Abstract
Background
Cancer immunotherapy, particularly using immune checkpoint inhibitors, has revolutionized cancer treatment; however, its efficacy remains limited to a subset of patients. Nanoparticles have potential in cancer treatment because they offer advantages such as biocompatibility, greater stability, and precise targeting capabilities.
Method
We synthesized an integrated glyco-nanovaccine (iGN) comprising gold nanoparticles conjugated with a synthetic Toll-like receptor 7 (TLR7) ligand, sugar chains, and peptide antigens for cancer immunotherapy. The potential of iGN was investigated using a therapeutic animal model.
Results
In murine models, iGN effectively induces antigen-specific cytotoxic T cells, demonstrating prophylactic and therapeutic efficacy against tumor growth. iGN stimulates antigen-presenting cells via the TLR7–MYD88 pathway, enhancing antigen presentation and priming of cytotoxic T cells. Combination therapy with iGN and anti-PD-1 antibodies improves survival of tumor-bearing mice.
Conclusions
These findings underscore the potential of iGN as a strategy to enhance cancer immunotherapy, particularly when used in combination with immune checkpoint blockade, to bolster anti-tumor immune responses and improve therapeutic outcomes.
Plain language summary
Cancer immunotherapy, particularly using immune checkpoint inhibitors, has revolutionized cancer treatment; however, its efficacy remains limited to a subset of patients. To address this issue, we developed integrated glyco-nanovaccine (iGN) comprising gold nanoparticles conjugated with an antigen (target) and an adjuvant (increases potency), and evaluated its potential using a tumor-bearing mouse model. iGN therapy alone reduced tumor growth, and combinating this therapy with immunotherapy (anti-PD-1 antibodies) improved the survival of tumor-bearing mice. These findings underscore the potential of iGN as a strategy to enhance cancer immunotherapy, particularly when used in combination with immune checkpoint blockade, to bolster anti-tumor immune responses and improve therapeutic outcomes.
Similar content being viewed by others
Introduction
Cancer cells develop mechanisms to evade the immune system by exploiting immune checkpoints, which allow them to persist and proliferate within the tumor microenvironment. The use of immune checkpoint inhibitors (ICIs) has emerged as a highly effective approach for cancer immunotherapy. Patients who display a significant response to ICI therapy may achieve complete remission by eliminating primary as well as metastatic tumors1,2. However, these ICIs show effectiveness in a subset of patients3 depending on whether the tumor is composed of a “hot” or “cold” tumor microenvironment. A “hot” microenvironment is characterized by a high tumor mutation burden, which give rise to neoantigens and a high level of CD8+ T cell infiltration, often resulting in a favorable response to ICIs. In contrast, a “cold” microenvironment is characterized by a low level of infiltrating CD8+ T cells and a poor response to ICIs. Therefore, it is expected that the effectiveness of ICIs can be enhanced by converting a “cold” tumor to “hot” tumor4,5. Thus, enhancing immunogenicity, i.e., the ability of an antigen to provoke an immune response, may improve effectiveness of ICIs. Indeed, immunogenic cancer cell death induced by specific anti-cancer treatments, including chemotherapy and low-dose radiation therapy, leads to the release of damage-associated molecular patterns (DAMPs), triggering an immune response to enhance anti-tumor immunity6,7. Necroptosis is a programmed form of cell death that releases DAMPs into the extracellular space8,9. These DAMPs stimulate endogenous pattern recognition receptors (PRRs) signaling pathways, including the cGAS-STING pathway, which respond to self-DNA to enhance anti-tumor immunity and function as adjuvants in the initiation of an immune response against cancer10. Therefore, signaling pathways in cancer cells may modulate cancer cell immunogenicity and affect anti-tumor immunity11. We previously revealed that inhibition of the Hippo pathway, an evolutionarily conserved signal that plays pivotal functions in development and cancer biology12, suppresses tumor formation by enhancing the adaptive immune response13. Mechanistically, cancer cells having Hippo pathway mutations secrete nucleic acid-rich extracellular vesicles, which can stimulate the host Toll-like receptor (TLR)–type I interferon (IFN) pathway to activate anti-tumor immunity. Genetic evidence utilizing MYD88 (myeloid differentiation primary response 88), TRIF (TIR-domain-containing adaptor-inducing interferon-β, also known as TICAM1), and TLR knockout mice suggested that the host TLRs–MYD88/TRIF signal is important to induce anti-tumor immunity, prompting us to develop TLR ligand-containing nanoparticles to explore potential therapeutic strategies against cancer.
Given that systemic administration of TLR ligands often causes severe immune-related systemic adverse events14, it is important to develop a targeted delivery system to administer them in vivo. Gold nanoparticles (GNPs) have versatile biomedical applications as drug carriers because of their chemical stability, ease of surface modification, and safety in administration to humans15,16; these have been employed in radiotherapy, photothermal therapy, and gene therapy17,18,19. In previous studies, we have developed methods to conjugate a synthetic TLR7 ligand (1V209; 2-methoxyethoxy-8- oxo-9-(4-carboxy benzyl)adenine)20 and sugars to GNPs, and demonstrated that the resulting GNPs were highly dispersible under physiological conditions21. Administration of these GNPs showed potent adjuvant functions by inducing humoral immunity in mice; however, their effects on cellular immunity, especially against cancer, remain to be elucidated. In the present study, we aimed to investigate the therapeutic potential of an integrated glyco-nanovaccine consisting of a tumor antigen and adjuvant for cancer treatment.
Methods
Animals
Eight-week-old male and female C57BL/6 mice were purchased from Kyudo Co., Ltd (Saga, Japan) and maintained by in-house breeding. Myd88F/F (stock no. 8888)22 and Cd11c-Cre (also known as Itgax-Cre) (stock no. 8068)23 transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and crossed to generate Cd11c-Cre/Myd88F/F mice. These mice were backcrossed to a C57BL/6 background for over 10 generations. All animal experiments involved 7–12 weeks old male and female mice. All the animals were maintained under specific pathogen-free (SPF) conditions, and all the animal experiments were approved and performed in accordance with the guidelines of the Institutional Animal Committee of Kumamoto University.
Cell culture
Mouse T-lymphoblast EG7-OVA cells were kindly provided by Dr. H. Tsukamoto from the Department of Immunology, Kumamoto University. EG7-OVA cells were cultured in RPMI-1640 medium (Wako, 189-02025) supplemented with heat-inactivated 10% fetal bovine serum (FBS; Nichirei, 175012), penicillin (10,000 μ/ml), and streptomycin (10 mg/ml; Wako, 168-23191) under 5% CO2 at 37 °C. Mouse melanoma B16-OVA cells were kindly provided by Dr. Kenneth Rock from the Department of Pathology, UMass Chan Medical School24. B16-OVA cells were cultured in DMEM medium (Wako, 044-29765) supplemented with heat-inactivated 10% FBS, penicillin (10,000 μ/ml), and streptomycin (10 mg/ml) under 5% CO2 at 37 °C. Primary bone marrow-derived dendritic cells (BMDCs) were generated from C57BL/6, Myd88F/F, or Cd11c-Cre/Myd88F/F mice after 9 days of differentiation using 20 ng/ml granulocyte macrophage colony-stimulating factor (GM-CSF; Peprotech, AF-315-03-20)25. These cells were cultured using the same conditions mentioned above.
Nanoparticle synthesis
Thioctic acid (TA)-modified “ESIINFEKL” peptide (TA-ESIINFEKL), TA-SGLEQLESIINFEKL, and “HHHHHHSGLEQLESIINFEKL” peptides were purchased from Genscript Japan (Tokyo, Japan). TA with α-mannose (Manα1-6Glc-mPDA-TA), TA with 1V209 (1V209-TTDDA-TA), and TA with nitrilotriacetic acid (NTA-TA) were synthesized in the laboratory. For preparation of peptide-antigen-conjugated 1V209–αMan–GNPs [ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs) and SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs)], an aqueous solution of gold (III) sodium chloride (2.2 mM, 3450 µl; Nacalai Tesque) was dissolved in the aqueous solution of sodium borohydride (50 mM, 750 μl; Nacalai Tesque) and vigorously stirred for 10 min at room temperature. N,N-dimethylformamide (DMF, 1800 μl) was added to the solution, followed by addition of a 30% DMF aqueous solution of TA-ESIINFEKL or TA-SGLEQLESIINFEKL (0.15 mM, 750 μl). Subsequently, a 30% DMF aqueous solution containing 1V209-TTDDA-TA and Manα1-6Glc-mPDA-TA (1.35 mM, 750 μl, molar ratio of 1:8) was added, and the reaction mixture was further stirred for 30 min at room temperature. For preparation of αMan-GNPs or SGLEQLESIINFEKL-αMan-GNPs, a 30% DMF aqueous solution of TA-SGLEQLESIINFEKL or 1V209-TTDDA-TA was not added in above processes. The resulting solution of peptide-antigen-conjugated 1V209–αMan–GNPs was dialyzed in water using Visking Tubing 12,000–14,000 MWCO (molecular weight cutoff) to remove excess reagents21,26,27.
For preparation of [(HHHHHHSGLEQLESIINFEKL)Ni-NTA–1V209–αMan–GNPs (long peptide Ni-GNPs)], an aqueous solution of gold (III) sodium chloride (1.25 mM, 6000 μl) was transferred into a conical tube and an aqueous solution of sodium borohydride (50 mM, 750 μl) was added. After vigorously stirring for 10 min at room temperature, aqueous solution containing 1V209-TTDDA-TA, NTA-TA, and Manα1-6Glc-mPDA-TA (1.5 mM, 750 μl, molar ratio of 1:1:8) was added to the reaction mixture. After vigorously stirring for 30 min at room temperature, the resulting solution of NTA–1V209–αMan–GNPs was dialyzed in water with Visking Tubing (MWCO 12,000–14,000) to remove excess reagents. Next, a HEPES buffer solution of Nickel (II) chloride (2.75 mM, 750 μl) was added to the aqueous solution of NTA–1V209–αMan–GNPs. After stirring overnight at room temperature, the resulting solution of Ni-NTA–1V209–αMan–GNPs was purified by dialyzing in water using Visking Tubing (MWCO 12,000–14,000) to remove excess Nickel (II) chloride. The purified aqueous solution of Ni-NTA–1V209–αMan–GNPs was concentrated using a centrifugal evaporator. Next, the aqueous solution of “HHHHHHSGLEQLESIINFEKL” peptide was mixed with aqueous solution of Ni-NTA–1V209–αMan–GNPs. The molar ratio of 1V209 to peptide was 1:1.
1V209–αMan–GNPs were prepared as follows: an aqueous solution of borohydride solution (50 mM, 250 μl) was added to aqueous solution of gold (III) sodium chloride (NaAuCl4,1.8 mM, 1375 μl) with vigorous stirring, and the mixture was stirred at room temperature for 10 min. Next, DMF (625 μl) was added to the reaction mixture before adding a 30% DMF aqueous solution containing 1V209-TTDDA-TA and Manα1-6Glc-mPDA-TA (1.5 mM, 250 μl, molar ration of 1:9). The reaction mixture was further stirred for 30 min at room temperature. Crude 1V209–αMan–GNPs were purified by dialysis using Spectra/Por3 (MWCO 3,500) in 10% DMSO aqueous solution, followed by dialysis in water using Visking Tubing (MWCO 12,000–14,000)21.
For preparation of Alexa633–1V209–αMan–GNPs, an aqueous solution of NaAuCl4 (1.25 mM, 6000 μl) was transferred into a conical tube and aqueous solution of sodium borohydride (50 mM, 750 μl) was added to the solution. After vigorously stirring for 10 min at room temperature, the aqueous solution containing 1V209-TTDDA-TA, Alexa633-PEG23-TA, and Manα1-6Glc-mPDA-TA (1.5 mM, 750 μl, molar ratio of 1:1:8) was added to the reaction mixture. After vigorously stirring for 30 min at room temperature, the resulting solution of Alexa633–1V209–αMan–GNPs was dialyzed in water using Visking Tubing (MWCO 12,000–14,000) to remove excess reagents.
Fluorescence imaging of GNPs
BMDCs from C57BL/6 mice were seeded at 1.5 × 106 cells in a 24-well glass bottom plate (Iwaki, 5822-024), incubated for 2 h, and then treated with Alexa633–1V209–αMan–GNPs (containing 0.5 μM 1V209 and 4 µM α-mannose) for 15 min, 2 h, or 20 h. After removing excess GNPs, the cells were incubated with 50 nM LysoTracker Green DND-26 (Invitrogen, L7526) and 5 μg/ml Hoechst33342 (Sigma-Aldrich, B2261) in RPMI-1640, and then subjected to live cell imaging under 5% CO2 at 37 °C using a live-cell microscope incubation system (Tokai Hit, INUBG2TF) and FLUOVIEW FV3000 microscope (Olympus, 60 × oil objective lens).
Reverse transcription and quantitative PCR (RT-qPCR) analysis
BMDCs from Myd88F/F or Cd11c-Cre/Myd88F/F mice were seeded at 1 × 106 cells in a 12-well plate, incubated for 2 h, and then treated with 1V209–αMan–GNPs (containing 1 μM 1V209 and 8 µM α-mannose) for 4 h. Total RNA was extracted from floating and adherent cells using ISOGEN (Nippon Gene, 319-90211), and complementary DNA (cDNA) was synthesized using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, FSQ-301) following the manufacturer’s protocol. The cDNA was quantified by real-time PCR (Applied Biosystems, Step One Plus) using Thunderbird Next SYBR (Toyobo, QPX-201). The sequences of qPCR primers (forward and reverse, respectively) used were: 5′-GCCTCCTTCTTGGGTATGG-3′ and 5′-AGGTCTTTACGGATGTCAACG-3′ for mouse Actb; 5′-TCGCTCAGGGTCACAAGAAA-3′ and 5′-ATCAGAGGCAAGGAGGAAAACAC-3′ for mouse Il1b; 5′-CCAGAGATACAAAGAAATGATGG-3′ and 5′-ACTCCAGAAGACCAGAGGAAAT-3′ for mouse Il6; 5′-CCACAGATGACATGGTGAAGACG-3′ and 5′-TGGTTTGGTCCCGTGTGATG-3′ for mouse Il12a; 5′-TGAAACCAGCAGCCTTTGCTC-3′ and 5′-AGGCATTCAGTTCCAGGTCAGTG-3′ for mouse Ccl3; Expression of the target genes was normalized to the corresponding expression of Actb mRNA.
Flow cytometry analysis
For analysis of CD86 expression, BMDCs from C57BL/6 mice were seeded at 1 × 106 cells in a 12-well plate, incubated for 2 h, and then treated with 1V209–αMan–GNPs (containing 1 μM 1V209 and 8 µM α-mannose) for 24 h, followed by staining to perform flow cytometry analysis.
For analysis of IFNγ+CD8+ cells, C57BL/6 mice were injected intradermally at the base of the tail with each GNPs (containing 0.4 nmol 1V209, 0.4 nmol peptides, and 3.2 nmol α-mannose). Seven days post-immunization, spleens were dissected, and the splenocytes were incubated in RPMI-1640 medium with or without 10 μg/ml SIINFEKL peptide (Anaspec, AS-60193-1) for 5 h. One hour after incubation began, a protein transport inhibitor (1:1500; BD Biosciences, 555029) was added, and incubation continued for an additional 4 h. Cells were then stained for flow cytometry analysis.
For analysis of CD8+ T cell activation marker, C57BL/6 mice were injected intradermally at the base of the tail with each GNPs (containing 0.4 nmol 1V209, 0.4 nmol peptides, and 3.2 nmol α-mannose). Seven days post-immunization, spleens were dissected and treated with ACK lysing buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA pH8.6) for 5 min on ice, followed by staining for flow cytometry analysis.
Cell suspensions from the spleen, tumor tissue, and BMDCs were stained using the LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Invitrogen, L34976, 1:2000) for 15 min on ice. Next, the cells were washed with PBS and incubated with anti-CD16/CD32 antibody (Tonbo Biosciences, 70-0161, 1:200) for 15 min on ice to block the Fc receptor. The cells were then kept for 30 min on ice and stained to detect cellular surface markers using the following antibodies: anti-CD45 (PerCP-Cy5.5, BD Pharmingen, 550994, 1:200), anti-CD45 (FITC, BD Pharmingen, 553080, 1:200), anti-CD8a (eFluor450, eBioscience, 48-0081-82, 1:100), anti-CD44 (BrilliantViolet421, BioLegend, 103040, 1:200), anti-CD62L (PE/Cyanine5, BioLegend, 104410, 1:1600), anti-CD8a (FITC, eBioscience, 11-0081-82, 1:100), anti-CD3e (PE/Cyanine7, BioLegend, 100319, 1:300), anti-CD3 (PE, BioLegend, 100205, 1:300), anti-CD11c (eFluor450, eBioscience, 48-0114-82, 1:200), anti-CD11c (PE, eBioscience, 12-0114-81, 1:200), anti-CD86 (PE, Biolegend, 105007, 1:200). For intracellular staining, the cells were fixed and permeabilized with Foxp3/Transcription Factor Staining Buffer Set (eBioscience) at room temperature for 30 min (Invitrogen, 00-5523), followed by staining at 4 °C with overnight incubation using the following antibodies: anti-IFNγ (APC, eBioscience, 17-7311-82, 1:300) and anti-Granzyme B (FITC, BioLegend, 515403, 1:200). For the confirmation of Myd88 deletion in CD11c+ cells, dead cells were stained with DAPI (Dojindo, 340-07971, 1:5000). Flow cytometry analysis was performed using BD FACSVerse (BD Biosciences) or CytoFLEX S (Beckman Coulter).
In vivo cytotoxicity assay
C57BL/6, Myd88F/F, or Cd11c-Cre/Myd88F/F mice were injected intradermally at the base of the tail with PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.4 nmol 1V209, 0.4 nmol SIINFEKL, and 3.2 nmol α-mannose). Seven days post immunization, spleens from naive C57BL/6 mice were dissected and treated with ACK lysis buffer for 5 min on ice. The resulting splenocytes (3 × 108) were pulsed with 8 μg/ml SIINFEKL peptide or 8 μg/ml of an irrelevant peptide (SARS-Cov-2-CD8, T cell epitope 4, GenScript) under 5% CO2 at 37 °C for 2 h. The pulsed cells were washed with PBS. SIINFEKL-pulsed cells and the irrelevant peptide-pulsed cells were labeled with 0.25 mM CFSE (Carboxyfluorescein succinimidyl ester; Thermo Fisher Scientific, C34554) in PBS and incubated under 5% CO2 at 37 °C for 10 min. FBS was added to each cell suspension to stop the labeling reaction with CFSE, followed by washing with PBS. The two types of pulsed cells were mixed at a 1:1 ratio, and 5 × 106 cells from the mixed cell suspension were intravenously injected into the immunized mice via the retro-orbital vein. Five hours after injection, spleens were dissected and treated with ACK lysis buffer for 5 min on ice. The splenocytes thus obtained were stained using the LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit and analyzed using CytoFLEX S. Percentage of specific killing was calculated as follows: Specific killing (%) = [1-“Sample ratio (GNP)”/“Average negative control ratio (PBS)”] ×100; “Sample ratio (GNP)”= CFSElow(SIINFEKL)/CFSEhigh (irrelevant peptide), which denotes individual value for each mouse immunized with GNP; “Average negative control ratio (PBS)” = CFSElow(SIINFEKL)/CFSEhigh(irrelevant peptide), which denotes average value for total number of mice immunized with PBS.
Tumor prevention by inoculation with vaccine
C57BL/6 mice were injected intradermally at the base of the tail with either PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.4 nmol 1V209, 0.4 nmol SIINFEKL, and 3.2 nmol α-mannose). Seven days post-immunization, EG7-OVA cells (5 × 105 cells) were subcutaneously transplanted in the right flank on the back of the immunized mice. Twelve days post-transplantation of EG7-OVA cells, SIINFEKL-specific IFNγ+CD8+ T cells derived from splenocytes were analyzed by flow cytometry as shown in “Flow cytometry analysis”. Tumor height and width were measured using a caliper every alternate day to calculate tumor volume (width2 × height × 0.523).
Therapeutic experiments
For therapeutic evaluation in EG7-OVA tumor-bearing mice, EG7-OVA cells (5 × 105 cells) were subcutaneously transplanted in the right flank on the back of C57BL/6 mice to establish a solid tumor (on day 0). To evaluate the therapeutic efficacy of iGN, mice were randomly divided into two groups (PBS and iGN), and treatment was started 5 days after tumor transplantation (average tumor size, ~20–30 mm3). Mice were injected intradermally at the base of the tail with PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.4 nmol 1V209, 0.4 nmol SIINFEKL, and 3.2 nmol α-mannose) thrice every 3 days (on days 5, 8, and 11). Tumor height and width were measured using a caliper every alternate day to calculate the tumor volume (tumor volume = width2 × height × 0.523). Tumor tissues were collected on days 14 and 17 to assess tumor-infiltrating CD8+ T cells or tumor cell death, respectively. To evaluate the combined therapeutic efficacy of iGN and anti-PD-1 antibodies, mice were randomized into four groups: PBS, anti-PD-1 alone, iGN alone, and iGN plus anti-PD-1). Treatments were initiated 5 days after tumor transplantation. Mice were injected intradermally at the base of the tail with PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.4 nmol 1V209, 0.4 nmol SIINFEKL, and 3.2 nmol α-mannose) thrice every 3 days (on days 5, 8, and 11). Anti-mouse PD-1 (100 μg/mouse) (BioXCell, BE0273, Clone: 29F.1A12) was intraperitoneally injected at day 7 and 11. Tumor height and width were measured using a caliper every other day to calculate tumor volume. For therapeutic experiments in B16-OVA tumor-bearing mice, B16-OVA cells (5 × 105 cells) were subcutaneously transplanted in the right flank on the back of C57BL/6 mice on day 0 to establish a solid tumor. To evaluate the therapeutic efficacy of iGN, mice were randomly divided into two groups (PBS and iGN), and treatment was started 5 days after tumor transplantation, when the average tumor size was ~20–30 mm3. Mice were injected intradermally at the base of the tail with PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.8 nmol 1V209, 0.8 nmol SIINFEKL, and 6.4 nmol α-mannose) five times every other day (on days 5, 7, 9, 11, and 13). Tumor height and width were measured using a caliper every alternate day to calculate the tumor volume (tumor volume = width2 × height × 0.523). The mice were sacrificed when the tumors reached 15 mm in diameter.
Analysis of tumor-infiltrating CD8+ T cells
Fourteen days post-transplantation, EG7-OVA tumor tissues were excised from PBS- or iGN-treated mice. Tumor samples pooled from 2 or 3 tumor-bearing mice were minced with scissors and dissociated employing MACS Octo Dissociator with Heaters (Miltenyi Biotec, 130-096-427) a tumor dissociation buffer comprising 30 μg/ml DNase I (Worthington, LS002139), 1 mg/ml collagenase type I (Wako, 035-17604), 0.6 μ/ml dispase II (Gibco, 17105041) in RPMI-1640 medium supplemented with 2% heat-inactivated FBS. The dissociated samples were passed through a 100 μm cell strainer (Sarstedt, 83.3945.070) and incubated with ACK lysis buffer for 5 min on ice. For analysis of IFNγ+CD8+ T cells, the tumor cells were incubated in the presence of 10 μg/ml SIINFEKL peptide in RPMI-1640 medium for 5 h at 37 °C under 5% CO2. A protein transport inhibitor (1:1500) was added 1 h after stimulation with the peptide. Tumor cells were stained as described in “Flow cytometry analysis”.
Histological analysis of iGN target cells
C57BL/6 mice were injected intradermally at the base of the tail with SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; containing 0.4 nmol 1V209, 0.4 nmol SIINFEKL, and 3.2 nmol α-mannose). Inguinal lymph nodes (LNs) were resected 72 h after the injection and embedded in O.C.T. compound (Sakura Finetek Japan, 45833). LN tissues were cut into 5 μm sections using a cryostat (Leica, CM1950). The sections were fixed with 10% formalin neutral buffer solution (Wako, 062-01661) for 15 min at room temperature and incubated with 1% BSA/PBS containing 0.3% TritonX-100 for 30 min at room temperature for blocking and permeabilization. Next, the sections were incubated with anti-CD11c antibody (final conc. 2.5 mg/ml, eBioscience, 14-0114-82) overnight at 4 °C, followed by detection using secondary antibodies conjugated with Alexa Fluor 594 (1:500 dilution, Jackson ImmunoResearch Laboratories, 127-585-160) and DAPI (1:2000 dilution, Dojindo Laboratories, D523) for 1 h at room temperature. Additionally, the sections were stained with hematoxylin (Sakura Finetek Japan, 8656) and eosin (Sakura Finetek Japan, 8659) (H&E) according to standard procedures. Images were acquired using the KEYENCE BZ-X800 fluorescence microscope equipped with 20× and 60× oil objective lenses, or using the FLUOVIEW FV3000 microscope (60× oil objective lens). The images were processed using Photoshop 2024 (Adobe) and a BZ-X800 Analyzer (KEYENCE).
RNA sequencing
BMDCs obtained from C57BL/6 mice were seeded at 1 × 106 cells in a 12-well plate and incubated at 37 °C for 2 h under 5% CO2. Next, the BMDCs were either treated or not treated with 1V209-αMan-GNPs 1V209–αMan–GNPs (containing 1 μM 1V209 and 8 µM α-mannose) for 4 h, followed by extraction of total RNA using RNeasy Mini Kit (Qiagen, 74104). The purity of RNA was measured using the 4150 TapeStation System (Agilent, G2992AA). Library preparation and sequencing were performed using Novogene (Tokyo, Japan). The libraries were sequenced using 150 bp paired end reads on the Illumina NovaSeq 6000 sequencing system. Fastp (version 0.23.2) was used to ensure high per-base-sequence quality of the reads. The sequenced reads were mapped to the Mus musculus genome (mm10) using STAR (version 2.7.10b). Raw count values were then quantified and filtered to obtain uniquely mapped reads. DEGs (adjusted p-value < 0.01, log2 fold change >2) were identified using DESeq2 (v.1.42.1). A volcano plot was constructed using Enhanced Volcano software (v.1.20.0). Gene Ontology (GO) analysis was performed using clusterProfiler software (version 4.10.1). Parameters were set to a p-value cutoff of 0.01 and q-value cutoff of 0.05 to identify significantly enriched GO terms. Gene Set Enrichment Analysis (GSEA) was conducted using clusterProfiler (version 4.10.1), with parameters set to a p-value cutoff of 0.05 to identify significantly enriched gene sets.
Immunoblot analysis
BMDCs from C57BL/6 mice were seeded at 1 × 106 cells in a 12-well plate, incubated for 2 h, and then treated with 1V209–αMan–GNPs (containing 1 μM 1V209 and 8 µM α-mannose) for 0, 15, 30, and 60 min. Equal amount of protein samples were resolved in SDS- PAGE under reducing conditions. Antibodies to phospho-IKKα/β (Ser176/180) (CST, 2697, 1:1000), IKKα (CST, 11930, 1:1000), IKKβ (CST, 8943, 1:1000), and β-Actin (CST, 12620, 1:2000) were used. Immunoblot signals were detected with chemiluminescent HRP substrate (Millipore, WBKLS0500), and images were obtained using ChemiDoc Touch imaging system (Bio-Rad Laboratories, 17001401JA).
Histological analyses of tumors
Tumor tissues were fixed with 10% formalin neutral buffer solution (Wako, 062-01661) overnight at 4 °C, followed by incubation in 70% ethanol overnight at 4 °C. The samples were embedded in paraffin using CT-Pro20 (GenoStaff). For H&E staining, the sections were deparaffinized and stained with hematoxylin (Sakura Finetek Japan, 8656) and eosin (Sakura Finetek Japan, 8659), according to standard procedures. For staining with the anti-CD8 antibody, the deparaffinized sections were incubated in a device (Decloaking Chamber NxGen, BIOCARE Medical, DC2012) in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH 8) for 15 min for antigen retrieval. The sections were then incubated with 0.3% H2O2 in methanol for 30 min at room temperature, followed by blocking with 1% bovine serum albumin (BSA,Wako, 018-15154) in PBS. The sections were stained with anti-CD8 antibody (1:400 dilution, CST, 98941) overnight at 4 °C, and then subjected to signal detection using VECTASTAIN Elite ABC HRP Kit (VECTOR, PK-6101) and Peroxidase Stain DAB Kit (Nacalai Tesque, 25985-50). Images were acquired using the KEYENCE BZ-X800 microscope (KEYENCE).
Evaluation of the efficacy of Myd88 deletion
Spleens were harvested from Myd88F/F or Cd11c-Cre/Myd88F/F mice and treated with ACK lysis buffer for 5 min on ice. Cells were then stained as described in the “Flow cytometry analysis” section. CD11c+ cells were sorted by gating the top ~1% of CD11c-expressing cells using a Cell Sorter SH800S (SONY). RNA extraction, RT, and qPCR analysis were subsequently performed on the isolated CD11c+ cells to assess the efficacy of Myd88 deletion, following the protocol descrived in the “RT-qPCR analysis” section.
Statistics
Statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, Inc., La Jolla, CA, USA). The statistical parameters and methods are described in the Figures and Figure legends. Statistical significance was set at p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Long peptide-antigen covalently conjugated to glyco-nanovaccine induces cytotoxic immune responses
We have previously developed adjuvant- and sugar-conjugated gold nanoparticles, denoted as 1V209–αMan–GNPs, containing a synthetic TLR7 ligand, 1V209, and a sugar chain, α-mannose21. 1V209 was immobilized on GNP via 4,7,10-Trioxa-1,13-tridecandiamine (TTDDA) and thioctic acid (TA), referred to as 1V209-TTDDA-TA (Supplementary Fig. 1a). α-Mannose was immobilized on GNP via glucose, m-phenylendiamine (mPDA) and TA, referred to as Manα1-6Glc-mPDA-TA (Supplementary Fig. 1b). α-Mannose on GNP serves two functions: First, it enhances the uptake of nanoparticles by antigen-presenting cells (APCs)21, which express PRRs for sugar chains commonly found on the surface of viruses and bacteria28. Second, while unmodified GNPs without α-mannose cannot disperse in aqueous media, the abundant hydroxyl groups on α-mannose confer hydrophilicity, thereby enhancing nanoparticle dispersibility21. We previously demonstrated that 1V209–αMan–GNPs exhibit potent adjuvant activity in mice21, prompting us to hypothesize that these GNPs may be utilized as a cancer vaccine. To test this hypothesis, we newly synthesized a peptide-antigen-conjugated 1V209–αMan–GNPs to enhance an antigen-specific immune response. We utilized the chicken ovalbumin (OVA) peptide “SIINFEKL”, which binds to the mouse H-2Kb major histocompatibility complex (MHC) class I molecule, as a model antigen to monitor immune responses directed against a specific antigen.
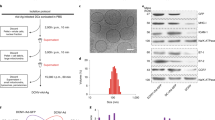
Given that both peptide length and formulation have a significant impact on the immune response29,30, we first examined the effects of peptide length and its formulation methods on vaccine efficacy. We used two different lengths of “SIINFEKL” peptides, consisting of the short peptide sequence “ESIINFEKL” and the long peptide sequence “SGLEQLESIINFEKL”. These peptides were conjugated to 1V209–αMan–GNPs using TA (Supplementary Fig. 1c, d), resulting in the formation of two distinct GNPs: ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs) and SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs) (Fig. 1a). To ensure proper dispersibility in aqueous media, the molar ratio of the components on each GNP was set as follows: peptide (ESIINFEKL or SGLEQLESIINFEKL): 1V209: α-Mannose = 1: 1: 8 (Supplementary Fig. 2a). Transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses revealed that the SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs) were slightly larger than the ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs), with an average diameter of 13.7 nm and 9.3 nm, respectively, as determined by DLS (Supplementary Fig. 2b and c). To evaluate vaccine efficacy, C57BL/6 mice were immunized with these GNPs intradermally at the base of the tail. On day 7 post immunization, splenocytes were isolated, stimulated ex vivo with the “SIINFEKL” peptide, and analyzed by flow cytometry (Fig. 1b). Live cells were gated on CD45+CD8+, and antigen-specific CD8+ T cell responses were assessed by measuring interferon-gamma (IFNγ) expression upon antigen stimulation (Supplementary Fig. 3a). We found a marked increase in the population of IFNγ+CD8+ T cells in the SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs) group compared to the ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs) group (Fig. 1c), indicating that the longer peptide antigen was more effective in inducing antigen-specific CD8+ T cells. Importantly, antigen peptide pulsing was essential for IFNγ induction, confirming that the observed responses were antigen-specific and not due to bystander activation (Supplementary Fig. 3b).
a Schematic diagram of nanoparticles ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs) and SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs). Schematic diagrams of components conjugated to each nanoparticle are shown in the gray box. b Experimental schedule for evaluating immune response induced by nanoparticles. Splenocytes were analyzed by flow cytometry at 7 days after vaccination with each type of nanoparticle (0.4 nmol 1V209/injection). c Expression of interferon-gamma (IFNγ) in CD8+ T cells derived from splenocytes stimulated with SIINFEKL peptide (10 μg/ml) was analyzed by flow cytometry 7 days after vaccination in mice immunized with ESIINFEKL–1V209–αMan–GNPs (short peptide GNPs) or SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs). Representative scatterplots of the gated CD8+ T cells are shown in the right panel. Data are presented as means ± SEM; n = 6 mice for the short peptide GNPs group, n = 7 mice for the PBS or long peptide GNPs group. ***p < 0.001, One-way ANOVA test followed by Tukey’s multiple comparison test. d Schematic diagram of (HHHHHHSGLEQLESIINFEKL)Ni-NTA–1V209–αMan–GNPs (long peptide Ni-GNPs). e Expression of IFNγ in CD8+ T cells derived from splenocytes stimulated with SIINFEKL peptide (10 μg/ml) was analyzed by flow cytometry 7 days after vaccination in mice immunized with (HHHHHHSGLEQLESIINFEKL)Ni-NTA–1V209–αMan–GNPs (long peptide Ni-GNPs). The percentage of IFNγ+CD8+ T cells in PBS or SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs) in Fig. 1c is shown in light gray or light pink, respectively, for reference. Data are presented as means ± SEM; n = 7 mice for each group. ***p < 0.001, One-way ANOVA test followed by Tukey’s multiple comparison test. f Schematic diagram of nanoparticles: αMan–GNPs, SGLEQLESIINFEKL–αMan–GNPs, and 1V209–αMan–GNPs. g Expression of IFNγ in CD8+ T cells derived from splenocytes stimulated with SIINFEKL peptide (10 μg/ml) was analyzed by flow cytometry at 7 days post vaccination in mice immunized with αMan–GNPs, SGLEQLESIINFEKL–αMan–GNPs, 1V209–αMan–GNPs, or SGLEQLESIINFEKL–1V209–αMan–GNPs (iGN; integrated glyco-nanovaccine). Data are presented as means ± SEM; n = 6 mice for each group. ***p < 0.001, One-way ANOVA test followed by Tukey’s multiple comparison test. h Mice were immunized with PBS or iGN (0.4 nmol 1V209/injection) as described in Fig. 1c and were then subjected to flow cytometry to analyze CD44 and CD62L expression at 7 days after vaccination. Representative scatterplots of the gated CD8+ T cells are shown in the right panel. Data are presented as means ± SEM; n = 7 mice for each group. **p < 0.01, unpaired t test. i Schematic representation of in vivo cytotoxicity assay using carboxyfluorescein succinimidyl ester (CFSE) labeling is shown in the gray box. Splenocytes pulsed with SIINFEKL peptide or irrelevant peptide were labeled with low or high concentration of CFSE, respectively. The two types of CFSE-labeled splenocytes were mixed at a 1: 1 ratio, followed by injection into mice immunized with PBS or iGN (0.4 nmol 1V209/injection). Five hours after the injection, splenocytes from immunized mice were analyzed to measure the ratio of CFSE-low and -high by flow cytometry. Antigen-specific killing activity was calculated and is shown at the right. Data are presented as means ± SEM; n = 5 mice for PBS group, n = 10 mice for iGN group. ***p < 0.001, unpaired t test.
We next investigated the effects of peptide formulation methods on vaccine efficacy. To this end, the longer peptide antigen “SGLEQLESIINFEKL” was either covalently conjugated to GNPs using TA or non-covalently bound to GNPs via nickel–histidine interaction. Nickel ions can bind to the metal chelator nitrilotriacetic acid (NTA), with the unoccupied coordination sites available for hexahistidine binding31. To formulate the antigen peptide on GNPs, the nickel-NTA complex is covalently conjugated to GNPs using TA, referred to as Ni-NTA-TA, while the peptide sequence “HHHHHHSGLEQLESIINFEKL” was non-covalently bound to GNPs via nickel–histidine interaction (Supplementary Fig. 4a). The proportions of each component on GNPs were identical to those in the short and long peptide GNPs (Supplementary Fig. 4b). The resulting (HHHHHHSGLEQLESIINFEKL)Ni-NTA–1V209–αMan–GNPs (long peptide Ni-GNPs) were used for further evaluation (Fig. 1d). While the size of (HHHHHHSGLEQLESIINFEKL)Ni-NTA–1V209–αMan–GNPs (long peptide Ni-GNPs) was almost comparable to that of SGLEQLESIINFEKL–1V209–αMan–GNPs (long peptide GNPs) (Supplementary Fig. 4c), vaccination experiments revealed that induction of antigen-specific CD8+ T cells was reduced by approximately tenfold in the long peptide Ni-GNPs group compared with the long peptide GNPs group (Fig. 1e). Therefore, our preliminary experiments suggest that the longer peptide antigen covalently conjugated to 1V209–αMan–GNPs using TA, SGLEQLESIINFEKL–1V209–αMan–GNPs, is more potent in inducing antigen-specific CD8+ T cells in vivo. Consequently, SGLEQLESIINFEKL–1V209–αMan–GNPs was selected for further investigation and is referred to as the integrated glyco-nanovaccine (iGN) hereafter (Fig. 1a, right).
To evaluate the contribution of each iGN component (SGLEQLESIINFEKL, 1V209, αMan, and GNPs) to antigen-specific CD8+ T cell responses, we compared immune responses induced by different GNP formulations, including αMan–GNPs (Fig. 1f left), SGLEQLESIINFEKL–αMan–GNPs (Fig.1f middle), and 1V209–αMan-GNPs (Fig.1f right). Since unmodified GNPs without α-mannose tend to aggregate in aqueous solution (Supplementary Fig. 5a), all GNP formulations included α-mannose. Our results showed that only the iGN increased significantly increased IFNγ+CD8+ population (Fig. 1g), indicating that both 1V209 and “SGLEQLESIINFEKL” peptides are necessary for inducing antigen-specific CD8+ T cell responses. Notably, the immune response induced by αMan–GNP, SGLEQLESIINFEKL–αMan–GNP, or 1V209–αMan–GNP were comparable to those induced by PBS, prompting us to use the PBS group as a control in subsequent experiments.
We subsequently investigated the effects of iGN on cytotoxic T cell activity. Consistent with high IFNγ production in CD8+ T cells isolated from mice treated with iGN (Fig. 1c), the effector CD8+ T cell population, as represented by CD44+CD62L– expression (Supplementary Fig. 5b), was increased in the iGN-treated group compared with the control PBS-treated group (Fig. 1h). Consistently, in vivo cytotoxicity assays using carboxyfluorescein succinimidyl ester (CFSE) labeling revealed a significant increase in antigen-specific killing activity of CD8+ T cells in iGN-immunized mice (Fig. 1i). Taken together, our data indicate that the adjuvant- and antigen-integrated vaccine, iGN, is a potent inducer of antigen-specific cytotoxic T cells in vivo.
Inoculation with iGN prevents tumor growth
Given that inoculation with iGN enhanced host antigen-specific cellular immunity in mice, we hypothesized that iGN might protect the host from a challenge with cancer cells expressing the same antigen. To test this hypothesis, we took advantage of the murine syngeneic tumor model of EG7-OVA, a mouse T lymphoma cell line expressing OVA32. C57BL/6 mice were injected intradermally at the base of the tail with iGN, and 7 days after inoculation, EG7-OVA T lymphoblasts were transplanted subcutaneously in the right flank on the back of the mice (Fig. 2a). Twelve days post-inoculation (corresponding to 5 days post transplantation), tumor engraftment was evident in all the PBS-treated mice but not in iGN-inoculated mice (Fig. 2b). Until day 19 (corresponding to 12 days post transplantation), no apparent tumor growth was observed in mice immunized with iGN (Fig. 2b, c). These results suggest that iGN inoculation enhanced host anti-tumor immunity and prevented tumor growth in mice. Notably, in our experimental setting, a single dose of vaccination with iGN was not sufficient to completely protect against tumor growth. Approximately 33% of the mice immunized with iGN remained tumor-free for 40 days when challenged with EG7-OVA cells. In contrast, all the mice in the PBS control group died before day 27 (Fig. 2d). A possible explanation for incomplete protection by iGN would be gradual reduction in antigen-specific immune response; while we consistently observed a significant increase in the IFNγ+CD8+ T cell population in the iGN-inoculated group compared to the control PBS-treated group at 19 days post inoculation (Fig. 2e), the proportion of IFNγ+CD8+ T cells decreased compared to that observed at 7 days post inoculation (Fig. 1c), approximately from 5% to 1.5%. Nevertheless, these observations suggest that iGN strongly enhances host anti-tumor immunity and prevents tumor growth in vivo.
a Experimental schedule for evaluating vaccine efficacy of iGN in the murine syngeneic tumor model of EG7-OVA. Seven days after vaccination with iGN (0.4 nmol1V209/injection) or PBS control, EG7-OVA cells (5 × 105) were subcutaneously (s.c.) transplanted into the right flank on the back of C57BL/6 mice. Nineteen days after vaccination, tumor weight was determined, and splenocytes were subjected to flow cytometry analysis. b Tumor growth was monitored in each group from 12 to 19 days post inoculation. n = 12 mice per group. c Tumor weights are demonstrated in the top panel and representative images of EG7-OVA tumors on day 19 post inoculation are shown in the bottom panel. Data are presented as means ± SEM; n = 12 mice for each group. ***p < 0.001, Mann Whitney test. Scale bar, 10 mm. d Kaplan–Meier survival curves of mice vaccinated and challenged as depicted in Fig. 2a are shown. n = 9 for each group. ***p < 0.001, log-rank test. e Expression of IFNγ in CD8+ T cells derived from splenocytes stimulated with SIINFEKL peptide (10 μg/ml) was analyzed by flow cytometry on day 19 post inoculation in mice immunized with iGN and challenged with EG7-OVA cells. Representative scatterplots of the gated CD8+ T cells are shown in the right panel. Data are presented as means ± SEM; n = 12 for each group. ***p < 0.001, unpaired t test.
Treatment with iGN increases tumor-infiltrating CD8+ T cells and inhibits tumor growth
Next, we explored the anti-tumor effects of iGN using a therapeutic model. Five days post-transplantation of EG7-OVA cells, iGN was administered intradermally at the tail base thrice every 3 days (on days 5, 8, and 11) (Fig. 3a). In the iGN-treated group, tumor size began to decrease by day 11, with significant tumor reduction observed by day 17 compared to the PBS-treated group (Fig. 3b), suggesting the therapeutic potential of iGN. Notably, it took ~7 days from the initiation of therapy to observe tumor reduction, suggesting that iGN treatment requires a certain amount of time to establish an adaptive immune response against cancer, typically taking about a week33. Consistent with tumor growth inhibition, histological analysis of tumor tissues revealed extensive tumor destruction (Fig. 3c) as well as infiltration of CD8+ T cells (Fig. 3d) in the iGN-treated group compared to controls. To further characterize CD8+ T cell infiltration and activation, we analyzed the tumor microenvironment on day 14 post transplantation, when tumor suppression in the iGN-treated group became apparent. Immune cells were distinguished from tumor cells using CD45 antibody, and cytotoxic T cells were identified by IFNγ and granzyme B (GzmB) expression (Supplementary Fig. 6a). Flow cytometry analysis revealed an increased number of tumor-infiltrating CD8+ T cells in the iGN-treated group compared to controls (Fig. 3e). Upon stimulation with the surrogate tumor antigen “SIINFEKL”, a marked increase in antigen-responding IFNγ+CD8+ T cells was observed iGN-treated tumors compared to controls (Fig. 3f). Similarly, the number of GzmB—key mediators of tumor cell apoptosis—was elevated in iGN-treated group compared to controls (Fig. 3g). These results demonstrate that iGN promotes the infiltration of tumor antigen-specific CD8+ T cells capable of mediating tumor destruction. To assess the broader applicability of iGN, we tested its therapeutic effect in the B16-OVA melanoma model using a modified protocol based on the EG7-OVA model (Supplementary Fig. 6b). Consistent with its efficacy in the EG7-OVA model, similar anti-tumor effects were observed in the B16-OVA model (Supplementary Fig. 6c), suggesting that iGN is effective across multiple tumor types, including more immunologically “cold” tumors.
a Experimental schedule for evaluating anti-tumor effects of iGN in a therapeutic model. EG7-OVA cells (5 × 105) were subcutaneously (s.c.) transplanted in the right flank on the back of C57BL/6 mice. Five days after transplantation, the mice were injected intradermally with either PBS or iGN (0.4 nmol 1V209/ injection) at the base of the tail thrice every 3 days (at day 5, 8, and 11). On day 14, tumors in each group were subjected to flow cytometry analysis for evaluating tumor-infiltrating CD8+ T cells. At day 17, the tumors were subjected to immunohistochemical analysis. b Tumor growth in each group was monitored from day 5 to day 17. Data are presented as means ± SEM; n = 14 mice per group. **p < 0.01, two-way ANOVA test. c Tumors were embedded in paraffin and stained with hematoxylin and eosin (H&E) 17 days after transplantation. Scale bars, 50 μm. d Paraffin sections of tumors were subjected to immunohistochemistry (IHC) analysis using anti-CD8 antibodies 17 days after transplantation. Immunocomplex signals (brown color) developed using 3,3′-Diaminobenzidine (DAB) were visualized; hematoxylin was used as a counterstain. Scale bars, 50 μm. e Percentage of tumor-infiltrating CD8+ T cells was quantified 14 days after transplantation. Data are presented as means ± SEM; n = 4 samples per group; each sample represents a pool of tumors obtained from 2–3 mice. *p < 0.05, unpaired t test. f Percentage of IFNγ expression in tumor-infiltrating CD8+ T cells was quantified 14 days after transplantation. Data are presented as means ± SEM; n = 4 samples per group; each sample represents a pool of tumors obtained from 2–3 mice. **p < 0.01, unpaired t test. g Percentage of granzyme B (GzmB) expression in tumor-infiltrating CD8+ T cells was quantified 14 days after transplantation. Data are presented as means ± SEM; n = 4 samples per group; each sample represents a pool of tumors obtained from 2–3 mice. *p < 0.05, unpaired t test.
iGN enhances transcriptional programs for cross-presentation in antigen-presenting cells
We next explored how iGN stimulates host anti-tumor immune responses. Because we observed a significant induction of antigen-specific cytotoxic T cells in mice injected with iGN (Fig. 1i), we hypothesized that iGN stimulates antigen presentation by antigen-presenting cells (APCs). Therefore, we first explored in vivo targets of iGN, especially in tumor-draining inguinal lymph nodes (LNs), where APCs promote antigenic priming of CD8+ T cells34. To this end, iGN was inoculated intradermally at the tail base of C57BL/6 mice, and the inguinal LNs were collected 3 days later. Histological analysis of inguinal LN tissues revealed that iGN accumulated in large cells, reminiscent of APCs (Fig. 4a). Indeed, immunofluorescence analysis confirmed that some iGN-incorporated cells express CD11c (also known as ITGAX), a typical marker of conventional dendritic cells (DCs)35 (Fig. 4b). These observations suggest that GNPs were partly incorporated into CD11c+ DCs in the inguinal LNs, thereby stimulating these cells to promote antigenic priming of CD8+ T cells.
a Hematoxylin and Eosin (H&E) staining of inguinal lymph nodes from C57BL/6 mice injected with iGN. The inset shows a corresponding higher-magnification view, and the arrowhead indicates accumulation of iGN. Scale bars, 50 μm. b Sections of inguinal lymph nodes from mice treated as in (a) were subjected to fluorescent immunohistochemical analysis using antibodies to CD11c. Arrowheads indicate cells incorporating iGN. Scale bars, 5 μm. c Differentially expressed genes (DEGs; fold change >2 and adjusted p-value < 0.01) in bone marrow-derived dendritic cells (BMDCs) treated with 1V209–αMan–GNPs (GNPs) compared to those in non-treated control BMDCs are displayed in a volcano plot (blue, downregulated; red, upregulated). Selected genes associated with dendritic cell activation are annotated. d Gene ontology (GO) analysis using the upregulated genes shown in (c). e Gene set enrichment analysis (GSEA) of the antigen processing- and cross-presentation-related gene set (MM14526). f BMDCs were treated (or not treated) with GNPs, followed by flow cytometry analysis for CD86 expression. Percentage of CD86 expression in CD11c+ cells was quantified. Data are presented as means ± SEM; n = 3 biologically independent samples per group. ***p < 0.001, unpaired t test.
Next, we investigated the effects of iGN on DC activation status. We obtained bone marrow-derived dendritic cells (BMDCs) from C57BL/6 mice and performed ex vivo experiments. Given that the synthetic TLR7 ligand 1V209 and the sugar moiety α-mannose dictate the major adjuvant function of iGN (SGLEQLESIINFEKL–1V209–αMan–GNPs)21, we used 1V209–αMan–GNPs (iGN without “SIINFEKL” peptide) (Supplementary Fig. 7a, b) for ex vivo experiments for the sake of convenience. BMDCs were stimulated with 1V209–αMan–GNPs and their transcriptome was characterized by RNA sequencing (RNA-seq) analysis. Our RNA-seq data are concisely depicted in a volcano plot (Fig. 4c), which highlights the genes exhibiting significant differential expression between GNP-treated BMDCs and their non-treated counterparts. The analysis revealed a substantial number of differentially expressed genes (DEGs), with 323 genes upregulated (Supplementary Data 1) and 179 genes downregulated (Supplementary Data 2) in GNP-treated BMDCs relative to control cells, all marked by a fold change threshold of >2 and an adjusted p-value threshold of <0.01. Among the upregulated genes were Il1b36, Il636, Il1237, and Ccl338,39, all associated with DC activation (Fig. 4c). Gene ontology (GO) analysis of the upregulated genes revealed enrichment in pathways related to cytokine production and immune activation (Fig. 4d), indicating that GNPs triggered immune-stimulatory gene programs in BMDCs. Furthermore, gene set enrichment analysis (GSEA) revealed that antigen processing and cross-presentation-related gene set (MM14526) was enriched in GNP-treated BMDCs (Fig. 4e), suggesting enhanced antigen-presenting capacity. Consistent with these findings, flow cytometry analysis revealed increased surface expression of CD86, a co-stimulatory molecule important for antigen presentation40, in BMDCs following GNP stimulation (Fig. 4f). Together, these results demonstrate that 1V209–αMan–GNPs activate BMDCs and enhance transcriptional programs associated with cross-presentation and immune activation.
iGN activates CD11c+ APCs via the TLR7–MYD88 signaling pathway
We next investigated the molecular mechanisms underlying the GNP-mediated DC activation. TLR7 ligands primarily initiate immune responses by binding to their cognate receptor, which signals through the adaptor protein myeloid differentiation primary response 88 (MYD88)36. Activation of the TLR7–MYD88 signaling pathway leads to the phosphorylation of IκB kinase (IKK) α/β, followed by the activation of transcriptional factors NF-κB and IRF741,42. Indeed, GSEA, using a NF-κB pathway-related gene set (MM5039), revealed that NF-κB signal transduction is enhanced in BMDCs treated with 1V209–αMan–GNPs (Fig. 5a). Consistently, immunoblot analysis showed that phosphorylation of IKKα/β increased as early as 30 min after stimulation (Fig. 5b and Supplementary Fig. 8a). Collectively, these results suggests that iGN activates the TLR7 signaling pathway in DCs.
a GSEA showing enrichment of genes related to the NF-κB signaling pathway (MM5039). b Immunoblot analysis of BMDC lysates stimulated with 1V209–αMan–GNPs for 0, 15, 30, and 60 min. Blots were probed with antibodies against the indicated proteins. c BMDCs were incubated with Alexa633–1V209–αMan–GNPs for 15 min, 2 h, or 18 h, followed by live imaging using Lysotracker and Hoechst. Arrowheads indicate BMDCs displaying GNP accumulation in lysosomes. Scale bars, 5 μm. d BMDCs derived from Myd88F/F mice (control) or Cd11c-Cre/Myd88F/F (Myd88ΔCD11c) mice were stimulated with 1V209–αMan–GNPs, and then subjected to reverse transcription and quantitative PCR (RT-qPCR) analysis for Il1b, Il6, Il12a, and Ccl3. Gene expression was normalized to that of Actb mRNA and displayed relative to GNP-stimulated control BMDCs. Data are presented as means ± SEM; n = 3 biologically independent samples per group. ns, not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, One-way ANOVA test followed by Tukey’s multiple comparison test. nd, not detected. e Control mice or Myd88ΔCD11c mice were immunized with either PBS or iGN, followed by in vivo cytotoxicity assay. Schematic representation of the experimental protocol is shown in the gray box. Data are presented as means ± SEM; n = 10 mice were used for PBS in control, iGN in control, and iGN in Myd88ΔCD11c groups; n = 9 mice for PBS in Myd88ΔCD11c group. *p < 0.05, ***p < 0.001, One-way ANOVA test followed by Tukey’s multiple comparison test.
NF-κB activation by TLR7 ligands is associated with their lysosomal localization, where TLR7 ligands, TLR7, and MYD88 together form a complex for downstream signal transduction43. To clarify the intracellular localization of GNPs in DCs, we synthesized an Alexa 633 fluorophore-conjugated 1V209–αMan–GNPs, namely Alexa633–1V209–αMan–GNPs (Supplementary Fig. 9a and 9b). DCs began internalizing the GNPs as early as 15 min post incubation, with prominent lysosomal accumulation evident by 2 h (Fig. 5c). By 18 h, most of the GNPs remained localized within lysosomes. These findings indicate that TLR7 ligand-conjugated GNPs are efficiently delivered to lysosomes, providing a platform for TLR signal activation. To determine whether GNP-induced NF-κB activation is mediated via MYD88, we generated mice lacking MYD88 specifically in CD11c+ DCs by crossing of Myd88F/F mice (which harbor floxed alleles of Myd88) with Cd11c-Cre transgenic mice (which express Cre recombinase under the regulation of the Cd11c promoter). The efficacy of Myd88 deletion was assessed in splenic CD11chigh cells from Cd11c-Cre/Myd88F/F (hereafter referred to as Myd88ΔCD11c) mice (Supplementary Fig. 10a). Reverse transcription and quantitative PCR (RT-qPCR) analysis revealed an 83% reduction in Myd88 mRNA levels, indicating partial but effective gene deletion (Supplementary Fig. 10b). We next generated BMDCs from Myd88ΔCD11c mice and evaluated the expression of NF-κB target genes following GNP stimulation. RT-qPCR analysis showed that the induction of Il1b and Il6 in response to iGN treatment was reduced in Myd88ΔCD11c BMDCs compared to control (Myd88F/F) BMDCs (Fig. 5d). Similarly, expression of Il12a and Ccl3—cytokines associated with DC activation—was also decreased in MYD88-deficient BMDCs. These results suggest that MYD88, if not solely, plays an important role in GNP-induced NF-κB signaling activation and downstream gene expression in DCs. To confirm these findings in vivo, we performed cytotoxicity assays in Myd88ΔCD11c and control mice. While iGN treatment induced strong cytotoxic T cell responses in control (Myd88F/F) mice, with an average specific killing of 95%, Myd88ΔCD11c mice exhibited a modest but significant reduction in cytotoxic activity, averaging 75% specific killing (Fig. 5e). We speculate that this residual activity may result from remaining MYD88-expressing APCs (Supplementary Fig. 10b), which are still capable of activating CD8+ T cells. Taken together, these observations suggest that iGN stimulates host anti-tumor immune responses, at least in part, by activating CD11c+ APCs via the TLR7–MYD88 signaling axis, thereby promoting efficient antigen priming and cytotoxic T cell activation.
Treatment with iGN improves survival of tumor-bearing mice
We next investigated whether iGN treatment contributes to a better prognosis in tumor-bearing mice. Five days after transplantation of EG7-OVA cells, iGN was administered intradermally at the base of the tail three times at 3-day intervals (on days 5, 8, and 11) (Fig. 6a). All mice in the PBS-treated group died before day 25, whereas treatment with iGN significantly improved survival and resulted in tumor-free survival in 7.7% (1 out of 13) of the mice (Fig. 6b). It is well characterized that IFNγ secreted by tumor-infiltrating CD8+ T cells upregulates the expression of programmed death-ligand 1 (PD-L1) on cancer cells44, thereby suppressing T cell activity through engagement with its receptor, programmed death-1 (PD-1). Given that treatment with iGN strongly induces IFNγ-expressing tumor-specific T cells to destroy cancer cells, we hypothesized that combinaing iGN with anti-PD-1 antibodies (αPD-1) might enhance its therapeutic efficacy. Indeed, co-administration of iGN and αPD-1 resulted in tumor-free survival in 46% of mice (6 out of 13) over a 60-day observation period (Fig. 6b). Collectively, these observations suggest that iGN not only suppresses tumor growth but also improves long-term survival in tumor-bearing mice, particularly when combined with immune checkpoint blockade.
a Experimental schedule for evaluating a combination therapy using iGN and anti-PD-1 (αPD-1) antibodies. EG7-OVA cells (5 × 105) were subcutaneously (s.c.) transplanted into the right flank on the back of C57BL/6 mice. Five days after transplantation, the mice were injected with PBS or iGN (0.4 nmol 1V209/injection) at the base of the tail thrice every 3 days (at days 5, 8, and 11). Anti-PD-1 antibodies (100 μg/mouse) were intraperitoneally administered (or not administered) on days 7 and 11. b Kaplan–Meier survival curves of mice treated with PBS, αPD-1 antibodies, iGN, or iGN in combination with anti-PD-1 antibodies. n = 12 mice for PBS and αPD-1 antibodies group; n = 13 mice for iGN and iGN + αPD-1 group. ***p < 0.001, log-rank test.
Discussion
In this study, we developed an integrated glyco-nanoadjuvant (iGN), SGLEQLESIINFEKL–1V209–αMan–GNPs, comprising gold nanoparticles conjugated with a synthetic TLR7 ligand, sugar chains, and peptide antigens. We showed that intradermal inoculation of iGN stimulates tumor-specific immune responses, demonstrating prophylactic and therapeutic effects in an EG7-OVA murine syngeneic tumor model. Mechanistically, we propose that iGN associates with antigen-presenting cells in tumor-draining lymph nodes to promote antigenic priming of CD8+ T cells, resulting in the induction of antigen-specific cytotoxic T cells and subsequent tumor destruction (Fig. 7). Furthermore, the therapeutic effect of iGN was enhanced by combined administration of ICIs, suggesting that a combination of cytotoxic T cell inducers and immune checkpoint blockers would be an ideal approach to enhance cancer immunotherapy. However, it is important to note that the immune system of mice differs significantly from that of humans. Thus, the extent to which our findings in mice can be applied directly to humans is yet to be determined. Nevertheless, future studies including material optimization, acquisition of pharmacokinetics in vivo, and therapeutic investigations of the human immune system, which may expand the therapeutic potential of integrated glyco-nanoadjuvants, will have important clinical implications.
Recently, nanoparticle-based drug delivery systems have been extensively employed for the management, prevention, and detection of various diseases. Nanoparticles provide distinct benefits, including biocompatibility, enhanced stability, lower toxicity, improved retention in the body, and precise targeting. Nanoparticles can be conjugated with various substances, including proteins, drugs, antibodies, enzymes, and nucleic acids. Additionally, the material, size, molecular weight, and surface charge can be varied. Therefore, modulation of physical characteristics in nanoparticles affects pharmacokinetics as well as the target cells of those particles45. GNPs stand out as promising drug carriers owing to their highly adaptable surface modification functionality. In our previous studies, we investigated the impact of the lengths of conjugating linkers and the types of sugar chains on GNP activity. We found that GNPs conjugated with 1V209 using polyethylene glycol (PEG) 23 showed better in vitro cytokine-inducing activity than those using TTDDA46. Similarly, a study suggested that the type of sugar structures on GNPs affects their immunostimulatory activities in vitro, with α-fucose showing higher type I IFN-inducing activity than α-mannose in J774A.1 cells28. As the spacer molecule and type of sugar chain used in our study were TTDDA and α-mannose, respectively, it would be interesting to investigate whether further optimization of the design of iGN may enhance its anti-tumor effects in future studies.
The method of drug administration plays a pivotal role in determining drug effectiveness as it directly influences the speed and extent of drug absorption. In the present study, we achieved both preventive and therapeutic effects of iGN through intradermal injection, a method known for its ability to efficiently trigger immune responses47,48. Our study confirmed the uptake of iGN by CD11c+ cells in inguinal LNs. We speculate that the GNPs introduced into the skin likely traveled to the inguinal LNs via lymphatic vessels, where they were taken up by resident APCs49. Alternatively, it is also possible that APCs abundant in the skin may have engulfed the GNPs, migrated to the LNs, and induced antigenic priming in the LNs50. To optimize the application of GNPs in cancer therapy in the future, it is important to explore alternative routes, such as intravenous administration. Baharom et al. recently demonstrated that the route of administration of a TLR7/8 agonist using self-assembling nanoparticles affects the nature of CD8+ T cells; intradermal injection tends to produce more terminally differentiated CD8+ T cells, whereas intravenous injection leads to the generation of stem-like CD8+ T cells51. Moreover, intravenous injection of nanoparticles diminishes the number of immunosuppressive monocytes, thereby reshaping tumor microenvironment and bolstering anti-tumor effects51. Therefore, the structure and properties of iGN need to be further optimized to confer robust therapeutic effects through systemic administration.
CD8+ T cells are activated by APCs, including DCs52 and macrophages53. In this study, we found that CD11c+ DCs preferentially internalize iGN in the mouse inguinal LNs (Fig. 4b). Additionally, our previous work demonstrated that mouse BMDCs, but not the murine macrophage cell line J774A.1, effciently accumulate 1V209–αMan–GNPs in the cytoplasm28. Given that DCs are the most potent and dominant APCs for CD8+ T cell induction, we utilized BMDCs in our ex vivo experiments. While our ex vivo data support that BMDCs can uptake iGN and activate the TLR7-MYD88 signaling pathway, we do not exclude the potential role of macrophages in vivo. Previous studies have shown that macrophages can internalize GNPs and mannose-labeled nanomaterials54, suggesting that our observations may reflect a context-dependent, rather than a general, pattern of uptake. Indeed, nanoparticle uptake efficiency is influenced not only by cell type but also by physicochemical properties of the particles, such as size, shape, and surface modifications. Further investigations employing in vivo deletion strategies—such as macrophage-specific diphtheria toxin expression—or adoptive transfer of specific APC populations will be valuable for identifying the specific APC subsets responsible for iGN uptake and for elucidating their distinct functional roles in iGN-mediated immune activation.
In conclusion, our findings highlight the potential of iGN as a promising tool in cancer immunotherapy. Further research and clinical trials are warranted to fully elucidate the therapeutic benefits and advance its application in the treatment of patients with cancer.
Data availability
The RNA-seq data generated in this study have been deposited in the DNA Data Bank of Japan (DDBJ) under accession number PRJDB18257. The source data are presented in Supplementary Data 3. All other raw data generated in this study are available from the corresponding author upon request.
References
Rizell, M. et al. Checkpoint Inhibition causing complete remission of metastatic combined hepatocellular-cholangiocarcinoma after hepatic resection. Case Rep. Oncol. 13, 478–484 (2020).
Camacho, L. H. et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 27, 1075–1081 (2009).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Kaczmarek, A., Vandenabeele, P. & Krysko, D. V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013).
Gao, W., Wang, X., Zhou, Y., Wang, X. & Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target Ther. 7, 196 (2022).
Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov. 10, 26–39 (2020).
Zhu, Y., Duong, L., Lu, X. & Lu, X. Cancer-cell-intrinsic mechanisms shaping the immunosuppressive landscape of prostate cancer. Asian J. Androl. 25, 171–178 (2023).
Zheng, Y. & Pan, D. The Hippo signaling pathway in development and disease. Dev. Cell 50, 264–282 (2019).
Moroishi, T. et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell 167, 1525–1539.e1517 (2016).
Gunzer, M. et al. Systemic administration of a TLR7 ligand leads to transient immune incompetence due to peripheral-blood leukocyte depletion. Blood 106, 2424–2432 (2005).
Mateu Ferrando, R., Lay, L. & Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 38, 57–67 (2020).
Ramalingam, V. Multifunctionality of gold nanoparticles: plausible and convincing properties. Adv. Colloid Interface Sci. 271, 101989 (2019).
Her, S., Jaffray, D. A. & Allen, C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv. Drug Deliv. Rev. 109, 84–101 (2017).
Shahbazi, R. et al. Targeted homology-directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat. Mater. 18, 1124–1132 (2019).
Peng, C. et al. Tuning the in vivo transport of anticancer drugs using renal-clearable gold nanoparticles. Angew. Chem. Int. Ed. Engl. 58, 8479–8483 (2019).
Chan, M. et al. Synthesis and immunological characterization of toll-like receptor 7 agonistic conjugates. Bioconjugate Chem. 20, 1194–1200 (2009).
Shinchi, H. et al. Gold nanoparticles coimmobilized with small molecule toll-like receptor 7 ligand and alpha-mannose as adjuvants. Bioconjugate Chem. 30, 2811–2821 (2019).
Hou, B., Reizis, B. & DeFranco, A. L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282 (2008).
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007).
Falo, L. D. Jr., Kovacsovics-Bankowski, M., Thompson, K. & Rock, K. L. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med.1, 649–653 (1995).
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Shinchi, H. et al. Cadmium-free sugar-chain-immobilized fluorescent nanoparticles containing low-toxicity ZnS-AgInS2 cores for probing lectin and cells. Bioconjugate Chem. 25, 286–295 (2014).
Tateo, S. et al. Optimized immobilization of single chain variable fragment antibody onto non-toxic fluorescent nanoparticles for efficient preparation of a bioprobe. Colloids Surf. B Biointerfaces 224, 113192 (2023).
Shinchi, H. et al. Glyco-nanoadjuvants: sugar structures on carriers of a small molecule TLR7 ligand affect their immunostimulatory activities. ACS Appl. Biol. Mater. 4, 2732–2741 (2021).
Lee, J., Oldham, M. L., Manon, V. & Chen, J. Principles of peptide selection by the transporter associated with antigen processing. Proc. Natl Acad. Sci. USA 121, e2320879121 (2024).
Pham, C. D., Woo, M. Y., Kim, Y. S., Park, S. & Kwon, M. H. An anti-nucleic acid antibody delivers antigen to the cross-presentation pathway in dendritic cells and potentiates therapeutic antitumor effects. J. Immunol. 189, 5755–5763 (2012).
Jin, L. et al. Use of alpha-N,N-bis[carboxymethyl]lysine-modified peroxidase in immunoassays. Anal. Biochem. 229, 54–60 (1995).
Moore, M. W., Carbone, F. R. & Bevan, M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54, 777–785 (1988).
Sun, L., Su, Y., Jiao, A., Wang, X. & Zhang, B. T cells in health and disease. Signal Transduct. Target Ther. 8, 235 (2023).
Philip, M. & Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Eisenbarth, S. C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89–103 (2019).
Guo, Q. et al. NF-kappaB in biology and targeted therapy: new insights and translational implications. Signal Transduct. Target Ther. 9, 53 (2024).
Tugues, S. et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 22, 237–246 (2015).
Allen, F. et al. CCL3 augments tumor rejection and enhances CD8(+) T cell infiltration through NK and CD103(+) dendritic cell recruitment via IFNgamma. Oncoimmunology 7, e1393598 (2018).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Edner, N. M., Carlesso, G., Rush, J. S. & Walker, L. S. K. Publisher Correction: Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 20, 82 (2021).
Hoshino, K. et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440, 949–953 (2006).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Mancuso, G. et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 10, 587–594 (2009).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med 5, 200ra116 (2013).
Irvine, D. J. & Dane, E. L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20, 321–334 (2020).
Shinchi, H. et al. Glyco-nanoadjuvants: impact of linker length for conjugating a synthetic small-molecule TLR7 ligand to glyco-nanoparticles on immunostimulatory effects. ACS Chem. Biol. 17, 957–968 (2022).
Liang, F. et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in Rhesus Macaques. Mol. Ther. 25, 2635–2647 (2017).
Roth, G. A. et al. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 7, 174–195 (2022).
Tozuka, M. et al. Efficient antigen delivery to the draining lymph nodes is a key component in the immunogenic pathway of the intradermal vaccine. J. Dermatol. Sci. 82, 38–45 (2016).
He, R., Zang, J., Zhao, Y., Dong, H. & Li, Y. Nanotechnology-based approaches to promote lymph node targeted delivery of cancer vaccines. ACS Biomater. Sci. Eng. 8, 406–423 (2022).
Baharom, F. et al. Systemic vaccination induces CD8(+) T cells and remodels the tumor microenvironment. Cell 185, 4317–4332.e4315 (2022).
Joffre, O. P., Segura, E., Savina, A. & Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569 (2012).
Martinez-Pomares, L. & Gordon, S. Antigen presentation the macrophage way. Cell 131, 641–643 (2007).
Liu, H. et al. Mannose-coated nanozyme for relief from chemotherapy-induced peripheral neuropathic pain. iScience 26, 106414 (2023).
Acknowledgements
We thank Hirotake Tsukamoto for the EG7-OVA cells; Kenneth Rock for the B16-OVA cells; Hao Li, Yanliang Liu, Mohamed Fathi Saleh, Yudai Ohta, Paweenapon Chunthaboon, Samson Wainaina Ngurari, Komen Joan Jepkosgei, Wattanapongpitak Sakornniya, David Nduru, Shuran Li, Suzu Yoshitomo, Shoko Sashiyama, Tomoko Matsunaga, and Kozue Terada for their technical assistance. This study was supported by the Japan Agency for Medical Research and Development (AMED) Project for Cancer Research and Therapeutic Evolution (P-CREATE) (JP22cm0106382), AMED PRIME (JP22gm6210030), Japan Science and Technology Agency (JST) PRESTO (grant JPMJPR17HA), JST FOREST (JPMJFR226J), Japan Society for the Promotion of Science (JSPS) KAKENHI grants (24H00864 and 24H00865), Takeda Science Foundation, and Hoansha Foundation (all to T.M.). Additional support, including financial and technical assistance, was provided by Medical Research Center Initiative for High Depth Omics (Science Tokyo), Nanken-Kyoten (Science Tokyo), and Multilayered Stress Diseases (JPMXP1323015483; Science Tokyo).
Author information
Authors and Affiliations
Contributions
M.N. and T.M. contributed to conceptualization. M.N., T.M., H.S., T.H., and D.A.C. contributed to the methodology. H.S., S.W., K.M., and H. Ohara contributed to synthesis of all types of gold nanoparticles. N.H. and S.L. performed animal experiments. A.M. contributed to transcriptome analysis. C.T. performed immunoblot analysis. M.N., Y. Sakamoto, and T.M. contributed to writing the original draft. Y. Sakamoto, M.S., Y.K., A.N., A.I., H. Oshiumi., M.W., H.S., and Y. Suda provided technical and intellectual support. Y. Sakamoto, M. S., and T. M. supervised the study. T.M. contributed to acquisition of funds.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Pakhi Birla and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niimura, M., Sakamoto, Y., Shimoda, M. et al. Harnessing an integrated glyco-nanovaccine technology for enhanced cancer immunotherapy. Commun Med 5, 378 (2025). https://doi.org/10.1038/s43856-025-01102-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01102-3