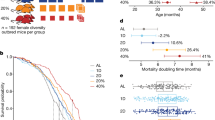
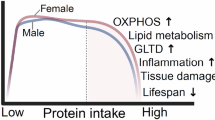
Abstract
Restricting the intake of protein or the branched-chain amino acid isoleucine promotes healthspan and extends lifespan in young or adult mice. However, their effects when initiated in aged animals are unknown. Here we investigate the consequences of consuming a diet with 67% reduction of all amino acids (low AA) or of isoleucine alone (low Ile), in male and female C57BL/6J.Nia mice starting at 20 months of age. Both dietary regimens effectively promote overall metabolic health without reducing calorie intake. Both low AA and low Ile diets improve aspects of frailty and slow multiple molecular indicators of aging rate; however, the low Ile diet reduces grip strength in both sexes and has mixed, sexually dimorphic effects on the heart. These results demonstrate that low AA and low Ile diets can promote aspects of healthy aging in aged mice and suggest that similar interventions might promote healthy aging in older adults.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA sequencing data have been deposited with Gene Expression Omnibus, accession GSE254721. Heart lipidomics data have been deposited with MassIVE, identifier MSV000095324. The data that support the plots within this article and other findings of this study, including full scans of western blot images, are provided as Source data files. All data supporting the findings of the study are available from the corresponding author upon request. Source data are provided with this paper.
References
Mihaylova, M. M. et al. When a calorie is not just a calorie: diet quality and timing as mediators of metabolism and healthy aging. Cell Metab. 35, 1114–1131 (2023).
Levine, M. E. et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 (2014).
Sluijs, I. et al. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33, 43–48 (2010).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
Ferraz-Bannitz, R. et al. Dietary protein restriction improves metabolic dysfunction in patients with metabolic syndrome in a randomized, controlled trial. Nutrients 14, 2670 (2022).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Mair, W., Piper, M. D. & Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3, e223 (2005).
Solon-Biet, S. M. et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 11, 1529–1534 (2015).
Hill, C. M. et al. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat. Commun. 13, 1897 (2022).
Richardson, N. E. et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat. Aging 1, 73–86 (2021).
White, P. J. et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 5, 538–551 (2016).
Cummings, N. E. et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 596, 623–645 (2018).
Yu, D. et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 33, 905–922.e6 (2021).
Solon-Biet, S. M. et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 1, 532–545 (2019).
Green, C. L. et al. Dietary restriction of isoleucine increases healthspan and lifespan of genetically heterogeneous mice. Cell Metab. 35, 1976–1995.e6 (2023).
Deelen, J. et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 10, 3346 (2019).
Hahn, O. et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. 1, 1059–1073 (2019).
Flurkey, K., Currer, J. & Harrison, D. in The Mouse in Biomedical Research 2nd edn (ed. Fox, J. G.) 637–672 (American College Laboratory Animal Medicine, 2007).
Whitehead, J. C. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. A 69, 621–632 (2014).
Miller, R. A., Li, X. & Garcia, G. Aging rate indicators: speedometers for aging research in mice. Aging Biol. https://doi.org/10.59368/agingbio.20230003 (2023).
Simcox, J. & Lamming, D. W. The central moTOR of metabolism. Dev. Cell 57, 691–706 (2022).
Baar, E. L., Carbajal, K. A., Ong, I. M. & Lamming, D. W. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 15, 155–166 (2016).
Mannick, J. B. & Lamming, D. W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 3, 642–660 (2023).
Jiang, E., Dinesh, A., Jadhav, S., Miller, R. A. & Garcia, G. G. Canagliflozin shares common mTOR and MAPK signaling mechanisms with other lifespan extension treatments. Life Sci. 328, 121904 (2023).
Miller, R. A. et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight 5, e140019 (2020).
Shen, Z., Hinson, A., Miller, R. A. & Garcia, G. G. Cap-independent translation: a shared mechanism for lifespan extension by rapamycin, acarbose, and 17α-estradiol. Aging Cell 20, e13345 (2021).
Watanabe, K. et al. Lifespan-extending interventions induce consistent patterns of fatty acid oxidation in mouse livers. Commun. Biol. 6, 768 (2023).
Roopra, A. MAGIC: a tool for predicting transcription factors and cofactors driving gene sets using ENCODE data. PLoS Comput. Biol. 16, e1007800 (2020).
Matthew, S. et al. in Hepatocellular Carcinoma (eds Streba, C. T., Vere, C. C. & Rogoveanu, I.) Ch. 4 (IntechOpen, 2017).
Dorn, G. Mitochondrial fission/fusion and cardiomyopathy. Curr. Opin. Genet. Dev. 38, 38–44 (2016).
Bellantuono, I. et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat. Protoc. 15, 540–574 (2020).
Norman, K., Haß, U. & Pirlich, M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients 13, 2764 (2021).
Nasimi, N. et al. Whey protein supplementation with or without vitamin D on sarcopenia-related measures: a systematic review and meta-analysis. Adv. Nutr. 14, 762–773 (2023).
Ni Lochlainn, M., Bowyer, R. C. E., Welch, A. A., Whelan, K. & Steves, C. J. Higher dietary protein intake is associated with sarcopenia in older British twins. Age Ageing 52, afad018 (2023).
Castets, P., Ham, D. J. & Rüegg, M. A. The TOR pathway at the neuromuscular junction: more than a metabolic player? Front. Mol. Neurosci. 13, 162 (2020).
Joseph, G. A. et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol. Cell. Biol. 39, e00141–19 (2019).
Tournissac, M. et al. Dietary intake of branched-chain amino acids in a mouse model of Alzheimer’s disease: effects on survival, behavior, and neuropathology. Alzheimers Dement. 4, 677–687 (2018).
Babygirija, R. et al. Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer’s disease. Nat. Commun. 15, 5217 (2024).
Harris, S. P. et al. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ. Res. 90, 594–601 (2002).
Calubag, M. F. et al. FGF21 has a sex-specific role in calorie-restriction-induced beiging of white adipose tissue in mice. Aging Biol. 1, 3 (2022).
Jain, R., Wade, G., Ong, I., Chaurasia, B. & Simcox, J. Determination of tissue contributions to the circulating lipid pool in cold exposure via systematic assessment of lipid profiles. J. Lipid Res. 63, 100197 (2022).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2017).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Acknowledgements
We thank J. Chen and J. Berg at the University of Michigan for assistance with molecular analysis of tissues, as well as all members of the Lamming laboratory for their assistance and input. The Lamming laboratory is supported in part by the National Institutes of Health (NIH)/NIA (AG056771, AG062328, AG081482, AG084156 and AG085898 to D.W.L.), National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK125859 to D.W.L.) and startup funds from the University of Wisconsin–Madison School of Medicine and Public Health and Department of Medicine to D.W.L. C.-Y.Y. was supported in part by a training grant (T32AG000213), a National Institute of Aging F32 postdoctoral fellowship (F32AG077916) and a National Institute of Aging K99 award (K99AG084921). M.F.C. is supported by F31AG082504. R.B. is supported by F31AG081115. M.E.T. is supported by F99AG083290. C.L.G. is supported by HF AGE-009 from the Hevolution Foundation. M.M.S. is supported in part by a Supplement to Promote Diversity in Health‐Related Research RF1AG056771-06S1. H.H.P. is supported in part by F31AG066311. J.A.S. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK133479) and Juvenile Diabetes Research Foundation (JDRF201309442) and J.W.D. is supported by an National Science Foundation Graduate Research Fellowship Program. J.A.S. is a Howard Hughes Medical Institute Freeman Hrabowski Scholar and is an American Federation for Aging Research grant recipient (A22068). R.A.M. and G.G.G. are supported by the Glenn Foundation for Medical Research. Support for this research was provided by the University of Wisconsin–Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. The authors used the University of Wisconsin–Madison Biotechnology Center Gene Expression Center (RRID:SCR_017757) and the University of Wisconsin Carbone Cancer Center Experimental Animal Pathology Laboratory (P30CA014520). The Lamming laboratory was supported in part by the US Department of Veterans Affairs (I01-BX004031 and IS1-BX005524), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
C.-Y.Y., M.S.G., T.A.H., R.A.M. and D.W.L. conceived and designed the experiments. C.-Y.Y., L.C.S.C., J.W.D., G.G.G., M.S.G., I.T.F., M.F.C., A.C.R., C.L.G., R.B., M.M.S., H.H.P. and M.E.T. performed the experiments. C.-Y.Y., M.S.G., T.A.H., G.G.G., R.A.M., J.A.S. and D.W.L. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.W.L. has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Stephen Simpson and the other, anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The effect of late-life feeding of a Low Ile or a Low AA diet on fat and lean mass, food consumption, fasting blood glucose, insulin tolerance, and glucose tolerance at 25 months of age.
(A-B) Changes in (A) fat and (B) lean mass in males over time. (A-B) n varies over time, maximum n = 10, 11, 10, and 10. (C) Food consumption of male mice throughout the experiment; n varies over time, maximum n = 5, 5, 6, and 6 cages. (D) Fasting blood glucose of 21-month-old male after 3 weeks on the indicated diets; n = 12, 13, 12, and 10. (E) Insulin tolerance test of 21-month-old male after 4 weeks on the indicated diets; n = 9, 9, 11, and 10. (F) Glucose tolerance test in a separate group of 25 months old mice; n = 6, 7, 6, and 5. (G-H) Changes in (G) fat and (H) lean mass in females over time. (G-H) maximum n = 10, 11, 10, and 10. (I) Food consumption of female mice throughout the experiment. (I) n varies over time, maximum n = 4 cages/group. (J) Fasting blood glucose of 21-month-old female mice after 3 weeks on the indicated diets; n = 10, 11, 10, and 10. (K) Insulin tolerance test; n = 10, 11, 10, and 10. (L) Glucose tolerance test in a separate group of 25 months old mice.; n = 9, 8, 10, and 5. n-value indicate biologically independent animals (or cages if indicated). ANOVA followed by two-sided Dunnett’s test vs. Aged Control-fed mice. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. N-values denote biologically independent animals unless otherwise specified and are listed in the order of Aged Control, Aged Low Ile, Aged Low AA, and Young Control. Data presented as mean ± SEM. The exact p-values are provided in Source data.
Extended Data Fig. 2 Effects of late-life Low AA and Low Ile diets on energy balance.
(A-B) ANCOVA of energy expenditure with lean mass as a covariate in (C) male and (D) female mice. (C-D) Spontaneous activity of (E) male and (F) female mice during the metabolic chambers experiments. Shaded period indicates dark phase. (E-F) Metabolic chambers were used to determine the respiratory exchange ratio of (A) male and (B) female mice. Shaded period indicates dark phase. (A-F) Male n = 8, 7, 10, and 10; Female n = 9, 10, 10, and 10. N-values denote biologically independent animals unless otherwise specified and are listed in the order of Aged Control, Aged Low Ile, Aged Low AA, and Young Control. *p < 0.05, ANOVA followed by two-sided Dunnett’s test; ANOVA conducted separately for the light and dark cycles. Data represented as mean ± SEM. The exact p-values are provided in Source data.
Extended Data Fig. 3 Western blotting of aging markers in the gastrocnemius muscle.
(A-B) Quantification of (A) total and (B) phosphorylated S6 in male muscle. (C-D) Quantification of (C) total and (D) phosphorylated AKT in male muscle. (E-F) Quantification of (E) total and (F) phosphorylated S6 in female muscle. (G-H) Quantification of (G) total and (H) phosphorylated AKT in female muscle. (I-J) Representative Western blots for (K) male and (J) female mice muscles. For all groups, n = 6. n-value indicates biologically independent animals. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Aged Control mice, ANOVA followed by two-sided Dunnett’s test. Data presented as mean ± SEM. The exact p-values are provided in Source data.
Extended Data Fig. 4 Volcano plots of hepatic DEGs after Low Ile feeding in aged mice.
(A-B) Volcano plots of differentially expressed genes in the liver of (blue) male and (red) female mice with (A) age and (B) diet. DEGs were identified using an empirical Bayes moderated linear model, and log coefficients and Benjamini-Hochberg (BH) adjusted p-values were generated for each comparison of interest (α = 0.10).
Extended Data Fig. 5 Venn diagram and enrichment analysis of differentially expressed hepatic genes.
(A) Venn diagram showing the number of overlapping and non-overlapping (left) age-driven and (right) diet-driven DEGs between male and females. (B-C) Significantly enriched KEGG pathways by (B) age and (C) diet in male mice. (D-E) Significantly enriched GO terms by (D) age and (E) diet in male mice. (F-G) Significantly enriched KEGG pathways by (F) age and (G) diet in female mice. (H-I) Significantly enriched GO terms by (H) age and (I) diet in female mice.
Extended Data Fig. 6 Expression analysis of senescence markers in the aged male liver.
Expression of the indicated genes in the livers of 20-month-old mice on the indicated diets for 4 months was determined by qPCR. n = 6 biologically independent animals (P21 Aged Control, Il-6 Aged Control, Il-6 Aged Low Ile), 8 biologically independent animals (P21 Aged Low AA, P16 Aged Low AA, Il1-b Aged Low AA, Mcp-1 Aged Low AA, Il-10 Aged Low AA), 5 biologically independent animals (Il-6 Aged Low AA), and 7 biologically independent animals for all other groups and genes, *p < 0.05, 2-way mixed-effects analysis (REML) followed by two-sided Dunnett’s test vs. Aged Control. Data presented as mean ± SEM. The exact p-values are provided in Source data.
Extended Data Fig. 7 H&E and F4/80 staining of the mice liver.
(A) Representative hematoxylin and eosin (H&E) staining of the mouse liver. Black arrows mark extramedullary hematopoiesis, indicative of lobular inflammation. (B-I) Quantification of (B-E) male and (F-I) female H&E staining in Nonalcoholic Fatty Liver Disease Activity Score (NAS) score and its component scores: steatosis, lobular inflammation, and hepatocyte ballooning. (J) Positive and negative control (without antibody) staining of the mouse liver with the F4/80 antibody. (K) Representative F4/80 staining of the mouse liver. Black arrows mark loci of extramedullary hematopoiesis, indicative of lobular inflammation, similar to the H&E staining. (L-M) Quantification of (L) male and (M) female F4/80 staining mean intensity. For all groups n = 4. *p < 0.05, **p < 0.01, ANOVA followed by two-sided Dunnett’s test vs. Aged Control. Data presented as mean ± SEM. A blinded evaluator scored the sections three times and the average score for each animal was taken for statistical analysis. The exact p-values are provided in Source data.
Extended Data Fig. 8 Oil Red O and Sirius Red staining of the mice liver.
(A) Representative oil red O (ORO) staining of the mouse liver. (B-E) Quantification of (B-C) male and (D-E) female ORO staining in % area and mean intensity. (F) Representative polarized light microscopy of the Sirius Red (SR) staining birefringence in the mouse liver. White arrow highlights bridging, an indication of advanced liver fibrosis. (G-H) Quantification of male (G) and female (H) liver fibrosis score. For all groups n = 4. ANOVA followed by two-sided Dunnett’s test vs. Aged Control. *p < 0.05. Data presented as mean ± SEM. A blinded evaluator scored the sections three times and the average score for each animal was taken for statistical analysis. The exact p-values are provided in Source data.
Supplementary information
Supplementary Information
Supplementary Figs. 15 and captions of Supplementary Tables 1–6.
Supplementary Data
Source data file for Supplementary Figs. 1–5.
Supplementary Tables 1–6
Supplementary information containing all supplementary data tables.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Table 2
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeh, CY., Chini, L.C.S., Davidson, J.W. et al. Late-life protein or isoleucine restriction impacts physiological and molecular signatures of aging. Nat Aging 4, 1760–1771 (2024). https://doi.org/10.1038/s43587-024-00744-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43587-024-00744-7
This article is cited by
-
Ammonia – an old metabolite brings new insights into Alzheimer’s disease
npj Dementia (2025)