Abstract
Background
The adenovirus-vaccine platform has come to prominence with the COVID-19 vaccination campaigns. The objective of this study was to validate a formulation that was suitable for lyophilisation and long-term storage at 5 (2–8) °C.
Methods
Vaccine stability was assessed up to five years at 5 °C using a lyophilised formulation of the chimpanzee-adenovirus vector ChAd155 encoding a respiratory syncytial virus (RSV) antigen. Vaccine potency was assessed by functional infectivity assay. Other assessments of vaccine stability included those for capsid integrity, particle content, and DNA release. Vaccine efficacy and safety were assessed after two years in a murine model of RSV challenge and a rabbit toxicology model, respectively.
Results
Here, we show that the potency loss from lyophilisation was 0.12 log10. The potency loss over five years at 5 °C was estimated at 0.21 log10 (95%CI 0.10–0.30). This coincides with a 25% increase in the ratio of non-infectious particles/infectious particles. After two years of storage at 5 °C, (i) the loss of infectivity was 0.17 log10; (ii) the vaccine remained immunogenic and effective at clearing RSV from the lungs in a mouse-challenge model; and (iii) the vaccine was not associated with any adverse safety signal in a rabbit toxicology model.
Conclusions
The 5-year stability of the lyophilised adenovirus-vector vaccine is within our acceptable limit ( < 0.3 log10 decrease). Its formulation process is amenable to manufacturing scale-up and should help in providing adenovirus-based vaccines where the cold chain is problematic, such as in low-income countries, and in pre-epidemic stockpiling.
Plain language summary
Being able to store vaccines for several years in the fridge rather than the freezer should increase their availability, especially in low-income countries, and enable more immediate use in epidemics. The challenge is greater for vaccines that are based on functional viruses. Here we evaluate a freeze-dried formulation of a vaccine that uses a genetically manipulated adenovirus, a similar design to that of many of the successful COVID-19 vaccines. We tested how stable the vaccine was in various ways and checked that it still worked in animal models. Our method could be used to store vaccines for up to five years in a fridge, which would enable them to be used more quickly across the world when required.
Similar content being viewed by others
Introduction
Overcoming the reliance on the cold chain for vaccine distribution and storage can help vaccination campaigns in low-income countries and for stockpiling in anticipation of epidemics1,2,3,4,5,6. A greater vaccine stability over the long term could also lead to a benefit in dose sparing by reducing the need to replenish vaccine stocks. Minimising the cold chain has been recognised in the production of next-generation SARS-CoV-2 vaccines7. Three of the early SARS-CoV-2 vaccines to obtain approval were based on the adenoviral vaccine platform and include the modified human adenovirus type 26 (Ad26) vector and/or the Ad5 vector. These adenovirus-based vaccines are stored and transported frozen, although one of them can be kept at 2 to 8 °C in liquid form for 11 months after thawing8. Another one has also been produced in a lyophilised format, and phase 1/2 clinical evaluations showed the lyophilised and frozen formats of the vaccine were similarly immunogenic, although the stability of the lyophilized product was not reported9.
The adenoviral vaccine platform consists of the adenovirus particle with a modified DNA genome containing the sequence encoding the vaccine antigen. After injection in the vaccine recipient, the adenovirus particles infect cells leading to the expression of the antigen. Hence the physical properties of the vector are not altered significantly by encoding different antigen sequencies in contrast to vaccines formulated with the actual antigen, implying that the parameters for vaccine stabilisation should be applicable to all vaccines based on the platform. Although the COVID-19 pandemic brought to prominence the adenovirus platform, other indications have been evaluated in the clinic, including Ebola and respiratory syncytial virus (RSV)-associated respiratory disease10,11,12.
The challenge for the development of a lyophilised format, is to identify a formulation that minimises potency loss both during the lyophilisation process and during long-term storage. Therefore, an important aspect of formulation is the selection of excipients, notably excipients that may regulate ice-crystal formation and sublimation4. Recent studies on the chimpanzee adenovirus (ChAdOx) vaccine vectors have identified formulations and methodologies that have brought the viral-titre loss from lyophilisation to <0.5 log10 and approaching what was identified with a formulation of hAd5-E10A in 2012 (0.12 log10)13,14,15. The results for long-term storage of both ChAd and hAd vaccine vectors at most up to 1 year at 4 °C, have also been encouraging with viral-titre loss ranging from negligible to 0.2 log1013,14,15. Other protocols have evaluated spray drying technology16,17,18,19,20. However, this approach is also reliant on identifying a suitable support matrix that is compatible with vaccine reconstitution for injection21, or as a potential means to administer the vaccine by inhalation or skin patch22,23.
The objective of this study was to demonstrate long-term stability of a lyophilised formulation of the ChAd vaccine vector ChAd155 in which the loss of vaccine-virus infectivity would be within 0.3 log10 after lyophilisation and five years of storage at +5 °C. This study used a candidate RSV vaccine for the evaluations11. Vaccine potency was assessed by functional infectivity assay. Other assessments of vaccine stability included those for capsid integrity, particle content, and DNA release. Vaccine efficacy and safety were assessed after two years in a murine model of RSV challenge and a rabbit toxicology model, respectively. Here we show that the potency loss from lyophilisation is 0.12 log10. The potency loss over five years at 5 °C is estimated at 0.21 log10 (95%CI 0.10–0.30). This coincides with a 25% increase in the ratio of non-infectious particles/infectious particles. After two years of storage at 5 °C, (i) the loss of infectivity is 0.17 log10; (ii) the vaccine remains immunogenic and effective at clearing RSV from the lungs in a mouse-challenge model; and (iii) the vaccine is not associated with any adverse safety signal in a rabbit toxicology model. Therefore, the five-year stability of the lyophilised adenovirus-vector vaccine is within our acceptable limit and meets our objective.
Methods
Formulation, filling and lyophilisation
The virus vector platform was based on a replication-defective (E1 gene deleted) chimpanzee adenovirus carrying the gene encoding for antigen(s). The basic matrix composition comprised sucrose (2% w/v), Tris (10 mM, pH8.5), histidine (10 mM), PS80 (0.02% w/v), and MgCl2 (1 mM). This matrix composition contained two other excipients (23% w/v trehalose and 5 mM NaCl)24,25.
Vaccines were stored in 3-ml glass vials (Müller & Müller, Holzminden, Germany), which had been siliconised (using a standard protocol) and sterilised by incubation at 260 °C for five hours. The vials were filled manually with 0.5 ml of the liquid vaccine formulations and partially stoppered with Datwyler stoppers. The stoppers included a vent that allowed the vapor to escape. Then, the vials were arranged on bottomless trays in a hexagonal configuration and loaded into the freeze-dryer. The bottom of the metallic tray was removed to allow a direct contact between the shelf and the vial.
A pilot-scale freeze dryer (Epsilon 2-25D, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) was used. The freeze dryer had seven shelves each measuring 0.27 m2, with a distance between shelves of 57 mm, a drying chamber volume of 0.38 m3, a duct between the chamber and the condenser, closed by a mushroom valve, a capacitance manometer to monitor the pressure inside the chamber, and a Pirani probe to follow the sublimation end point during primary drying. Tempris wireless temperature probes (iQ-mobil solution GmbH, Holzkirchen, Germany) were used to register the ice temperature.
The lyophilisation process included four phases (i) the freezing/annealing; (ii) primary drying; (iii) secondary drying, and (iv) unloading. The three main parameters that control the process include shelf temperature, step duration, and chamber pressure. Freezing (i) and unloading (iv) were performed at 1 bar, whereas the drying (ii and iii) was performed at low pressure (80 µbar and 40 µbar, respectively; Table 1)26,27.
The annealing step (− 10 °C for 2 h) in (i) was used to increase the size of ice crystals through structural relaxation of the frozen matrix by increasing the shelves temperature above the glass transition temperature of the product during the freezing phase (the transition point from amorphous material to crystalline material)28. This in turn should generate larger and spherical pores in the dry layer of the vaccine sample to facilitate the sublimation phase and improve the cake appearance.
Assessments of vaccine-virus integrity
The overall integrity of the vaccine-virus particles was assessed by three parameters: (i) capsid integrity (absence of released dsDNA), (ii) particle content, and (iii) functional infectivity. The third parameter also set the criterion for overall stability, including storage (≤ 0.3 log [twofold] loss in infectivity).
Three different vaccine batches were used in the analysis, The first batch (LS1) has been used at 5 °C (long term stability) and high temperature (25 °C and 37 °C). The second and third batches (respectively LS2 and LS3) have been used for long term stability (five years at 5 °C; Table 2). At each timepoint, six to nine samples (cakes in vials) were removed from the incubation and stored at −20 °C, to block the kinetics and prevent any further degradation. For a given time-series, all samples were analysed in the laboratory at the same time.
For the LS1 and LS3 batches, the dosage was 9 × 1010 pU/ml corresponding to high dosage and 50% overage. For the LS2 batch, the dosage was 1.2 × 1011 pU/ml corresponding to high dosage and 100% overage.
Capsid integrity
Double-stranded DNA concentration was measured using the Quant-IT Picogreen ds DNA Test Kit (Invitrogen) and multi-96 UV–bottom-transparent well plates (Corning, NY 14831, USA). Values were normalised in relation to a linear scale in which 0% was set as the batch at t = 0 was diluted to the same concentration of the sample analysed, and 100% was set as the value after that diluted batch had been incubated at 60 °C for 45 min.
Functional infectivity
A flow cytometry cell-based assay was used to quantify the number of infectious viral particles, in which 96-well plates (Thermo Scientific; Ref: 167008) pre-coated with poly-L-lysine (Corning; Ref: 354516) and seeded cells were incubated with serial dilutions of vaccine samples. Fluorescent (positively infected) cells were detected using MACSQuant VYB (Miltenyi Biotec) flow cytometer.
Particle content
The AEX-HPLC fluorometry (Waters Acquity UPLC H-class and Thermofisher U3000) was used to quantify viral particles in pU/ml. Viral particles were detected by light diffusion with a fluorescent detector and considered proportional to the concentration of the particles.
Quantitative polymerase chain reaction (qPCR)
The total amount of viral-vector DNA was measured by quantitative PCR (qPCR) using Roche LC480v2 SAP313054 CALPEN100903, MicroAmp Optical 96-well Reaction Plate with Barcode (Life Technologies).
Accelerated stability modelling
The modelling of vaccine infectivity (log scale) over time, considered the three different storage temperatures evaluated, and was based on the approach of Šesták-Berggren29. The integrated form of the ordinary differential equation was used (which resulted in a non-linear model), and three kinetic parameters were obtained after optimisation by Levenberg-Marquardt algorithm. The statistical intervals (confidence and prediction) were obtained by analytical formulae using the delta method. The fit was obtained by least squares using data from the LS1 batch, and validation used data from LS2 and LS3 batches. The intercept was also optimised for LS1. The three estimated kinetic parameters were then used to predict the loss of infectivity on batches LS2 and LS3 at 5 °C.
RSV challenge model
Animal husbandry and experiments were conducted by Aragen Bioscience (Morgan Hill, CA, USA) in accredited animal facilities following the protocol #17-0323, titled: “Non-GLP studies: Evaluating efficacy of antiviral biotherapeutics or the efficacy of vaccine candidates against RSV infection in mice approved by the local Institutional Animal Care and Use Committee (IACUC)”. Husbandry and experiments were ethically reviewed and performed in accordance with US laws/guidelines/policies for animal experimentation, housing, and care, and GSK’s Policy on the Care, Welfare, and Treatment of Animals.
The BALB/c mouse RSV-challenge model has been widely used to study the human respiratory disease and effects of vaccination30.
Female BALB/c aged 6–7 weeks old were purchased from Charles River. Only female mice were used to minimise territorial and aggressive behavior, which can lead to injuries and added stress and make it more challenging to maintain a stable and controlled environment for the study. The animals were housed in a temperature-controlled room with a 12 h light/dark cycle, with ad libitum access to autoclaved water and irradiated laboratory chow throughout the study. During the acclimation period, mice were house either up to ten per cage in large mouse cages or up to five per cage in small mouse cages that comply with the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) standards. During the in-life portion of the study, the mice were housed five per cage (same group). All animals received nesting material throughout the study. The animals were individually identified by ear tags. There was no evidence of aggression or cannibalism during the study. No criteria were set for excluding an animal from the study and no animal was excluded. No procedures were adopted to minimise potential confounders such as the order of treatments and measurements. No blind was administered.
In total 140 mice were used and randomly allocated to one of 12 groups (Table 3). This included eight groups of 15 mice and four groups of five mice. In the groups of 15 mice, five mice were used in the immunogenicity analysis and ten mice were used for the RSV challenge; six of which were used for assessing virus titres (right lung) and lung pathology (left lung). The remaining four mice were used for other analyses (not shown). The groups of five mice were used for the ELISpot analysis only. All vaccines and the PBS control were administered as two doses 3-weeks apart. Lyophilised ChAd155-RSV vaccines were resuspended in water for injection (WFI), and frozen ChAd155-RSV vaccine was thawed to ambient temperature. For the ChAd155-RSV vaccines, 50 μl was injected into right quadricep by an intramuscular route. For the formalin-inactivated RSV vaccine (FI-RSV Sigmovir Biosystems, Inc. MD, USA), 50 μl was injected to both quadriceps. RSV challenge was performed 2-weeks after the second dose, whereby 1 × 106 pfu of RSVA2 (ATCC- 1540, stock expanded at Aragen) in 70 μl was administered to both nares in animals that were lightly anesthetised with isoflurane.
Whole blood was drawn via cardiac puncture when the animals were deeply anesthetised and transferred to serum collection tubes (BD Biosciences cat# 365967; Table 3). Blood was allowed to clot for at least 15 min and then centrifuged to separate out serum. Serum was collected and divided into two aliquots and stored at –20 °C. After exsanguination by cardiac bleed and cervical dislocation, mouse spleens were collected and processed by grinding with a syringe plunger into single cell suspension. Red cells were depleted by incubating the spleen cells with PharmLyse lysing buffer. Cells were washed with RPMI-1640 + 3% HI-FBS and then were resuspended in complete culture medium to 107 cells/ml.
Collection of lungs for histology and viral quantification
Lungs were perfused through the right ventricle with PBS to remove blood from the lung vessels (Table 3). The right lungs were stored at −80 °C until analysed for virus load. The left lung was inflated with neutral buffered formalin for histology.
Viral quantification in lung
Lungs (0.2 g/ml) in ice-cold 1×MEM were homogenised in for 15 s and cleared by centrifugation. Serial dilutions of homogenates were titred on to Vero cells (ATCC; CCL-81) for 5-6 days incubation. Plaques were identified with a goat anti-RSV polyclonal antibody (Meridian), and subsequent incubation with a peroxidase-conjugated rabbit anti-goat IgG (Rockland) and TrueBlue peroxidase.
Lung pathology and data analysis
Prior to staining, paraffin-embedded lung-tissue sections (~ 5 μm thick) were rehydrated. Goblet cells were identified by periodic acid-Schiff (PAS) staining (five minutes in 0.5% periodic acid (Sigma P7875), two washes in deionised [d] H2O, 15 min in Schiff Reagent [Sigma 3952016], and a final rinse for five minutes in dH2O). For the staining of eosinophils, the sections were treated with pepsin for 15 min, prior to 60 min incubation with rabbit anti-MBP (Dr James Lee Mayo Clinic, 1:250). The primary antibody was detected by subsequent incubation with biotinylated goat anti-rabbit secondary, streptavidin peroxidase and AEC Single Solution. For quantitative analysis of PAS-positive tissue (goblet cells), the area of PAS-positive segmented tissue was normalised by the perimeter of the bronchi and bronchiole epithelium and expressed as the mean PAS load per mm basement membrane. Eosinophil count was expressed as mean number per volume of total lung tissue.
RSV-A neutralizing assay
Serial dilutions of serum (Table 3) in DMEM/3% FBS were incubated for 120 min at 35 °C with RSV A Long (ATCC, VR-26; 100 pfu/well). Samples were then transferred to plates previously seeded with Vero cells (ATCC; CCL-81) and incubated for two hours at 35 °C 5% CO2. Samples were then replaced by RSV medium containing 0.5% carboxymethylcellulose and incubated for 42 hours at 35 °C before staining. Staining was performed with mouse anti-RSV N and anti-RSV F monoclonal antibodies (AbD Serotec, MCA490 and MCA491G, 1:1000 dilution) followed by rabbit anti-goat IgG-horseradish peroxidase (HRP)-conjugated (Rockland 605-403-B69, diluted 1:1000) and TrueBlue substrate (KPL 71-00-68). Foci were counted using a CTL Immunospot S5 UV Analyzer. The neutralization titer was defined as the reciprocal of the highest interpolated serum dilution producing a 60% reduction in foci (ED50), relative to virus-control wells without serum. If a sample’s titer was below the first serum dilution tested, 1:20, it was assigned a titer of 10.
ELISpot
Single-cell suspension of splenocytes (Table 3) were analysed for IFN-γ–secreting cell number using an ELISpot assay (Mabtech 3321-4APW) according to the manufacturer’s protocol. Briefly, 2.5 × 105 cells/well were incubated with (i) media alone; (ii) F peptide pool; or (iii) N pool + M2 pool. Peptide pools were each used at 5 μg/ml. ConA at 4 μg/ml (Sigma, cat#CO412) was utilised as a positive control for IFN-γ expression and detection. Plates were incubated at 37 °C, 5% CO2 at least 36 h, followed by spot detection. Spot counts per well were obtained with an ELISpot reader.
Mycoplasma testing
The method used for mycoplasma testing aimed to confirm or deny the presence of mycoplasma in the culture supernatants being evaluated. The detection method relies on identifying certain intracellular enzymes, such as ATP synthases or adenylate kinase, which are present in high quantities in mycoplasmas. The presence of these enzymes in the evaluated culture supernatants indicates potential contamination. The procedure involves adding the “MycoAlert Reagent” from the MycoAlert Mycoplasma Detection Kit (Lonza catalog LT07-218), which causes lysis of any mycoplasmas present in the supernatants, thereby releasing their enzymes into the medium. Subsequently, the “MycoAlert substrate” is added. The combination of mycoplasma enzymes and the substrate catalyzes the conversion of ADP to ATP. By measuring ATP levels before (Read A) and after (Read B) the addition of the “MycoAlert substrate,” a ratio indicating the presence or absence of mycoplasma can be obtained. If these enzymes are not present, the second reading will show no increase compared to the first, whereas the reaction of mycoplasma enzymes with their specific substrates in the “MycoAlert Substrate” leads to elevated ATP levels.
Repeat-dose toxicology
Animal husbandry and experiments were ethically reviewed by the CiToxLAB France Ethical Committee and carried out in accordance with the European Directive 2010/63/EU and the repeat-dose toxicology study was performed in accordance with the OECD Principles of Good Laboratory Practice using male and female (1:1) rabbits obtained from Charles River Labs International Inc. (Raleigh, NC, USA). Twenty animals were randomly allocated to the two groups (PBS-control or lyophilised ChAd155-RSV resuspended in WFI). Male and female animals in each group were randomly allocated (1:1) to two subgroups (Subgroups A and B). Animals in Subgroup A were subject to necropsy three days after Dose 2, whereas animals in Subgroup B were subject to necropsy 28 days after Dose 2.
Animals were group-housed (same sex and same group) in polyethylene cages containing appropriate bedding, with the temperature at 18 °C to 24 °C, humidity at 30% to 70%, and under a cycle of 12 h light and 12 h dark. Where possible, control group animals were housed on a separate rack from the animals of the test-item groups. Animals were fed ad libitum with access to tap water, treated by reverse osmosis and ultraviolet irradiation. For psychological/environmental enrichment, animals were provided with items such as a hiding device, a chewing object, and occasional treats.
Rabbits received two doses two weeks apart of the lyophilised formulation. Each dose was administered as two 500 µl intramuscular injections in the right and left thigh muscles of the hind limbs, respectively, whereby the Dose 2 injection sites were more proximal than the Dose 1 injection sites. The injection site was shaved not more than one day before administration and disinfected with 70% ethanol just prior to injection. The dose administered to the rabbits was considered as the planned human dose (7 × 1010 pU), equating to an exposure of approximately 16-fold the highest planned human dose. Macroscopic and microscopic examinations were performed as standard. Vaccine and control groups each included 20 animals (male and female at 1:1 ratio) which subjected to detailed post-necropsy examinations, either three days (10 animals) or four weeks (10 animals) after dosing. No criteria were set for excluding an animal from the study and no animal was excluded. No procedures were adopted to minimise potential confounders such as the order of treatments and measurements. No blind was administered.
Statistics and reproducibility
All statistics used in the study were descriptive (with point estimates and confidence intervals when appropriate), and no formal hypotheses were evaluated. Hence the sample sizes/replicates used in the vaccine-stability assessments and animal models are in line with those used in earlier studies.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Potency loss with storage up to five years
The lyophilization process led to an average loss of viral infectivity (IU) of 24% (95%CI 20–29) and loss of viral-particle (pU) content of 27% (95%CI 25–29). This corresponded to a log10 loss in infectivity of 0.12 (95%CI 0.10–0.15). For each time series, a further loss of viral infectivity and particle content occurred over the storage period. As expected, for incubations at 37 °C and 25 °C, these losses were more rapid than at 5 °C (Fig. 1a). When the different time series were considered together and normalized with respect to baseline infectivity of the lyophilized formulations being 9 × 109 IU/ml then the average log10 loss over five years at 5 °C was estimated at 0.21 (95%CI 0.10–0.30). Therefore, the log10 loss of infectivity from five-year storage at 5 °C was below the 0.3 acceptable margin, and the log10 loss of infectivity from lyophilisation and two-year storage at 5 °C was estimated at 0.17. For incubations at 37 °C and 25 °C, a combined log10 loss in infectivity of 0.3 was estimated to have occurred within one month and three months, respectively (Fig. 1b).
Vaccine infectivity was modelled over time by a non-linear statistical fit of vaccine infectivity data collected at different storage time points and storage temperatures. a Individual plots for each of the three batches (LS1, LS2 and LS3; including 25 °C and 37 °C for LS1). b A summary plot where data from all batches are superimposed (after rescaling on the intercept of LS1). Vaccine infectivity was modelled over time by a non-linear statistical fit using the data from batch LS1. The 95% prediction and confidence intervals are illustrated by lighter and darker shading, respectively (N = 6–9; see Table 2 for numbers of replicates). Closed symbols represent the data points used for defining the models. Opened symbols represent the data points at later time points not used in the modelling. The symbol colours reflect the experimental series described in (a).
The loss in infectivity over five years at 5 °C coincided with an average 7% increase in free DNA concentration, 10% decrease in total virus particles, and a 25% increase in the ratio of non-infectious particles/infectious particles (Fig. 2). These rates appeared unaffected by the baseline concentration of infectivity, given that the two batches analysed were at 1.2 × 1011 pU/ml (LS2) and 9 × 1010 pU/ml (LS3).
The assessment was based on samples from batches LS2 (red triangles) and LS3 (blue circles), by evaluation of (a) the concentration of virus particles; (b) the ratio of free DNA (in supernatant after centrifugation) over total DNA; and (c) the ratio of all virus particles over infectious particles. Darker symbols represent mean values (N = 5–6; see Table 2 for numbers of replicates).
Lyophilised adenovirus vaccine was effective and immunogenic in murine RSV-challenge model
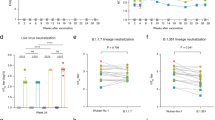
The lyophilised formulation of the ChAd155-RSV viral vaccine reconstituted prior to injection, either used directly after lyophilisation or after being stored at 5 °C for two years, was as effective and immunogenic as the unprocessed (control) formulation that had been stored frozen and thawed prior to injection (Fig. 3). This was shown in a mouse model of RSV challenge30, in which groups of mice were administered two intramuscular doses of ChAd155-RSV vaccine preparations (maximum dosage 1 × 108 pU), formalin-inactivated RSV vaccine formulation (FI-RSV), or PBS, three weeks apart, and challenged with intranasal dose of RSV two weeks later. Six mice per group were used in the analysis of vaccine effectiveness, and five mice per group were used in the analysis of immunogenicity.
The evaluation of vaccine integrity (ChAd155-RSV; 1 × 108 particles) after two years of storage at 5 °C (T24) was performed in comparison to the vaccine immediately after lyophilisation (T0), frozen at −80 °C and, in comparison to a formaldehyde-inactivated respiratory syncytial virus (RSV) vaccine (FI-RSV) and challenge RSV (RSVA2). Evaluations were performed in the RSV-challenge model in mice, where mice were vaccinated on Day 0 and 21 and challenged on Day 35 with RSVA2. (a) Viral load in the lung tissue at Day 40; (b) RSV-neutralizing titres on Day 35 and Day 40 (by effective dilution for 50% neutralization [ED50]) where each dot-plot corresponds to individual animals and the horizontal line represents the geometric mean for each group with the 95% confidence intervals; (c) RSV-specific IFN-γ-ELISpots from stimulated splenocytes harvested at Day 40 where each dot-plot corresponds to individual animals and the horizontal line represents the mean for each group with the standard deviation. (d) Pathology analysis of the lung tissue at Day 40, based on eosinophil staining (left graph) and mucus-producing goblet-cell induction (periodic acid Schiff [PAS] staining of basement membrane [BM], right graph) where each dot-plot corresponds to individual animals and the horizontal line represents the mean for each group with the standard deviation. Six mice per group were used in the analysis of vaccine effectiveness (a and d), and five mice per group were used in the analysis of immunogenicity (b and c).
ChAd155-RSV vaccine effectiveness was confirmed by clearance of challenge-RSV from the lungs (Fig. 3a). Among the four groups of lyophilised-vaccine recipients and frozen vaccine recipients, challenge-RSV was only detected in only one animal, at a level at least 10-fold lower than in the unvaccinated controls. Moreover, that single animal was a recipient of the higher dose of vaccine that was administered directly after lyophilisation was completed, rather than a recipient of a lower dose or a dose after two years of storage, that would have been anticipated if either of these factors were detrimental to vaccine potency. By contrast, challenge-RSV was detected in 4/6 FI-RSV recipients, illustrating the relatively poor effectiveness for virus removal of that vaccine.
ChAd155-RSV vaccine immunogenicity was confirmed by the induction of RSV-specific neutralizing titres (Fig. 3b), RSV-specific cell-mediated immune (CMI) activity in splenocytes measured by IFN-γ ELISpots (Fig. 3c) and was unaffected by lyophilisation or lyophilisation plus storage. Moreover, geometric mean neutralizing titres and geometric mean ELISpot frequencies were higher in the lyophilised and frozen ChAd155-RSV groups than in the FI-RSV group.
There was no evidence to suggest that the lyophilised or frozen ChAd155-RSV formulations could result in vaccine-enhanced respiratory disease, as has been identified with the FI-RSV formulation31. In contrast to FI-RSV, frozen and lyophilised formulations were not associated with inducing eosinophil recruitment or mucus production by goblet cell in the lungs (Fig. 3d).
Lyophilised adenovirus vaccine had an acceptable safety profile in a standard toxicology assessment in rabbits
The vaccine-related findings were transient and commensurate with a mild acute inflammatory reaction. These findings included transient elevated neutrophil count, fibrinogen concentration, and C-reactive protein concentration. Additional non-adverse findings included a transient elevated level of follicular germinal centre lymphocytes in the spleen and right iliac lymph nodes and a transient appearance of mixed cell infiltrate at the injection site. All observed changes had either fully or partially resolved by the end of the 28-day treatment-free (recovery) period.
Discussion
Having the option to prepare lyophilised forms of adenovirus-vector-based vaccines should increase the versatility of this vaccine platform, notably in providing vaccines in low-income countries where the cold chain is problematic, and for stockpiling vaccines in precautionary anticipation of epidemics1,2,3,4,5,6,7. Here, we identified an excipient composition and lyophilisation protocol that can provide up to five-year shelf-life for a simian-adenovirus–based vaccine.
The loss of potency from long-term storage of five years at 5 °C of 0.21 log10, primarily in terms infectivity and physical integrity, was within our acceptable threshold of 0.3 log10. This was also achieved with excipients that resulted in a relatively small potency loss (0.12 log10) from lyophilisation process, especially as certain excipients that stabilise lyophilisation can be incompatible with stabilising storage, reflecting two different properties required of the excipients4,32. Using our formulation protocol and after two years of storage at 5 °C, vaccine effectiveness, immunogenicity, and safety profile appeared unaffected in the preclinical challenge and toxicology models.
Our results compare favourably with three other studies on the lyophilisation and long-term storage of adenovirus vectors (ChAdOx13,14 and hAd515; Table 4). For lyophilisation, the ChAd155 formulation was at the better end of the log10-loss range, with a stability equivalent to that identified with the L1 formulation of hAd5-E10A (0.12 log10). For long-term storage, all vector formulations were within the 0.3 log10 loss-threshold. For lyophilised ChAdOx1 (-RVF and -luc), and ChAdOx2-RabG after one year of storage at 4 °C, infectivity losses were negligible and ≈0.2 log10, respectively13,14. For hAd5 after six months of storage at 4 °C, infectivity loss was 0.07 log1015.
Overall, the comparison of Ad-vector stabilities across the four studies suggested that the approaches to formulation are converging on the use of similar blends of excipients. It is also likely that the differences in excipient formulation, rather than the types of adenoviruses used in ours and the other studies, determined long-term stability, given that ChAd155 is derived from subtype C as with hAd5 and hAd6, and ChAdOx is derived from subtype E13,14,15,33. All approaches used excipients comprising inulin, mannitol, magnesium chloride and Tris buffer13,14,15. The combination of mannitol and inulin was considered as the major contributor to preserving vector infectivity during long-term storage. Our excipient formulation also privileged sugar components over sodium chloride and was similar to the more recent investigation of the ChAdOx vectors14, in which sodium-chloride omission and higher disaccharide concentration were identified as beneficial for lyophilisation. We considered that because of the known negative impact of sodium chloride on the glass transition temperature34, this aspect controlled the detrimental effects of osmotic pressure. Moreover, the use of trehalose disaccharide (di-glucose), which has shown promise as a thermostabilising agent35,36,37,38, (i) increased the melting temperature of the dried formulation ( ≈ 90 °C, data not shown), potentially providing a strong ability to resist elevated temperature excursions (stronger than that from sucrose32; data not shown), and (ii) functioned as a water replacement and cryoprotectant during the freeze-drying process.
Our protocol for adenovirus-vector formulation has been developed using facilities and techniques that can be immediately applied to producing Phase 2 clinical material. Although further steps would be required, notably, the optimisation of the primary and secondary drying parameters of the lyophilisation process39, the scale-up to manufacturing of the final commercial product should be relatively straightforward.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author (or other sources, as applicable) on reasonable request.
References
Lydon, P. et al. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull. World Health Organ 92, 86–92 (2014).
Ashok, A., Brison, M. & LeTallec, Y. Improving cold chain systems: Challenges and solutions. Vaccine 35, 2217–2223 (2017).
Pelliccia, M. et al. Additives for vaccine storage to improve thermal stability of adenoviruses from hours to months. Nat. Commun. 7, 13520 (2016).
Hansen, L. J. J., Daoussi, R., Vervaet, C., Remon, J. P. & De Beer, T. R. M. Freeze-drying of live virus vaccines: A review. Vaccine 33, 5507–5519 (2015).
Jarrett, S. et al. The importance of vaccine stockpiling to respond to epidemics and remediate global supply shortages affecting immunization: strategic challenges and risks identified by manufacturers. Vaccin. X 9, 100119 (2021).
Hosangadi, D., Martin, E. K., Watson, M., Bruns, R. & Connell, N. Supporting use of thermostable vaccines during public health emergencies: Considerations and recommendations for the future. Vaccine 39, 6972–6974 (2021).
AboulFotouh, K., Cui, Z. & Williams, R. O. 3rd Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration. AAPS PharmSciTech 22, 126 (2021).
Janssen-Cilag International NV. Jcovden (previously COVID-19 Vaccine Janssen): EPAR - Medicine overview. https://www.ema.europa.eu/en/documents/product-information/jcovden-previously-covid-19-vaccine-janssen-epar-product-information_en.pdf (2022).
Logunov, D. Y. et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396, 887–897 (2020).
Tapia, M. D. et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in adults in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 20, 707–718 (2020).
Cicconi, P. et al. First-in-Human Randomized Study to Assess the Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus (RSV) Vaccine Based on Chimpanzee-Adenovirus-155 Viral Vector-Expressing RSV Fusion, Nucleocapsid, and Antitermination Viral Proteins in Healthy Adults. Clin. Infect. Dis. 70, 2073–2081 (2020).
De Santis, O. et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 16, 311–320 (2016).
Berg, A. et al. Stability of Chimpanzee Adenovirus Vectored Vaccines (ChAdOx1 and ChAdOx2) in Liquid and Lyophilised Formulations. Vaccines (Basel) 9, https://doi.org/10.3390/vaccines9111249 (2021).
Zhang, C., Berg, A., Joe, C. C. D., Dalby, P. A. & Douglas, A. D. Lyophilization to enable distribution of ChAdOx1 and ChAdOx2 adenovirus-vectored vaccines without refrigeration. NPJ Vaccines 8, 85 (2023).
Chen, S. et al. Investigation on formulation and preparation of adenovirus encoding human endostatin lyophilized powders. Int J. Pharm. 427, 145–152 (2012).
Wang, C. et al. A simian-adenovirus-vectored rabies vaccine suitable for thermostabilisation and clinical development for low-cost single-dose pre-exposure prophylaxis. PLoS Negl. Trop. Dis. 12, e0006870 (2018).
Dulal, P. et al. Potency of a thermostabilised chimpanzee adenovirus Rift Valley Fever vaccine in cattle. Vaccine 34, 2296–2298 (2016).
Alcock, R. et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci. Transl. Med. 2, 19ra12 (2010).
LeClair, D. A., Cranston, E. D., Xing, Z. & Thompson, M. R. Evaluation of excipients for enhanced thermal stabilization of a human type 5 adenoviral vector through spray drying. Int J. Pharm. 506, 289–301 (2016).
Afkhami, S. et al. Spray dried human and chimpanzee adenoviral-vectored vaccines are thermally stable and immunogenic in vivo. Vaccine 35, 2916–2924 (2017).
Dulal, P. et al. Characterisation of factors contributing to the performance of nonwoven fibrous matrices as substrates for adenovirus vectored vaccine stabilisation. Sci. Rep. 11, 20877 (2021).
Jin, T. H., Tsao, E., Goudsmit, J., Dheenadhayalan, V. & Sadoff, J. Stabilizing formulations for inhalable powders of an adenovirus 35-vectored tuberculosis (TB) vaccine (AERAS-402). Vaccine 28, 4369–4375 (2010).
Pearson, F. E. et al. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One 8, e67888 (2013).
Mathot, F. S. & Vasselle, M. EP3814511 - Formulations For Simian Adenoviral Vectors Having Enhanced Stability. (2019)
Bourlès, E., Despas, O., Guillaume, D., Mathot, F. S. & Vasselle, M. WO2018138667A1 Novel Formulation for Stabilizing Adenovectors. (2018).
Bourlès, E. & Mathot, F. S. WO2017013169A1 Pharmaceutical Composition Comprising an Adenoviral Vector. (2017).
Levet, V. E., Mathot, F. S. & Vuylsteke, B. EP3833382 - Processes and Vaccines (2019).
Searles, J. A. in Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products (ed L. Rey) Ch. 3, 52-81 (Taylor & Francis Group, 2010).
Šesták, J. & Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 3, 1–12 (1971).
Castilow, E. M., Olson, M. R. & Varga, S. M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 39, 225–239 (2007).
Graham, B. S., Modjarrad, K. & McLellan, J. S. Novel antigens for RSV vaccines. Curr. Opin. Immunol. 35, 30–38 (2015).
Stewart, M., Ward, S. J. & Drew, J. Use of adenovirus as a model system to illustrate a simple method using standard equipment and inexpensive excipients to remove live virus dependence on the cold-chain. Vaccine 32, 2931–2938 (2014).
Colloca, S. et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4, 115ra112 (2012).
Nesarikar, V. V. & Nassar, M. N. Effect of cations and anions on glass transition temperatures in excipient solutions. Pharm. Dev. Technol. 12, 259–264 (2007).
Leung, V. et al. Thermal Stabilization of Viral Vaccines in Low-Cost Sugar Films. Sci. Rep. 9, 7631 (2019).
Gomez, M. et al. Evaluation of the stability of a spray-dried tuberculosis vaccine candidate designed for dry powder respiratory delivery. Vaccine 39, 5025–5036 (2021).
de la Torre Arrieta, J., Briceno, D., de Castro, I. G. & Roser, B. A thermostable tetanus/diphtheria (Td) vaccine in the StablevaX pre-filled delivery system. Vaccine 41, 3413–3421 (2023).
Archer, M. C. et al. Stressed stability and protective efficacy of lead lyophilized formulations of ID93+GLA-SE tuberculosis vaccine. Heliyon 9, e17325 (2023).
Scutella, B. & Bourles, E. Development of freeze-drying cycle via design space approach: a case study on vaccines. Pharm. Dev. Technol. 25, 1302–1313 (2020).
Acknowledgements
This work was sponsored by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study conduct and analysis. The costs associated with the development and publishing of the manuscript, including scientific-writing assistance, were provided by GlaxoSmithKline Biologicals SA. The authors thank Haifeng Song, Naveen Surendran and Ellen Kuta, Kunal Tungare, and Glomil Corbin (former employees of GSK, USA) who contributed to generating the preclinical data; Michel Protz (GSK, Belgium) for conducting the viral-infectivity assays; and Benjamin Dock, Brigitte Claes and Catherine Van Loo (all GSK, Belgium) for general laboratory technical support, Matthieu Vasselle (GSK, Belgium) who contributed to the design of the study, and Anne Mary (former employee of GSK, Belgium) for coordinating the analytical testing. Matthew Morgan (MG Science Communications, Belgium) and Badiaa Bouzya (GSK, Belgium) provided scientific writing services and advice. Ulrike Krause and Pascal Cadot (GSK, Belgium) provided editorial assistance and manuscript coordination.
Author information
Authors and Affiliations
Contributions
F.M., E.B., E.L. were involved in the conception/design of the study. D.G., E.L., B.F. participated in the acquisition and analysis of the data. F.M., E.B., E.L., D.G. supervised the design and analysis of experiments. B.F. created the core algorithm of the statistical model of the decline in infectivity over time. F.M., E.B., E.L., D.G. participated in the interpretation of data. All authors were involved in drafting the manuscript or revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
All authors are or were at the time of the study, employees of the GSK group of companies and may hold shares in GSK.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mathot, F., Lefebvre, E., Francq, B.G. et al. A lyophilised formulation of chimpanzee adenovirus vector for long-term stability outside the deep-freeze cold chain. Commun Med 5, 23 (2025). https://doi.org/10.1038/s43856-025-00740-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-00740-x