Abstract
Northern forests (forests north of 30°N) are major terrestrial carbon dioxide (CO2) sinks, while rapid warming can disturb their CO2 sink function. Here we use multi-year net CO2 exchange observations from 65 northern forest sites to show that the increased net CO2 uptake during warmer springs was more pronounced in old forests (>90 years old) compared to young (<40 years old) and mid-aged (40–90 years old) forests. In addition, the decreased net CO2 uptake during warmer summers and autumns was more pronounced in young forests compared to mid- and old-aged forests. Annually, this resulted in an increase in net CO2 uptake due to seasonal warming for old forests (4.8 g C m−2 yr-1) and a decrease in young- and mid-aged forests (3.2 and 0.8 g C m−2 yr-1, respectively). In future projections, increasingly uneven seasonal warming may amplify the impacts of stand age on CO2 sinks of northern forests.
Similar content being viewed by others
Introduction
At northern mid- and high latitudes (>30°N), air temperatures are increasing rapidly, but are not spatially and seasonally uniform1. Forests in these regions (i.e., northern forests) sequester large amounts of carbon (C) and tend to be long-term carbon dioxide (CO2) sinks2,3. Previous studies have found that warming-induced northern temperate and boreal tree growth has largely increased the northern CO2 sinks2,4,5 and dominated the global increase in biomass C stock over the past decade6,7. The observed “northern greening” over the past decades is viewed as a sign that northern forests will continue to grow more and thus accumulate more C in a warmer world5,7,8. However, spatially non-uniform warming, with temperature rising faster in some areas, may also cause vegetation browning in some northern forests due to warming induced increasing evaporative demand and water deficit9,10. A comprehensive assessment of impacts of non-uniform warming on CO2 sinks in northern forests is essential.
Seasonally non-uniform warming may influence ecosystem photosynthesis and respiration differently. Generally, increasing air temperature in spring triggers an earlier start of the growing season and increases photosynthesis more than respiration early in the growing season5,7,8,11. However, some studies have also identified instances of high respiration during warm springs, when limited carbon uptake occurs due to phenological limitations12. In the summer, although increasing temperature has positive effects on both photosynthesis and respiration, the net effect on the CO2 sink depends on the co-varying negative effect of water limitation at higher temperatures10,13,14. Some studies have found that increasing summer temperature has inhibited tree growth and decreased forest CO2 sink strength15,16,17. Autumn warming has been shown to prolong photosynthetic period by delaying the decline in photosynthetic capacity18. However, this increase in carbon uptake is offset by a concurrent rise in soil respiration, potentially leading to a net carbon loss18,19,20,21. Thus, the overall seasonal warming impacts on photosynthesis and respiration, and the resultant trajectories in CO2 sink strength of northern forests remain controversial.
The CO2 sink strength of forests is also related to stand age, and its dynamic pattern for northern forests has been well established over the past decade22,23,24. Based on tree-ring observations and manipulative experiments, substantial progress has been made in understanding the warming response of boreal tree growth and photosynthesis25,26,27,28,29,30,31,32, but there is no consensus regarding whether older forests or younger forests were more resilient to the non-uniform seasonal warming. Currently, our mechanistic understanding of warming induced responses of net CO2 uptake is mostly derived from short- and long-term uniform warming experiments conducted under laboratory conditions using seedlings or field experiments in which individual trees (or plant tissues) are warmed (but see the Spruce and Peatland Responses under Changing Environments (SPRUCE), a whole-ecosystem warming experiment in northern Minnesota as well as Flakaliden experiments which advanced how whole tree responded to warming)20,27,31,33,34,35. These experimental studies demonstrate that photosynthetic CO2 uptake by tree seedlings is influenced by how warming alleviates low temperature limitations versus limitations due to decreased water availability. While much of the existing literature has focused on young trees’ thermal responses, there has been limited information on mature forests (except for recent work which examines mature trees27). Old trees are reported to be less sensitive to drought than young trees36, but old trees seem to be thermally acclimate photosynthesis to maintain carbon uptake under warming26,27,33. It is unclear whether these thermal responses of young trees accurately represent mature trees growing in natural conditions in the field27,37. Furthermore, historical air-temperature records reveal seasonal and spatial differences in warming trends (Supplementary Fig. 1), which are challenging to replicate in experimental warming studies. Limited evidence from disturbance chronosequence studies hints that the response of northern forests to climate variations is age-dependent38. Therefore, it is crucial to consider the combined effects of non-uniform seasonal warming and forest age when assessing the thermal responses of forests.
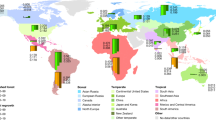
This study aims to better understand how non-uniform seasonal warming and stand age affect the annual net ecosystem CO2 exchange (NEE) of northern forests. To quantify the effect of warmer air temperatures on the interannual variability of NEE, we analyzed in situ, multi-year (≥5 years), ecosystem-scale NEE observations for 65 northern forest sites obtained from FLUXNET, AmeriFlux, Chinaflux and ICOS (Integrated Carbon Observation System) (831 site-years; Fig. 1 and Supplementary Table 1) networks. First, monthly temperature sensitivities of NEE anomalies (difference from mean monthly NEE during observation period) of northern forests were the estimated fixed effect slopes for seasonal relationships between NEE and air temperature in linear mixed-effects regression models with sites as the random effect. The related sensitivities of NEE to underlying drivers, including the Enhanced Vegetation Index (EVI) as a proxy for vegetation productivity, the Priestley-Taylor coefficient (α) as a proxy for water availability, and incoming shortwave radiation as a proxy for light availability, were derived in the similar way. Second, we compared the sensitivity of seasonal NEE anomalies with air-temperature anomalies derived from different stand age groups (stand age <40 years, stand age between 40 and 90 years, and stand age >90 years). Since the sensitivities of northern forests to warming will depend on vegetation type39,40, we also compared the sensitivity of seasonal NEE anomalies with air-temperature anomalies separately for evergreen-dominated forests (EF) and deciduous broadleaved and mixed forests (DBMF). Third, we combined empirical monthly temperature sensitivities of NEE with monthly resolved observation-based air temperature change between 1981–2020 and 1951–1980 across northern forest areas (>30oN) to quantify the effect of seasonal warming on decadal changes in the CO2 sink strength of northern forests.
Results
Climate sensitivities of NEE across northern forests sites
Correlations between anomalies in monthly NEE of northern forests and air temperature varied seasonally. Specifically, temperature anomalies during warm springs were associated with an increase in net CO2 uptake, while warm temperature anomalies during summer and autumn months were associated with a decrease in net CO2 uptake (Fig. 2a). During winter, the NEE response to warming was insignificant (p > 0.1), positive (decreasing net CO2 uptake with warming) and small (<0.3 g C m−2 month−1 °C−1). The largest decrease in net CO2 uptake (i.e., increase in NEE) with positive air-temperature anomalies was observed for the summer months, with values of 4 g C m−2 month−1 °C−1.
a–c, Slopes of ΔNEE versus air-temperature anomalies (ΔTa) a, ΔNEE versus Enhanced Vegetation Index (EVI) anomalies (ΔEVI) b, and ΔNEE versus α anomalies (Δα) c as derived from a linear mixed-effects regression model for seasonal periods. Error bars show 95% CIs of estimated slope parameters, all statistical analysis were significant at 0.05 except for winter seasons (p > 0.1). Mean NEE values are represented by the color scale. The size of data points corresponds to the sensitivity values.
During spring months, positive EVI and α anomalies were correlated with increased net CO2 uptake (Fig. 2b, c). But in contrast to positive temperature anomalies, positive EVI anomalies (i.e., enhanced vegetation productivity) and positive α anomalies (i.e., favorable water conditions) during summer months were correlated with increased net CO2 uptake (Fig. 2b, c). Monthly α was negatively correlated with air temperature for the summer months, indicating drier conditions in warmer years in the summer (Supplementary Fig. 2). When using GPP as a proxy for vegetation productivity or SPEI as an indicator of water availability, the results were consistent with those obtained using EVI and α (Supplementary Fig. 3). Furthermore, the results obtained from the multiple linear regression model were comparable to those from the linear mixed-effects models (Supplementary Figs. 4, 5). Net CO2 uptake increased with positive incoming shortwave radiation anomalies in autumn (Supplementary Fig. 3), indicating an enhanced net CO2 sink during warm and cloudless autumn periods. Similarly, reduced terrestrial CO2 uptake during periods with reduced light availability in the late growing season has also been found for other northern ecosystems41.
Comparison of climate sensitivities of NEE among age groups and forest types
NEE response to warming differed significantly among young forests, mid-aged forests and old forests (p < 0.05, Fig. 3a). Net CO2 uptake in old forests increased more with warmer springs compared to that of both young forests and mid-aged forests. Net CO2 uptake decreased more during warmer summers and autumns in young forests compared to mid-aged and old forests. During spring, the sensitivity of net CO2 uptake to enhanced vegetation productivity was similar among age groups (Fig. 3b), while net CO2 uptake in mid-aged forests was more sensitive to favorable water conditions (i.e., positive α anomalies) than that of young- and old forest (Fig. 3c). In summer and autumn, net CO2 uptake in young forests was more sensitive to enhanced vegetation productivity and favorable water conditions compared to mid-aged and old forests. During winter months, net CO2 uptake response to EVI and α was insignificant, negative and small (p > 0.1).
a–c, Slopes of ΔNEE versus air-temperature anomalies (ΔTa) a, ΔNEE versus EVI anomalies (ΔEVI) b, and ΔNEE versus α anomalies (Δα) c as derived from a linear mixed-effects regression model for seasonal periods and stand age groups. Error bars show 95% CIs of estimated slope parameters, all statistical analysis were significant at 0.05 except for winter seasons (p > 0.1). The red star inside circles indicate statistical significance at 0.05. Mean NEE values are represented by the color scale.
Monthly net CO2 uptake of northern temperate and boreal forests showed very similar responses to air-temperature anomalies across seasons with the exception of summer months (Supplementary Fig. 6a). Net CO2 uptake in northern temperate forests was more sensitive to warmer summers compared to boreal forests. Although enhanced vegetation productivity and favorable water conditions were associated with increased net CO2 uptake in both northern temperate and boreal forests, the increase was much less for boreal forests than temperate forests (Supplementary Fig. 6b, c).
When considering the forest types (i.e., evergreen forests, EF and deciduous broadleaved and mixed forests, DBMF), the temperature responses of net CO2 uptake for both types showed very similar seasonal patterns: they were negative in response to spring warming (i.e., increased uptake with increased temperature) and positive in response to summer and autumn warming (Supplementary Fig. 7a). The net CO2 uptake of DBMF showed much stronger responses to enhanced vegetation productivity and favorable water conditions across seasons (Supplementary Fig. 7b, c).
NEE responses to seasonally uniform and variable warming
Seasonal warming trends and stand age have substantial impacts on estimated NEE changes across northern forests (Fig. 4). We linked the monthly resolved temperature sensitivity of NEE (Supplementary Fig. 8) to monthly resolved observed warming between the periods 2001–2020 and 1951–1970 (Supplementary Fig. 1) across northern forest areas. When assuming seasonally uniform warming (same warming rate across all seasons), estimated changes in NEE scale linearly with warming rates, with a reduction in net CO2 uptake of northern forests of about 12 g C m−2 yr−1 across the northern forest area (Fig. 4a). The net CO2 uptake on average decreased by about 5 g C m−2 yr−1 for seasonal warming effects without considering stand age impacts (Fig. 4b). The decrease in net CO2 uptake (i.e., increase in NEE) for variable seasonal warming using young forests temperature sensitivities is ~60% larger (with 8–30 g C m−2 yr−1) than that of uniform warming scenario (Fig. 4c). We observed an increase in net CO2 uptake for variable seasonal warming considering stand age impacts, which is on average 1 g C m−2 yr−1 (Fig. 4d). Our simulations show that the largest NEE differences between uniform warming and variable seasonal warming when considering stand age impacts occur in regions, such as central Siberia, where the largest spring and least summer warming is observed (decline in NEE, i.e., ΔNEE = -10 g C m−2 y−1, Figs. 4 and S1). About 80% of the increased net CO2 uptake during warmer springs is compensated by decreased net CO2 uptake during warmer summers in the seasonal warming scenario simulations considering stand age impacts (Warming3), compared to 95% for simulations that do not consider stand age impacts (Warming1) (Fig. 5). Under non-uniform seasonal warming scenario, the carbon sink of northern forests would be underestimated by approximately 8% (ΔNEE over mean NEE) without considering the stand age impacts.
Maps of ΔNEE (between the periods 1951–1970 and 2001–2020) for seasonally uniform warming (same warming rate across all seasons) (a), for seasonally varying warming (b), for seasonally varying warming using young forests temperature sensitivities (c), and for seasonally varying warming considering stand age impacts on NEE temperature (d).
Box plot of ΔNEE (between the periods 1951–1970 and 2001–2020) during different seasons for seasonally uniform warming (Annual warming), for seasonally varying warming (Seasonal warming1), for seasonally varying warming using young forests temperature sensitivities (Seasonal warming2), and for seasonally varying warming considering stand age (Seasonal warming3) (a); Stacked plot of ΔNEE during different seasons for seasonally varying warming considering stand age (b).
Discussion
Our results show that the response of net CO2 uptake to warming in northern forests was sensitive to seasonality in warming trends and stand age. Warmer springs were correlated with enhanced net CO2 uptake by northern forests. This positive effect was consistent across various biomes or vegetation types. However, significant differences among age groups were observed, as indicated by the lack of overlap in their confidence intervals; the impact was more pronounced in old forests compared to younger ones. A previous study revealed that warmer springs stimulate tree growth in cold wet regions by mitigating thermal limitations but not in dry regions42. Our results show that photosynthesis increased more than respiration in warmer springs, a phenomenon that is more pronounced in cold wet regions than cold dry regions (Supplementary Fig. 9c, d). Nonetheless, the impact of spring warming on net CO2 uptake did not show significant differences between wet and dry areas within the boreal forest biome across seasons (Supplementary Fig. 9a). The more pronounced impacts of spring warming on net CO2 uptake in old forests compared to the younger ones was due to greater enhancement of photosynthesis than respiration. Stand age can influence the response of photosynthesis to spring warming by enhancing soil conditions over time in different ways (for example, a thicker humus layer with favorable microbial communities, increased water storage capacity and enhanced site fertility following the nutrient losses occurring during major disturbances).
Warmer summers correlated with decreased net CO2 uptake by northern forests regardless of biome type, vegetation type or stand age (Figs. 2, 3, S4, S5). Warmer summers have been reported to have negative impacts on summer net CO2 uptake in the past two decades in ecosystems north of 50 oN16. The emerging negative impacts of warmer summers has been attributed to the diminished positive effect of warming on photosynthesis37. In the current study, water availability is found to have a relatively limited impact on summer net CO2 uptake of boreal forests (Supplementary Fig. 6c). Photosynthesis showed a larger decrease than respiration in warm summers in boreal forests (Supplementary Fig. 11), which is likely due to the higher optimum temperature for respiration compared to photosynthesis43,44. When considering stand age, decreased net CO2 uptake due to summer warming was less pronounced in old and mid-age forests compared to young ones (Fig. 3). This can be attributed to a more prononnced decrease in photosynthesis and a greater increase in respiration in young forests compared to old forests (Supplementary Fig. 10). Compared with old forests, young forests established after a stand-replacing disturbance may experience greater competition for space and nutrients among young trees that tend to occupy the same ecological niche, resulting in them being more vulnerable to external stressors such as climate change-associated drought. While structurally complex, forests (usually old forests) suffer less from water limitation45,46.
Autumn warming correlated with decreased net CO2 uptake in northern forests. Generally, autumn warming is more important for enhancing litter decomposition and has no clear influence on photosynthesis, probably due to the photoperiod limitation during this season29,46,47. But this may not be the case for old forests. Our results showed the increases in photosynthesis and respiration were similar in old forests during warmer autumns (Supplementary Fig. 10). Old forests seem to be more efficient than younger ones in using light48. Anthropogenic disturbances combined with climate-induced threats, have led to the decline of old forests. Projected autumn warming, together with changes in forest structure, would result in greater CO2 loss under future temperature scenarios. Leaving forests intact and protecting old forests is a potential strategy for maintaining forest carbon sinks.
Furthermore, the net CO2 uptake of northern forests from seasonal warming trends is compensated by opposite effects in different seasons (Fig. 5). Similar compensatory effects on net CO2 uptake have been found in other boreal and temperate ecosystems experiencing increased net CO2 uptake in response to warmer springs, and decreased uptake with warming in the late growing season and the related reductions in soil water availability and enhanced water stress49,50,51,52. Northern forests will accumulate more carbon only if their CO2 sink capability can be maintained under the pressure of climate change. On decadal timescales, northern forests that experience pronounced spring warming (for example, Central Siberia) appear to be more responsive to seasonal warming regarding their CO2 sink capability (Fig. 4a, b). Forests in regions that are susceptible to increasing aridity during the summer months (for example, western Europe) may experience decreasing net CO2 uptake or even net CO2 loss. However, on longer timescales from decades to centuries, when temperatures are expected to exceed the current historical records, changes in the CO2 sink strength may be nonlinear due to slower changes in ecosystem processes and structure such as plant and microbial species and trait composition adjusting to new climate conditions. Contemporary NEE observations or short-term manipulation experiments (<10 years) probably cannot capture these slow changes.
Much of the existing literature has focused on young trees’ thermal responses, with limited attention to mature forests (except for recent works which examines mature trees27), our study contributes by providing a comparative analysis. We found that considering net CO2 uptake from seasonal warming, old forests were more resilient. In contrast, regions where young forests are dominant may experience decreasing net CO2 uptake with warming (Fig. 4 and S10). Previous studies25,27,53 have shown shifts in thermal optimum of photosynthesis (Topt) of old trees in response to warming is relatively limited compared to young trees, which does not fully match increase in air temperature. But shifts in Topt does not necessarily involves the increase in net CO2 uptake. Because the autotrophic respiration usually acclimates to warming such that the temperature sensitivity is reduced in warming plants, decreasing the carbon losses54,55. Therefore, the net CO2 uptake of old trees, which was generally larger than that of young trees56,57, is maintained at the prevailing temperature through a combination of thermal acclimation and changes in instantaneous temperature responses of photosynthetic and respiratory processes25,27. Furthermore, as trees grow older the development of root systems became more extensive, allowing better access to subsurface water and thus being less suppressed by drought than younger trees36,37. This may be the reason for less depressed net CO2 uptake of older forests during warm summers and autumns than younger forests. In addition, results of a uniform warming scenario as opposed to a variable seasonal warming scenario showed that the former biases the annual net CO2 uptake toward a greater decrease (Fig. 4). Results from current warming experiments without considering the seasonality of warming trends or the impacts of stand age on net CO2 uptake may underestimate the potential CO2 sinks of northern forests in a warmer future.
The temperature response in current Terrestrial Biosphere Models has been largely developed using data from young trees grown in greenhouse warming experiments27,30. It remains unclear how well these temperature responses represent old forests growing under natural conditions in the field. Our results reveal how stand age interacts with non-uniform seasonal warming trends to affect the response of NEE in northern forests to changing temperatures. We demonstrate that the CO2 sinks in old forests exhibit greater resilience to non-uniform seasonal warming compared to young and mid-aged forests. These results suggest that old forests may be able to maintain their CO2 sink capacity under future air temperature increases. Although we have included as many sites as possible, our dataset consists of only 65 extant sites. Notably, there are no sites in certain regions such as Anatolia, the Balkans, Iberia, and North Asia. Consequently, certain forest types, such as dry Mediterranean forests, are underrepresented. Thus, the geographical gap introduces potential uncertainty when attempting to upscale our results to represent the entire northern forest region. We acknowledge that this limitation may affect the generalizability of our findings, and thus, we caution against overgeneralizing the results without further corroborating evidence. Additionally, we acknowledge that applying a tree cover threshold of >10% may result in differences in the age effects observed in ecosystems with lower tree cover compared to denser forests. This threshold was chosen to strike a balance between data availability and ecological representativeness. Future studies should aim to expand the dataset by incorporating more sites, particularly in underrepresented regions, to either validate or refine the conclusions drawn from this study.
Materials and methods
Field- and satellite-based observations
We analyzed multiyear monthly NEE observations from FLUXNET, AmeriFlux, Chinaflux and ICOS (Integrated Carbon Observation System)56,57 dataset obtained at 65 northern forest sites (n = 9972 site-months; Supplementary Table 1). The sites were chosen based on the following criteria: (1) no severe disturbances had occurred since the last stand-replacing disturbance, (2) stand age information was available and at least 5 years of data were available. Of all 65 forest sites with age ranging from two to 475 years, 44 were evergreen forests (EF), 19 were deciduous broadleaved forests (DBF) and 2 were mixed forests (MF). NEE data had already been processed following a consistent and uniform processing pipeline58. This data processing pipeline mainly included: (1) thorough data quality control checks; (2) calculation of a range of friction velocity thresholds; (3) gap-filling of meteorological and flux measurements using the marginal distribution sampling (MDS) methods; (4) partitioning of CO2 fluxes into respiration and photosynthesis components. We used the GPP estimated by a nighttime approach based on short-term temperature sensitivity59. Here, we use the micrometeorological sign convention that negative NEE indicates net ecosystem CO2 uptake and positive NEE indicates net ecosystem CO2 loss to the atmosphere.
Air temperature was measured at the flux tower sites and was aggregated into monthly means. Monthly Priestley Taylor coefficient (α) was used as a proxy for water availability60. Average stand age of each forest site was obtained from the Biological, Ancillary, Disturbance and Metadata (BADM) and the published literature, and it refers to the time since the last severe disturbance event. Stand age reported in the BADM is the average tree age of the stand since the last major disturbance and was reported for different sites. Satellite-based monthly EVI (n = 8952 site-months) was taken from the MODIS (moderate-resolution spectroradiometer) vegetation indices 16-day MOD13Q1 product (250 m resolution) and used as a proxy for vegetation productivity. The MOD13Q1 product covers the period 2000–2020, overlapping with most (>90%) of the NEE observation periods.
NEE sensitivity to environmental drivers across sites
We estimated NEE sensitivities to air temperature, α, vegetation productivity and incoming short-wave radiation anomalies for different seasonal periods. Sensitivity was defined as the slope of the relationship between anomalies of monthly NEE and respective explanatory variable. Seasonal periods were defined on the basis of standardized mean monthly air-temperature seasonality. For each site, the mean annual air temperature was subtracted from the mean monthly air temperatures and then divided by the standard deviation of monthly air temperatures to make seasonality comparable between sites with different temperature amplitudes. Springs were defined for each site to be from the first month with a standardized air temperature above 0 °C to end of May. Summers were defined for each site to be from June to August. Autumns were defined for each site to be from September to the last month with a standardized air temperature above 0 °C. The remaining months were considered to be winter. We applied linear mixed-effects models using the fitlme and fixedEffects functions in Matlab (R2020) to estimate NEE sensitivities.
Where β represents the fixed effect, X represents the independent variables (e.g., Ta, α, EVI or incoming shortwave radiation). The models were fitted to each response variable separately to characterize seasonal changes in NEE sensitivity to different driver variables. First, for each variable, monthly anomalies were derived by subtracting the mean variable value for the corresponding month from the annual mean value for each site. Second, the anomalies dataset was divided into different groups according to the seasonal periods, age groups, biome types and vegetation types. Last, for each seasonal period, age group, biome type and forest type, a linear mixed-effects model was separately fitted for NEE anomalies, with fixed effects for monthly air temperature, α, EVI and incoming shortwave radiation anomalies, and the uncorrelated random effect for intercept and air temperature, α, EVI or incoming shortwave radiation anomalies, grouped by site. Alternative variables, including GPP (an efficient indicator of vegetation productivity) and the standardized precipitation evapotranspiration index (SPEI, as indicator of water stress), were also considered by applying the linear mixed-effects models (Supplementary Fig. 3).
To assess collinearity between Ta and other climatic variables, we applied linear mixed models for Ta in relation to incoming shortwave radiation, EVI, α and SPEI, respectively (Supplementary Fig. 2). Additionally, we adopted a multiple regression model to quantify the sensitivity of ΔNEE at specific sites to inter-annual variations in air temperature, water availability (α) and vegetation productivity (EVI). The sensitivities obtained from the multiple regression model were comparable to those derived from the linear mixed-effects models (Supplementary Fig. 4).
Warming trends and simulated NEE responses
Monthly warming trends were assessed by computing the difference in mean monthly air temperatures between 1951–1970 and 2001–2020 using data from CRU TS v.4.0761. Spatial resolution of the dataset was 0.5° × 0.5° and covered land areas north of 30°N. Monthly NEE temperature sensitivities across all forest sites were derived by fitting linear mixed-effects models to each month (Supplementary Fig. 8). We mapped the empirical sensitivities of the 65 northern forest sites. When mapping the sensitivities of NEE to air temperature across different age groups, we utilized the forest age distribution, operating under the assumption that the warming response of forests varies primarily between different age groups while remaining relatively uniform within each age group. Furthermore, to account for uncertainties in monthly NEE temperature sensitivities, we ran Monte Carlo simulations 1000 times, randomly sampling for each month and for each age group from a normal distribution around the mean monthly NEE sensitivities (derived using the fixedEffects function in Matlab). And then, the median of the 1000 NEE simulations were used to estimate the impacts of seasonal warming and stand age on NEE.
The global forest distribution and forest-stand age was obtained from GFAD62. GFAD provides a distribution of stand age in 10-y age bins up to an age of 140 y from a base year of 2010 on a 0.5° grid. Our results are restricted to the forested area shown in Fig. 1 with a reference year of 2010. This was defined based on European Space Agency Climate Change Initiative (ESA CCI) land cover, with all forest land-cover types with at least 15% canopy cover being included. The land cover at a nominal 300-m resolution was aggregated to give fractional coverage of forest at 0.5° resolution63. Relative forest cover fractions from GFAD were used to break down the forest area into young- (<40 years), mid-aged (40–90 years), and old forests (>90 years)64.
To quantify the impact of seasonally uniform warming, the monthly NEE temperature sensitivity was multiplied by the mean annual warming rate for each month and grid cell (Fig. 4a). Annual NEE changes were then derived by summing monthly NEE changes from January to December. To quantify the effect of seasonally varying warming on forest NEE responses, the monthly NEE temperature sensitivity was multiplied by the warming rate for each month and grid cell and then summed to annual NEE changes (Fig. 4b). To quantify the effect of stand age on responses of forest NEE to seasonally varying warming, we used two procedures. First, the monthly NEE temperature sensitivity of young forests was multiplied by the warming rate for each month and grid cell, and then summed to annual NEE changes (Fig. 4c). Second, the monthly NEE temperature sensitivities of young-, mid- and old-aged forests were multiplied by the warming rate for each month and corresponding forest grid cell of each respective forest age group, subsequently summing these values to estimate annual NEE changes (Fig. 4d).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in this study are openly available in the following databases: The eddy covariance measurements are obtained from the ICOS, FLUXNET2015 datasets (https://fluxnet.org/login/?redirect_to=/data/download-data/) and AmeriFlux (https://ameriflux.lbl.gov/data/download-data/). The global forest age dataset was download from https://doi.pangaea.de/10.1594/PANGAEA.889943. MOD13Q1 EVI dataset was download from https://appeears.earthdatacloud.nasa.gov/task/point. Global warming trends was extracted from the CRU TS v.4.07 dataset which is available from https://crudata.uea.ac.uk/cru/data/hrg/cru_ts_4.07/cruts.2304141047.v4.07/. Global SPEI data (SPEIbase v2.10) was downloaded from https://spei.csic.es/database.html. The data to produce the figures can be found on Zenodo, https://doi.org/10.5281/zenodo.10865368.
References
Xia, J. et al. Terrestrial carbon cycle affected by non-uniform climate warming. Nat. Geosci. 7, 173–180 (2014).
Graven, H. D. et al. Enhanced seasonal exchange of CO2 by northern ecosystems since 1960. Science 341, 1085–1089 (2013).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Forkel, M. et al. Enhanced seasonal CO2 exchange caused by amplified plant productivity in northern ecosystems. Science 351, 696–699 (2016).
Keenan, T. F. et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Change 4, 598–604 (2014).
Nemani, R. R. et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563 (2003).
Yang, H. et al. Global increase in biomass carbon stock dominated by growth of northern young forests over past decade. Nat. Geosci. 16, 886–892 (2023).
Piao, S. et al. Weakening temperature control on the interannual variations of spring carbon uptake across northern lands. Nat. Clim. Change 7, 359–363 (2017).
Liu, Q. et al. Vegetation browning: global drivers, impacts, and feedbacks. Trends Plant Sci. 28, 1014–1032 (2023).
Mirabel, A., Girardin, M. P., Metsaranta, J., Way, D. & Reich, P. B. Increasing atmospheric dryness reduces boreal forest tree growth. Nat. Commun. 14, 6901 (2023).
Black, T. A. et al. Increased carbon sequestration by a boreal deciduous forest in years with a warm spring. Geophys. Res. Lett. 27, 1271–1274 (2000).
Sanders‐DeMott, R. et al. Divergent carbon cycle response of forest and grass‐dominated northern temperate ecosystems to record winter warming. Glob. Change Biol. 26, 1519–1531 (2020).
He, H. et al. Altered trends in carbon uptake in China’s terrestrial ecosystems under the enhanced summer monsoon and warming hiatus. Natl Sci. Rev. 6, 505–514 (2019).
Lin, D., Xia, J. & Wan, S. Climate warming and biomass accumulation of terrestrial plants: a meta‐analysis. N. Phytol. 188, 187–198 (2010).
Duveneck, M. J. & Thompson, J. R. Climate change imposes phenological trade‐offs on forest net primary productivity. J. Geophys. Res. Biogeosci. 122, 2298–2313 (2017).
Wang, T. et al. Emerging negative impact of warming on summer carbon uptake in northern ecosystems. Nat. Commun. 9, 5391 (2018).
Zhang, Y. et al. Future reversal of warming-enhanced vegetation productivity in the Northern Hemisphere. Nat. Clim. Change 12, 581–586 (2022).
Stinziano, J. R. & Way, D. A. Autumn photosynthetic decline and growth cessation in seedlings of white spruce are decoupled under warming and photoperiod manipulations. Plant Cell Environ. 40, 1296–1316 (2017).
Piao, S. et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 (2008).
Ryan, M. G. Three decades of research at Flakaliden advancing whole-tree physiology, forest ecosystem and global change research. Tree Physiol. 33, 1123–1131 (2013).
Zhang, Y., Commane, R., Zhou, S., Williams, A. P. & Gentine, P. Light limitation regulates the response of autumn terrestrial carbon uptake to warming. Nat. Clim. Change 10, 739–743 (2020).
Curtis, P. S. & Gough, C. M. Forest aging, disturbance and the carbon cycle. N. Phytol. 219, 1188–1193 (2018).
Goulden, M. L. et al. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Change Biol. 17, 855–871 (2011).
Krishnan, P., Black, T. A., Jassal, R. S., Chen, B., & Nesic, Z. Interannual variability of the carbon balance of three different‐aged Douglas‐fir stands in the Pacific Northwest. J. Geophys. Res. Biogeosci. 114, G04011 (2009).
Dusenge, M. E., Madhavji, S. & Way, D. A. Contrasting acclimation responses to elevated CO2 and warming between an evergreen and a deciduous boreal conifer. Glob. Change Biol. 26, 3639–3657 (2020).
Dusenge, M. E. et al. Warming induces divergent stomatal dynamics in co‐occurring boreal trees. Glob. Change Biol. 27, 3079–3094 (2021).
Dusenge, M. E. et al. Boreal conifers maintain carbon uptake with warming despite failure to track optimal temperatures. Nat. Commun. 14, 4667 (2023).
Girardin, M. P. et al. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming. Glob. Change Biol. 22, 627–643 (2016).
Marchand, W., Girardin, M. P., Hartmann, H., Gauthier, S. & Bergeron, Y. Taxonomy, together with ontogeny and growing conditions, drives needleleaf species’ sensitivity to climate in boreal North America. Glob. Change Biol. 25, 2793–2809 (2019).
Mirabel, A. et al. New tree-ring data from Canadian boreal and hemi-boreal forests provide insight for improving the climate sensitivity of terrestrial biosphere models. Sci. Total Environ. 851, 158062 (2022).
Reich, P. B. et al. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263–267 (2018).
Reich, P. B. et al. Even modest climate change may lead to major transitions in boreal forests. Nature 608, 540–545 (2022).
Dusenge, M. E. et al. Photosynthetic capacity in middle‐aged larch and spruce acclimates independently to experimental warming and elevated CO2. Plant Cell Environ. 47, 4886–4902 (2024).
Hanson, P. J. et al. Rapid net carbon loss from a whole‐ecosystem warmed peatland. Agu Adv. 1, e2020AV000163 (2020).
Saxe, H., Cannell, M. G., Johnsen, Ø., Ryan, M. G. & Vourlitis, G. Tree and forest functioning in response to global warming. N. Phytol. 149, 369–399 (2001).
Au, T. F. et al. Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat. Clim. Change 12, 1168–1174 (2022).
Girardin, M. P. et al. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proc. Natl Acad. Sci. USA 113, E8406–E8414 (2016).
Xu, B. et al. Seasonal variability of forest sensitivity to heat and drought stresses: a synthesis based on carbon fluxes from North American forest ecosystems. Glob. Change Biol. 26, 901–918 (2020).
McMillan, A. M., Winston, G. C. & Goulden, M. L. Age‐dependent response of boreal forest to temperature and rainfall variability. Glob. Change Biol. 14, 1904–1916 (2008).
Welp, L. R., Randerson, J. T. & Liu, H. P. The sensitivity of carbon fluxes to spring warming and summer drought depends on plant functional type in boreal forest ecosystems. Agric. For. Meteorol. 147, 172–185 (2007).
Helbig, M. et al. Warming response of peatland CO2 sink is sensitive to seasonality in warming trends. Nat. Clim. Change 12, 743–749 (2022).
Gao, S. et al. An earlier start of the thermal growing season enhances tree growth in cold humid areas but not in dry areas. Nat. Ecol. Evol. 6, 397–404 (2022).
Chen, W. et al. Evidence for widespread thermal optimality of ecosystem respiration. Nat. Ecol. Evol. 7, 1379–1387 (2023).
Huang, M. et al. Air temperature optima of vegetation productivity across global biomes. Nat. Ecol. Evol. 3, 772–779 (2019).
Ma, Q. et al. Tree mortality during long-term droughts is lower in structurally complex forest stands. Nat. Commun. 14, 7467 (2023).
Martínez-García, E. et al. Drought response of the boreal forest carbon sink is driven by understorey–tree composition. Nat. Geosci. 17, 197–204 (2024).
Bauerle, W. L. et al. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Natl Acad. Sci. USA 109, 8612–8617 (2012).
Gough, C. M., Atkins, J. W., Fahey, R. T. & Hardiman, B. S. High rates of primary production in structurally complex forests. Ecology 100, e02864 (2019).
Buermann, W. et al. Widespread seasonal compensation effects of spring warming on northern plant productivity. Nature 562, 110–114 (2018).
Wolf, S. et al. Warm spring reduced carbon cycle impact of the 2012 US summer drought. Proc. Natl Acad. Sci. USA 113, 5880–5885 (2016).
Yu, Z., Griffis, T. J. & Baker, J. M. Warming temperatures lead to reduced summer carbon sequestration in the US Corn Belt. Commun. Earth Environ. 2, 53 (2021).
Zani, D., Crowther, T. W., Mo, L., Renner, S. S. & Zohner, C. M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 370, 1066–1071 (2020).
Jiang, M. et al. Microbial competition for phosphorus limits the CO2 response of a mature forest. Nature 630, 660–665 (2024).
Reich, P. B. et al. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531, 633–636 (2016).
Smith, N. G. & Dukes, J. S. Short‐term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Glob. Change Biol. 23, 4840–4853 (2017).
Girardin, M. P. et al. Annual aboveground carbon uptake enhancements from assisted gene flow in boreal black spruce forests are not long-lasting. Nat. Commun. 12, 1169 (2021).
Warm Winter 2020 Team, & ICOS Ecosystem Thematic Centre (2022). Warm Winter 2020 ecosystem eddy covariance flux product for 73 stations in FLUXNET-Archive format-release 2022−1 (Version 1.0). ICOS Carbon Portal. https://doi.org/10.18160/2G60-ZHAK.
Pastorello, G. et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 7, 1–27 (2020).
Reichstein, M. et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob. Change Biol. 11, 1424–1439 (2005).
Priestley, C. H. B. & Taylor, R. J. On the assessment of surface heat flux and evaporation using large-scale parameters. Monthly Weather Rev. 100, 81–92 (1972).
Harris, I., Osborn, T. J., Jones, P. & Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 7, 109 (2020).
Poulter, B. et al. The global forest age dataset and its uncertainties (GFADv1. 1). NASA National Aeronautics and Space Administration, https://doi.org/10.1594/PANGAEA.897392 (PANGAEA, 2019).
Pugh, T. A. et al. Role of forest regrowth in global carbon sink dynamics. Proc. Natl Acad. Sci. USA 116, 4382–4387 (2019).
Kuuluvainen, T. & Gauthier, S. Young and old forest in the boreal: critical stages of ecosystem dynamics and management under global change. For. Ecosyst. 5, 1–15 (2018).
Acknowledgements
This study was supported by National Key R&D Program of China grant 2020YFA0608100 and the National Natural Science Foundation of China grant 32101588.
Author information
Authors and Affiliations
Contributions
Peng Liu led the research conception, data analysis, interpretation, and the writing and revision of the manuscript. Tianshan Zha contributed to the conception and design of the study. T. Andrew Black and Rachhpal S. Jassal provided critical revisions of the manuscript and offered guidance as academic advisors throughout the project. Asko Noormets and Andrew Ouimette conducted field research, managed data collection, and revised the manuscript. Xin Jia contributed to manuscript editing, while Tian Yun and Xinhao Li assisted with data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Jinquan Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dushan Kumarathunge and Alice Drinkwater. [A peer review file is available.].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, P., Zha, T., Black, T.A. et al. Seasonal warming responses of the carbon dioxide sink from northern forests are sensitive to stand age. Commun Earth Environ 6, 43 (2025). https://doi.org/10.1038/s43247-025-02008-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02008-7
This article is cited by
-
Insights into terrestrial carbon and water cycling from the global eddy covariance network
Nature Reviews Earth & Environment (2025)