Abstract
Background
Neurological conditions account for millions of deaths per year and induce long-lasting cognitive impairments. The disruption of structural brain networks predicts the emergence of cognitive impairments in stroke cases, but the role of the white matter in modeling longitudinal behavioral trajectories in glioma patients is understudied.
Methods
We analyzed 486 intracranial brain stimulations from 297 patients (age range 37–40, male ratio 53–64% depending on the functional categories) along with functional and structural brain connectivity data from over 1750 healthy individuals, to create a network mapping method able to identify the neural substrate causally involved in language production. We tested the validity of our procedure by (i) quantifying the spatial correspondence between white matter metabolic and hemodynamic spontaneous activity, measured via resting-state functional Magnetic Resonance Imaging and [18 F]-fluorodeoxyglucose functional Positron Emission Tomography (respectively); (ii) predicting unseen intracranial stimulations points; (iii) modeling the severity of stroke-induced aphasia (n = 105) and the longitudinal recovery of language abilities in glioma patients (n = 42, 3 timepoints).
Results
We show that spontaneous white matter hemodynamic oscillations map into metabolic fluctuations. We also demonstrate that the integration of patient-specific intracranial stimulation points and normative human connectivity data (i) is predictive of unseen stimulation points; (ii) provides better estimates than total lesion volume in predicting the severity of stroke-induced aphasia symptoms; (iii) models post-operative language recovery trajectories better than state-of-the-art clinical measures in glioma patients.
Conclusions
This work presents a data-driven and neurobiologically grounded tool for modeling cognitive and neurological impairments in terms of network disruption, demonstrating improved precision over existing approaches.
Plain language summary
Understanding how connections in the brain can affect the recovery of language skills after stroke or surgery for brain tumors is of critical importance. Here, we developed a method that combines non-invasive brain scans with direct brain stimulation to identify the exact brain connections important for language production. Our findings reveal a brain map that could predict the severity of language problems after a stroke and how well people recovered their language skills following surgery for brain tumors, better than traditional ways of predicting language problems. We created an effective way to predict and track language difficulties caused by damage in the brain. This could help doctors plan better treatments and support for people recovering from brain injuries or surgery.
Similar content being viewed by others
Introduction
Neurological conditions such as stroke and glioma represent leading causes of disability worldwide, accounting for millions of deaths per year and long-lasting cognitive impairments1. In all countries, both conditions are widespread and undertreated.
Recent evidence suggests that the disruption of structural brain networks—as derived from the normative modeling of magnetic resonance imaging (MRI) data— is a promising predictor of survival rates in glioma and efficiently models cognitive impairment in stroke2,3. Brain tumors overlapping with regions characterized by a low density of white matter tracts in a reference wiring diagram of the human brain —a so-called normative structural connectome—were predictive of overall higher survival rates2. Similarly, advanced analytical techniques revealed that white matter disconnections reliably predict neuropsychological scores 1 year after stroke3.
Crucially, a similar connectivity framework to model cognitive functioning after surgery is currently lacking, even though the emergence of post-operative cognitive and/or neurological deficits has a dramatic impact on both the patients’ lives and those one of their families4. The impossibility of canonical MRI-derived measures to delineate the neural substrates causally implicated in a given cognitive function is the pivotal reason behind such a limitation5,6. In addition, the intrinsic difficulties in acquiring long MRI sessions in patients with a brain tumor are also slowing down the discovery of better neuroimaging-derived prognostic indicators.
In the context of glioma treatment, direct electrical brain stimulation (DES) during cognitive and neurological monitoring in awake surgery represents the current reference therapeutic approach7. DES is an invasive mapping technique that provides causal, reliable, and patient-tailored evidence for the functional role of the stimulated brain regions. Patient-specific mapping results in the preservation of eloquent brain structures, together with increased survival rates7,8,9. However, DES alone provides only focal and, as such, incomplete insights into the brain’s functional architecture, given that complex cognitive functions are subserved by the orchestrated activity of spatially distributed but connected regions, so-called macroscale functional networks10. Establishing a causal link between focal electrical stimulation, behavior, and the wiring of such functional ensembles is therefore an urgent clinical need6.
Building upon a recent study probing the clinical validity and translation potential of integrating cortical DES with normative MRI-driven functional network mapping11, here we present a data-driven method able to characterize and recover normative maps delineating the causal white matter substrate associated with a given cognitive function. Intriguingly, recent findings suggest that spontaneous brain oscillations in the white matter map into distributed functional networks with distinct genetic, electrophysiological, and structural signatures12,13,14, potentially supported by the presence of excitatory synapses15,16. By leveraging the unique combination of white matter DES stimulation and normative white matter functional connectivity, we first recovered the functional white matter networks underlying semantics, phonology, and speech arrest, key functions subtending language production. Showing that white matter functional networks defined on the basis of hemodynamic spontaneous fluctuations may have a metabolic origin and are predictive of unseen stimulation points allowed us to define white matter functional hubs. Being localized in the deep white matter of the brain, functional hubs can be seen as fiduciary waypoints for a given cognitive function. By leveraging such waypoints as filters to obtain the wiring diagram related to a specific language functional domain, we show that it is possible not only to unveil the causal structural substrate underlying semantics, phonology, and speech arrest, but also to easily compare commonalities and differences in the spatial organization of such macro-scale networks.
Critically, we provide robust evidence suggesting that our multimodal derivation of DES-derived structural networks can be leveraged as a tool to effectively model lesion-induced impairments in stroke and glioma patients, highlighting its translational potential. We show that our results compare favorably to state-of-the-art normative approaches in predicting the severity of stroke-induced aphasia symptoms (N = 105). Even more critically, we demonstrate that our network mapping procedure describes post-operative language recovery trajectories in glioma patients (N = 42, 3 time points) better than genetic and molecular read-outs, potentially elucidating the neural substrates causally underlying surgery-induced plasticity phenomena. The normative maps of the white matter are publicly available, with the intention of offering a transdiagnostic resource to both medical and research communities.
Materials and methods
Direct electrical stimulation (DES) dataset
Cortical mapping refers to the gray matter electrically stimulated after craniotomy and before/during tumor removal, while subcortical mapping refers to the white matter bundles and/or caudate/basal ganglia that was exposed at the depth of the surgical resection cavity7. We used 280, 106, and 100 subcortical DES points corresponding to the functional categories of semantics, phonology, and speech articulation (respectively) to provide a multimodal mapping of the functional and structural scaffold underlying language production. The DES points used in the current study are part of a larger dataset consisting of 2906 cortical and 1231 subcortical DES points derived from 592 low (WHO grade II) and 20 high-grade (WHO grade III) glioma patients originally used to validate the integration of cortical DES with functional connectome mapping11. Intraoperative mapping and behavioral tasks description are reported below. Mean patient age (min-max: 37–40) and the proportion of right-handers (min-max: 85–87%) were stable across cognitive categories, while the male-to-female ratio (min-max: 53–64%) showed a more pronounced variability (Supplementary Table 1).
Direct electrical stimulation (DES) intraoperative mapping
We followed the principle of functionally guided tumor removal7,17. Practically, the principle of functionally guided tumor removal entails that the tumor removal is initiated only after completion of the direct electrical stimulation (DES) cortical mapping, and administered until positive DES subcortical responses are obtained. Of note, every stimulation site is repeated three times during the surgery, and the stimulation is categorized as “functional”—and annotated with a tag placed on the brain’ surface—if it induces a behavioral disturbance at least 2/3 times in a nonconsecutive manner. Stimulation sites that do not reach this level of reproducibility are categorized as “non-functional”. In doing so, the technique avoids possible cognitive deficits even in territories not classically and properly considered functional, such as the frontal pole and the mesial surfaces.
Neuropsychological testing
Concerning the description of the behavioral tasks, we are reporting here the details of the three functional categories7.
Speech articulation
Spontaneous language production was monitored by a counting task (series from 0 to 10) and a repetition test, with positive functional responses being involuntary cessation of counting or inability to repeat, respectively, during stimulation.
Phonology, semantics
Phonological and semantic aspects of language processing (i.e. semantic and phonological paraphasias or pure anomia, not related to motor/praxis/visual disturbances) were assessed with picture naming test [denomination object 80 (DO 80)] Examples of positive functional responses included semantic paraphasias (e.g. patient says “dog” instead of cat during presentation of a picture of a cat), phonemic paraphasias (e.g. patient says “kite” instead of cat after presentation of a picture of a cat), and anomias (e.g. patient states “this is a...” but cannot come up with “cat” during presentation of a cat picture).
Importantly, during these tasks, both the patient and neuropsychologist/speech therapist were blinded to the timing of DES performed by the surgeon.
Functional network construction
We derived the DES-driven functional networks using a fully preprocessed resting-state functional MRI dataset consisting of 1000 healthy subjects (Brain Genomics Superstruct Project dataset)18. The acquisition parameters and pre-processing steps have been described elsewhere19. Here, we report only the choice of performing global signal regression as part of the denoising pipeline. Of note, functional network mapping via resting state functional MRI assumes that the degree of synchronization—assessed via Pearson’s correlation—of the spontaneous gray matter oscillations measured when a subject/patient is not actively engaged in a cognitive task can be taken as a proxy for a shared cognitive function, and it is called functional connectivity5.
In line with recent observations suggesting that also spontaneous white matter BOLD oscillations map into functional networks12,13,14 and might have appealing translational properties20, we seeded white matter BOLD signal from subcortical DES points to derive whole-brain functional networks in the white matter. As per our previous work11, we obtained a whole-brain functional connectivity map per DES point. Next, we tested for the statistical significance of the single DES point functional network topography separately for the positive (i.e., correlated) and negative (i.e., anti-correlated) networks by using a non-parametric procedure (randomize as implemented in FSL, v6.0.5.121). Next, and for each of the three functional categories independently, we obtained a single brain map expressing at each voxel location the number (expressed as a frequency) of DES-derived seeds with statistically significant functional connectivity. The spatial distribution of the white matter functional networks is presented in Supplementary Fig. 1). The detailed procedure—known as lesion network mapping (LNM)—is described below.
From direct electrical stimulation to white matter functional networks
Our multimodal causal network mapping procedure aims at integrating DES with resting state fMRI (rsfMRI) via LNM5. By using the lesions location associated with a certain syndrome as seeds to derive group representative functional maps obtained from a large cohort of healthy subjects, LNM is able to leverage the spatial heterogeneity in lesions location to extract a common neural substrate associated with that syndrome. Similarly, we treated each cortical stimulation point as a transient lesion, and used it as a seed for performing a seed based analysis (SBA)22 in a normative connectome derived from 1000 healthy individuals (Brain Genomics Superstruct Project dataset)18,19.
Data preprocessing
We used the subcortical DES coordinates published in a recent study11. More details on the preprocessing of the DES coordinates can be found in the corresponding publication. Briefly,
structural gadolinium-based T1 MR images were acquired either in Montpellier (306 patients) or Trento (50 patients), 3 months after surgery. The subject native T1 images were realigned with fslreorient2std (FSL v6.0.5.1)23 to match the approximate orientation of the standard template image (MNI152 as provided by FSL, 1 mm3 isotropic spatial resolution), automatically skull stripped using mri_synthstrip (Freesurfer v7.3.2)24, and linearly registered to the MNI template (1 mm3 isotropic spatial resolution, trilinear interpolation) using FSL flirt (flirt v6.0). Due to the unavailability of the tumors’ segmentations, an affine registration was used. Both skull stripping and registration outputs were visually inspected to detect gross abnormalities.
Next, the stimulation coordinates were extracted from a CSV file and written into a nifti file—1 at the stimulated voxel, 0 otherwise—in the subject native space via nibabel and numpy (v3.2.2 and v1.21.5, respectively)25,26. Similarly to the preprocessing applied to the corresponding subject native T1, the nifti file was reoriented using fslreorient2std and linearly registered to the MNI template using the registration matrices obtained from the subject-to-template alignment. Finally, the coordinates (in mm) of the stimulated voxel in the MNI space were extracted with fslstats and stored in a CSV file.
Realignment of subcortical points to white matter and subcortical nuclei
Subcortical stimulation points were realigned to a brain mask encompassing the white matter and the deep subcortical nuclei and obtained by automatically segmenting the T1 image of the MNI template via FreeSurfer’s SynthSeg tool27. Subcortical stimulation points that had to be moved ≥5 mm were discarded from the analysis.
Seed-based analysis (SBA) with the cortical stimulation points
For each tested function separately, cortical stimulation points were used as seeds for performing a SBA analysis28,29 in the GSP dataset. For each subject and stimulation point, the average rsfMRI BOLD signal was extracted within a sphere of 5 mm radius—corresponding to spacing of the bipolar electrode used for stimulating the subcortical tissues—centered on the stimulation coordinates, and cross-correlated with every other voxel of the brain—and as such encompassing both gray and white matter—to obtain a subject and DES points specific functional map. Before statistics, Pearson’s r scores were transformed to z scores using Fisher’s r-to-z transform via AFNI7. For each seed, representative group maps—one for positive correlations, and one for negative correlations—were obtained with a non-parametric one sample t-test (FSL’s tool randomize with the Threshold-Free Cluster enhancement option enabled)21. Due to the high number of both subjects and seeds, the number of permutations was reduced to 1000. By providing connectivity maps at the voxel level, SBA allows a spatially unbiased mapping of whole brain connectivity, and combined with seeds derived from DES, it potentially allows to causally map the spatial topography of putative large scale functional networks subtending each tested functional category.
From seed maps to functional networks via lesion network mapping
Traditional LNM consists of three steps30. First, a group representative functional connectivity map—usually obtained with a parametric approach (e.g., one-sample t-test)—is computed for each seed. Subsequently, a high threshold (e.g., T-score) is applied to each image, resulting in a binary representation of functional connectivity. As a last step, binary images are aggregated, and a single frequency map is computed, with high concordance brain regions delineating the network of interest. The second step is where our approach and the traditional LNM differ the most, as we did not rely on an arbitrary and universal hard threshold for all DES-derived functional maps but employed a non-parametric procedure to threshold each DES connectivity map separately. Of note, lesion size represents a possible confound in traditional LNM, a methodological artifact that is circumvented by using DES, as DES-derived seeds have equal volume.
Validation
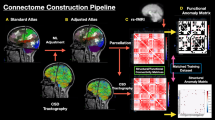
Due to its recent introduction, the adoption of white matter functional connectivity to probe the functional architecture of the brain is still controversial. For this reason, we performed a series of validation analyses to corroborate the integration of subcortical DES mapping with white matter resting state fMRI (Fig. 1). To provide a link between white matter hemodynamic fluctuations and underlying neural activity, we compared the spatial organization of white matter functional networks with the spatial organization of white matter metabolic networks as measured with [18F]-fluorodeoxyglucose functional positron emission tomography (FDG-fPET), a proxy for dynamic neural activity. The FDG-fPET dataset consisted of 26 healthy control subjects (77% female, 18–23 years old, N = 25 for the current study) scanned with a protocol that allows the tracking of metabolic oscillations similarly to resting state fMRI31,32. For each subject, we partitioned the white matter in 50 regions of interest using ICBM-DTI-81 white-matter labels atlas33 as provided by FSL, extracting region-averaged time series from both the FDG-fPET and resting state fMRI datasets using nilearn (v0.10.034). We then transformed Pearson’s r scores to z scores using Fisher’s r-to-z transform, and we finally obtained metabolic and functional connectivity at the group level by averaging single subject matrices. Averaged matrices were converted back to Pearson’s r value via the tanh function (numpy v1.21.526). We assessed the degree of similarity in the spatial distribution of metabolic and functional connectivity in the white matter by correlating the two matrices row-wise, correcting for spatial autocorrelation using parametrized null models (10,000 instantiations per region with default parameters using the python implementation of BrainSMASH35) and multiple comparisons with a false discovery rate procedure (python package statsmodel v0.9.0)36. We removed 10 regions of interest for which the null model preserving spatial autocorrelation returned a non-optimal fit to the empirical data (Pearson’s r between one average null model and empirical data <0.75, 1000 null realizations, scipy v 1.7.3), as recently suggested37.
We used lesion network mapping to derive functional networks from the set of both DES cortical and subcortical points (rendered on the surfaces reconstruction of the gray and white matter, respectively), mapping functional network hubs in both white and gray matter in a normative sample of 1000 healthy individuals (see main text). We used functional hubs as waypoints for tractography filtering in an additional independent cohort of 753 healthy subjects, obtaining in a data driven manner function specific wiring diagrams that were segmented in a semi-automated manner to extract white matter pathways, and converted to a volumetric representation for the clinical analyses. We also probed the robustness and biological foundation of white matter DES-derived functional networks by comparing cerebral oxygenation dynamics and metabolic fluctuations in the white matter, as well as by investigating whether white matter DES-derived functional networks are predictive of unseen stimulation points. A.U arbitrary units, DES direct electrical stimulation, FC functional connectivity, FDG-fPET [18 F]-fluorodeoxyglucose functional positron emission tomography, GM gray matter, WM white matter.
As an additional validation, we tested whether DES-derived networks are predictive of unseen subcortical stimulation points via cross-validation11. For each of the three categories separately, we repeated the network construction procedure by iteratively leaving one DES point out of the network building procedure. Importantly, we used the left-out point as a benchmark to test the predictive power of both the DES positive and negative derived functional networks estimated using all the other stimulations. The left-out point not included in the network construction phase was used to contrast the DES positive (i.e., correlated) against the DES negative (i.e., anti-correlated) network. We reasoned that if functional connectivity is a proxy for a shared function, then the left-out point not included in the DES-derived networks should fall in a brain region characterized by high positive functional connectivity, and hence higher frequency for the DES positive network than for the negative network. The left-out point was used to compute the mean concordance rate for the positive and negative networks separately, and we classified the DES network as predictive if the average concordance rate for the positive network was greater than the average concordance rate of the negative network. We tested for between positive vs negative DES differences in the mean concordance rate of the left-out point via a one-sided t-test for dependent samples and quantified effect sizes using Cohen’s d. Finally, as we noticed that ca. 30% of the points across the three cognitive categories were derived from the same individual, we repeated the analysis by employing a leave-one-patient-out scheme. We carried out this analysis with the same set of custom scripts previously described and publicly available11.
Structural network derivation
To integrate functional mapping with structural connectivity, we used the whole brain tractograms in the standard reference space (MNI as provided by FSL)23 obtained via probabilistic tractography of 753 healthy control subjects derived from the Human Connectome Project (s1200 release), publicly available via brainlife.io38. To derive the wiring diagrams of semantic, phonological, and speech articulation networks, we filtered the whole brain tractograms on a per subject basis (Fig. 1), using fiduciary points in both white and gray matter as defined by DES-derived functional networks. Within the scope of the structural network derivation analysis, we equated fiduciary points with the notion of hubs, the latter defined as all the brain regions exceeding the 90th percentile of the correctly classified DES points only. To preserve consistency with the subcortical mapping, we derived cortical hubs —and hence fiduciary termination points in the gray matter—with the exact same procedure11, computing their gray/white matter interface using Mrtrix (v3.04)39. In summary, the set of cognitive category-specific subcortical/cortical hubs defined fiduciary waypoints and terminations (respectively) that were derived from the integration of DES and resting state fMRI.
Next, for each subject and each cognitive category separately, we obtained a filtered tractogram. Filtered tractograms were used to obtain a two-fold representation of the white matter: as a volumetric map for the clinical analyses (Fig. 2) and as white matter pathways of interest for visualization (Fig. 3).
To obtain normative and completely data-driven normative volumetric maps for the clinical analyses, we computed for each subject and each functional category separately the number of fibers crossing any given voxel in the standard reference space (1 mm3 resolution) with MRtrix. Subsequently, we normalized the voxel’s value in 0–1 range, aggregated the volumetric images across subjects, and normalized again in 0–1 range, obtaining one volumetric map for the functional category. For each functional category, the voxels’ values of the volumetric map reflect whether and to what extent a given portion of white matter represents a common—across subjects—salient point in the corresponding network’s architecture, building a robust and causally derived normative reference to map disconnection impairments. As preparation for the clinical analyses, we separately thresholded the volumetric structural maps to retain only the values exceeding the 95th percentile.
To gain insights into the white matter architecture of the brain, we extracted white matter bundles of interest. We clustered single subject filtered tractograms using QuickBundle (with a clustering threshold of 20 mm), as implemented in dipy (v1.7.0)40,41. Finally, for each category separately we aggregated the clusters’ centroids across the 753 participants into a single tractogram, and we applied Tractome42 to manually identify the main components of the pathways resulting from the previous filtering and aggregation process. Segmented and manually cleaned bundles are presented in Fig. 3, while in Supplementary Fig. 2 we reported the bundles before segmentation.
Modeling lesion induced impairments and recovery with DES derived structural network
To provide a clinical validation of our multimodal mapping procedure, we used a publicly available dataset of aphasic stroke patients43 (upper panel of Fig. 2) and re-analyzed a sample of glioma patients previously characterized (lower panel of Fig. 2)44. Details on the acquisition parameters in the stroke cohort can be consulted in the accompanying publication43. The acquisition parameters of the glioma sample used in the clinical analysis are reported in the original work44. Briefly, each patient underwent an MRI examination as a standard protocol for glioma surgery at “Santa Chiara Hospital” in Trento, with the acquisition of structural for pre-operative planning of glioma resection on a clinical Optima MR450w GE 1.5T scanner (GE Healthcare, Milwaukee, WI, United States), equipped with an 8-channel receive head RF coil. A T1-weighted volumetric sequence (axial acquisition, TR/TI/TE = 10.64/450/4.23 ms, FA = 12°, square field of view (FOV) = 256 mm, voxel size = 1 × 1 × 1 mm3) was performed for each patient. A second early post-operative T1 with gadolinium acquired 24 h after surgery according to the protocol defined above was used for the characterization of the surgical cavity.
With respect to stroke patients, we focused on the first MRI and behavioral assessment post stroke, retaining only the patients in the chronic phase (i.e., measured at least 6 months after the stroke) and those for which the behavioral and MRI assessments were max 10 days apart. We excluded patients with missing values and with no aphasia diagnosis. For the selected patients (N = 105, 67 male, mean age at stroke = 56.7, SD = ± 10.84, all lesions localize in the left hemisphere), we linearly registered the T1-weighted and lesions’ masks in MNI space (as provided by FSL) using FLIRT45 (lesions’ heat map shown in Supplementary Fig. 3). For each patient, we computed the percentage of the lesion’s volume overlapping with the thresholded volumetric white matter maps of semantics and phonology and used it to predict via quantile regression—as implemented in scikit-learn (v1.1.2)46—the overall severity of the disorder. The degree of overlap between the lesion and the structural networks was computed as the intersection over the total volume of the reference network. The severity of the disorder was as assessed by the aphasia quotient score from the Western Aphasia Battery (revised version47). To avoid data leakage between training and test sets, we implemented a nested cross validation scheme to perform hyperparameter optimization (quantile to be predicted and the strength of regularization to be applied), with an outer leave one out cross validation scheme and 10-fold random split as inner cross validation strategy. To avoid overfitting, we assigned 50% of the patients to the training set and the remaining 50% as validation set in the inner cross validation loop. We selected the coefficient of determination (r2) as a metric to be optimized and tested the significance of our results via permutations (1000 shuffles). To account for the randomness of the internal splitting, we estimated the empirical r2 100 times. As a readout, we also computed mean absolute percentage error3. Finally, we repeated the whole procedure by using total lesion volume as a predictor (Fig. 2).
Given that all lesions were localized in the left hemisphere, and all patients were diagnosed with aphasia, it follows that the lesions were already localized in brain regions crucial to language preservation. As such, the cohort of stroke patients did not allow us to completely disentangle the contribution of total lesion volume from the overlap between the DES derived structural networks and language eloquent brain regions. To circumvent this issue, we retrospectively re-analyzed an already characterized sample of glioma patients (lower panel of Fig. 2)44. In their original investigation, the authors found that left hemisphere lesions encompassing the left superior frontal or temporal gyri—and associated white matter bundles—were related to worse verbal fluency performances than lesions located in the right hemisphere. The clinical sample consisted of 42 patients (26 male, mean age = 52.5 years, SD = ± 13.3) who underwent awake (54.8%) or asleep (45.2%) surgery for low-grade (16.7%) or high-grade-glioma (83.3%) in the frontal (64.3%) or temporal lobe (35.7%) of the left (50%) or right (50%) hemisphere. After linear registration to the MNI template using FSL flirt (tumors’ heat map shown in Supplementary Fig. 4), we computed the overlap between the tumor’s cavity obtained one week after surgery and the structural white matter maps of semantic, phonological, and used it to predict post-operative longitudinal language recovery. Semantic and phonological fluency scores were assessed before surgery, 1 week, and 1 month after surgery. We did not include the structural speech articulation network, as speech arrest was measured intra-operatively only. To model the longitudinal data, we employed a piecewise linear mixed model using R (v4.3.2)48, lme4 (v1.1.35.1)49, performance (v0.10.8)50, tidyverse (v2.0.0)51, and the partR2 (v0.9.1)52 packages. The piecewise linear model was implemented by dividing the time axis into 2 segments centered around the time point “1 week after surgery”. We fitted a linear model in each of the two segments, where the overlap with the DES derived network was compared (in a pairwise manner) with eight control variables. The overlap between the lesion and DES derived structural network was computed as a binary variable (overlap/no overlap) to be consistent with the encoding of cellular biomarkers (MGMT methylation status and IDH mutation), sex education, tumor grade, and surgery type. Only the total lesion volume was encoded as a continuous variable. With the exception of SUB-IDs entered as random intercepts, all the other variables were entered as fixed effects. For each head-to-head comparison, we fitted three different models (i) only overlap with DES-derived structural network as a fixed effect; (ii) only the control clinical variable as a fixed effect; (iii) both the DES-derived structural network and the clinical variable as separate fixed effects. Due to the nested structure of the three models, p-values were computed via likelihood ratio tests. As we compared our primary variable of interest against eight other variables, we employed a Bonferroni correction to correct for the family-wise error rate. The p-values after Bonferroni correction—performed separately for the phonemic and semantic fluency scores—are reported in Supplementary Tables 2 and 3. In Fig. 2, we plotted for each comparison the relative increase in effect size achieved by adding the overlap between lesion and DES-derived structural network to a model containing the control variable only, and vice versa, the gain in effect size achieved by adding the control variable to the model containing only the overlap between the lesion and DES-derived structural network. For Fig. 2, we grouped the eight variables of interest in three macro categories: age, sex (M/W), and education under demographic variables; lesion size, tumor grade (high vs low grade), and surgery type (awake vs asleep) under clinical variables; MGMT methylation status and IDH mutations as genetic variables. Effect size comparison was implemented following the recommendation contained in ref. 53. Finally, we repeated the whole procedure using short-term visuo-spatial memory54 as a control task, probing the specificity of our functional mapping (Supplementary Fig. 5 and Supplementary Tables 4 and 5 for semantic and phonological white matter structural networks, respectively).
Statistics and reproducibility
Data were summarized with appropriate descriptive statistics, including mean with standard deviation and/or variable min max, and effect size (Cohen’s d). One sided t-tests were used throughout the manuscript and significance was assessed using α = 0.05. We employed state-of-the-art methods to account for spatial autocorrelation when comparing brain maps37 and to adjust for multiple comparison in all other analyses (FDR and/or Bonferroni). To assess the reliability of our nested cross validation in the stroke analysis, we estimated the empirical r2 100 times to account for the randomness of the internal splitting.
Ethical statement
For the glioma cohort, all participants provided informed consent in accordance with the Declaration of Helsinki. The study was approved by the ethical committee of the Azienda Provinciale per i Servizi Sanitari (APSS) located in Trento, Italy (Project: NeuSurPlan, code A734, 29/10/2021). All the data were collected according to the procedure approved by the Internal Review Boards. For the stroke cohort, the study was approved by the Institutional Review Board, located in Columbia, SC, at the University of South Carolina. All participants provided informed consent43. For the normative functional data (GSP dataset), participants provided written informed consent in accordance with guidelines established by the Partners Health Care Institutional Review Board and the Harvard University Committee on the Use of Human Subjects in Research55. For the normative structural data (HCP dataset), the study was approved by the Washington University Institutional Review Board (IRB)56.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
The spatial arrangement of the white matter functional networks is presented in Supplementary Fig. 1. Below, we present the results pertaining the validation, clinical analyses, and visual inspection of the DES-derived structural networks.
White matter hemodynamic fluctuations map into metabolic oscillations
To gain insights into the neurophysiological basis of white matter functional connectivity, we investigated whether the spatial organization of spontaneous metabolic oscillations in the white matter measured with [18F]-fluorodeoxyglucose functional positron emission tomography (FDG-fPET, metabolic connectivity) resembled the spontaneous oscillation measured via resting state fMRI. After accounting for spatial autocorrelation and correcting for multiple comparisons, we found that 62.5% of the regions (25/40) showed a statistically significant (p-values < 0.05) correlation between the spatial organization of spontaneous resting state fMRI brain oscillations and FDG-fPET derived glucose metabolism in the white matter. An additional 17.5% of the regions (7/40) showed either significant correlations that did not survive the correction for multiple comparisons or a statistical trend (p-value < 0.1) towards significance. Finally, for 20% of the regions (8/40) our analysis yielded p-values above 0.1. Supplementary Data File 1 reports on a region-by-region basis the fit with the set of null models, uncorrected, and FDR-corrected p-values.
DES-derived networks predict stimulation points in the white matter
To further probe the validity of our mapping procedure, we tested whether DES-derived networks are predictive of unseen subcortical stimulation points. For each category, we repeated the network construction procedure by iteratively leaving one subcortical DES point out of the network building procedure, using it as a benchmark to test the predictive power of both the DES positive (i.e., correlated) and negative (i.e., anticorrelated) derived functional networks estimated using all the other DES points. We found that DES-driven functional white matter networks are predictive of unseen subcortical stimulations (94.98%, 94.34%, and 96% accuracy for semantics, phonology, and speech articulation, respectively). The t-tests for dependent samples yielded significant results and large effect size for semantics (mean concordance in DES positive network = 0.55, mean concordance in DES negative network = 0.2, t(278) = 29.3, p-value < 0.0001, Cohens’d = 0.94), phonology (mean concordance in DES positive network = 0.52, mean concordance in DES negative network = 0.26, t(105) = 14.1, p-value < 0.0001, Cohens’d = 1.21), and speech articulation (mean concordance in DES positive network = 0.58, mean concordance in DES negative network = 0.18, t(99) = 18.1, p-value < 0.0001, Cohens’d = 0.85). Repeating the analysis with a leave one patient out scheme to account for repeated sampling within a single individual yielded similar results (93.97%, 94.65%, and 96.3% accuracy for semantics, phonology, and speech articulation, respectively).
DES-derived structural networks model the severity of stroke induced aphasia symptoms and the recovery of language abilities after glioma resection
We investigated two translational applications of our mapping procedure by characterizing language deficits in a cohort of stroke-induced aphasia patients (N = 105) and post-operative longitudinal language recovery in glioma patients (42 patients, 3 timepoints). We found that the volume of overlap between the stroke lesion and the volumetric normative maps for semantic and phonological processing significantly correlates with symptom severity (upper panel of Fig. 2). Indeed, the larger the overlap with the volumetric map, the more severe the symptoms, expressed as low aphasia quotient score (Pearson’s r = −0.6, p-value < 0.0001, Person’s r = −0.61, p-value < 0.0001 for semantic and phonological, respectively). Of note, we also found a strong—albeit 10% smaller compared to the volumetric maps —negative correlation between total lesion volume and the aphasia quotient score (Pearson’s r = −0.55, p-value < 0.0001). Interestingly, the difference in predictive capacity of the volumetric maps compared to total lesion size was more apparent in the quantile regression, as we found that DES derived structural networks predicted symptom severity in aphasic stroke patients more reliably than total lesion size (mean r2 = 0.34, min-max = 0.31–0.36 and 0.31–0.37 for semantics and phonology, respectively, mean r2 = 0.17, min-max = 0.1–0.25 for total lesion size over the 100 repetitions). Both DES derived networks and total lesion volume yielded statistically significant predictions (mean absolute percentage error = 17% and p-value < 0.001 after permutation testing for both semantics and phonology, and a mean absolute percentage error = 20%, 0.14, together with p-value < 0.001 after permutation testing for total lesion size, upper panel of Fig. 2). Importantly, the model containing only the network overlap predictor resulted in statistically significant smaller residuals than a model containing total lesion volume as predictor. We repeated this analysis 100 times to account for the randomness of the internal splitting of the nested cross validation, using a one-sided t-test for paired samples and the absolute values of the residuals57. For semantics, 82% of the times the p-value was statistically significant (100% of the times the p-values were below 0.1). For phonology, 99/100, the p-value was statistically significant (100% the p-values were below 0.1). Effect sizes—as per Cohen’s d—ranged between 0.10 and 0.28 for both semantics and phonology.
(Upper panel) We found that the volume of overlap between the stroke lesion and the volumetric normative maps for semantics and phonological processing significantly correlates with symptom severity—as assessed by the aphasia quotient score from the Western Aphasia Battery (revised version)—and yields better predictions than total lesion size. (lower panel) We compared DES-derived structural networks against state-of-the-art clinical and demographic variables (see main text) in predicting the longitudinal semantic and phonemic fluencies measured pre-, 1 week, and 1 month after surgery in glioma patients using a series of piecewise linear mixed models. In a head-to-head comparison, we found that adding the overlap between the tumor cavity and the normative structural maps to a model containing only a clinical control variable explained significantly more variance than adding the clinical control variable to a model containing only the overlap between the tumor cavity and the normative structural maps. For each comparison, the green bar recapitulates the increase in variance explained—expressed as effect size—by adding the overlap between the tumor cavity and the normative structural maps, the non-green bar illustrates the effect size increase achieved by adding the clinical control variable. We sorted the comparison by increasing magnitude of the difference in variance explained (dashed lines).
Similarly, we investigated whether the volumetric normative maps for semantic and phonological processing provide reliable estimates in modeling longitudinal language recovery in glioma patients. We found that the overlap between the tumor cavity and the normative volumetric maps to be the best predictor of longitudinal language recovery (lower panel of Fig. 2). The pairwise comparison with all the other predictors revealed that adding network overlap to a model containing one of the control variables significantly increased model fit, while the opposite operation —i.e., adding one control variable to a model containing the network overlap— resulted in marginal and often statistically non-significant gains (p-values reported in Supplementary Tables 2 and 3). Of note, modeling the recovery of short-term visuo-spatial abilities with the language-specific normative maps revealed a dramatically decreased prediction capacity (Supplementary Tables 4 and 5).
Language production is subserved by a highly integrated and spatially distributed set of networks
To gain further insights into the wiring diagram of the rich and complex organization underlying speech production, we tried to recover both function specific and shared architectural features across semantics, phonology, and speech arrest (Fig. 3). Specifically, we observed a clear involvement of ventral pathways for semantic processing, and a much more marked presence of dorsal structural connections subtending phonological abilities and speech articulation (Fig. 3). Even more importantly, we found striking overlaps and a tight cross talk across the three cognitive categories, as highlighted by the semi-automated white matter pathways segmentation presented in Fig. 3. Notably, we observed a robust presence of integrative transversal white matter connections—mainly on U-fibers (in blue, Fig. 3a)—enabling constant communication both within the dorsal or ventral stream (Fig. 3a, red and green pathways, respectively) as well as between them. Finally, we highlighted the integrative nature of the neural substrate underlying language production by extracting fibers’ termination points that were shared by at least two cognitive categories (or at least between two different pathways within a given function, Fig. 3b). We found several language integration areas in the left hemisphere—inferior frontal gyrus, ventral premotor cortex, temporo-parietal junction, and to a lesser extent in the left middle temporal gyrus and left supplementary motor area—facilitating a non-relayed structural crosstalk between distinct cognitive categories and/or ventral and dorsal processing streams.
a Semi-automated bundles segmentation revealed striking overlaps and the presence of several integration points across semantics, phonology, and speech articulation. We observed a pronounced presence of integrative transversal white matter pathways (blue) connecting ventral (green) and dorsal (red) portions of the brain. b We mapped shared streamlines termination points both within connectional subdivisions (left panels) and between functional categories (right panel). We found integration points in the left inferior frontal gyrus, left ventral premotor cortex, left temporo-parietal junction, and to a lesser extent in the left middle temporal gyrus and left supplementary motor area, highlighting the distributed, integrated, and redundant nature of the neural substrate underlying language production.
Discussion
In the present report, we propose a data-driven approach that integrates causal mapping with functional and structural connectomics. With respect to the previous work of11—where the authors focused on cortical mapping—the present set of analysis targets explicitly the white matter of the human brain. We introduce the use of white matter functional connectivity, validating it against a neurobiologically grounded metabolic readout, and providing a much more in-depth clinical validation. Taken together, here we provide a multimodal characterization of the large-scale structural networks of the human brain, delineating for the first time the white matter scaffold causally subtending critical cognitive functions. Applied to language production, we showed how to derive the structuro-functional brain networks underlying semantic, phonological, and speech articulation, highlighting their distinct but at the same time integrative and overlapping spatial distribution. Showcasing concrete clinical applications, we investigated language deficits in a cohort of stroke-induced aphasia patients and characterized post-operative longitudinal language recovery in glioma patients. We found that DES-derived networks predicted the severity of stroke-induced aphasia symptoms better than total lesion volume and are able to capture the variation in post-operative language recovery more accurately than state-of-the-art prognostic factors in glioma patients.
Prompted by the promising results in the clinical populations, we argue that the most notable contribution of the present work is the presentation of a data-driven and neurobiologically grounded tool to investigate lesion-induced cognitive/neurological impairments. Of note, our network mapping procedure better estimates the severity of stroke-induced aphasia symptoms compared to total lesion volume. While the improvement in model fit given by the DES-derived structural networks may appear negligible compared to the estimate obtained with total lesion volume only, we argue that the analysis conducted in the stroke cohort is particularly challenging for our network mapping procedure. As reported in the “Materials and methods” section, all lesions were localized in the left hemisphere, and we only included patients with a diagnosis of aphasia, suggesting that the lesions were already localized in brain regions crucial to language preservation. Nonetheless, focusing on DES-derived structural networks rather than total lesion size yielded 10–15% improvement in the overall quality of the predictions, together with a much more pronounced capacity in predicting new unseen samples, as highlighted by the substantial improvement in the coefficient of determination. Moreover, our mean absolute percentage error estimate is in line with a recent study3 showing that stroke induces white matter disconnections derived from a normative population are predictive of neuropsychological deficits in multiple and large cohorts of stroke patients (17% vs 20%, respectively), while our goodness of fit estimate was higher (r2 of 0.35 and 0.2, respectively, but see limitations’ paragraph).
To further corroborate the potential of our network mapping procedure, we conducted a second additional analysis in a completely independent cohort of glioma patients. Moving away from using total lesion volume as the only control factor, we compared DES-derived structural networks against a wide array of state-of-the-art clinical measures, ranging from tumor grade to genetic biomarkers. Our head-to-head comparison revealed a striking superiority of DES-derived networks in modeling post-operative language recovery trajectories in glioma patients. As shown in Fig. 3, adding the overlap between surgical cavities and DES-derived networks as an additional regressor to the clinical measures significantly increased model fit and yielded a marked increase in the model’s effect size, while the opposite—adding clinical measures to the overlap between surgical cavities and DES-derived networks—resulted in marginal and negligible improvements. Of note, recovery of short-term visuo-spatial abilities via the language-specific normative maps revealed a dramatically decreased prediction capacity, highlighting the specificity of our approach. To the best of our knowledge, our study is the first to perform such direct comparison with respect to the characterization of language recovery trajectories in this clinical population. Remarkably, our findings greatly complement recent evidence highlighting the clinical relevance and translational potential of the white matter architecture of the brain2,3,58.
Re-analyzing the sample in ref. 44 allows to tap into the vivid and ongoing debate on the suitability of normative measures in modeling patient-specific clinical trajectories. By investigating patient-specific dysconnectivity indices, the authors concluded that the integrity of white matter pathways in the ventral and dorsal left hemisphere was predictive of semantic and phonemic verbal fluencies (respectively). By resorting to normative indices, we came to similar conclusions. While we expect patient-specific metrics to be more sensitive and specific than normative measures, here we argue that data availability and the possibility of scaling up with respect to sample size should play a pivotal role in the debate. Of note, the clinical analyses reported in the current work necessitate only a T1w image of the brain and segmented lesion. In contrast, in the original work44 the authors had to collect diffusion weighted imaging data in patients, perform fiber tracking, and manually dissect the bundles of interest. All these operations require a high degree of expertise, manual tweaking of several parameters, are operator dependent, and nonetheless require a normative reference. Moreover, fiber tracking in physiologically altered tissues is highly problematic and not always feasible, posing hard constraints towards scalability. Not scaling up with respect to sample size means to cast serious doubts on the robustness of single study observations, as thousands of subjects are needed to establish a reliable link between the brain’s properties and the underlying behavior59. By replicating the original work of the authors, we argue that our procedure may represent a viable and less resource-consuming alternative that enables both scientific discovery and clinical applications, as it can be used to investigate other complex cognitive functions for which a-priori knowledge is scarce. Importantly, normative measures can be combined with patient specific information to boost the predictive capacity of prognostic models.
Complementing clinical analyses, we argue that the integration of DES and connectome mapping offers a unique window into the intrinsic architecture of the human brain, enabling a real holistic exploration of complex cognitive functions. Concretely, it delineates and allows to empirically test a more integrative hypothesis of brain functioning, away from the “one bundle one function” perspective. Consequently—and as depicted in Fig. 3b—we found several overlapping territories and integrative areas in the white matter of semantic, phonological and speech articulatory networks. Specifically, we found three major clusters of overlapping fibers’ termination points: the left inferior frontal gyrus, left ventral premotor cortex, and left temporo-parietal junction. To a lesser extent, we also observed possible points of integration in the left middle temporal gyrus and left supplementary motor area, suggesting the presence of an intimate—and not relayed by the transversal fibers—link between sensorimotor networks and language60. Speculatively, we argue that the presence of overlapping territories in the connectivity profiles of the three cognitive categories highlights a possible substrate implicated in the recovery of language abilities after surgery. This would be in agreement with the pronounced “V-shape” plot—i.e., worsening right after surgery followed by recovery within 1 month—describing language recovery in glioma patients whose tumor overlaps with the DES-derived networks (Fig. 3b).
While our multimodal procedure delineates a robust framework able to integrate DES and connectome mapping in the human brain, several limitations need to be addressed. First, DES mapping—in terms of deciding the exact brain location to be stimulated—is determined by clinical rather than research goals, and as such intrinsically associated with selection and sampling biases, limited brain coverage, and often missing experimental protocols for the acquisition of negative stimulation data. A more balanced acquisition of positive/negative responses and a more comprehensive spatial coverage of the DES points may be used to redefine the networks, leading to more accurate cortical hubs and subcortical way points estimation, and thus to a more accurate mapping of the white matter tracts. Second, the exact definition of the cognitive subdomains involved in a given complex cognitive function may not be straightforward, raising a compositional issue. In other words: how to define and investigate how many cognitive subdomains constitute a complex cognitive function? In the present work, we ideally split language into understanding and production, and tested the latter only, but language is highly intertwined with other broadly defined cognitive categories, such as declarative memory61. Nonetheless, we argue that the flexibility offered by DES mapping could overcome the compositional issue mentioned above. Third, we grouped both glioma and stroke under the same umbrella expression of “dysconnection syndromes”, even though their onset, clinical evolution, and pathophysiological underpinnings greatly differ. Fourth, we employed normative populations of healthy and young participants for both the functional and structural DES network derivations, while the stroke and glioma populations tested were significantly older. Age-specific normative connectomes may result in more sensitivity and specificity with respect to symptom modeling. Last, the sample size of both clinical populations—and especially of the glioma sample—was relatively modest in size, thus limiting the explanatory power of our analysis and raising legitimate questions about whether the findings would generalize to larger, more heterogeneous clinical data. Larger clinical samples and multicentre studies are needed to substantiate the results of our network mapping procedure and to perform fair comparisons between studies. Nonetheless, we validated our approach in two different, completely independent, and neurophysiologically distinct clinical cohorts.
In summary, we concretely show how to integrate DES and connectomics mapping to dissect and subsequently recompose the structuro-functional scaffold underlying language production, highlighting the clinical relevance and translational potential of our procedure. Our method can be applied in principle to investigate any cognitive function, with important scientific and clinical consequences for a broad range of fields, ranging from psychiatry and neurology up to neuropsychology, neurosurgery, and neurorehabilitation.
Data availability
Cortical and subcortical DES coordinates in MNI space and anonymized patients’ demographic were made available as part of our previous publication11 (https://zenodo.org/records/10439149). The white matter structural and functional maps described in the current report are available on Zenodo, together with the data and scripts to reproduce Fig. 2 (glioma panel only) and Fig. 3 (https://zenodo.org/records/16418628). Raw MRI data and derivatives, as well as clinical scores of glioma patients cannot be made publicly available. These restrictions are in accordance with institutional review board guidelines and the informed consent provided by the patients.
Code availability
LNM implementation was made available as part of our previous publication (https://github.com/FBK-NILab/intraopmap)11,62. The code used in the present report is available on Github63 (https://github.com/FBK-NILab/White-Matter-DES-Brain-Maps).
References
Brain health. https://www.who.int/health-topics/brain-health#tab=tab_2.
Salvalaggio, A. et al. White matter tract density index prediction model of overall survival in glioblastoma. JAMA Neurol. 80, 1222 (2023).
Talozzi, L. et al. Latent disconnectome prediction of long-term cognitive-behavioural symptoms in stroke. Brain 146, 1963–1978 (2023).
Duffau, H. New philosophy, clinical pearls, and methods for intraoperative cognition mapping and monitoring “à la carte” in brain tumor patients. Neurosurgery 88, 919–930 (2021).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Siddiqi, S. H., Kording, K. P., Parvizi, J. & Fox, M. D. Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375 (2022).
Sarubbo, S. et al. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. NeuroImage 205, 116237 (2020).
Sarubbo, S. et al. Towards a functional atlas of human white matter: Functional Atlas of White Matter. Hum. Brain Mapp. 36, 3117–3136 (2015).
Zacà, D. et al. Whole-brain network connectivity underlying the human speech articulation as emerged integrating direct electric stimulation, resting state fMRI and tractography. Front. Hum. Neurosci. 12, 405 (2018).
Fotiadis, P. et al. Structure–function coupling in macroscale human brain networks. Nat. Rev. Neurosci. 25, 688–704 (2024).
Coletta, L. et al. Integrating direct electrical brain stimulation with the human connectome. Brain 147, 1100–1111 (2024).
Huang, Y. et al. Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter. Nat. Commun. 14, 3414 (2023).
Li, J. et al. Transcriptomic and macroscopic architectures of intersubject functional variability in human brain white-matter. Commun. Biol. 4, 1417 (2021).
Zu, Z. et al. The missing third dimension—Functional correlations of BOLD signals incorporating white matter. Sci. Adv. 10, eadi0616 (2024).
Alix, J. J. P. & De Jesus Domingues, A. M. White matter synapses: form, function, and dysfunction. Neurology 76, 397–404 (2011).
Ji, G.-J. et al. White matter dysfunction in psychiatric disorders is associated with neurotransmitter and genetic profiles. Nat. Ment. Health 1, 655–666 (2023).
Ng, S. et al. Intraoperative functional remapping unveils evolving patterns of cortical plasticity. Brain 146, 3088–3100 (2023).
Buckner, R. L., Roffman, J. L. & Smoller, J. W. Brain genomics superstruct project (GSP). Harvard Dataverse https://doi.org/10.7910/DVN/25833 (2014).
Joutsa, J. et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat. Med. 28, 1249–1255 (2022).
Siddiqi, S. H. et al. Lesion network localization of depression in multiple sclerosis. Nat. Ment. Health 1, 36–44 (2023).
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. NeuroImage 92, 381–397 (2014).
Lv, H. et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. Am. J. Neuroradiol. ajnr;ajnr.A5527v1 https://doi.org/10.3174/ajnr.A5527 (2018).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. F. S. L. NeuroImage 62, 782–790 (2012).
Hoopes, A., Mora, J. S., Dalca, A. V., Fischl, B. & Hoffmann, M. SynthStrip: skull-stripping for any brain image. NeuroImage 260, 119474 (2022).
Brett, M. et al. nipy/nibabel: 3.2.2. Zenodo https://doi.org/10.5281/ZENODO.6617121 (2022).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Billot, B. et al. Robust machine learning segmentation for large-scale analysis of heterogeneous clinical brain MRI datasets. Proc. Natl. Acad. Sci. USA 120, e2216399120 (2023).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Rocchi, F. et al. Increased fMRI connectivity upon chemogenetic inhibition of the mouse prefrontal cortex. Nat. Commun. 13, 1056 (2022).
Boes, A. D. et al. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075 (2015).
Hansen, J. Y. et al. Integrating multimodal and multiscale connectivity blueprints of the human cerebral cortex in health and disease. PLOS Biol. 21, e3002314 (2023).
Jamadar, S. D. et al. Metabolic and hemodynamic resting-state connectivity of the human brain: a high-temporal resolution simultaneous BOLD-fMRI and FDG-fPET multimodality study. Cereb. Cortex 31, 2855–2867 (2021).
Mori, S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40, 570–582 (2008).
Nilearn contributors et al. nilearn. [object Object] https://doi.org/10.5281/ZENODO.8397156 (2024).
Burt, J. B., Helmer, M., Shinn, M., Anticevic, A. & Murray, J. D. Generative modeling of brain maps with spatial autocorrelation. NeuroImage 220, 117038 (2020).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995).
Markello, R. D. & Misic, B. Comparing spatial null models for brain maps. NeuroImage 236, 118052 (2021).
Hayashi, S. et al. brainlife.io: a decentralized and open-source cloud platform to support neuroscience research. Nat. Methods 21, 809–813 (2024).
Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Garyfallidis, E., Brett, M., Correia, M. M., Williams, G. B. & Nimmo-Smith, I. QuickBundles, a method for tractography simplification. Front. Neurosci. 6, 175 (2012).
Garyfallidis, E. et al. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinformatics 8, 8 (2014).
Porro-Muñoz, D. et al. Tractome: a visual data mining tool for brain connectivity analysis. Data Min. Knowl. Discov. 29, 1258–1279 (2015).
Gibson, M. et al. The Aphasia Recovery Cohort, an open-source chronic stroke repository. Sci. Data 11, 981 (2024).
Zigiotto, L. et al. Segregated circuits for phonemic and semantic fluency: a novel patient-tailored disconnection study. NeuroImage Clin. 36, 103149 (2022).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841 (2002).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Kertesz, A. Western aphasia battery–revised. https://doi.org/10.1037/t15168-000 (2012).
R: The R project for statistical computing. https://www.r-project.org/.
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Lüdecke, D., Ben-Shachar, M., Patil, I., Waggoner, P. & Makowski, D. Performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6, 3139 (2021).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Stoffel, M. A., Nakagawa, S. & Schielzeth, H. partR2: partitioning R2 in generalized linear mixed models. PeerJ 9, e11414 (2021).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Orsini, A. et al. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital. J. Neurol. Sci. 8, 537–548 (1987).
Holmes, A. J. et al. Brain genomics superstruct project initial data release with structural, functional, and behavioral measures. Sci. Data 2, 150031 (2015).
Van Essen, D. C. et al. The WU-Minn human connectome project: an overview. NeuroImage 80, 62–79 (2013).
Nadeau, C. & Bengio, Y. Oshua Inference for the generalization error. Mach. Learn. 52, 239–281 (2003).
Salvalaggio, A., Pini, L., Bertoldo, A. & Corbetta, M. Glioblastoma and brain connectivity: the need for a paradigm shift. Lancet Neurol. 23, 740–748 (2024).
Marek, S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022).
Pulvermüller, F. & Fadiga, L. Active perception: sensorimotor circuits as a cortical basis for language. Nat. Rev. Neurosci. 11, 351–360 (2010).
Roger, E., Banjac, S., Thiebaut De Schotten, M. & Baciu, M. Missing links: the functional unification of language and memory (L∪M). Neurosci. Biobehav. Rev. 133, 104489 (2022).
LudoConn & Paolo Avesani FBK-NILab/intraopmap: Intraopmap (Publication). Zenodo https://doi.org/10.5281/ZENODO.16911676 (2025) .
LudoConn & Paolo Avesani FBK-NILab/White-Matter-DES-Brain-Maps: Code for Publication. Zenodo https://doi.org/10.5281/ZENODO.16911660 (2025) .
Acknowledgements
Paolo Avesani acknowledges the support of the PNRR project FAIR—Future AI Research (PE00000013), under the NRRP MUR program funded by the NextGenerationEU. Ludovico Coletta and Laura Vavassori are partially supported by the grant PAT Reg. no. 764/2021 NeuSurPlan. S.D.J. is supported by a National Health and Medical Research Council of Australia Fellowship APP1174164.
Author information
Authors and Affiliations
Contributions
L.C. conceived of the presented idea, performed all analyses, and took the lead in writing the manuscript. P.A. conceived of the presented idea, supervised the project, and provided funding. L.Z. provided the neuroimaging and behavioral data of the glioma patients, was responsible for the neuropsychological monitoring during awake surgery, and provided critical consultation on interpreting the results. M.V. was involved in the annotation of the stimulation points and provided critical consultation on interpreting the results. L.A performed surgery, was involved in the annotation of the stimulation points, and provided critical consultation on interpreting the results. L.V. provided critical consultation on interpreting the results and in drafting the manuscript. S.D., E.L., J.Y., and B.V. provided the preprocessed FDG-fPET dataset and contributed to drafting the manuscript. S.N. and H.D. performed surgery, collected stimulation points, and provided critical consultation on interpreting the results. S.S. performed surgery, collected stimulation points, conceived of the presented idea, and provided critical consultation on interpreting the results. All authors provided critical feedback and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Coletta, L., Avesani, P., Zigiotto, L. et al. Integrating direct electrical stimulation with brain connectivity predicts lesion-induced language impairment and recovery. Commun Med 5, 416 (2025). https://doi.org/10.1038/s43856-025-01121-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01121-0