & Statham, P. J. Iron and zinc enrichments in the northeastern subarctic Pacific: Ligand production and zinc availability in response to phytoplankton growth. Limnol. Oceanogr. 50, 1427–1437 (2005).
Xu, Y., Shi, D. L., Aristilde, L. & Morel, F. M. M. The effect of pH on the uptake of zinc and cadmium in marine phytoplankton: Possible role of weak complexes. Limnol. Oceanogr. 57, 293–304 (2012).
Kim, J. M., Baars, O. & Morel, F. M. M. The effect of acidification on the bioavailability and electrochemical lability of zinc in seawater. Philos. Trans. R. Soc. A 374, 20150296 (2016).
Wei, H., Xie, D. M., Wang, D. Z. & Wang, M. H. A meta-analysis reveals global change stressors potentially aggravate mercury toxicity in marine biota. Environ. Sci. Technol. 58, 219–230 (2023).
Cao, R. et al. Seawater acidification aggravated cadmium toxicity in the oyster Crassostrea gigas: metal bioaccumulation, subcellular distribution and multiple physiological responses. Sci. Total Environ. 642, 809–823 (2018).
Romero-Freire, A. et al. Trace metal accumulation in the commercial mussel M. galloprovincialis under future climate change scenarios. Mar. Chem. https://doi.org/10.1016/j.marchem.2020.103840 (2020).
Leal, P. P. et al. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Sci. Rep. https://doi.org/10.1038/s41598-018-32899-w (2018).
Figueiredo et al. A triple threat: ocean warming, acidification, and rare earth elements exposure triggers a superior antioxidant response and pigment production in the adaptable Ulva rigida. Environ. Adv. 8, 100235 (2022).
Nardi, A., Benedetti, M., d’Errico, G., Fattorini, D. & Regoli, F. Effects of ocean warming and acidification on accumulation and cellular responsiveness to cadmium in mussels Mytilus galloprovincialis: Importance of the seasonal status. Aquat. Toxicol. 204, 171–179 (2018).
Portner, H. O. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893 (2010).
Cabral, H., Fonseca, V., Sousa, T. & Leal, M. C. Synergistic effects of climate change and marine pollution: an overlooked interaction in coastal and estuarine areas. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph16152737 (2019).
Horstmann, C., Buttigieg, P. L., Simpson, P., Pearlman, J. & Waite, A. M. Perspectives on documenting methods to create ocean best practices. Front. Mar. Sci. https://doi.org/10.3389/fmars.2020.556234 (2021).
Pearlman, J. et al. Evolving and Sustaining Ocean Best Practices to Enable Interoperability in the UN Decade of Ocean Science for Sustainable Development. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.619685 (2021).
Pearlman, J. et al. Evolving and sustaining ocean best practices and standards for the next decade. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00277 (2019).
German, C. R. et al. Hydrothermal impacts on trace element and isotope ocean biogeochemistry. Philos. Trans. R. Soc. A https://doi.org/10.1098/rsta.2016.0035 (2016).
Li, C. M. et al. Heavy metal pollution in coastal wetlands: a systematic review of studies globally over the past three decades. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2021.127312 (2022).
Cobelo-Garcia, A. et al. COST action TD1407: network on technology-critical elements (NOTICE)-from environmental processes to human health threats. Environ. Sci. Pollut. Res. 22, 15188–15194 (2015).
Kennish, M. J. Drivers of change in estuarine and coastal marine environments: an overview. Open J. Ecol. 11, 224–239 (2021).
Grand, M. M. et al. Developing autonomous observing systems for micronutrient trace metals. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00035 (2019).
Tercier-Waeber, M. L. et al. Advanced multichannel submersible probe for autonomous high-resolution in situ monitoring of the cycling of the potentially bioavailable fraction of a range of trace metals. Chemosphere https://doi.org/10.1016/j.chemosphere.2021.131014 (2021).
Penezic, A. et al. Spatial variability of arsenic speciation in the Gironde Estuary: emphasis on dynamic (potentially bioavailable) inorganic arsenite and arsenate fractions. Mar. Chem. https://doi.org/10.1016/j.marchem.2020.103804 (2020).
Tercier-Waeber, M. L. et al. In situ voltammetric sensor of potentially bioavailable inorganic mercury in marine aquatic systems based on gel-integrated nanostructured gold-based microelectrode arrays. ACS Sens. 6, 925–937 (2021).
Umbria-Salinas, K., Valero, A., Martins, S. E. & Wallner-Kersanach, M. Copper ecological risk assessment using DGT technique and PNEC: a case study in the Brazilian coast. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2020.123918 (2021).
Gonzalez, J. L. et al. An international intercomparison exercise on passive samplers (DGT) for monitoring metals in marine waters under a regulatory context. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2022.157499 (2022).
Amouroux, I. et al. A new approach to using Diffusive Gradient in Thin-films (DGT) labile concentration for water framework directive chemical status assessment: adaptation of environmental quality standard to DGT for cadmium, nickel and lead. Environ. Sci. Eur. https://doi.org/10.1186/s12302-023-00733-4 (2023).
MarChemSpec (Marine Chemical Speciation Model) v. 1.01 (Zenodos, 2023).
Millero, F. J., Feistel, R., Wright, D. G. & McDougall, T. J. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. I 55, 50–72 (2008).
Desboeufs, K. V., Sofikitis, A., Losno, R., Colin, J. L. & Ausset, P. Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter. Chemosphere 58, 195–203 (2005).
Tovar-Sanchez, A. et al. Characterizing the surface microlayer in the Mediterranean Sea: trace metal concentrations and microbial plankton abundance. Biogeosciences 17, 2349–2364 (2020).
Eiriksdottir, E. S., Gislason, S. R. & Oelkers, E. H. Direct evidence of the feedback between climate and nutrient, major, and trace element transport to the oceans. Geochim. Cosmochim. Acta 166, 249–266 (2015).
Visser, A. et al. Climate change impacts on the leaching of a heavy metal contamination in a small lowland catchment. J. Contam. Hydrol. 127, 47–64 (2012).
Knee, K. L. & Paytan, A. in Treatise on Estuarine and Coastal Science (eds Wolanski, E. & McLusky, D.) 205–233 (Academic Press, 2011).
Augustsson, A., Bergbäck, B. & Åström, M. Trace metals in recharge and discharge ground waters at two sites at the Baltic coast of Sweden. Appl. Geochem. 24, 1640–1652 (2009).
Malakar, A. et al. Occurrence of arsenite in surface and groundwater associated with a perennial stream located in Western Nebraska, USA. J. Hazard. Mater. 416, 126170–126170 (2021).
Cotovicz, L. C., Marins, R. V. & da Silva, A. R. F. Eutrophication amplifies the diel variability of carbonate chemistry in an equatorial, semi-arid, and negative estuary. Front. Mar. Sci. https://doi.org/10.3389/fmars.2022.767632 (2022).
Ponting, J., Kelly, T. J., Verhoef, A., Watts, M. J. & Sizmur, T. The impact of increased flooding occurrence on the mobility of potentially toxic elements in floodplain soil - a review. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.142040 (2021).
Simpson, S. L., Apte, S. C. & Batley, G. E. Effect of short-term resuspension events on the oxidation of cadmium, lead, and zinc sulfide phases in anoxic estuarine sediments. Environ. Sci. Technol. 34, 4533–4537 (2000).
de Lacerda, L. D., Marins, R. V. & Dias, F. J. D. An Arctic Paradox: response of fluvial hg inputs and bioavailability to global climate change in an extreme coastal environment. Front. Earth Sci. https://doi.org/10.3389/feart.2020.00093 (2020).
Cobb, G. P. et al. Metal distributions in New Orleans following Hurricanes Katrina and Rita: a continuation study. Environ. Sci. Technol. 40, 4571–4577 (2006).
Presley, S. M. et al. Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environ. Sci. Technol. 40, 468–474 (2006).
Horowitz, A. J., Elrick, K. A., Smith, J. J. & Stephens, V. C. The effects of Hurricane Irene and Tropical Storm Lee on the bed sediment geochemistry of US Atlantic coastal rivers. Hydrol. Process. 28, 1250–1259 (2014).
Whelan, T., Van Tussenbroek, B. I. & Santos, M. G. B. Changes in trace metals in Thalassia testudinum after hurricane impacts. Mar. Pollut. Bull. 62, 2797–2802 (2011).
Mashio, A. S., Obata, H., Shimazaki, T., Fukuda, H. & Ogawa, H. Spatiotemporal variations of platinum in seawater in Otsuchi Bay, Japan after the 2011 tsunami. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134659 (2020).
Monserrat, S., Vilibic, I. & Rabinovich, A. B. Meteotsunamis: atmospherically induced destructive ocean waves in the tsunami frequency band. Nat. Hazard. Earth Syst. 6, 1035–1051 (2006).
Vilibic, I., Rabinovich, A. B. & Anderson, E. J. Special issue on the global perspective on meteotsunami science: editorial. Nat. Hazards 106, 1087–1104 (2021).
Denamiel, C., Belusic, D., Zemunik, P. & Vilibic, I. Climate projections of meteotsunami hazards. Front. Mar. Sci. https://doi.org/10.3389/fmars.2023.1167863 (2023).
Leblanc, M., Tweed, S., Van Dijk, A. & Timbal, B. A review of historic and future hydrological changes in the Murray-Darling Basin. Glob. Planet. Chang. 80-81, 226–246 (2012).
Job, T., Penny, D. & Morgan, B. Geochemical signatures of acidic drainage recorded in estuarine sediments after an extreme drought. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.141435 (2020).
LeMonte, J. J. et al. Sea level rise induced arsenic release from historically contaminated coastal soils. Environ. Sci. Technol. 51, 5913–5922 (2017).
Miner, K. R. et al. Emergent biogeochemical risks from Arctic permafrost degradation. Nat. Clim. Chang. 11, 809–819 (2021).
Lim, A. G. et al. Dispersed ground ice of permafrost peatlands: Potential unaccounted carbon, nutrient and metal sources. Chemosphere 266, 128953–128953 (2021).
Langer, M. et al. Thawing permafrost poses environmental threat to thousands of sites with legacy industrial contamination. Nat. Commun. 14, 1721 (2023).
Gwynn, J. P., Hatje, V., Casacuberta, N., Sarin, M. & Osvath, I. The effect of climate change on sources of radionuclides to the marine environment. Commun. Earth Environ. https://doi.org/10.1038/s43247-024-01241-w (2024).
Loiko, S. V. et al. Abrupt permafrost collapse enhances organic carbon, CO2, nutrient and metal release into surface waters. Chem. Geol. 471, 153–165 (2017).
Payandi-Rolland, D., Shirokova, L. S., Labonne, F., Bénézeth, P. & Pokrovsky, O. S. Impact of freeze-thaw cycles on organic carbon and metals in waters of permafrost peatlands. Chemosphere 279, 130510–130510 (2021).
Raudina, T. V. et al. Permafrost thaw and climate warming may decrease the CO2, carbon, and metal concentration in peat soil waters of the Western Siberia Lowland. Sci. Total Environ. 634, 1004–1023 (2018).
Krickov, I. V. et al. Colloidal transport of carbon and metals by western Siberian rivers during different seasons across a permafrost gradient. Geochim. Cosmochim. Acta 265, 221–241 (2019).
Pokrovsky, O. S. et al. Impact of permafrost thaw and climate warming on riverine export fluxes of carbon, nutrients and metals in Western Siberia. Water 12, 1817–1817 (2020).
Chételat, J. et al. Climate change and mercury in the Arctic: abiotic interactions. Sci. Total Environ. 824, 153715–153715 (2022).
Stern, G. A. et al. How does climate change influence Arctic mercury? Sci. Total Environ. 414, 22–42 (2012).
Loseto, L. L., Lean, D. R. S. & Siciliano, S. D. Snowmelt sources of methylmercury to high arctic ecosystems. Environ. Sci. Technol. 38, 3004–3010 (2004).
Søndergaard, J. et al. Mercury exports from a High-Arctic river basin in Northeast Greenland (74°N) largely controlled by glacial lake outburst floods. Sci. Total Environ. 514, 83–91 (2015).
Lim, A. G. et al. A revised pan-Arctic permafrost soil Hg pool based on Western Siberian peat Hg and carbon observations. Biogeosciences 17, 3083–3097 (2020).
Schuster, P. F. et al. Permafrost stores a globally significant amount of mercury. Geophys. Res. Lett. 45, 1463–1471 (2018).
Dietz, R. et al. A risk assessment review of mercury exposure in Arctic marine and terrestrial mammals. Sci. Total Environ. 829, 154445–154445 (2022).
Booth, S. & Zeller, D. Mercury, food webs, and marine mammals: Implications of diet and climate change for human health. Environ. Health Perspect. 113, 521–526 (2005).
Fisher, J. A. et al. A synthesis of mercury research in the Southern Hemisphere, part 2: anthropogenic perturbations. Ambio https://doi.org/10.1007/s13280-023-01840-5 (2023).
Wang, F. Y. et al. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? - Implications for evaluating the effectiveness of the Minamata Convention. Sci. Total Environ. 674, 58–70 (2019).
Schartup, A. T. et al. Climate change and overfishing increase neurotoxicant in marine predators. Nature 572, 648 (2019).
Campbell, A. L., Mangan, S., Ellis, R. P. & Lewis, C. Ocean acidification increases copper toxicity to the early life history stages of the polychaete Arenicola marina in artificial seawater. Environ. Sci. Technol. 48, 9745–9753 (2014).
Yu, X., Liu, J., Qiu, T., Cao, L. & Dou, S. Ocean acidification induces tissue-specific interactions with copper toxicity on antioxidant defences in viscera and gills of Asiatic hard clam Meretrix petechialis (Lamarck, 1818). Sci. Total Environ. 875, 162634 (2023).
Dong, F. et al. Mitigation effects of CO2-driven ocean acidification on Cd toxicity to the marine diatom Skeletonema costatum. Environ. Pollut. 259, 113850–113850 (2020).
Belivermis, M. et al. Influence of pH on Pb accumulation in the blue mussel, Mytilus edulis. Mar. Pollut. Bull. https://doi.org/10.1016/j.marpolbul.2020.111203 (2020).
López, I. R., Kalman, J., Vale, C. & Blasco, J. Influence of sediment acidification on the bioaccumulation of metals in Ruditapes philippinarum. Environ. Sci. Pollut. Res. 17, 1519–1528 (2010).
Rodríguez-Romero, A. et al. Predicting the impacts of CO2 leakage from subseabed storage: effects of metal accumulation and toxicity on the model benthic organism Ruditapes philippinarum. Environ. Sci. Technol. 48, 12292–12301 (2014).
Gentès, S. et al. In vivo mercury (de)methylation metabolism in cephalopods under different pCO2 scenarios. Environ. Sci. Technol. 57, 5761–5770 (2023).
Freitas, R. et al. Does pre-exposure to warming conditions increase Mytilus galloprovincialis tolerance to Hg contamination? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 203, 1–11 (2017).
Bai, Z. A. & Wang, M. H. Warmer temperature increases mercury toxicity in a marine copepod. Ecotoxicol. Environ. Saf. https://doi.org/10.1016/j.ecoenv.2020.110861 (2020).
Gomiero, A. & Viarengo, A. Effects of elevated temperature on the toxicity of copper and oxytetracycline in the marine model, Euplotes crassus: a climate change perspective. Environ. Pollut. 194, 262–271 (2014).
Lannig, G., Cherkasov, A. S. & Sokolova, I. M. Temperature-dependent effects of cadmium on mitochondrial and whole-organism bioenergetics of oysters (Crassostrea virginica). Mar. Environ. Res. 62, S79–S82 (2006).
Tsui, M. T. K. & Wang, W. X. Acute toxicity of mercury to Daphnia magna under different conditions. Environ. Sci. Technol. 40, 4025–4030 (2006).
Figueiredo, C. et al. Warming enhances lanthanum accumulation and toxicity promoting cellular damage in glass eels (Anguilla anguilla). Environ. Res. https://doi.org/10.1016/j.envres.2020.110051 (2020).
Blanckaert, A. C. A., Omanovic, D., Fine, M., Grover, R. & Ferrier-Pages, C. Desert dust deposition supplies essential bioelements to Red Sea corals. Glob. Chang. Biol. 28, 2341–2359 (2022).
Acknowledgements
Authors are part of the Working Group 45 on Climate Change and Greenhouse Gas Related Impacts on Contaminants in the Ocean (WG45) of the Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP), supported by the International Atomic Energy Agency (IAEA), and co-sponsored by the United Nations Environmental Program (UNEP), International Oceanographic Commission (IOC-UNESCO), the World Meteorological Organization (WMO), and the International Maritime Organization (IMO). We thank Marcelo Ketzer (Linnaeus University, Sweden) and Chris Vivian (Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP)) for their valuable comments on the draft of this manuscript, and David Turner (University of Gothenburg) for his help with the MarChemSpec calculations.
Author information
Authors and Affiliations
Contributions
All authors (R.Z., S.M., V.H., S.G.S., C.V., M.S., and D.O.) contributed to the systematic review, engaged in the preliminary discussion that led to this work and participated in the drafting and editing of the manuscript. All authors thoroughly discussed all topics covered in this review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Clifton Buck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis and Alice Drinkwater. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zitoun, R., Marcinek, S., Hatje, V. et al. Climate change driven effects on transport, fate and biogeochemistry of trace element contaminants in coastal marine ecosystems. Commun Earth Environ 5, 560 (2024). https://doi.org/10.1038/s43247-024-01679-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-024-01679-y
This article is cited by
-
Seabed Microtopography Modulates Vertical Turbulent Transport and Porewater Exchange Processes Under Wave Forcing
Estuaries and Coasts (2026)
-
Welcome to Clean Oceans: a platform for solutions to ocean pollution and sustainability challenges
Clean Oceans (2025)
-
Impact of land-use conversion from a long-term paddy to abandoned field on the distribution of C, N, and trace elements: a study in central Viet Nam
Environmental Geochemistry and Health (2025)
-
Hydrogeochemical sources and enrichment mechanisms of trace elements in river water of a typical endorheic headwater region on Tibetan Plateau
Applied Water Science (2025)
-
Effects of Agricultural Drainage on Plant Community in Bolboschoenus planiculmis Wetlands in Songnen Plain, China
Chinese Geographical Science (2025)