Abstract
Background
Klebsiella pneumoniae is ubiquitous in animals, humans, and the environment, facilitating the dissemination of antimicrobial resistance (AMR) and virulence traits. Most studies are primarily focused on human clinical isolates, leaving critical gaps in understanding non-human reservoirs and cross-species transmission risks.
Methods
We combined large-scale genomic analyses with in vitro and in vivo infection models to characterize the evolutionary dynamics of 2809 K. pneumoniae isolates sourced from 8 host species across 57 countries. We examined the potential for cross-host transmission of K. pneumoniae, explored its AMR and virulence characteristics across different hosts, and evaluated the temporal evolution of AMR and virulence.
Results
Here, we demonstrate that the rise in AMR strongly correlates with the global expansion of multidrug-resistant (MDR) sequence types, while the increase in virulence is partially driven by the acquisition of key virulence loci in certain MDR clones. Population structure analyses show no distinct genetic boundaries between human- and animal-derived strains, strengthening the evidence for cross-species transmission potential.
Conclusions
These findings underscore the urgent need for a One Health approach to address the dual threat of AMR and hypervirulence, providing critical insights to guide global surveillance and public health interventions.
Plain language summary
Klebsiella pneumoniae is a bacterium that can be found in different hosts, including livestock, humans and the environment. They can develop antibiotic resistance (making medicines less effective) and make infections harder to treat. Past studies mainly focused on bacterial strains that infect humans, leaving a knowledge gap about their presence in animals or whether they can spread between animals and humans. Here, we studied over 2800 bacterial strains from 8 hosts (including cows, pigs, and humans) across 57 countries, examining how they change, spread, and become more harmful. We found that antibiotic-resistant strains are spreading globally, fueling resistance. Some antibiotic-resistant bacteria acquire genes that render them more infectious. We also found that K. pneumoniae strains found in humans and animals are genetically similar, suggesting potential for cross-host transmission. This calls for careful surveillance of K. pneumoniae strains to prevent crossover.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae, classified as a critical priority healthcare-associated pathogen by the World Health Organization (WHO), serves as a major driver of antimicrobial resistance (AMR) worldwide1,2. In recent years, the emergence of highly antimicrobial-resistant strains of K. pneumoniae producing carbapenemases has posed a serious public health threat. These strains exhibit extensive resistance to multiple antibiotics, making infections increasingly challenging to treat3,4. K. pneumoniae is classified into two major virulence phenotypes: hypervirulent and classical strains5. Classical K. pneumoniae is the most common phenotype, typically associated with nosocomial infections in patients with underlying comorbidities. In contrast, hypervirulent K. pneumoniae (hvKp), although less frequent, is characterized by its ability to cause severe infections in healthy individuals in community settings, with a propensity for rapid dissemination6. While these hypervirulent isolates generally retain sensitivity to antibiotics, the increasing convergence of multidrug resistance and high virulence in specific strains represents a growing global concern7,8.
K. pneumoniae is transmitted not only among humans but also is commonly detected in animals and shared environments9,10,11,12. This widespread presence facilitates the co-dissemination of AMR and virulence factors across different species, amplifying the risk to human health. Recent One Health studies show that identical sequence types (STs) and shared mobile elements are present in both human and animal isolates, highlighting two-way transmission across species13,14. However, most existing studies primarily focus on clinical K. pneumoniae isolates from humans, resulting in gaps in our comprehension of the distribution of AMR and virulence in non-human populations. Moreover, it is unclear whether the lineages and AMR or virulence elements common in human infections are equally represented in animal and environmental populations. Understanding the transmission pathways of these pathogens from animals to humans is crucial, as animals can act as reservoirs for K. pneumoniae strains accountable for human clinical infections15.
To address this issue, we conducted a comprehensive investigation into 2809 K. pneumoniae strains isolated from eight different hosts across 57 countries and regions. This work was achieved through a combination of in vivo and in vitro experiments, coupled with bioinformatics analysis. Specifically, we delved into the potential for cross-host transmission of these strains. We also explored the evolutionary trends of AMR and virulence from the 1900s to the 2020s.
This study reveals the epidemiological patterns of antimicrobial resistance genes (ARGs) and virulence factor genes (VFGs) across various hosts, while also analyzes their occurrence in plasmids. These findings enrich our understanding of K. pneumoniae transmission, AMR, and virulence development, providing actionable insights to guide effective public health interventions. Within the “One Health” framework, our findings contribute to global surveillance and prevention of AMR, offering fresh perspectives and practical guidelines to mitigate threats to human health.
Methods
Ethical statement, bacterial strains, cell lines, culture conditions, and whole genome sequences
K. pneumoniae strains used for whole genome sequencing in this study included 91 strains isolated from nasal swabs of pigs and eight from nasal swabs of dogs (Supplementary Data 1). The collection and use of these samples, as well as the animal experiment described below, were approved by the University Ethical Committee at Huazhong Agricultural University (Approval ID: HZAUSW-2023-0039). Unless otherwise notified, bacterial strains were incubated on tryptic soy agar (TSA; Becton, Dickinson and Company, Franklin Lakes, USA) or in tryptic soy broth (TSB) at 37 °C. Cell lines used in this study included mammalian respiratory epithelial cells (A549, BEAS-2B, NPTr) and intestinal epithelial cells (Caco-2, IPEC). Among these cell lines, A549, BEAS-2B, Caco-2, and NPTr are maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Waltham, USA) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco). while Caco-2 cells are cultured in DMEM containing 20% heat-inactivated FBS. All cells were incubated at 37 °C under a 5% CO2 atmosphere.
Whole genome sequences (WGS) of K. pneumoniae downloaded from NCBI included 2369 ones from human-origin strains, 44 ones from pig strains, 126 ones from poultry-origin strains, 54 ones from cattle-origin strains, 40 ones from dog-origin strains, 23 ones from cat-origin strains, 33 ones from environmental samples, as well as 21 ones from blowfly-origin strains (Supplementary Date 2).
Illumina sequencing and bioinformatical analysis
Whole genome sequences of the 99 K. pneumoniae strains isolated from pigs and/or dogs were generated by Illumina sequencing. Briefly, genomic DNAs are extracted using a commercial TIANamp Bacteria DNA Kit (Tiangen, Beijing, China) following the manufactory’s manual. The quality and quantity of bacterial genomic DNAs were valuated using electrophoresis on a 1% agarose gel and a Qubit 4 Fluorometer (Thermo Scientific, Waltham, USA). Subsequently, a NEBNext® UltraTM II DNA Library Prep Kit (New England BioLabs, Ipswich, USA) was used to construct sequencing libraries. The libraries were then sequenced on the NovaSeq 6000 platform using the paired-end 150 bp protocol. Raw reads with low quality were removed as described previously16. High-quality reads were de novo assembled with SPAdesv3.9.017. Whole genome sequences of the 99 laboratory strains have been deposited in the NCBI GenBank under BioProject PRJNA99821316.
Single-nucleotide polymorphisms (SNPs) between specific genome sequences against the reference genome sequence (GenBank accession no. PDLW01000000) were analyzed using MUMmer v3.118, revealing a total of 105,059,827 SNPs. Phylogenetic trees were constructed using Gubbins (v2.4.0)19 and RAxML-NG (GTRGAMMA model)20. Bayesian analysis of population structure (BAPS) groups was determined using Fastbaps21. Multilocus sequence typing (MLST) was conducted using fastMLST (v0.0.15)22, and a minimum spanning tree was generated using PHYLOViZ 2.023. ARGs and plasmid replicons were identified using ResFinder (v.4.5.0)24 and PlasmidFinder (v.2.1)25, respectively. ARG variants were matched with their alleles based on the CDC & FDA Antimicrobial Resistance Isolate Bank (https://wwwn.cdc.gov/ARIsolateBank/GeneGlossary). Correlations between ARGs and plasmid replicons were assessed using the Pearson correlation coefficient test26. VFGs were identified using Abricate (v1.0.1) by comparing genome assemblies against the Virulence Factor Database. Additionally, we used Kleborate to complement species identification and systematically assess the presence of resistance and virulence determinants, as well as STs and virulence scores (https://github.com/katholt/Kleborate)27.
Cell adherence and invasion assays
Cell adherence and invasion assays were conducted as described previously28. Briefly, cells (A549, NPTr, BEAS-2B, IPEC, Caco-2) were seeded 1 × 106 cells/well in a 12-well plate with 1 ml of medium in each well. The plate was then placed in a 37 °C incubator with 5% CO2 until the cells formed a confluent monolayer. Thereafter, K. pneumoniae strains (KP9, KP61, KP214) were added to each well at a multiplicity of infection (MOI) of 100. The plate was then incubated at 37 °C for 2 h to allow the bacteria to adhere to the cells. After that, the wells were washed five times with PBS to remove non-adherent bacteria. Subsequently, the cells were lysed by adding 200 μl of sterile water to each well, and the plate was incubated at room temperature. Finally, the lysate was serially diluted and plated on agar plates for bacterial counting.
To calculate intracellular bacteria, cell lysis was replaced by adding 1 ml of medium containing either 180 μg/ml tigecycline or 100 μg/ml gentamicin into the wells, and incubating at 4 °C for 1 h to kill extracellular bacteria. After the antibiotic treatment, the wells were washed three times with PBS to remove the antibiotics and non-adherent bacteria. Subsequently, 200 μl of sterile water was added to each well and incubated at room temperature to lyse the cells. The lysate was serially diluted and plated on agar plates for bacterial counting. The adhesion and/or invasion rate was calculated as follows: Adhesion rate = (number of adhered bacteria + number of invaded bacteria)/initial inoculated bacteria. Invasion rate = number of invaded bacteria/initial inoculated bacteria.
Experimental infectious assays
Animal experiments were conducted at the Laboratory Animal Center at Huazhong Agricultural University, Wuhan, China. During the experiments, animals were treated following the ARRIVE guidelines29. Briefly, 4-week-old specific pathogen-free pigs (n = 20) were randomly divided into 4 groups (Gi-Giv) and each group contained 5 pigs. Randomization was performed using a random number table prior to the start of the experiment. Subsequently, pigs in Gi-Giii were challenged with KP9, KP61, and KP214 at 109 CFU per pig through the auricular vein, respectively. Pigs in Giv served as a negative control and received an injection of PBS through the auricular vein and were used as controls. The experimental unit was an individual pig. After the challenge, the body temperatures were monitored daily, and their clinical symptoms were observed and scored using a clinical symptom scoring chart was prepared according to previous publications30,31. The observers were blinded to group allocation when scoring clinical signs to minimize bias. At 3, 6, 9, 12, 24, 48, and 72 h after challenge, blood samples were collected and subjected to serial dilution. The diluted bacterial suspension was then plated on TSA plates for bacterial counting. On days 1, 2, and 7 after infection, one randomly selected pig from each group was euthanized for autopsy. Pig lungs, livers, spleens, kidneys, and lymph nodes were collected for histological examinations. Tissue samples, including hearts, lungs, livers, spleens, kidneys, and lymph nodes, were also collected for measuring bacterial loadings. For bacterial load quantification, three technical replicates were performed for each tissue sample. No animals or data points were excluded from the analysis. No formal sample size calculation was performed prior to the study. All efforts were made to minimize animal suffering and reduce the number of animals used.
Statistical analysis and reproducibility
Statistical analysis was performed using the Multiple t-tests strategy in GraphPad 254 Prism8.0 (GraphPad Software, San Diego, CA). The assumptions of normality and homogeneity of variance were assessed, and when these assumptions were violated, non-parametric alternatives such as the Mann–Whitney U test were employed. Data represent mean ± SD, and the significance level was set at p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Diversity of K. pneumoniae isolates from various host sources worldwide
This study encompasses 2809 strains of K. pneumoniae isolated from 8 different hosts across 57 countries and regions (Fig. 1a), spanning from the 1900s to the 2020 s (Fig. 1b). Their host origins included 2369 human strains, 135 pig strains, 126 poultry strains, 54 cattle strains, 48 dog strains, 23 cat strains, and 21 blowfly strains, with an additional 33 samples collected from the environment (Fig. 1c). Detailed sample information is available in Supplementary Data 1 and 2.
a Distribution of samples across 57 countries globally. b Temporal distributions of the samples. c Host distributions of the samples. d Proportions of the top 20 predominant sequence types (STs). Note: The names and materials used in this map do not represent any opinion of Research Square on the legal status of any country, region, city, or territory, nor do they involve the delineation of its borders or boundaries. This map is provided by the authors.
Although all 2809 isolates were originally labeled as K. pneumoniae based on their metadata, species re-identification using Kleborate revealed that 2788 strains (99.4%) were K. pneumoniae, while 8 strains (0.3%) were K. quasipneumoniae and another 8 strains (0.3%) were K. variicola (Supplementary Data 3). These findings indicate that a small number of isolates may have been inaccurately annotated in the NCBI database. Therefore, we did not restrict our analysis strictly to K. pneumoniae sensu stricto but included all isolates belonging to the K. pneumoniae complex.
MLST analysis of the 2809 strains revealed that despite being classified into over 500 STs, there were no significant distinctions between strains of human and non-human origin. Notably, known multidrug-resistant (MDR) STs and hypervirulent STs were found to be prevalent among both human and non-human isolates. Specifically, the 2809 isolates were distributed across 500 known STs, with ST11, ST258, ST15, and ST16 emerging as the predominant types, constituting 16.1%, 8.3%, 6.4%, and 4.1% of the total, respectively (Fig. 1d and Supplementary Data 3). The primary STs in human isolates were ST11 (16.1%) and ST258 (8.3%), while in non-human isolates were ST11 (7.3%) and ST37 (7.3%). Noteworthy MDR STs, including ST11, ST258, ST37, ST15, and ST307, are strains of global public health concern that rank among the top three STs across all animal sources. This observation indicates a significant trend in their prevalence (Supplementary Fig. 1). Moreover, hypervirulent STs that have recently garnered attention, including ST65 and ST86, were identified in samples from both human and non-human isolates, including cats and cattle, whereas hypervirulent ST23 was exclusively found in human isolates (Supplementary Data 3).
K. pneumoniae strains exhibit the potential for cross-species infection
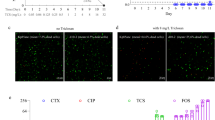
To investigate the cross-species infection potential of K. pneumoniae, we selected three representative strains from different hosts, KP9 (ST11, human origin), KP61 (ST258, canine origin), and KP214 (ST25, porcine origin), based on their MLST types and epidemiological relevance. ST11 is a globally disseminated lineage frequently associated with hypervirulence and multidrug resistance in human clinical infections, particularly in Asia, and has also been reported in animals, suggesting potential for cross-host transmission. ST258 is another globally prevalent lineage, primarily found in humans and associated with carbapenem resistance; its detection in a canine host in this study suggests potential host barrier crossing and adaptation. ST25, represented by KP214, has been linked to septicemia in piglets, making it a relevant pig-associated pathogenic strain. We evaluated the pathogenicity of these three strains in both human and pig cell lines as well as in a piglet infection model. Additionally, we investigated the genomic variances of K. pneumoniae strains from different host sources through population structure and phylogenetic analyses. Initially, adhesion and invasion assays were conducted on five human and pig cell lines, comprising human lung adenocarcinoma epithelial cells (A549), bronchial epithelial cells (BEAS-2B), colon adenocarcinoma cells (Caco-2), swine tracheal cells (NPTr), and intestinal porcine epithelial cells (IPEC). The experimental findings indicated that the three K. pneumoniae strains from diverse origins displayed no host preference in adhesion and invasion capabilities. Notably, there were no significant differences in the adhesion abilities of KP9, KP61, and KP214 to A549 and NPTr, or to IPEC and Caco-2. However, KP9 showed stronger adhesion to A549 compared to KP214 (Fig. 2a). Furthermore, the invasion abilities did not correlate with host origin. KP61 demonstrated higher invasion abilities towards A549 and BEAS-2B cells compared to KP9, while KP214 exhibited stronger invasion of Caco-2. In contrast, KP61 exhibited higher invasion abilities against IPEC cells than KP214 (Fig. 2b), suggesting the potential for cross-species transmission of K. pneumoniae strains.
a Bacterial adherence and invasion to respiratory and intestinal epithelial cells derived from humans and/or pigs. b Bacterial invasion of respiratory and intestinal epithelial cells derived from humans and/or pigs. Each experiment was performed with four technical replicates. c Clinical sign scores of pigs challenged with K. pneumoniae strains originating from humans, dogs, and pigs. d The number of K. pneumoniae strains recovered from different organs of challenged pigs at 48 hours post-challenge. e The number of K. pneumoniae strains recovered from different organs of challenged pigs at 7 days post-challenge. The error bar represents the standard deviation. The significance level was set at p < 0.05 (*), or p < 0.01 (**), non-significant comparisons (p > 0.05) are not labeled. The error bar represents the standard deviation. N = 5 biologically independent animals per group. f Histological examinations of different organs from challenged pigs are shown. (Scale bars = 50 μm) The lungs of challenged pigs exhibit extensive thickening of the alveolar walls, with unclear alveolar wall structure. A small amount of inflammatory cell infiltration is observed on the alveolar walls. The bronchial structures appeared normal without any evident abnormalities. There were no significant interstitial proliferations or other notable abnormalities observed in the interstitium. The livers showed hepatocellular focal necrosis with hemorrhage, accompanied by a small amount of inflammatory cell infiltration within the necrotic lesion. Additionally, there was a small amount of hepatocellular hydropic degeneration, cellular swelling, and pale staining of the cytoplasm. The spleens of challenged pigs exhibited a decrease in white pulp volume, occasionally accompanied by mild hemorrhage within the white pulp, along with a small amount of extravasated red blood cells. There was no significant abnormality in the number of parenchymal cells within the red pulp, and the splenic sinuses do not show significant dilation. However, there was notable infiltration of granulocytes. The renal tissue of the kidneys showed widespread tubular necrosis, with necrosis and dissolution of the renal tubular epithelial cells, resulting in an unclear tubular structure. Acidophilic material can be seen within the glomerular capillaries. Focal hemorrhage was observed in multiple areas of the interstitium, along with scattered inflammatory cell infiltration. The lymph nodes exhibited abundant and well-defined lymph follicles within the cortex, without any evident abnormalities. Focal hemorrhage is observed in multiple areas of the medulla.
Subsequent to the cell experiments, pathogenicity assessments were performed using a pig model. When infected at equal doses, the three K. pneumoniae strains infiltrated multiple organs in pigs and induced similar clinical symptoms, including elevated body temperature (Supplementary Fig. 2a), depression, reduced appetite and activity, coughing, difficulty breathing, and diarrhea. In terms of clinical symptom scores, no significant statistical differences were observed between KP9 and KP214, while KP61 exhibited higher scores after one day of inoculation (Fig. 2c). Additionally, except at 6- and 24-hours post-inoculation, the three K. pneumoniae strains displayed no significant differences in bacterial loads in the blood (Supplementary Fig. 2b). Further analysis of bacterial loads at different time points and in various organs revealed significant differences in the invasion abilities of the three strains across diverse organs, with no host dependency observed (Fig. 2d, e). Histological examinations affirmed that these strains inflicted notable damage to the lungs, liver, kidneys, spleen, and lymph nodes (Fig. 2f).
The population structure analysis based on Bayesian models also unveiled similar population structures between human and non-human strains. The analysis categorized the strains into 23 BAPS groups (Fig. 3a, b), with only BAPS groups 1, 3, and 5 exclusively containing human strains, while the remaining 20 groups comprised a mix of human and non-human strains. Additionally, phylogenetic analysis using whole-genome sequences indicated no significant correlation between the phylogenetic relationships of the strains and host preferences (Fig. 3a). These results suggest that there is no distinct genetic boundary separating human and non-human strains of K. pneumoniae. These findings collectively demonstrated no host specificity or dependency in the pathogenicity and infectivity of the K. pneumoniae strains. Genomic analysis further indicated that these strains did not exhibit significant host preferences at the genetic level. These results support the potential for K. pneumoniae to have cross-species infection capabilities.
a The whole genome sequence single nucleotide polymorphisms are utilized to generate a phylogenetic tree. From the inner to the outer circles are 1. Sample source, 2. Baps group, 3. Hypervirulent K. pneumoniae (hvKP), 4. Carbapenem-resistant (CRKP), 5. Hypervirulent carbapenem-resistant (hv-CRKP), 6. Top 10 predominant sequence types (STs). b The number of K. pneumoniae strains from different host species belonging to different BAPS groups.
Temporal trends in K. pneumoniae antimicrobial resistance
To investigate the trends in AMR in K. pneumoniae over time and the impact of policy implementation, we analyzed ARGs in 2809 K. pneumoniae strains spanning from the 1900s to the 2020s using whole genome sequencing data. A total of 414 AMR genotypes associated with 15 antimicrobial agents, including quaternary ammonium salts (QAS), were identified (Supplementary Data 4). Overall, the resistance burden in K. pneumoniae exhibited an upward trend over this period, influenced by both the expansion of MDR clones and the antimicrobial regulation policies adopted in multiple countries.
In terms of ARG carriage, the average number of ARGs carried by K. pneumoniae strains reached its peak in the 2000s, subsequently declined, and then stabilized throughout the 2010s and 2020 s (Fig. 4a). This trend coincided with the introduction of major antimicrobial restriction policies, such as the European Union’s 2006 ban on antibiotic growth promoters in animal feed, the 2015 Global Action Plan on AMR issued by the WHO, and national-level guidelines implemented in the United States (2007) and China (2019) (Supplementary Fig. 3a). Statistical analyses indicated a significant reduction in the average number of ARGs per strain across different regions following the introduction of these policies (p ≤ 0.05; Supplementary Fig. 3b–d). Notably, the reduction in ARG carriage occurred during a period when several antibiotic stewardship policies were introduced globally, although the direct impact of these interventions remains to be determined. These findings suggest that policy-driven reductions in antibiotic usage may help curb the horizontal spread of ARGs and potentially delay the emergence of novel resistance mechanisms. However, this ostensibly “positive trend” requires careful and nuanced interpretation. Although the number of ARGs per strain has decreased, the overall level of AMR has continued to escalate, most notably characterized by high resistance rates to critical antibiotics such as extended-spectrum β-lactamases (ESBLs) and carbapenems.
a Distribution of numbers of ARGs per genome in different sampling periods: b AMR scores in different sampling periods, calculated using Kleborate in different sampling periods. Before 2000 (n = 121), 2000s (n = 393), 2010s (n = 1959), and 2020 s (n = 336). The error bar represents the standard deviation. The error bar represents the standard deviation. The significance level was set at p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***). c Proportions of hypervirulent sequence types (hvSTs) and MDR STs in each sampling period (n = 2809). d Temporal changes in AMR genotypes. Different colors represent the different antimicrobial agents (n = 2809). AMR genotypes were annotated based on the NCBI Bacterial Antimicrobial Resistance Reference Gene Database (BioProject ID: PRJNA313047). The values are presented in Supplementary Data 5. e Temporal variation in the prevalence and abundance of ARGs (n = 2809). The heatmap displays ARG prevalence (left blue) and ARG abundance within categories (right green), categorized by sampling period. Only ARGs with a prevalence exceeding 5% in any sampling group are shown. Colored bars indicate the predicted resistance phenotypes associated with the ARGs.
To further assess resistance levels comprehensively, we reanalyzed all isolates using Kleborate. The results indicate that calculated resistance scores have exhibited a consistent year-on-year increase (Fig. 4b). This upward trend aligns with the proliferation of specific highly antibiotic-resistant clones (Fig. 4c; Supplementary Fig. 4). Additionally, temporal variations in antibiotic resistance patterns were observed to differ significantly across various antibiotic classes. For instance, resistance to commonly used antibiotics, including aminoglycosides, macrolides, tetracyclines, sulfonamides, and trimethoprim, remained relatively low (<20%) prior to the 2000s (Supplementary Data 5). However, it increased sharply thereafter and stabilized at elevated levels (25–80%) in subsequent decades. Resistance to extended-spectrum ESBLs and carbapenems has risen continuously since the 2010s, reaching 60.4% and 50.6%, respectively. In contrast, resistance to β-lactams, quinolones, and fosfomycin remained consistently high (80.7–100%) throughout the entire period. Notably, polymyxin resistance, despite its classification as a last-resort antibiotic, has persisted at low levels (0–3.4%) across all examined timeframes (Fig. 4d).
Furthermore, we examined the variations in the prevalence of specific ARGs among strains over this period and their relative proportions within the same ARG category (Fig. 4e). Notably, the aminoglycoside resistance gene aac(6’)-Ib-cr showed a continuous increase, whereas aadA1 exhibited a decline in the 2010s and 2020s. The predominant aminoglycoside resistance genes transitioned from aadA1 (conferring streptomycin resistance) to aac(6’)-Ib-cr (conferring amikacin resistance). Chloramphenicol has been prohibited in developed nations due to severe adverse effects, except for its topical or ophthalmic applications, leading to a reduction in its clinical use in human medicine in recent years32. However, its derivative florfenicol remains extensively utilized in veterinary medicine33. The prevailing chloramphenicol resistance genes shifted from catA1 and catB4 to floR, which confers resistance to both chloramphenicol and florfenicol. The primary resistance genes associated with fosfomycin shifted from fosA to fosA6.
Temporal dynamics of virulence in K. pneumoniae
By identifying virulence-associated genes in K. pneumoniae (Supplementary Data 6), we observed a progressive increase in the overall virulence potential of the K. pneumoniae population over time. This trend was primarily driven by the expansion of MDR clones (e.g., ST11) that have acquired key virulence factors, rather than by the spread of classical hypervirulent clones. To investigate the basis of this temporal shift, we systematically evaluated virulence levels across time periods. Both the virulence gene burden (Supplementary Fig. 5a) and Kleborate-assigned virulence scores (Supplementary Fig. 5b) showed a marked upward trend from the 2000s to the 2020s. Notably, a subset of isolates collected before 2000 displayed unexpectedly high virulence scores, potentially due to sampling bias in earlier studies. We further examined the temporal dynamics of key virulence determinants, including siderophore systems (yersiniabactin, aerobactin, enterobactin, salmochelin), the “regulators of mucoid phenotype” (rmpA and rmpA2), and the genotoxin colibactin. With the exception of colibactin, carriage rates of these factors steadily increased over time (Fig. 5a), in parallel with a rise in predicted siderophore gene counts (Fig. 5b).
a Frequency of virulence gene clusters across different sampling periods. b Distribution of the number of siderophore gene clusters per isolate across sampling periods. The siderophore gene clusters include yersiniabactin, aerobactin, salmochelin, and enterobactin. Before 2000 (n = 121), 2000s (n = 393), 2010s (n = 1959), and 2020 s (n = 336). c Temporal dynamics in the carriage of virulence scores predicted by Kleborate for strains belonging among major K. pneumoniae sequence types. Only the top 24 most prevalent STs are shown (n = 2809). d Temporal prevalence of the top 24 most common STs across the four sampling periods. VFGs categories were annotated based on the Virulence Factor Database (VFDB), n = 2809.
Despite the rising virulence trend, the prevalence of traditional hypervirulent clones (e.g., ST23) remained relatively stable (Fig. 4c), suggesting that the observed increase in population-level virulence may be attributable to the stepwise acquisition of virulence genes within certain STs. Supporting this, several MDR clones (e.g., ST11, ST15, ST16, ST101, ST147) demonstrated significant concurrent increases in both Kleborate scores (Fig. 5c), enhanced carriage of key virulence genes (aerobactin, yersiniabactin, salmochelin, and rmpA/rmpA2; Supplementary Fig. 6), and a progressive rise in population-wide relative abundance (Fig. 5d). These findings suggest that population-wide virulence enhancement is not being driven by the expansion of traditional hypervirulent clones, but rather by the expansion of MDR clones that have acquired virulence traits, reflecting convergent evolution driving the emergence of dual-risk clones with both resistance and virulence.
To further evaluate this convergence and its epidemiological implications, we focused on a representative convergent phenotype: carbapenem-resistant hypervirulent K. pneumoniae (CR-hvKP). Based on previously established criteria involving the co-presence of rmpA/rmpA2 and iucABCD-iutA virulence markers34, we identified 415 hvKP strains across the dataset. To validate this classification, we assessed their virulence scores, siderophore gene content, capsule serotypes (notably K1 and K2), and the representation of hypervirulent STs (e.g., ST23 and ST65) among the identified hvKP strains. These strains consistently exhibited classical hypervirulence features, confirming the robustness of our definition (Supplementary Fig. 7). Among these, 193 strains were further classified as CR-hvKP. The number of CR-hvKP isolates showed a clear upward trend from the 2000s to the 2020s, despite a minor drop in 2021, likely due to sampling bias. Clonal distribution analysis revealed striking geographic differences in the clonal structure of CR-hvKP: ST11 dominated in China (78.6%), while ST23 was more prevalent outside China (25%) (Supplementary Fig. 8).
Human-derived strains exhibit higher antimicrobial resistance and virulence than non-human strains
To examine the distribution patterns of ARGs, VFGs, and plasmids in K. pneumoniae strains, we compared WGS data from strains originating from eight distinct hosts: humans, cats, dogs, poultry, pigs, cattle, blowflies, and the environment. Overall, non-human isolates exhibited lower levels of antibiotic resistance and virulence abundance compared to human-derived strains. In terms of ARG counts, isolates from poultry and cats harbored significantly more ARGs than those from humans, while cattle-derived strains exhibited the lowest ARG counts (Fig. 6a). Isolates from dogs, pigs, blowflies, and the environment showed no significant differences compared to human strains. However, AMR scores predicted from WGS data using Kleborate revealed that, with the exception of blowfly isolates, all non-human strains exhibited significantly lower resistance scores than human isolates (Fig. 6c). This discrepancy between ARG count and AMR score is largely due to the fact that AMR scores reflect only resistance to carbapenems, ESBLs, and colistin. Consistent with this, non-human isolates showed higher prevalence of resistance to tetracyclines, macrolides, and rifampicin, while human-derived strains exhibited markedly higher resistance to carbapenems and ESBLs (Fig. 6e). Epidemiological trends of specific ARGs also varied by host source, with blaKPC-2 being predominant in human isolates, while blaNDM-5 (in pigs, poultry, and the environment), blaNDM-7 (in blowflies), and blaOXA-48 (in dogs and cats) were prevalent in non-human isolates. Colistin ARGs predominantly included mcr-1 in human strains, mcr-8 in poultry and blowfly strains, and chloramphenicol ARGs circulated as floR in farm animals and the environment, with catA/B predominantly found in human and pet isolates (Supplementary Fig. 9).
a The distribution of the number of ARGs in different sample hosts. b The distribution of the number of VFGs in different sample hosts. c AMR scores predicted by Kleborate for isolates from different host sources. d Virulence scores predicted by Kleborate for isolates from different host sources. The error bar represents the standard deviation. The significance level was set at p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***). e Distribution of AMR and hypervirulence-associated genes among K. pneumoniae. fim type I fimbriae, mrk type 3 fimbriae, ent enterobactin, K-locus capsule, O-locus lipopolysaccharide (LPS), clb colibactin locus; iro salmochelin locus, iuc aerobactin locus, rmpA/A2 regulators of mucoid phenotype genes, ybt yersiniabactin locus. The numbers of strains isolated from different hosts are: humans (n = 2369), pigs (n = 135), poultry (n = 126), cattle (n = 54), dogs (n = 48), environments (n = 33), cats (n = 23), blowflies (n = 21).
The distribution of VFGs also differed markedly between human and non-human sources. Both the number of VFGs and virulence scores predicted by Kleborate indicated that human-derived strains exhibited higher virulence than those from non-human hosts (Fig. 6b, d). This difference was particularly evident in accessory virulence-associated genes such as clb, ybt, iro, rmpA/A2, and iuc (Fig. 6e). The clb gene was exclusively detected in human isolates. In addition, ybt, iro, and rmpA/A2 were significantly more prevalent in human strains than in non-human strains. As for hvKP strains, the vast majority were identified in human-derived strains (408 strains), with only a few detected in strains from pigs (5 strains), cats (1 strain), and cattle (1 strain). Notably, CR-hvKP strains were exclusively found in human-derived isolates.
Transfer of antimicrobial resistance genes and virulence factor genes
We investigated the correlations among plasmids, ARGs, and VFGs in isolates sourced from various hosts. A total of 9621 plasmid replicons were identified and classified into 111 distinct types. Notably, IncFIB(K), IncR, ColRNAI, IncHI1B(pNDM-MAR), and IncFII(pHN7A8) emerged as the most prevalent, constituting 40.62%, 35.39%, 34.46%, 20.68%, and 20.08%, respectively, of the 2809 isolates (Supplementary Data 7). To explore potential associations among plasmid replicons, ARGs, and VFGs, we conducted a Pearson correlation analysis. Although short-read sequencing data are insufficient for inferring direct physical linkage or horizontal transmission events, the co-occurrence patterns provide preliminary indications of potential relationships between certain plasmid types and ARGs or virulence genes. Notably, several plasmid replicons exhibited concurrent associations with both ARGs and major VFGs. For instance, IncFIB plasmids were correlated with the high virulence marker gene iroE and ARGs such as blaACT-16, catB4, and fosA, whereas IncHI1 plasmids were significantly associated with the high virulence marker gene rmpA and ARGs like tet(X4), lnu(G), and rmtB2 (Fig. 7). In our analysis grouping strains by human and non-human origins, we observed that the relationships among ARGs, VFGs, and plasmids in human isolates reflected the broader trends (Supplementary Fig. 10). Conversely, non-human isolates displayed a higher number of plasmid incompatibility groups associated with colistin resistance genes mcr-1 or mcr-8, including ColRNAI, IncFIB, and IncX4 (Supplementary Fig. 10).
Pairwise values with Pearson correlation coefficients greater than 0.5 (red) or less than −0.5 (blue) are displayed. The blue box labeled “ARG-ARG” illustrates the pairwise co-occurrence and mutual exclusivity among ARGs, while the green box labeled “VFG-VFG” showcases the same for VFGs. The green box marked “Plasmid-Plasmid” represents the pairwise co-occurrence and mutual exclusivity among plasmid replicons. Gold boxes with numerical labels denote the co-occurrence and mutual exclusivity between ARGs/VFGs and plasmid replicons, with annotations provided in the bottom left corner.
Overall, a positive correlation was evident between the count of ARGs and the number of plasmid replicons (rPearson = 0.39, p < 0.0001, Supplementary Fig. 11a), while no significant correlation was observed between VFGs and the count of plasmid replicons (rPearson = 0.07, p < 0.0001, Supplementary Fig. 11b). Additionally, there was no substantial correlation detected between ARGs and VFGs (rPearson = −0.07, p < 0.0001, Supplementary Fig. 11c).
Discussion
In this study, we provided a comprehensive and systematic investigation of K. pneumoniae, analyzing isolates from both human and non-human sources, including pigs, poultry, cattle, dogs, cats, blowflies, and environmental samples, collected across 57 countries since the 1900s. While numerous genomic studies have focused on K. pneumoniae populations originating from humans, there is a scarcity of data regarding the distribution of genetic lineages, AMR, and virulence in non-human sources. This study marks the initial systematic exploration of extensive genomic data encompassing both human and non-human sources.
K. pneumoniae is widely distributed across livestock, companion animals, and environmental reservoirs, constituting potential reservoirs for high-risk lineages capable of causing human infections35. The proximity between animals and humans in contemporary society heightens the risk of interspecies transmission of bacteria. Moreover, humans might directly encounter these pathogens through the food chain when consuming animal-derived products. Reports suggest that humans have exchanged K. pneumoniae strains with closely associated pets35, highlighting the possibility of transmission between human and animal populations11. In alignment with these studies, we also revealed an alignment in the genetic attributes of K. pneumoniae sourced from both human and non-human origins. Our subsequent in vivo and in vitro experiments additionally illustrate that K. pneumoniae exhibits cross-species pathogenicity.
The dissemination of antimicrobial resistant K. pneumoniae poses a significant public health concern4,6. Similar to other Enterobacteriaceae, K. pneumoniae can serve as an indicator of changes in AMR profiles36. Our analysis shows that since the 2000s, each strain has harbored an increasing burden of AMR determinants, driven by two parallel dynamics. On the one hand, regulatory restrictions on the use of conventional antibiotics, such as tetracyclines, sulfonamides, and early β-lactams, implemented in many countries have curtailed the expansion of “low-level” resistance genes in environmental and community settings. This has contributed to a decline and subsequent stabilization in the overall number of ARGs per genome following a historical peak. On the other hand, the proliferation of MDR high-risk clones, such as ST11, ST258, and ST15, carrying ESBLs and carbapenemase genes has accelerated in hospitals and surrounding environments37. These clones possess clinically advantageous “high-value” resistance traits, driving a continuous increase in AMR burden. Notably, while antibiotic stewardship policies have effectively reduced exposure to broad-spectrum conventional drugs, they have had limited impact on the clinical use of critical last-resort antibiotics, such as ESBL inhibitors and carbapenems, thus maintaining the selective pressure for resistance against these agents.
We compared the variances in ARGs between strains from human and non-human origins and noted that non-human strains generally harbor more ARGs, displaying elevated resistance phenotypes. This observation may be linked to the extensive agricultural use of antibiotics, with approximately 70% of antibiotics worldwide being utilized in agriculture, notably in livestock farming38,39,40. Our specific findings on ARGs carried by strains from diverse farm animals corroborate this trend. Notably, K. pneumoniae strains isolated from poultry exhibited the highest proportions of ARG carriage, while those from cattle displayed the lowest proportions. This aligns with the increased antibiotic usage in poultry farming compared to cattle farming41. The AMR profiles of K. pneumoniae strains from pets, blowflies, and environmental sources revealed concerning conditions. Many of these strains exhibited similar genetic traits and close associations with human strains, emphasizing the necessity to consider these species within the One Health framework to address the global AMR challenge.
Although carbapenems are not licensed for use in livestock and companion animals, we identified several carbapenemase-coding genes in strains originating from non-human species. Previous studies have reported the presence of Enterobacteriaceae carrying carbapenem resistance genes, particularly blaNDM and blaOXA, in poultry and pig farms16,42. The contamination within the in-house environment could explain this occurrence, as demonstrated by the high detection rates (26.8–31.4%) of blaNDM in environmental samples from poultry farms despite standard cleaning and disinfection practices42. The identification of blaNDM as the main carbapenemase gene in strains from farm animals, blowflies, and environments may also highlight a possible explanation for contamination within the in-house environment. The identification of carbapenemase genes in strains from cats and dogs might result from transmission between humans and cats due to their daily contact. In addition, the legally prescribed extralabel use of several carbapenem agents, such as imipenem and meropenem, primarily for the treating of resistant infections among pets in some regions, may also induce the development of carbapenem resistance strains in pets43.
Our study also revealed a continuous increase in the virulence potential of K. pneumoniae over time. Importantly, this trend was not driven by the expansion of classical hypervirulent clones (e.g., ST23, ST65), but rather by convergent evolution in MDR clones (e.g., ST11, ST15, ST258) that acquired key virulence factors such as siderophore systems and rmpA/rmpA2. Although hypervirulent strains have historically been regarded as antibiotic-sensitive44, the extensive use of antimicrobials and environmental selective pressures has created opportunities for MDR clones to acquire hypervirulence determinants5. These virulence genes, in turn, enhance colonization and dissemination within the host, forming a dual threat to public health. Recent studies have reported the convergence of AMR and hypervirulence in specific clones, such as ST11, ST147, and ST307, which carry both carbapenemase genes and hypervirulence plasmids. Whole-genome analyses have further indicated that insertion sequences and mobilizable plasmids play a central role in facilitating this convergence45,46. Consistent with these findings, we observed several plasmid replicons co-harboring both high-priority resistance genes and key virulence factors, supporting the global emergence of MDR-hypervirulent convergent clones.
While the majority of hvKP isolates in our dataset originated from human sources, this distribution may reflect a degree of sampling and reporting bias. HvKP strains are often pan-susceptible and lack distinct molecular signatures, making them less likely to be prioritized in AMR-focused surveillance. Consequently, these strains are underrepresented in public genomic databases, especially from animal and environmental sources, potentially leading to an underestimation of their true prevalence in non-human hosts. Nevertheless, emerging evidence indicates that animals can serve as potential reservoirs of hvKP47. In this study, we also found several hvKP strains in animals such as pigs, cattle, and cats, suggesting a potential transmission risk between animals and humans. K. pneumoniae has been shown to cause severe diseases in various animals, including sepsis in piglets and mastitis in sows12,48, affecting not only animal health but also potentially leading to economic losses in agriculture34,48. In cattle, K. pneumoniae can also cause pneumonia and mastitis49,50, while in cats, it has been confirmed to be associated with urinary tract infections50.
This study has two main limitations. First, the relatively small number of genome sequences from K. pneumoniae strains originating from non-human species that were used for analysis. This is partly due to the fact that K. pneumoniae strains from non-human species have received less attention in previous years. However, our study emphasizes the potential public health risk posed by K. pneumoniae strains originating from animals. Moving forward, we will focus on enhancing our monitoring efforts of K. pneumoniae strains originating from animals to gain a better understanding of their impact and implications. Second, our reliance on short-read sequencing for plasmid analysis is a limitation as well. While Pearson correlation of plasmid replicons with ARGs and VFGs offers preliminary insights, short reads often fragment complex plasmid structures and cannot confirm physical linkage or horizontal transfer. Nonetheless, our results establish a valuable foundation for future work; integrating long-read or hybrid assembly strategies will be crucial to reconstruct complete plasmid architectures and rigorously determine their roles in the dissemination of AMR and virulence genes.
In conclusion, our study presents comprehensive global genomic surveillance data on K. pneumoniae strains originating from various hosts. This research demonstrates through in vivo and in vitro experiments and genomic analyses that K. pneumoniae exhibits cross-species infection capacities. Our analyses reveal that increasing antibiotic resistance is closely linked to the expansion of MDR clones, while the rise in virulence over time is driven by the acquisition of key virulence determinants by these same clones. Comparative analyses of AMR and virulence across different hosts indicate that non-human isolates harbor a greater number of ARGs, while human isolates demonstrate heightened virulence. Furthermore, we observed significant correlations between certain plasmids and specific K. pneumoniae ARGs and VFGs. This research enhances our understanding of K. pneumoniae transmission, AMR, and virulence evolution, offering valuable insights to inform effective public health intervention strategies.
Data availability
Whole genome sequences of K. pneumoniae strains generated in this study have been deposited in the NCBI GenBank database under BioProject PRJNA998213. Accession numbers are provided in Supplementary Data 1 (JAUUFE000000000–JAUUIG000000000). Source data underlying the figures are available in Supplementary Data 8 and Supplementary Data 9. Other data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Murray, Christopher J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Jin, X. et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine 37, 100986 (2021).
Budia-Silva, M. et al. International and regional spread of carbapenem-resistant Klebsiella pneumoniae in Europe. Nat. Commun. 15, 5092 (2024).
Hu, F. et al. Carbapenem-resistant Klebsiella pneumoniae capsular types, antibiotic resistance and virulence factors in China: a longitudinal, multi-centre study. Nat. Microbiol. 9, 814–829 (2024).
Shon, A. S., Bajwa, R. P. & Russo, T. A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4, 107–118 (2013).
Paczosa, M. K. & Mecsas, J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 80, 629–661 (2016).
Struve, C. et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio 6, e00630 (2015).
Monteiro, A. S. S., Cordeiro, S. M. & Reis, J. N. Virulence factors in Klebsiella pneumoniae: a literature review. Indian J. Microbiol. 64, 389–401 (2024).
Franklin-Alming, F. V. et al. Exploring Klebsiella pneumoniae in healthy poultry reveals high genetic diversity, good biofilm-forming abilities and higher prevalence in Turkeys than broilers. Front. Microbiol. 12, 725414 (2021).
Wareth, G. & Neubauer, H. The animal-foods-environment interface of Klebsiella pneumoniae in Germany: an observational study on pathogenicity, resistance development and the current situation. Vet. Res. 52, 16 (2021).
Leangapichart, T. et al. Characterization of Klebsiella pneumoniae complex isolates from pigs and humans in farms in Thailand: population genomic structure, antibiotic resistance and virulence genes. J. Antimicrob. Chemother. 76, 2012–2016 (2021).
Bidewell, C. A. et al. Emergence of Klebsiella pneumoniae subspecies pneumoniae as a cause of septicaemia in pigs in England. PLoS ONE 13, e0191958 (2018).
Hetland, M. A. K. et al. A genome-wide one health study of Klebsiella pneumoniae in Norway reveals overlapping populations but few recent transmission events across reservoirs. Genome Med. 17, 42 (2025).
Calland, J. K. et al. Population structure and antimicrobial resistance among Klebsiella isolates sampled from human, animal, and environmental sources in Ghana: a cross-sectional genomic one health study. Lancet Microbe 4, e943–e952 (2023).
Holt, K. E. et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 112, E3574–E3581 (2015).
Peng, Z. et al. Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat. Commun. 13, 1116 (2022).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Kurtz, S. et al. Versatile and open software for comparing large genomes. Genome Biol. 5, R12 (2004).
Croucher, N. J. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 43, e15 (2015).
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019).
Tonkin-Hill, G., Lees, J. A., Bentley, S. D., Frost, S. D. W. & Corander, J. Fast hierarchical Bayesian analysis of population structure. Nucleic Acids Res. 47, 5539–5549 (2019).
Guerrero-Araya, E., Muñoz, M., Rodríguez, C. & Paredes-Sabja, D. FastMLST: a multi-core tool for multilocus sequence typing of draft genome assemblies. Bioinform. Biol. Insights 15, 11779322211059238 (2021).
Nascimento, M. et al. PHYLOViZ 2.0: providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics 33, 128–129 (2017).
Florensa, A. F., Kaas, R. S., Clausen, P., Aytan-Aktug, D. & Aarestrup, F. M. ResFinder—an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb. Genom. 8, https://doi.org/10.1099/mgen.0.000748 (2022).
Carattoli, A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58, 3895–3903 (2014).
Rovetta, A. Raiders of the lost correlation: a guide on using Pearson and Spearman coefficients to detect hidden correlations in medical sciences. Cureus 12, e11794 (2020).
Lam, M. M. C. et al. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 12, 4188 (2021).
Zhang, J. et al. Genomic characterization of Salmonella enterica serovar Weltevreden associated with human diarrhea. Microbiol. Spectr. 11, e0354222 (2023).
Percie du Sert, N. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411 (2020).
Mittelholzer, C., Moser, C., Tratschin, J. D. & Hofmann, M. A. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 74, 293–308 (2000).
Zhang, J. et al. Synergistic pathogenicity by coinfection and sequential infection with NADC30-like PRRSV and PCV2 in post-weaned pigs. Viruses 14, https://doi.org/10.3390/v14020193 (2022).
Lorenzo, D. Chloramphenicol resurrected: a journey from antibiotic resistance in eye infections to biofilm and ocular microbiota. Microorganisms 7, https://doi.org/10.3390/microorganisms7090278 (2019).
Trif, E. et al. Old antibiotics can learn new ways: a systematic review of florfenicol use in veterinary medicine and future perspectives using nanotechnology. Animals 13, https://doi.org/10.3390/ani13101695 (2023).
Tian, D. et al. Prevalence of hypervirulent and carbapenem-resistant Klebsiella pneumoniae under divergent evolutionary patterns. Emerg. Microbes Infect. 11, 1936–1949 (2022).
Marques, C. et al. Evidence of sharing of Klebsiella pneumoniae strains between healthy companion animals and cohabiting humans. J. Clin. Microbiol. 57, https://doi.org/10.1128/jcm.01537-18 (2019).
Navon-Venezia, S., Kondratyeva, K. & Carattoli, A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 41, 252–275 (2017).
Marsh, J. W. et al. Evolution of outbreak-causing carbapenem-resistant Klebsiella pneumoniae ST258 at a tertiary care hospital over 8 Years. mBio 10, https://doi.org/10.1128/mBio.01945-19 (2019).
Mulchandani, R., Wang, Y., Gilbert, M. & Van Boeckel, T. P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 3, e0001305 (2023).
Van Boeckel, T. P. et al. Reducing antimicrobial use in food animals. Science 357, 1350–1352 (2017).
Tiseo, K., Huber, L., Gilbert, M., Robinson, T. P. & Van Boeckel, T. P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 9, https://doi.org/10.3390/antibiotics9120918 (2020).
Van Boeckel, T. P. et al. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 112, 5649–5654 (2015).
Zhai, R. et al. Contaminated in-house environment contributes to the persistence and transmission of NDM-producing bacteria in a Chinese poultry farm. Environ. Int. 139, 105715 (2020).
Smith, A., Wayne, A. S., Fellman, C. L. & Rosenbaum, M. H. Usage patterns of carbapenem antimicrobials in dogs and cats at a veterinary tertiary care hospital. J. Vet. Intern. Med. 33, 1677–1685 (2019).
Vandhana, V., Saralaya, K. V., Bhat, S., Shenoy Mulki, S. & Bhat, A. K. Characterization of hypervirulent Klebsiella pneumoniae (Hv-Kp): correlation of virulence with antimicrobial susceptibility. Int. J. Microbiol. 2022, 4532707 (2022).
Silvester, R. et al. Global surveillance of antimicrobial resistance and hypervirulence in Klebsiella pneumoniae from LMICs: an in-silico approach. Sci. Total Environ. 802, 149859 (2022).
Hala, S. et al. The emergence of highly resistant and hypervirulent Klebsiella pneumoniae CC14 clone in a tertiary hospital over 8 years. Genome Med. 16, 58 (2024).
Al Ismail, D., Campos-Madueno, E. I., Donà, V. & Endimiani, A. Hypervirulent Klebsiella pneumoniae (hvKp): overview, epidemiology, and laboratory detection. Pathog. Immun. 10, 80–119 (2024).
Bowring, B. G., Fahy, V. A., Morris, A. & Collins, A. M. An unusual culprit: Klebsiella pneumoniae causing septicaemia outbreaks in neonatal pigs? Vet. Microbiol. 203, 267–270 (2017).
Yang, J. et al. Comparative genomic analyses of Klebsiella pneumoniae K57 capsule serotypes isolated from bovine mastitis in China. J. Dairy Sci. 107, 3114–3126 (2024).
Ribeiro, M. G. et al. Klebsiella-induced infections in domestic species: a case-series study in 697 animals (1997-2019). Braz. J. Microbiol. 53, 455–464 (2022).
Acknowledgements
This study was funded in part by the National Key Research and Development Program of China (No. 2022YFD1800905), Hubei Provincial Natural Foundation of China (No. 2023AFA094), the Fundamental Research Funds for the Central Universities (Grant·No. 2662025DKPY004), Yingzi Tech & Huazhong Agricultural University Intelligent Research Institute of Food Health (No. IRIFH202209), Walmart Foundation (Project # 61626817), and Hubei Hongshan Laboratory & Huazhong Agricultural University Startup fund. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Z.P. and B.W. designed the study and contributed to the revision of the manuscript. X.H. and X.Y. performed the bioinformatical analysis and data management. X.H., Y.H., D.Z., R.X., C.S., Y.S., H.B., W.S. and L.H. performed laboratory experiments and animal tests. C.L. helped to collect clinical bacterial strains. Z.P. and X.H. drafted the first version of the manuscript, while Z.P., B.W. and H.C. participated in the discussion and manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Elias Eger, Nelly M Mohamed and Harapriya Mohapatra for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, X., Yao, X., Hou, Y. et al. Global trends of antimicrobial resistance and virulence of Klebsiella pneumoniae from different host sources. Commun Med 5, 383 (2025). https://doi.org/10.1038/s43856-025-01112-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-01112-1