Abstract
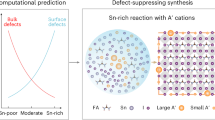
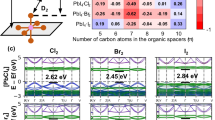
Organic–inorganic hybrid perovskite nanocrystals (PNCs) (APbX3, A = formamidinium, methylammonium, X = Cl, Br, I) are semiconductor materials with important implications for fundamental research and optoelectronic applications. However, the development of hybrid PNCs lags behind their all-inorganic counterparts (CsPbX3), primarily due to their fast growth time (tens of seconds) caused by the uncontrollable kinetics of their synthesis. Here we present a diffusion-mediated synthesis approach by selecting lead precursors with desired solubility in the reaction solvent. Pb(SCN)2, which has limited solubility, serves as a lead reservoir, providing a continuous source of lead throughout the reaction process. This strategy significantly slows down the reaction kinetics. The synthesis time for hybrid PNCs can be drastically prolonged to 180 min, while maintaining the size-focusing stage. As a result, the diffusion-mediated kinetics enables the scalable synthesis of high-quality hybrid PNCs with high monodispersity and near-unity photoluminescence quantum yield. The high-quality hybrid PNCs obtained by this method will stimulate explorations into their properties and drive the development of efficient optoelectronic devices.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All necessary data generated or analysed during this study are included in this published article, and other auxiliary data are available from the corresponding authors upon request. Source data are provided with this paper.
Change history
13 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s44160-025-00739-1
References
Dey, A. et al. State of the art and prospects for halide perovskite nanocrystals. ACS Nano 15, 10775–10981 (2021).
Kovalenko, M. V., Protesescu, L. & Bodnarchuk, M. I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 358, 745–750 (2017).
Akkerman, Q. A., Raino, G., Kovalenko, M. V. & Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 17, 394–405 (2018).
Shamsi, J., Urban, A. S., Imran, M., De Trizio, L. & Manna, L. Metal halide perovskite nanocrystals: synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 119, 3296–3348 (2019).
Kim, Y.-H. et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 15, 148–155 (2021).
Hassan, Y. et al. Ligand-engineered bandgap stability in mixed-halide perovskite LEDs. Nature 591, 72–77 (2021).
Kim, J. S. et al. Ultra-bright, efficient and stable perovskite light-emitting diodes. Nature 611, 688–694 (2022).
Song, J. et al. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 27, 7162–7167 (2015).
Liu, X. K. et al. Metal halide perovskites for light-emitting diodes. Nat. Mater. 20, 10–21 (2021).
Chen, Q. et al. All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018).
Zhang, Q., Shang, Q., Su, R., Do, T. T. H. & Xiong, Q. Halide perovskite semiconductor lasers: materials, cavity design, and low threshold. Nano Lett. 21, 1903–1914 (2021).
Yakunin, S. et al. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 6, 8056 (2015).
Zhang, X. et al. Ligand-assisted coupling manipulation for efficient and stable FAPbI3 colloidal quantum dot solar cells. Angew. Chem. Int. Ed. 62, e202214241 (2023).
Swarnkar, A. et al. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92–95 (2016).
Yuan, J. et al. Metal halide perovskites in quantum dot solar cells: progress and prospects. Joule 4, 1160–1185 (2020).
Jia, D. et al. Tailoring solvent-mediated ligand exchange for CsPbI3 perovskite quantum dot solar cells with efficiency exceeding 16.5%. Joule 6, 1632–1653 (2022).
Yang, W. et al. Overcoming charge confinement in perovskite nanocrystal solar cells. Adv. Mater. 35, 2304533 (2023).
Jia, D., Chen, J., Zhuang, R., Hua, Y. & Zhang, X. Antisolvent-assisted in situ cation exchange of perovskite quantum dots for efficient solar cells. Adv. Mater. 35, 2212160 (2023).
Chen, B. et al. Tin dioxide buffer layer-assisted efficiency and stability of wide-bandgap inverted perovskite solar cells. J. Semicond. 43, 052201 (2022).
Nguyen, H. A. et al. Design rules for obtaining narrow luminescence from semiconductors made in solution. Chem. Rev. 123, 7890–7952 (2023).
Li, D. et al. Enhancing performance of inverted quantum-dot light-emitting diodes based on a solution-processed hole transport layer via ligand treatment. J. Semicond. 44, 092603 (2023).
Peng, X. An essay on synthetic chemistry of colloidal nanocrystals. Nano Res. 2, 425–447 (2009).
Murray, C., Norris, D. & Bawendi, M. G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Park, J., Joo, J., Kwon, S. G., Jang, Y. & Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. Int. Ed. 46, 4630–4660 (2007).
Calvin, J. J., Brewer, A. S. & Alivisatos, A. P. The role of organic ligand shell structures in colloidal nanocrystal synthesis. Nat. Synth. 1, 127–137 (2022).
Kwon, S. G. & Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 7, 2685–2702 (2011).
Sun, C., Jiang, Y., Zhang, L., Wei, K. & Yuan, M. Toward the controlled synthesis of lead halide perovskite nanocrystals. ACS Nano 17, 17600–17609 (2023).
Pradhan, N. Growth of lead halide perovskite nanocrystals: still in mystery. ACS Phys. Chem Au 2, 268–276 (2022).
Koolyk, M., Amgar, D., Aharon, S. & Etgar, L. Kinetics of cesium lead halide perovskite nanoparticle growth; focusing and de-focusing of size distribution. Nanoscale 8, 6403–6409 (2016).
Das, S. & Samanta, A. Highly luminescent and phase-stable red/nir-emitting all-inorganic and hybrid perovskite nanocrystals. ACS Energy Lett. 6, 3780–3787 (2021).
Protesescu, L. et al. Dismantling the ‘red wall’ of colloidal perovskites: highly luminescent formamidinium and formamidinium–cesium lead iodide nanocrystals. ACS Nano 11, 3119–3134 (2017).
Akkerman, Q. A. et al. Controlling the nucleation and growth kinetics of lead halide perovskite quantum dots. Science 377, 1406–1412 (2022).
Lee, J. W., Tan, S., Seok, S. I., Yang, Y. & Park, N. G. Rethinking the A cation in halide perovskites. Science 375, eabj1186 (2022).
Quan, L. N. et al. Perovskites for next-generation optical sources. Chem. Rev. 119, 7444–7477 (2019).
Huang, J., Yuan, Y., Shao, Y. & Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2, 17042 (2017).
Li, W. et al. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2, 16099 (2017).
Yan, Y., Li, Z. & Lou, Z. Photodetector based on Ruddlesden–Popper perovskite microwires with broader band detection. J. Semicond. 44, 082201 (2023).
Liu, S. et al. Buried interface molecular hybrid for inverted perovskite solar cells. Nature 632, 536–542 (2024).
Aqoma, H. et al. Alkyl ammonium iodide-based ligand exchange strategy for high-efficiency organic-cation perovskite quantum dot solar cells. Nat. Energy 9, 324–332 (2024).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Ding, S. et al. In situ bonding regulation of surface ligands for efficient and stable FAPbI3 quantum dot solar cells. Adv. Sci. 9, 2204476 (2022).
Imran, M. et al. Benzoyl halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J. Am. Chem. Soc. 140, 2656–2664 (2018).
Tseng, Z.-L. et al. Aggregation control, surface passivation, and optimization of device structure toward near-infrared perovskite quantum-dot light-emitting diodes with an EQE up to 15.4%. Adv. Mater. 34, 2109785 (2022).
Xue, J. et al. Surface ligand management for stable FAPbI3 perovskite quantum dot solar cells. Joule 2, 1866–1878 (2018).
Lou, Y. et al. Rod-shaped thiocyanate-induced abnormal band gap broadening in SCN− doped CsPbBr3 perovskite nanocrystals. Nano Res. 11, 2715–2723 (2018).
Yang, S. et al. Thiocyanate assisted performance enhancement of formamidinium based planar perovskite solar cells through a single one-step solution process. J. Mater. Chem. A 4, 9430–9436 (2016).
Jiang, Q. et al. Pseudohalide-induced moisture tolerance in perovskite CH3NH3Pb(SCN)2I thin films. Angew. Chem. Int. Ed. 54, 7617–7620 (2015).
Maceiczyk, R. M. et al. Microfluidic reactors provide preparative and mechanistic insights into the synthesis of formamidinium lead halide perovskite nanocrystals. Chem. Mater. 29, 8433–8439 (2017).
Aldakov, D. & Reiss, P. Safer-by-design fluorescent nanocrystals: metal halide perovskites vs semiconductor quantum dots. J. Phys. Chem. C 123, 12527–12541 (2019).
Wright, A. D. et al. Intrinsic quantum confinement in formamidinium lead triiodide perovskite. Nat. Mater. 19, 1201–1206 (2020).
Peng, L., Dutta, A., Xie, R., Yang, W. & Pradhan, N. Dot–wire–platelet–cube: step growth and structural transformations in CsPbBr3 perovskite nanocrystals. ACS Energy Lett. 3, 2014–2020 (2018).
Talapin, D. V., Rogach, A. L., Haase, M. & Weller, H. Evolution of an ensemble of nanoparticles in a colloidal solution: theoretical study. J. Phys. Chem. B 105, 12278–12285 (2001).
Yin, Y. & Alivisatos, A. P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437, 664–670 (2005).
Luther, J. M. et al. Structural, optical, and electrical properties of self-assembled films of pbse nanocrystals treated with 1,2-ethanedithiol. ACS Nano 2, 271–280 (2008).
Toso, S., Baranov, D., Giannini, C., Marras, S. & Manna, L. Wide-angle X-ray diffraction evidence of structural coherence in CsPbBr3 nanocrystal superlattices. ACS Mater. Lett. 1, 272–276 (2019).
Toso, S., Baranov, D., Filippi, U., Giannini, C. & Manna, L. Collective diffraction effects in perovskite nanocrystal superlattices. Acc. Chem. Res. 56, 66–76 (2023).
Ohmi, T. et al. Thiocyanate-stabilized pseudo-cubic perovskite CH(NH2)2PbI3 from coincident columnar defect lattices. J. Am. Chem. Soc. 145, 19759–19767 (2023).
Koscher, B. A., Swabeck, J. K., Bronstein, N. D. & Alivisatos, A. P. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. J. Am. Chem. Soc. 139, 6566–6569 (2017).
Ding, C. et al. Photoexcited hot and cold electron and hole dynamics at FAPbI3 perovskite quantum dots/metal oxide heterojunctions used for stable perovskite quantum dot solar cells. Nano Energy 67, 104267 (2020).
Xue, J. et al. A small-molecule ‘charge driver’ enables perovskite quantum dot solar cells with efficiency approaching 13%. Adv. Mater. 31, 1900111 (2019).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (grant number 2022YFE0110300), the National Natural Science Foundation of China (grant numbers 92163114, 52372215 and 52002260), the Special Fund for the ‘Dual Carbon’ Science and Technology Innovation of Jiangsu Province (Industrial Prospect and Key Technology Research Program) (grant numbers BE2022021 and BE2022023), the Gusu Innovation and Entrepreneurship Leading Talent Program (grant number ZXL2022451) and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant number 21KJA430004). This work is supported by Suzhou Key Laboratory of Functional Nano & Soft Materials, Collaborative Innovation Center of Suzhou Nano Science & Technology, and the 111 Project.
Author information
Authors and Affiliations
Contributions
Z.L. and W.M. conceived the idea and supervised the project. X.S. designed the experiments. X.S., L.Y., Y.Z., C.Z. and X.Z. performed the experiments and data analysis. G.S. and Q.S. conducted the PL decay measurement. G.S., Yumin Wang and Yaxing Wang measured the TA spectra. M.M. and B.S. contributed to the high-resolution STEM measurement. Y.L. and C.L. contributed to in situ PL data analysis. X.S., Z.L. and W.M. co-wrote the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Tae-Woo Lee, Tao Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and additional experimental methods, Supplementary Figs. 1–29, Note 1, and Tables 1–6.
Source data
Source Data Fig. 1
Raw data of in situ PL spectra of FAPbI3 nanocrystals synthesized by conventional and diffusion-mediated approaches.
Source Data Fig. 2
Unprocessed TEM images of FAPbI3 nanocrystals with different reaction times synthesized by conventional and diffusion-mediated approaches.
Source Data Fig. 3
Raw data of in situ PL spectra of FAPbI3 nanocrystals.
Source Data Fig. 3
Unprocessed TEM images of FAPbI3 nanocrystals with different reaction times.
Source Data Fig. 4
Raw data of absorption, PL, PLQY and XRD spectra of FAPbX3 (X = I, Br or Cl) nanocrystals.
Source Data Fig. 4
Unprocessed TEM images of FAPbX3 (X = I, Br or Cl) nanocrystals.
Source Data Fig. 5
Raw data of absorption, PL, PLQY and XRD spectra of MAPbX3 (X = I, Br or Cl) nanocrystals.
Source Data Fig. 5
Unprocessed TEM images of MAPbX3 (X = I, Br or Cl) nanocrystals.
Source Data Fig. 6
Raw data of TA and PL mapping spectra of FAPbI3 nanocrystals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Yuan, L., Liu, Y. et al. Diffusion-mediated synthesis of high-quality organic–inorganic hybrid perovskite nanocrystals. Nat. Synth 4, 167–176 (2025). https://doi.org/10.1038/s44160-024-00678-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00678-3
This article is cited by
-
Thermally activated conduction and dielectric relaxation in the quasi-one-dimensional hybrid perovskite NH2(CH3)2CuCl3
Journal of Materials Science: Materials in Electronics (2025)
-
Uniform growth of perovskite nanocrystals
Nature Synthesis (2024)