Abstract
Despite advances in the field of 2D polymerization, the synthesis of high-quality, micrometre-thick films of oriented 2D covalent organic frameworks (COFs) remains challenging. Conventional approaches focusing on thermodynamic control of the polymerization pathway face a detrimental trade-off between orientation and thickness. Here we describe a straightforward method for preparing imine-linked 2D COF films with a near-perfect face-on orientation by leveraging kinetically trapped amorphous 3D covalent adaptable network (CAN) intermediates. These off-pathway intermediates are generated as coatings through solution casting, during which the CANs spontaneously align to relax tensile stresses induced by solvent evaporation. A subsequent lift-off process, followed by an amorphous-to-crystalline transformation under solvothermal conditions, converts the 3D-oriented polymer networks into thermodynamically stable, porous and free-standing 2D COF films. This versatile kinetic trapping strategy is suitable for a range of building blocks and network topologies, constituting a convenient synthetic tool for accessing high-quality, robust, large-area 2D COF films with a strongly aligned polycrystalline structure.

Similar content being viewed by others
Main
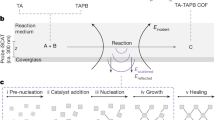
The advent of reticular chemistry has enabled the rational design and synthesis of crystalline two-dimensional (2D) polymers referred to as 2D covalent organic frameworks (COFs)1,2,3. These materials are composed of topologically planar, ordered polymeric networks that stack in the third dimension, giving rise to one-dimensional void channels ideally accessible only from directions perpendicular to the covalently linked 2D structure4,5,6. In principle, this anisotropic architecture facilitates both efficient charge and mass transport along the stacked columns and the pore channels, respectively, rendering these materials appealing for applications ranging from energy storage and conversion to high-efficiency separation, catalysis and sensing7,8,9,10,11,12. Besides, the difficulties in growing large single crystals13,14,15,16 and the poor processability of 2D COFs17 leave polycrystalline films with aligned domains as the most promising morphology for leveraging the anisotropic properties of these materials for their technological applications (Fig. 1a).
a, Differences between the single-crystalline, oriented polycrystalline and non-oriented polycrystalline film (side view of the 2D polymer layers). b, Well-established methods for the fabrication of oriented 2D COF films. The figure illustrates various interfaces used for the confinement, preorganization and preorientation of the precursors. c, A schematic representation of the two limiting mechanisms of growth of polyimine in solution. By following a purely kinetic (polymerization rate scaling with the 2/3 power of the number of incorporated monomers, n2/3) or purely thermodynamic pathway (rate proportional to n1/2), the selective formation of a gel or particles can be achieved38. Growth under typical solvothermal conditions follows an intertwined mechanism where π–π stacking favours the formation of the thermodynamic product, whereas in typical conditions the presence of non-solvents leads to the rapid precipitation of larger species, thereby trapping the defective polycrystalline product. d, The proposed method for the preparation of oriented 2D COF films by spontaneous alignment of a CAN followed by amorphous-to-crystalline transformation. The red arrows indicate the tensile stress acting on the droplet and the compressive stress acting on the rigid substrate.
Current syntheses of oriented 2D COF films exploit various interfacial interactions inducing the confinement and preorganization of the precursors to balance the entropic penalty associated with the formation of an oriented 2D network18,19. As a consequence of this spatial confinement, the 2D polymerizations carried at liquid–liquid20,21,22, liquid–air23,24,25, liquid–solid26,27,28,29,30 or vapour–solid31,32 interfaces yield ultrathin films, that is, sub- to several-nanometres-thick oriented fragile materials, which need to be supported on a solid substrate for any further manipulation (Fig. 1b). Meanwhile, colloidal printing methods provide thicker but poorly oriented and often discontinuous materials33,34,35,36. Therefore, none of these methods can yield large-area, robust, free-standing oriented 2D COFs films. Yet, mechanical stability is critical for the practical applications of these materials, and therefore, the development of a general synthetic strategy providing micrometre-thick oriented 2D COF films remains a fundamental challenge37.
Here, we report a widely applicable and facile method for the preparation of free-standing, micrometre-thick, highly oriented and crystalline films of imine-linked 2D COFs by a convenient solvent processing method. Capitalizing on the pathway complexity of dynamic polycondensation, our strategy disentangles the orientation and crystallization processes. In the first step, we impart an orientation to the film by leveraging the spontaneous alignment of three-dimensional (3D) covalent adaptable networks (CANs) in response to self-developing tensile stresses. In the second step, we introduce crystallinity by converting 3D CANs into 2D COFs in the solid state, while preserving their face-on orientation. Most notably, by circumventing the necessity of interfacial confinement of reactants, the fabrication of robust, micrometre-thick and large-area films by simple solution casting and subsequent solvothermal annealing becomes possible. The quality and thickness of the films enabled their structural characterization by conventional laboratory X-ray diffraction. We demonstrate that this strategy can be extended to various molecular building blocks, highlighting its general applicability.
Design and advantages of the kinetic polymerization pathway
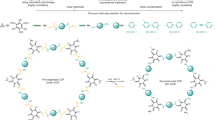
We assumed that the failure of interfacial polymerizations in delivering micrometre-thick oriented films of 2D COFs probably stems from the limited range of the interfacial bias. As the thickness increases, secondary nucleation and out-of-plane defects quickly compromise the orientation of the material. Therefore, to overcome this trade-off between orientation and thickness, we devised a film fabrication strategy relying on a macroscopic bias instead of the conventional short-range interfacial interactions. To this end, we aimed to detour the thermodynamic trajectory of polymerization towards the formation of a kinetically favoured 3D polymeric networks crosslinked by reversible bonds—that is, 3D CANs—which not only eliminates nucleation events but also allows convenient material alignment. Specifically, whenever single-bond rotation is enabled, 3D branching and out-of-plane growth of the polymer are expected to outpace 2D polymerization38. This feature gives rise to a pathway complexity of polycondensation (Fig. 1c) where the purely kinetic pathway leads to the formation of 3D amorphous CANs and the purely thermodynamic one yields single-crystalline 2D COF particles. As CANs are capable of relaxing external mechanical stress by rearrangement of the dynamic covalent bonds39,40,41, it is expected that the lateral macroscopic tensile stress42 developing in a drying solution-cast imine-linked CAN film could induce material creep, leading to the spontaneous alignment of polymer branches (Fig. 1d). The resulting amorphous oriented film would be transformed into an oriented crystalline 2D COF film under conditions enabling the crystallization at the solid state.
The practical realization of this approach poses several distinct challenges. First, this strategy necessitates stabilization of the kinetically favoured CAN throughout the film solidification. Furthermore, the conditions for the film formation must preclude the entropy-driven dynamic bonds reshuffling leading to isotropization of the aligned network. Likewise, the solvothermal crystallization of the CAN into oriented COF must preserve the anisotropy of the material.
Following these considerations, the appropriate system for the fabrication of oriented films by kinetic trapping of the metastable imine-linked CAN should feature (1) high solubility of oligomers to suppress stacking and nucleation, (2) a high rate of imine bond formation to ensure rapid incorporation of oligomers in the growing CAN during evaporation, (3) an adequate rate of imine bond hydrolysis, facilitating stress relaxation while retarding the equilibration of the CAN to the 2D COF, and (4) sufficient volatility of the solvent for convenient control over the evaporation rate. Note that conditions 2 and 3 together imply a high equilibrium constant. Under these premises, trifluoracetic acid (TFA) stands out as a promising solvent and catalyst for the preparation of imine-based CANs owing to its versatility, miscibility with water, volatility and strong acidity43 necessary to disrupt π–π stacking in the growing polymer through electrostatic repulsion between the charged species44,45.
Polymerization in solution leads to a metastable CAN gel
The equilibrium functional group conversion (p) for the solution polymerization of 1,3,5-tris(4-aminophenyl)benzene (TPB) with various rigid linear dialdehydes (1–10; Fig. 2a,b) in TFA-d/D2O at [FG]0 = 200 mM (where FG denotes the functional group, [FG]0 = 2 × [TPB]0 = 3 × [1]0) were determined via 1H nuclear magnetic resonance (NMR) spectroscopy (Supplementary Figs. 1–3; see ‘Material and methods’ section in the Supplementary Information for experimental details). The observed reactivity trend for aldehydes 1–10 was found to correlate with the increasing electrophilicity of the carbonyl carbon. The lowest conversion was observed for the electron-poor aldehydes, while the highest conversion was observed for the most electron-rich aldehydes, that is, for the 2,5-dialkoxy substituted 1 and 2 monomers, which produced deep-red solutions characteristic of the formation of a polyiminium salt12. This trend is consistent with increasing hydrolytic stability of the polymer10, which further translates to a low rate of iminium hydrolysis and renders aldehydes 1 and 2 the most suitable candidates for the stabilization of the metastable 3D CAN under the experimental conditions. More specifically, while the rate constant of imine formation of TPB-1 was found to be too high to be determined by routine experiments, hydrolysis proceeded relatively slowly, despite the excess of water (solvent quantity). The pseudo-first-order rate constant of hydrolysis was determined upon rapid dilution of concentrated polyiminium, yelding a half-life of t1/2 ≈ 440 s (Supplementary Figs. 4 and 5; see ‘Material and methods’ section in the Supplementary Information for experimental details). Consistently, the polymerization of TPB-1 was found to be highly exergonic with an apparent equilibrium constant of Keq(20 °C) = 183 ± 23 and a corresponding standard Gibbs free energy of polymerization of ΔGp0(20 °C) = −12.7 ± 0.4 kJ mol−1 (Supplementary Figs. 6–9). The van ‘t Hoff analysis, carried out at 0–60 °C, indicated that the polycondensation was both enthalpically and entropically driven with a standard enthalpy of polymerization of ΔHp0 = −7.7 ± 0.5 kJ mol−1 and a standard entropy of polymerization of ΔSp0 = 17 ± 2 J mol−1 K−1, the latter accounting for roughly a third of the Gibbs free energy at 20 °C. These values are remarkably high compared with those of typical equilibrium polycondensations, indicating the highly favourable formation of iminium moieties under these conditions46.
a, Polymerization of amine TPB with aldehydes 1–10. b, Structures of aldehydes 1–10 and the corresponding functional group conversion values (p, estimated by 1H NMR) for the polymerization with TPB in TFA/H2O (solvents: 90:10 v/v; monomers: [FG]0 = 200 mM, stoichiometric FG ratio; 23 °C). c, Concentration–temperature phase diagram describing the sol–gel equilibrium of TPB-1 in TFA/H2O (solvents: 95:5 v/v; monomers: stoichiometric FG ratio). The diagram was constructed via the test-tube inversion method. Note that the sol–gel transition could be reversed by varying the concentration or the temperature. d, A SEM image of the solid part of the TPB-1 gel phase, isolated by solvent exchange and lyophilization. e, Plot of the relative amount of each functional group estimated by 1H NMR, at increasing concentrations of TPB-1 in TFA/H2O (solvents: 95:5 v/v; monomers: stoichiometric FG ratio). Note that only the sol fraction is analysed in this experiment. f, A schematic depiction of the thermodynamic (on-pathway) and kinetic (off-pathway) reaction products for TBP-1 polymerization in TFA/H2O 95:5 v/v.
At concentrations exceeding 200 mM, a transition from liquid-like to solid-like behaviour was observed within 5 min after mixing the reactants (Supplementary Fig. 10). Gelation, that is, the emergence of a single sample-spanning molecule with, in principle, infinite molecular weight, occurs at the critical functional group conversion pc = 0.75 (Fig. 2e), which is consistent with predictions based on the Flory–Stockmayer model (A2 + B3 polycondensation)47. Similarly, good agreement between the theoretically predicted and experimental pc values was observed for the 1,3,6,8-tetrakis(4-aminophenyl)pyrene (TPPy)-1 system (A4 + B2 polycondensation; Supplementary Figs. 11–14). The sol–gel transition of TPB-1 was found to be temperature dependent and fully reversible, which is in line with the dynamic nature of the iminium bond (Fig. 2c). The lyophilized solid component of the gel, analysed via scanning electron microscopy (SEM), displayed a foam-like macroporous structure (Fig. 2d and Supplementary Fig. 15), while powder X-ray diffraction indicated a lack of structural order in the material (Supplementary Fig. 16). For the TPB-1 system, nucleation of COF particles was observed only after prolonged equilibration (15 days; Supplementary Figs. 10 and 17–20) of highly concentrated gels ([FG]0 ≥500 mM, 23 °C), indicating that the CAN is transitioning from deep to shallow kinetic trap (Fig. 2f). Similar pathway complexity is often observed in supramolecular polymerizations but remains unprecedented in their covalent counterparts48,49,50. By contrast, for the TPB-2 system, the CAN was found to be a shallow kinetic trap over the entire concentration range as nucleation of COF particles was observed even below the critical gelation concentration (Supplementary Figs. 10, 21 and 22). This lower kinetic stability of the metastable CAN probably stems from additional van der Waals interactions between long alkyl chains and renders 2 an unsuitable substrate for the fabrication of aligned polymeric networks.
Overall, the equilibrium concentrations of the participating species in the reaction between 1 and TPB (Fig. 2e) indicate a sequence of consecutive reactions qualitatively consistent with the step-growth polymerization mechanism. The apparent equilibrium constant decreases (by more than an order of magnitude) with increasing functional group conversion, indicating that the formation of shorter oligomers is thermodynamically favoured over the formation of larger species (Supplementary Figs. 23–27). Consequently, the equilibrium concentrations of monomers decrease rapidly in diluted solutions (0.1–10 mM; Fig. 2e, yellow line), whereas at higher concentrations, the decrease in concentration of branch terminating moieties becomes less steep, as the growth of the polymer proceeds by condensation of shorter species. All these observations, together with the large entropic contribution to the Gibbs free energy and the values of pc, strongly support the formation of a highly disordered 3D material with a minimal degree of intramolecular cyclization. Finally, in addition to the concentration, p and, thus, the molecular weight of the oligomers in the mixture, can be tuned by adjusting the TFA/H2O ratio (Supplementary Fig. 28).
Alignment of the CAN and conversion to 2D COF
Casting a concentrated solution of oligomers (single deposition) on flat, inert substrates and subsequent evaporation of the solvent at 40 °C in a closed Petri dish led to the formation of deep-red, uniform polymeric coatings without any lateral shrinkage (Supplementary Fig. 29). Lift-off and neutralization provided mechanically robust, yellow, transparent films with a clearly visible wrinkling pattern (Fig. 3c, inset, and Supplementary Fig. 30). The formation of similar wrinkling patterns is often observed in solvent-borne polymer coatings and is associated with the gradient of tensile stress developing upon drying and curing of the film51. The uniform films were 13–20 μm thick (thicker at the edges and thinner at the centre) with a highly layered structure in the cross-section as indicated by SEM imaging (Fig. 3a and Supplementary Fig. 31). Two-dimensional grazing-incidence wide-angle X-ray diffraction (GIWAXS) showed a broad reflection (~25°) localized along the out-of-plane direction, denoting an amorphous material with preferential orientation of the π–π stacking perpendicular to the plane of the films (Fig. 3b,c). Materials with various surface properties could be effectively used as substrates (Supplementary Fig. 32). The chemical composition of the TPB-1 CAN film was further confirmed by infrared and solid-state NMR spectroscopies (Supplementary Figs. 33–36).
a, Cross-sectional SEM images of the amorphous film. b, The 2D GIWAXS pattern of the amorphous film. c, In-plane (near 2θz = 0; 2θ is the diffraction angle) and out-of-plane (near 2θxy = 0) projections of the 2D GIWAXS pattern of the amorphous film. Inset: camera picture of the film. Note that the partial light transparency of the film reveals a characteristic drying pattern. d, A schematic illustration of the solvothermal annealing in MeCN and aqueous AcOH leading to the amorphous-to-crystalline transformation. e, Cross-sectional SEM images of the 2D COF film. f, The 2D GIWAXS pattern of the 2D COF. Note that the free-standing nature of the films enables the collection of diffraction data at negative 2θz angles (below the plane of the film). g, In-plane (near 2θz = 0) and out-of-plane (near 2θxy = 0) projections of the 2D GIWAXS pattern of the 2D COF film. Inset: camera picture of the film. Note that the film becomes opaque, which is consistent with the scattering of light on the ordered structure.
These amorphous, oriented CAN films were transformed into crystalline and highly oriented 2D COFs by solvothermal annealing in acetonitrile (MeCN) and aqueous acetic acid (AcOH) (Fig. 3d and Supplementary Fig. 37). Upon crystallization, the films maintained their chemical composition (as indicated by solid-state NMR; Supplementary Figs. 38 and 39), macroscopic shape and robustness but became more brittle, which is typical for crystalline structures (Supplementary Fig. 40). Atomic force microscopy indicated an increase of roughness of the films consistent with the formation of crystalline domains (Supplementary Fig. 41). Cross-sectional SEM images revealed a drastic change in the morphology to arrays of rod-like particles (up to 1 μm long) aligned perpendicularly to the film surface (Fig. 3e and Supplementary Fig. 42). Two-dimensional GIWAXS showed several sharp, intense diffraction spots localized along the in-plane direction and one, less intense, localized along the out-of-plane direction (Fig. 3f,g) indicating the formation of a highly oriented and crystalline structure with the [001] direction perpendicular to the macroscopic surface of the films. The near-perfect face-on alignment was further suggested by the Hermans orientation factor of 0.93 calculated for the (210) reflection (Supplementary Fig. 43). The in-plane and out-of-plane integrated patterns matched well with the simulated diffraction pattern of a structure based on a 2D network with a hexagonal P6/m space group and eclipsed AA-type stacking of the layers, as corroborated by Rietveld refinement (Supplementary Fig. 44). Scherrer analysis of the four most intense (hk0) reflections denoted a relatively large coherently scattering domain size of 42 nm. Similar analysis on the π–π stacking reflection indicated a domain size of 6.0 nm in the crystalline film, suggesting that the rod-like particles consisted of several slipped ordered pellet-like domains (Supplementary Fig. 45). Tracking the morphological evolution of the material during annealing revealed that, after just 4 h, the material becomes partially crystalline and well oriented. The degree of order then gradually increased up to 48 h, while the orientation remained constant throughout the process (Supplementary Figs. 46–49). The formation of rod-like crystallites may be attributed to the minimization of the stress associated with in-plane cross-linking upon transition from a 3D to a 2D topology. Indeed, infrared spectroscopy (Supplementary Figs. 50 and 51) indicated that this crystallization step is associated with a decrease in the amount of unreacted carbonyl and aniline moieties; however, further studies are needed to elucidate the origin of this layered rod-like morphology. In contrast to the virtually non-porous CAN films, the crystalline materials showed high Brunauer–Emmett–Teller surface areas (SBET), up to 1,060 m2 g−1 with a median pore size of 3.2 nm (Supplementary Figs. 52 and 53).
Mechanism of spontaneous alignment of the polymeric network
We propose that the observed spontaneous orientation of the CAN originates from the gradual dissipation of the lateral tensile stress associated with the unidimensional shrinkage of the film upon evaporation of the solvent51. Although similar in some aspects, this process is distinct from the mechanical alignment of liquid-crystalline CANs42 or the evaporation-induced self-assembly of liquid-crystalline amphiphiles52.
To verify the proposed mechanism, the morphological evolution of the material during the drying process was investigated by ex situ SEM and in situ 2D GIWAXS, which yielded consistent results. After deposition, the evaporation of the solvent and the consequent increase in concentration induce gelation of the coating (~10 min; Fig. 4a and Supplementary Figs. 54 and 55). Further evaporation produces a more compact material by vertical shrinkage, which is responsible for the development of lateral tensile stress (Supplementary Figs. 56–59). Substantial lateral shrinkage was observed for films lifted off at the early stages of the process (15 min; Supplementary Fig. 56), whereas samples lifted off after longer durations exhibited minimal deformation (Supplementary Fig. 58). These observations are consistent with the rapid development of lateral tensile stress in the film that is dissipated over longer timescales by the creep of the material. The morphological evolution from an inclined (15 min; Fig. 4a and Supplementary Fig. 57) to a horizontally layered texture (30 min; Fig. 4a and Supplementary Fig. 59) accompanied by the gradual transition from isotropic to well-aligned material (Fig. 4b and Supplementary Figs. 60 and 61) suggested that the relaxation proceeded through the structural alignment of the polymer branches. Furthermore, rheological studies on the bulk CAN gel indicated a viscoelastic liquid behaviour and further corroborated a dissociative exchange mechanism (Fig. 4c and Supplementary Figs. 62 and 63). The relaxation time in the gel (τ, storage-loss moduli crossover) was found to be timescale consistent (100 s) with the half-life of the iminium bond in this solvent mixture (440 s), strongly suggesting that the structural alignment in the polymer film is enabled by the hydrolysis and reformation of the iminium bonds39.
a, Ex situ SEM images of the drying polymer coating at different reaction times (10, 15 and 30 min). Insets: camera pictures of the isolated films. b, In situ time-dependent 2D GIWAXS patterns of the drying CAN film (10, 15, 30, 60 and 120 min). Note that the gradual evolution of the scattering maxima towards higher angles is in accordance with a reduction in the average interchain distance upon solvent evaporation. c, Frequency sweep of the TPB-1 gel ([FG]0 = 300 mM in TFA/H2O 95:5 v/v) at 25 °C under an oscillating shear strain of 1%. d, Left: SEM image of the polymer particles obtained by slow solvent evaporation. Right: SEM image of the polymer film obtained by fast and uncontrolled solvent evaporation. Insets: camera pictures of the films. Under slow evaporation, the film is inhomogeneous and characterized by an evident ‘coffee ring effect’. By contrast, under fast evaporation, the film cracks and delaminates from the substrate. e, A depiction of the proposed mechanism for stress relaxation in a drying CAN film.
Within the proposed mechanistic framework, neither too fast nor too slow evaporation of the solvent can be expected to lead to the formation of an oriented CAN (Fig. 4e). Indeed, slow evaporation at room temperature allowed the system to nucleate and equilibrate to the more thermodynamically favoured product leading to non-oriented discontinuous deposits of particles ~0.5 μm in size (Fig. 4d, left, and Supplementary Figs. 64–66). By contrast, rapid evaporation in an open Petri dish at 40 °C produced cracked and delaminated non-oriented film of collapsed CAN, as under these conditions the high rate of stress accumulation precluded efficient relaxation by alignment and led to macroscopic deformation of the film (Fig. 4d, right, and Supplementary Figs. 67–69). Accordingly, building blocks that are prone to stacking (2) or electron-poor aldehydes (6) tend to equilibrate rapidly, ultimately producing non-oriented or poorly oriented films upon solvent evaporation (see Supplementary Figs. 70–73 for examples).
All these observations are consistent with the proposed mechanism and highlight that matching the bond dynamics with the rate of stress development is crucial for spontaneous CAN orientation. Finally, in addition to generating the lateral tensile stress, evaporation of the solvent, including water, is arguably associated with a gradual decrease in the rate of imine exchange, which ultimately freezes the topology of the aligned network, thereby preventing entropy-driven isotropization.
Scope of the method
The generality of this two-stage approach was showcased by the synthesis of a range of oriented 2D COF films from various building blocks (Fig. 5; see Supplementary Figs. 74–95 for camera and SEM pictures, 2D GIWAXS and sorption analyses), including one based on a previously unreported framework derived from a methoxy-functionalized biphenyl dialdehyde (Fig. 5a). Within the current experimental framework, the required conditions for spontaneous CAN alignment are easily satisfied by various combinations of electron-rich aldehydes and rigid anilines, including Wurster-type-, pyrene- and porphyrin-based building blocks (BTA, TPPy and TPPor, respectively) with small modifications to the original procedure (see ‘Materials and methods’ section in the Supplementary Information). Nevertheless, the versatility of the method allows the fabrication of robust and oriented films with various topologies (hcb, kgm and sql), functionalities and pore sizes. All films could be analysed by laboratory-grade X-ray source 2D GIWAXS, demonstrating good to exceptionally high orientation, and crystallinities comparable to the reported isotropic powders53,54,55. Notably, TPB-11 achieved near-perfect face-on alignment as indicated by the Hermans orientation factor of 0.92 calculated for the (2 − 10) reflection (Supplementary Fig. 78). Finally, the ability to control the film thickness from a few micrometres to several tens of micrometres was demonstrated for TPB-1 by adjusting the concentration of the initial oligomer solutions (Supplementary Fig. 96).
Four additional pairs of amines and aldehydes were successfully polymerized into oriented CANs films and then converted to the corresponding oriented (face-on) 2D COF films by solvothermal treatment. a, TPB-11 (aldehyde 11 is 3,3′-dimethoxy-[1,1′-biphenyl]-4,4′-dicarbaldehyde) 2D GIWAXS pattern and in-plane (near 2θz = 0) projection. b, BTA-1 (amine BTA is N,N,N′,N′-tetra(4-aminophenyl)benzene-1,4-diamine) 2D GIWAXS pattern and in-plane projection. c, TPPy-1 2D GIWAXS pattern and in-plane projection. d, TPPor-1 2D GIWAXS pattern and in-plane projection. Insets: schematic representation of the 2D lattices of the respective 2D COF.
Conclusions
Kinetically trapped states are often undesired but typically emerge upon solution processing of molecular materials. We demonstrate that these metastable states may be exploited to produce free-standing, highly oriented and crystalline, large-area imine-linked 2D COF films. Our approach, following the kinetic-to-thermodynamic pathway, capitalizes on the processability, and dynamic nature of metastable 3D iminium-linked CANs generated in TFA/H2O, which enables fabrication via simple solution casting. The tensile stresses acting on the drying CAN film induce a structural rearrangement of the polymer branches assisted by the dynamic covalent nature of the iminium bond, eventually leading to the spontaneous formation of a free-standing, amorphous, oriented polymeric film. The subsequent 3D-to-2D topological transformation by solvothermal treatment yields a porous and crystalline 2D COF film with one-dimensional nanochannels oriented perpendicular to the macroscopic surface of the film. We expect that the exceptional morphological features of these materials, combined with their mechanical robustness will expand the relevance of 2D COFs for applications such as membranes for the separation of chemicals, water treatment, precise molecular sieving or separators in ion batteries. Furthermore, we anticipate that the phenomena described in this study are general. Further studies will enable the expansion of the present scope to other molecular building blocks including anilines, aldehydes and the related CAN and COF structures.
Our findings demonstrate that covalent polymerizations, like their supramolecular counterparts, can proceed through multiple pathways. The choice of pathway can be critical for determining the properties of the final material and can provide access to the desired material morphologies and structures that cannot be attained following the thermodynamically favoured trajectory. We believe that further endeavours in this direction will unveil a multitude of approaches using non-equilibrium intermediates as effective tools in the synthesis of polymeric materials.
Methods
General procedure for the polymerization in TFA/H2O
Concentrated solutions of the desired n-functional amine (An, n = 3–4) and difunctional aldehyde (B2) were prepared by dissolving the respective solids in a mixture of TFA and H2O. TFA/H2O solvent mixtures were prepared in volumetric flasks, stored well sealed and used within a few days from preparation. The solution of the amine and the solution of the aldehyde were mixed to reach the desired concentration in a 1:1 stoichiometric ratio of functional groups: [FG]0 = n × [An]0 = 2 × [B2]0, where [FG]0 is the concentration of functional groups, [An]0 is the initial concentration of the amine monomer (TPB or other amines) and [B2]0 is the initial concentration of the aldehyde monomer (1 or other aldehydes). The resulting polymer solutions were stored in sealed vials and used within a few hours of the preparation. As an example, to prepare 2 ml a TPB-1 solution of at [FG]0 = 200 mM in TFA/H2O 90:10 v/v, 46.9 mg of 1,3,5-tris(4-aminophenyl)benzene TPB (0.133 mmol) and 38.8 mg of 2,5-dimethoxyterephthalaldehyde 1 (0.200 mmol) were separately dissolved in 1 ml each of the TFA/H2O mixture. The two solutions were then combined. For the NMR studies, deuterated solvents were used in place of the protonated ones.
Preparation of the oriented amorphous TPB-1 CAN films
A 15 × 15 mm2 silicon substrate was placed in a Petri dish (50 mm 10 mm, diameter × height). The Petri dish was placed on a preheated hotplate at 40 °C. Before the deposition of the solution, the hotplate was levelled using a bubble level. Oriented films were prepared by carefully casting 0.15 ml of [FG]0 = 150 mM TPB-1 solution on the substrate. After the deposition, the Petri dish was quickly covered with the complementary glass cover. The film was left drying on surface for 4 h, resulting in a golden reflective coating that showed a symmetric pattern on the surface. Then, the cover was removed, and the substrate was quickly immersed in methanol (MeOH), which caused the delamination from the surface and the change of colour to dark red. The self-standing film was then picked up with tweezers and transferred into a triethylamine–MeOH (10:90 v/v) solution. The neutralization of the adsorbed TFA was indicated by the quick colour change to bright yellow. The film was then Soxhlet extracted with tetrahydrofuran (THF) for 12 h to remove low-molecular-weight oligomers. Next, THF was exchanged three times with n-pentane and the film was transferred on filter paper. The film was dried in ambient conditions and ensuring even evaporation of the solvent from both sides to avoid warping deformations. After 1 h, the film was transferred to a vacuum oven and the volatile residues of solvents were further removed at 100 °C for 12 h under reduced pressure. The material was finally isolated as robust, flexible, homogeneous, transparent, yellow film, with a yield of 95%.
Procedure for the solvothermal annealing to TPB-1 COF
The solvothermal annealing of the amorphous films was carried out in a 5 ml crimp seal vial using 0.4 ml of MeCN as solvent and 0.04 ml of 10 M aqueous acetic acid as catalyst for two films. The vial was then placed in an oven and heated to 100 °C for 48 h. After cooling down, the film was washed with MeCN and MeOH and Soxhlet extracted with THF for 12 h. Then, THF was exchanged with n-pentane and dried according to the procedure reported for the pristine film. The desired material was obtained as opaque-yellow film with a yield ranging between 80% and 90%.
Data availability
The data supporting this research are available within the Article and its Supplementary Information. Raw data files are available via figshare at https://doi.org/10.6084/m9.figshare.25599411 (ref. 56).
Change history
30 July 2025
A Correction to this paper has been published: https://doi.org/10.1038/s44160-025-00864-x
References
Yaghi, O. M., Kalmutzki, M. J. & Diercks, C. S. Introduction to Reticular Chemistry: Metal–Organic Frameworks and Covalent Organic Frameworks (Wiley, 2019).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Geng, K. et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020).
Ascherl, L. et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016).
Zhang, W. et al. Reconstructed covalent organic frameworks. Nature 604, 72–79 (2022).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Zhao, S. et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation. Nat. Mater. 20, 1551–1558 (2021).
Xu, H., Tao, S. & Jiang, D. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 15, 722–726 (2016).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Gao, H. et al. A pyrene-4,5,9,10-tetraone-based covalent organic framework delivers high specific capacity as a Li-ion positive electrode. J. Am. Chem. Soc. 144, 9434–9442 (2022).
Ascherl, L. et al. Perylene-based covalent organic frameworks for acid vapor sensing. J. Am. Chem. Soc. 141, 15693–15699 (2019).
Evans, A. M. et al. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 361, 52–57 (2018).
Han, J. et al. Fast growth of single-crystal covalent organic frameworks for laboratory X-ray diffraction. Science 383, 1014–1019 (2024).
Wang, S. et al. Single-crystal 2D covalent organic frameworks for plant biotechnology. J. Am. Chem. Soc. 145, 12155–12163 (2023).
Yi, L., Gao, Y., Luo, S., Wang, T. & Deng, H. Structure evolution of 2D covalent organic frameworks unveiled by single-crystal X-ray diffraction. J. Am. Chem. Soc. 146, 19643–19648 (2024).
Rodríguez-San-Miguel, D. & Zamora, F. Processing of covalent organic frameworks: an ingredient for a material to succeed. Chem. Soc. Rev. 48, 4375–4386 (2019).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Evans, A. M. et al. Two-dimensional polymers and polymerizations. Chem. Rev. 122, 442–564 (2022).
Hao, Q. et al. Confined synthesis of two-dimensional covalent organic framework thin films within superspreading water layer. J. Am. Chem. Soc. 140, 12152–12158 (2018).
Zhang, B. et al. 2D covalent organic framework thin films via interfacial self-polycondensation of an A2B2 type monomer. Chem. Commun. 56, 3253–3256 (2020).
Zhong, Y. et al. Wafer-scale synthesis of monolayer two-dimensional porphyrin polymers for hybrid superlattices. Science 366, 1379–1384 (2019).
Liu, K. et al. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 11, 994–1000 (2019).
Wang, Z. et al. On-water surface synthesis of charged two-dimensional polymer single crystals via the irreversible Katritzky reaction. Nat Synth 1, 69–76 (2021).
Ou, Z. et al. Oriented growth of thin films of covalent organic frameworks with large single-crystalline domains on the water surface. J. Am. Chem. Soc. 144, 3233–3241 (2022).
Colson, J. W. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011).
Evans, A. M. et al. Thermally conductive ultra-low-k dielectric layers based on two-dimensional covalent organic frameworks. Nat. Mater. 20, 1142–1148 (2021).
Medina, D. D. et al. Oriented thin films of a benzodithiophene covalent organic framework. ACS Nano 8, 4042–4052 (2014).
Fabozzi, F. G., Severin, N., Rabe, J. P. & Hecht, S. Room temperature on-surface synthesis of a vinylene-linked single layer covalent organic framework. J. Am. Chem. Soc. 145, 18205–18209 (2023).
Zhan, G. et al. Observing polymerization in 2D dynamic covalent polymers. Nature 603, 835–840 (2022).
Daum, J. P. et al. Solutions are the problem: ordered two-dimensional covalent organic framework films by chemical vapor deposition. ACS Nano 17, 21411–21419 (2023).
Khan, N. A. et al. Solid–vapor interface engineered covalent organic framework membranes for molecular separation. J. Am. Chem. Soc. 142, 13450–13458 (2020).
Yao, L. et al. Covalent organic framework nanoplates enable solution-processed crystalline nanofilms for photoelectrochemical hydrogen evolution. J. Am. Chem. Soc. 144, 10291–10300 (2022).
Smith, B. J. et al. Colloidal covalent organic frameworks. ACS Cent. Sci. 3, 58–65 (2017).
Wang, M. et al. Ultrafast seawater desalination with covalent organic framework membranes. Nat. Sustain. 5, 518–526 (2022).
Kandambeth, S. et al. Selective molecular sieving in self‐standing porous covalent‐organic‐framework membranes. Adv. Mater. 29, 1603945 (2017).
Burke, D. W., Jiang, Z., Livingston, A. G. & Dichtel, W. R. 2D covalent organic framework membranes for liquid‐phase molecular separations: state of the field, common pitfalls, and future opportunities. Adv. Mater. 36, 2300525 (2024).
Zhang, G., Zeng, Y., Gordiichuk, P. & Strano, M. S. Chemical kinetic mechanisms and scaling of two-dimensional polymers via irreversible solution-phase reactions. J. Chem. Phys. 154, 194901 (2021).
Kloxin, C. J. & Bowman, C. N. Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 42, 7161–7173 (2013).
Elling, B. R. & Dichtel, W. R. Reprocessable cross-linked polymer networks: are associative exchange mechanisms desirable? ACS Cent. Sci. 6, 1488–1496 (2020).
Zheng, N., Xu, Y., Zhao, Q. & Xie, T. Dynamic covalent polymer networks: a molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 121, 1716–1745 (2021).
Pei, Z. et al. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 13, 36–41 (2014).
Cox, R. A. & Yates, K. Acidity functions: an update. Can. J. Chem. 61, 2225–2243 (1983).
Burke, D. W. et al. Acid exfoliation of imine‐linked covalent organic frameworks enables solution processing into crystalline thin films. Angew. Chem. Int. Ed. 59, 5165–5171 (2020).
Barnes, M. G., McLeod, D. C. & Lambeth, R. H. Highly crystalline, free-standing covalent organic framework films produced directly from monomer solutions. ACS Appl. Polym. Mater. 4, 2017–2021 (2022).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Flory, P. J. Molecular size distribution in three dimensional polymers. I. Gelation. J. Am. Chem. Soc. 63, 3083–3090 (1941).
Aliprandi, A., Mauro, M. & De Cola, L. Controlling and imaging biomimetic self-assembly. Nat. Chem. 8, 10–15 (2016).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Wang, S. et al. Pathway complexity in the stacking of imine-linked macrocycles related to two-dimensional covalent organic frameworks. Chem. Mater. 31, 7104–7111 (2019).
Tirumkudulu, M. S. & Punati, V. S. Solventborne polymer coatings: drying, film formation, stress evolution, and failure. Langmuir 38, 2409–2414 (2022).
Lu, Y. et al. Continuous formation of supported cubic and hexagonal mesoporous films by sol–gel dip-coating. Nature 389, 364–368 (1997).
Krishnaraj, C. et al. Strongly reducing (diarylamino)benzene-based covalent organic framework for metal-free visible light photocatalytic H2O2 generation. J. Am. Chem. Soc. 142, 20107–20116 (2020).
Guo, M. et al. The promotion effect of π–π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 13, 1770 (2022).
Keller, N. et al. Enforcing extended porphyrin J-aggregate stacking in covalent organic frameworks. J. Am. Chem. Soc. 140, 16544–16552 (2018).
Cusin, L. et al. Data Repository for “Synthesis of micrometer-thick oriented 2D covalent organic framework films by a kinetic polymerization pathway”. figshare https://doi.org/10.6084/m9.figshare.25599411 (2025).
Acknowledgements
We acknowledge C. Antheaume for the support with the NMR experiments, I. Moudrakovski for assistance with the solid state NMR measurements, C. Melart and A. Lopez-Acosta for the support with the rheometry experiments. Insightful discussions with T. van Leeuwen and W. R. Browne are much appreciated. We acknowledge the financial support of the European Commission through the Marie Sklodowska-Curie projects ULTIMATE (GA-813036) (P.S.) and LA2DCOFS (GA-101027639) (W.D.), the Interdisciplinary Thematic Institute SysChem via the IdEx Unistra (ANR-10-IDEX-0002) within the Investissement d’Avenir programme, the International Center for Frontier Research in Chemistry (icFRC), the Institut Universitaire de France (IUF) (P.S.), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) via the SFB 1333/2 (Project-ID 358283783) (B.V.L. and S.K.), the DFG Cluster of Excellence e-conversion (EXC 2089/1–390776260) (B.V.L.), the Bavarian Research Network SOLTECH (B.V.L.), the Excellence Initiative – Research University from University of Warsaw (P.C.), Polish National Agency for Academic Exchange (BPN/PPO/2023/1/00014) (W.D.) and National Science Center Poland (reg. no. 2024/03/1/ST5/00003) (W.D.) for financial support.
Author information
Authors and Affiliations
Contributions
L.C. and W.D. conceived the project and the experiments; L.C. performed the study of the sol–gel system as well as the synthesis and characterization of the films and analysed the data; W.D. synthesized the monomers; P.C. and P.W.M. performed the GIWAXS experiments and analysed the data; S.V.G. performed the solid-state NMR, powder X-ray diffraction and structural refinements; F.H. performed the gas sorption measurements, L.C., W.D. and P.C. wrote the first draft; S.K., P.W.M. and B.V.L. supervised and supported parts of the project; W.D. and P.S. guided the project; all the authors discussed the results and participated in editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Rafael Verduzco, Dong Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ali Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–96, Schemes 1–13 and Dscussion.
Source data
Source Data Fig. 2
Integrated NMR data, numerical data for construction of plots; unprocessed SEM.
Source Data Fig. 3
Integrated GIWAX data; unprocessed SEM; 2D GIWAX maps.
Source Data Fig. 4
Rheology data; unprocessed SEM; 2D GIWAX maps.
Source Data Fig. 5
Integrated GIWAX data; 2D GIWAX maps.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cusin, L., Cieciórski, P., Van Gele, S. et al. Synthesis of micrometre-thick oriented 2D covalent organic framework films by a kinetic polymerization pathway. Nat. Synth 4, 632–641 (2025). https://doi.org/10.1038/s44160-025-00741-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00741-7
This article is cited by
-
Fundamentals of charge transport in two-dimensional framework materials
Nature Reviews Materials (2025)