Abstract
Sequence-defined oligomers have been synthesized with increasing complexity, but the participation of chiral centres in the oligomerization process remains challenging. Here we report the synthesis of sequence-defined oligosulfonimidates (up to thirteen-mers) obtained via a series of sulfur–fluoride exchange and sulfur–phenolate exchange reactions. Next, we demonstrate that the use of chiral sulfonimidoyl fluorides allows the construction of chiral oligomers, in which the chirality of each linking S-centred site can be tuned independently of the rest of the molecule, thereby opening a way to a potentially vast increase in the information density of such oligomers. Finally, by activating a dormant fluorous tag used for the separation of oligomers, a tail-to-head-type sulfur–phenolate exchange polymerization strategy was developed using these oligomers, resulting in sequence-controlled polymers. The high variability of sulfonimidates and the chiral control provided to macromolecules through this technique results in another step towards full control over nature-mimicking polymers.

Similar content being viewed by others
Main
Precise control over molar mass, sequence and chirality is crucial for the functioning of biological polymers, such as proteins and nucleic acids. Mimicking the characteristics of such natural sequence-defined polymers1 is considered as the holy grail in polymer science2. In addition, such endeavours are of substantial interest because of their potential applications in information storage3,4, catalysis5, self-assembly6,7,8 and molecular recognition9. In some respects such artificial polymers could even surpass natural biomolecules, as shown by DNA analogues like peptide nucleic acids (PNA) that overcome some of the vulnerability of DNA to hydrolysis10,11. Moreover, the introduction of specific functional groups in synthetic sequence-defined polymers could facilitate the interpretation of sequences and provide the capacity to store more information by incorporating large diversity of monomers12.
Due to the prominent advantages of synthetic sequence-defined polymers, the construction of various types of sequence-defined polymer has become a hot topic in polymer science in recent years. Iterative stepwise growth (ISG)13 and iterative exponential growth (IEG)14,15 are the two commonly used strategies to synthesize sequence-defined oligomers, and many reactions have been applied for this. Given the characteristics of click reactions16,17,18, evidently these are of interest in this field, and for example both the copper-catalysed azide–alkyne cycloaddition (CuAAC)14,19 and sulfur–fluoride exchange (SuFEx)20,21 reactions have been used. However, site-specific control over chirality has, to the best of our knowledge, not yet been reported. So far, the use of chiral monomers that incorporate non-reactive chiral moieties near the reactive site has been a common method to make chiral oligomers. In 2018, Boyer and co-workers developed stereospecific oligomers using alternating radical chain growth and sequential photoinduced reversible addition−fragmentation chain-transfer single unit monomer insertion (photo-RAFT SUMI). They found the stereochemistry of cyclic monomer insertion into the RAFT agents is trans-selective due to steric hindrance from the repeating monomer units22. More recently, Du Prez and co-workers developed stereocontrolled sequence-defined oligomers through the ring opening of chiral thiolactone isocyanate, in which the chiral carbon centre adjacent to the carbon reactive site of the ring-opening reaction can be functionalized23. However, full and enantiospecific control of chirality at the reactive site has not been reported, and this biomimetic goal of being able to define for each monomer in an oligomer both the composition and spatial orientation at will is still high on the wish list of this field.
We hypothesized that two developments from our group, however, could make this possible. In 2020, we developed an intrinsically enantiospecific click reaction, namely the SuFEx reaction, starting from chiral sulfonimidoyl fluorides (SFs, Fig. 1a)24. While highly effective it still relied on the availability of both enantiomers of the SFs—synthesized in five steps from commercial sources—either via two parallel syntheses or via preparative chiral high-performance liquid chromatography (HPLC). This limitation was overcome by the realization that with a good leaving group, the sulfonimidate formed from the SuFEx reaction may undergo another chirality-inverting S(VI) exchange reaction with another, more electron-rich phenol, leading to sulfur–phenolate exchange (SuPhenEx, Fig. 1a). This reaction was also shown to be both quantitative and enantiospecific25,26, and as such requires only one of the chiral SFs to be synthesized to still easily access both S-centred stereoisomers.
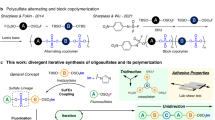
a, General scheme for chiral SuFEx and SuPhenEx reactions towards the synthesis of two enantiomeric sulfonimidates from one chiral sulfonimidoyl fluoride24,26. b, Representative monomers of di-sulfonimidoyl fluorides (di-SFs) and diphenols as used in this study. c, Overview of this work. SuFEx, sulfur–fluoride exchange reaction; SuPhenEx, sulfur–phenolate exchange reaction. Tol, p-tolyl.
In this Article, we report a synthetic strategy for the synthesis of SuFEx-based sequence-defined oligomers by a stepwise iterative approach, in which series of symmetric bisphenols and di-SFs (Fig. 1b) were used as two types of building blocks. Next, using chiral SFs and stereoinversion via the SuPhenEx reaction, we obtained sequence-defined oligomers in which the stereochemistry at the reactive site can be independently controlled during the synthetic procedure. Finally, a tail-to-head-type polymerization strategy was developed to synthesize SuPhenEx-based sequence-controlled polysulfonimidate polymers. This allows the transfer of sequence and chirality control from oligomers to polymers.
Results and discussion
Synthesis of sequence-defined oligomers
In our initial investigations, the symmetric building blocks N′,N″-[4,4′-oxybis(benzoyl)]bis(4-methylbenzenesulfonimidoyl fluoride) 1a and bisphenol A 2a were employed for a proof-of-concept synthesis of sequence-defined oligomers (Fig. 2a). The first critical choice in this synthesis was the introduction of a perfluoro-tagged phenol A1, in which the tag links to the phenol via an S atom. This tag allows for the easy purification of reaction products during the oligomerization27,28,29,30,31 but also (see below) provides access to further functionalization. The perfluoro-tagged phenol A1 (116 mg, 0.201 mmol, 1.0 equiv.) underwent a SuFEx reaction with excess 1a (568 mg, 1.000 mmol, 5 equiv.), to ensure monosubstitution, in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (45 µl, 0.301 mmol, 1.5 equiv.) as the base at room temperature for 30 min. After the reaction was completed, the reaction mixture was loaded onto fluorous silica gel. There the sequence of fluorophobic eluents (85% methanol/H2O) and fluorophilic eluents (tetrahydrofuran or acetonitrile) first washed out the excess of monomer 1a, which was followed by the purified product, dimer A2, which was obtained in an isolated yield of 85% (190 mg, 0.170 mmol) and di-substitution by-product M1 that was isolated in 3% yield (10 mg, 6 µmol, see Supplementary Section 3 for details). Subsequently, the remaining sulfonimidoyl fluoride of A2 (190 mg, 0.170 mmol, 1 equiv.) reacted with diphenol 2a (193 mg, 0.846 mmol, 5 equiv.), retaining a phenol reactive site, which allowed the oligomer to be further extended. Following four more steps of stepwise iterative growth, heptamer A7 (42 mg, 0.015 mmol) (Fig. 2b) was obtained with an overall yield of 29% over six steps. Purifying compounds A2 and A3 by normal-phase chromatography is extremely challenging due to the similar polarity between A2 and di-SF, as well as between A3 and bisphenol A. Therefore, using fluorous solid-phase extraction (FSPE) can significantly simplify the purification process. However, as the oligomer chain extends, the resulting longer oligomers (for example after the pentamer stage) have a very different polarity than the monomers, allowing excess monomers to be easily separated by normal-phase chromatography32. To demonstrate the versatility of this method, a variety of di-phenols was employed to elongate the oligomer chains. Analogous to the synthesis of A7, we aimed to synthesize sequence-defined oligosulfonimidates B7 and C7 to check on the scope of our approach. Whereas oligomer A7 has a repetitive structure with only one type of di-SF and one type of diphenol, in heptamer B7 the di-SF was still kept constant, but the diphenol was varied (Fig. 2c), while finally in heptamer C7 both the di-SF and the diphenol were varied (Fig. 2d). We were pleased to observe that this synthetic route displayed the versatility hoped for, with an average isolated yield of >80% in each step, ranging from 70 to 91%. This yield did not decrease with increasing oligomer length, so that the final products could be obtained in overall yields of 33% (83 mg, 0.027 mmol) and 29% (82 mg, 0.028 mmol), respectively. Such consistently high yields are specifically of interest, since in several routes towards sequence-defined oligomers the yields rapidly drop with increasing oligomer length, sometimes from already the trimer stage onwards27; that we do not observe that here demonstrates the potential of our approach. Structures of the obtained products were confirmed by a combination of NMR spectroscopy and high-resolution mass spectrometry. Gel permeation chromatography showed the expected mono-dispersity of the formed oligomers and the decrease in retention time with an increase of the oligomer chain length (Supplementary Section 8).
a, General synthesis of oligosulfonimidates: (i) Coupling of oligomers with di-sulfonimidoyl fluorides using DBU as a base (in molar ratio 1:5:1.5) at room temperature (r.t.); (ii) Coupling of oligomers with bisphenols using DBU as a base (in molar ratio 1:5:5) in THF at r.t. b, Synthesized oligosulfonimidate A7 consisting of 1a and 2a units. c, Synthesized oligosulfonimidate B7 with constant di-SF and three different di-phenols. d, Synthesized oligosulfonimidate C7 with three different di-phenols and di-SFs. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; THF, tetrahydrofuran; tol, p-tolyl.
After demonstrating that both di-phenols and di-SFs could be varied, we aimed to investigate modulation of the phenol-linked fluorous cap with the idea that, after having been extremely useful in the FSPE purification, variability of this end of the molecule would further expand the flexibility of our approach. Here, it is good to point out the dual role that was needed for the S–O linkage of the fluorous tag: it should be stable under all the SuFEx reaction conditions used to prepare the oligomers, yet still be easily removable in an orthogonal manner afterwards. The stability was not only demonstrated by its use in the aforementioned syntheses, in which this moiety was maintained over and over again. The first attempt to remove it was made by treating the reaction products under harsher basic conditions than used in these syntheses. To this aim, heptamer A7 was treated with 5 equiv. phenol (this excess was used to ensure substantial deprotonation of this molecule rather than the terminal phenol moiety on the A7 heptamer) and 1.1 equiv. NaH. Monitoring the reaction progress by thin-layer chromatography (TLC), however, showed the formation of multiple spots, indicative of cleavage reactions at multiple sites, not only at the terminus leading to a multitude of small degradation compounds. To substitute this stably bound fluorous tag in a chemoselective manner under much milder SuPhenEx conditions, we then reasoned that an oxidative reaction could transform the poor sulfide–phenolate leaving group into a much better sulfone–phenolate leaving group33,34. First, we optimized the reaction conditions using trimer A3 (Fig. 3b). After oxidizing the sulfur of the trimer using 30% H2O2 in the presence of ammonium molybdate tetrahydrate for 2 h at room temperature (r.t.), a SuPhenEx substitution was used to replace the now electron-deficient and easily leaving fluorous tag in D1 by phenol, chosen as a representative phenolic moiety. The oxidation was evident by characteristic downfield shifts of multiple signals in the 1H NMR spectra, and particularly from the downfield shift of the CH group linked to sulfur from 3.15–3.03 ppm to 3.35–3.27 ppm and the downfield shift of the CH group linked to the fluorine tag from 2.44–2.23 ppm to 2.74–2.53 ppm. Using DBU as a base, phenol-tagged trimer E1 was obtained in a yield of 86% (27 mg, 0.032 mmol) within 2 h at room temperature. This demonstrates the high reactivity and chemoselectivity of this SuPhenEx reaction and opens up the road to further modification of the oligomer chains. (Note: the oxidation step is rather mild, and thus allows many different functionalities in the monomers, but easy-to-oxidize moieties like aldehydes, disulfides, or free thiols are to be avoided.)
a, Synthesis of E1 from A3. b, Synthesis of E2 from A5. c, Synthesis of E3 from A7. Reaction conditions: (i) Oligomer with fluoro-tag (A3, A5 or A7, 1 equiv.), ammonium molybdate tetrahydrate (0.1 equiv.), 30% H2O2 (18 equiv.), THF, r.t., 2 h; (ii) Oxidized intermediate (D1–D3, 1 equiv.), phenol (10 equiv.), DBU (3 equiv.), THF, r.t., 2 h. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; THF, tetrahydrofuran; tol, p-tolyl.
As a next step in testing the versatility of this strategy, we focused on fluoro-tagged pentamer D2, obtained using such an oxidation step, which has four potentially SuPhenEx-reactive sites. When D2 (35 mg, 0.0165 mmol) was reacted with phenol at r.t. it gently gave phenol-tagged pentamer E2 (Fig. 3c) in a yield of 87% (23 mg, 0.0143 mmol), demonstrating that none of the internal S(VI) sites were reacting under these conditions. We finally applied this procedure to the sulfone-linked fluorous tag in heptamer D3, with six internal SuPhenEx-reactive sites, and chemoselectively exchanged only the fluorous tag for phenol, providing phenol-tagged heptamer E3 (Fig. 3d) in a yield of 77% (18 mg, 7.62 µmol). Given the electronics of the SuPhenEx reaction, we hypothesize that this oxidized perfluoro tag can be replaced by any phenol, alcohol or amine26,35,36 that is more electron-donating than the SO2-substituted phenol produced after oxidation. Given the relatively high Hammett σ-value of the –SO2– moiety (0.72 for para-SO2CH3 (ref. 37)), which becomes only more electronegative with the attached fluoro tag), this basically means almost any of these nucleophiles.
To obtain some more quantitative information, we calculated the barrier for the SuPhenEx reaction of phenoxide on an analogue of compound IV (Fig. 1a) with –O-phenyl-p-SCH3 or –O-phenyl-p-SO2CH3 replacing the green S–Ph in IV. We used ωB97XD/def2QZVPP//ωB97XD/6-311+G(d,p) density functional theory calculations, together with an SMD solvation model to mimic THF. In line with experiment, the barrier for loss of an –O-phenyl-p-SO2CH3 anion is indeed significantly lower, namely 3 kcal mol−1, than for loss of an –O-phenyl-p-SCH3 anion (ΔG# calculated to be 11 versus 14 kcal mol−1). This calculated difference supports the experimental observation that, under mild reaction conditions, the oxidation of the sulfur turns only this moiety into a leaving group and no discernible cleavage of the chain by the SuPhenEx reaction on the internal part of the oligomer chain is observed (Supplementary Section 11).
The efficiency of the SuPhenEx reaction also allows a final step in this synthesis after ‘waking up’ the dormant sulfide linker: rather than a terminal (mono-)phenol, one can also react a (fluoro-tagged) oligomer with a free phenolic moiety. In this manner, the fluorous-tag phenol can be considered as a protecting group for the SuPhenEx reaction, to be replaced by other phenols when necessary. This strategy also allows divergent routes to grow long oligomeric chains in a divergent manner and thus more efficiently (Fig. 4). First, the phenol-capped trimer E1 (30 mg, 35.3 µmol, 1.1 equiv.) was coupled with the fluoro-tagged tetramer A4 (60 mg, 32.0 µmol) through a SuFEx reaction, resulting in the synthesis of heptamer F1 with an isolated yield of 96% (83 mg, 30.6 µmol). Subsequently, the perfluoro-tag phenol on heptamer F1 was activated through an oxidation reaction, followed by coupling with the fluorous-tag trimer A3 via a SuPhenEx reaction. This yielded nonamer F3 with an isolated yield of 83% (19 mg, 5.48 µmol). Finally, the perfluoro-tag phenol on nonamer F3 was activated via oxidation and coupled with pentamer E2 to yield thirteen-mer F5 (26 mg, 5.8 µmol) with an isolated yield of 67%. For this reaction, a more reactive 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) base was employed in place of DBU and the reaction was complete within 2 h at room temperature. The molecular weight reached 4.50 kDa, which is approximately three times that of previously reported oligosulfates38. Given the continuously good yields of the SuPhenEx coupling steps, we believe that if the oligomer F5 could contain a latent fluorous-tag phenol, it would allow for further elongation of the oligomeric chain.
Reaction conditions: (i) A4 (1 equiv.), E1 (1.1 equiv.), DBU (1.2 equiv.), THF, r.t., 2 h; (ii) Oligomer with fluoro-tag (F1 or F3, 1 equiv.), ammonium molybdate tetrahydrate (0.1 equiv.), 30% H2O2 (18 equiv.), THF, r.t., 2 h; (iii) F2 (1 equiv.), A3 (5 equiv.), DBU (5 equiv.), THF, 60 °C, 16 h; (iv) Oxidized intermediate (1 equiv.), E2 (1.5 equiv.), TBD (1.6 equiv.), THF, r.t., 2 h. SuFEx, sulfur–fluoride exchange reaction; SuPhenEx, sulfur–phenolate exchange reaction; THF, tetrahydrofuran; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; TBD, 1,5,7-triazabicyclo[4.4.0]dec-5-ene; tol, p-tolyl.
Chiral sequence-defined oligomers
Both the SuFEx and the SuPhenEx reactions were previously shown to be enantiospecific click reactions24,26. After developing a strategy for sequence definition, we aimed for the next step by adding chiral information to the oligomers. This required a few changes to our strategy: for non-chiral SuFEx reactions as used above, DBU can be used nicely as an easy to add, readily available base. However, as shown by both our work24 and that of Bull and co-workers39, capture of the fluoride anion formed from the SuFEx reaction is essential to prevent racemization by re-attack onto the S(VI)–F centre. Given the frequently small amounts of the reacting phenolate, rather than trying to prepare the phenolate in advance by using an accurately determined but tiny amount of NaH (as we had done previously), we exploited Bull’s approach to capture a fluoride ion by the addition of excess lithium bromide. This caused the reaction to proceed in an enantiospecific manner to yield chiral sequence-defined oligomers. In addition, when a symmetric chiral di-SF was utilized as the monomer, the same configuration for the two chiral centres of the monomer would be obtained if only the SuFEx reaction was employed to grow chains. However, the combination of SuFEx and SuPhenEx reactions uniquely allows control of the configuration of each and every sulfur centre (Fig. 1a). To demonstrate the tunability of each chiral sulfur centre, we decided to design the configuration of the oligomer chain as SRSS (easily memorized as S(ha)R(ple)SS; Fig. 5). The fluoro-tagged phenol A1 (114 mg, 0.2 mmol) reacted with chiral di-SF compound 1d (341 mg, 0.6 mmol, 96% diastereomeric excess (d.e.)) in the presence of DBU (44.7 µl, 0.3 mmol) as the base and lithium bromide (3 equiv.) as the fluoride anion scavenger. Product G1 (S,R) was obtained with a yield of 81% (182 mg, 0.162 mmol) and virtually unchanged 96% d.e., demonstrating both high reactivity and the enantiospecificity of the coupling reaction. Subsequently, G1 (100 mg, 0.089 mmol) underwent a SuFEx reaction with phenol, producing (S,S)-G2 with a yield of 80% (85 mg, 0.071 mmol) and 94% d.e. Following this, the sulfur atom of the fluoro-tagged phenol on G1 was oxidized by H2O2 as described before and the resulting sulfone moiety substituted by 2a through a SuPhenEx reaction, resulting in the product (S,R)-G4 with a yield of 83% (23 mg, 0.027 mmol) and 94% d.e. Finally, (S,R)-G4 (21 mg, 24.7 µmol) reacted with G1 (33 mg, 29.5 µmol), producing (S,R,S,S)-G5 with a yield of 73% (35 mg, 17.5 µmol). In order to identify the diastereospecificity of the SuFEx reaction in the last step, the reaction was repeated without LiBr, producing a partially racemized (S,R,S/R,S)-G5. Peaks of two diastereomers were separated using chiral HPLC and only 3% total reduction of stereochemistry was observed during SuFEx coupling, which was less than 1% of racemization at each sulfur centre. In this it should be noted that reaching this (S,R,S,S) configuration for the oligosulfonimidate is not dependent on the stereochemistry of the starting di-SF: had the reaction started with the different configuration of the di-SF monomer, stereoinversion using SuPhenEx reactions would have paved the way towards the same compound.
Reaction conditions: (i) A1 (1 equiv.), 1d (3 equiv.), DBU (1.5 equiv.), LiBr (3 equiv.), THF, r.t., 2 h; (ii) G1 (1 equiv.), phenol (1.2 equiv.), LiBr (1.5 equiv.), DBU (1.2 equiv.), THF, r.t., 2 h; (iii) G2 (1 equiv.), ammonium molybdate tetrahydrate (0.1 equiv.), 30% H2O2 (18 equiv.), THF, r.t., 2 h; (iv) G3 (1 equiv.), bisphenol A (5 equiv.), DBU (5 equiv.), THF, r.t., 2 h; (v) G4 (1 equiv.), G1 (1 equiv.), LiBr (1.6 equiv.), DBU (1.2 equiv.), THF, r.t., 3 h. SuFEx, sulfur–fluoride exchange reaction; SuPhenEx, sulfur–phenolate exchange reaction; THF, tetrahydrofuran; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; tol, p-tolyl.
After demonstrating that the configuration of chiral sulfurs could be adjusted on demand, we next aimed to further extend the chiral oligomer chain length to demonstrate the generality of this synthetic approach. Therefore, the synthetic route towards the (S,R,S,S,S,S)-G8 heptamer was designed (Fig. 6). (S,R)-G1 (88 mg, 0.079 mmol, 1 equiv.) reacted with excess equiv. of bisphenol A to yield (S,S)-G6 (86 mg, 0.065 mmol, 82%, 96% d.e.). Subsequently, (S,S)-G6 (60 mg, 45 µmol) reacted with chiral di-SF compound 1d (77 mg, 135 µmol, 96% d.e., 3 equiv.) to give (S,S,S,R)-G7 (58 mg, 31 µmol) with a yield of 68%. Finally, (S,S,S,R)-G7 (20 mg, 10.7 µmol) coupled with (S,R)-G4 (10 mg, 11.8 µmol, 94% d.e.), produced (S,R,S,S,S,S)-G8 with a yield of 86%. In order to establish the diastereoselectivity of the last synthetic steps, partially racemized products (S,S,S/R,S/R)-G7 and (S,R,S/R,S,S,S)-G8 were synthesized and used to identify HPLC peaks corresponding to the racemization products. In this way we were able to confirm that the late-stage SuFEx reactions proceeded with maximally 1% racemization at each of the sulfur centres that are involved in the corresponding SuFEx reactions. Note that we employed 1d with a d.e. of 96% as the starting material; we believe an even higher d.e. of the chiral oligomers can be obtained by increasing the d.e. of 1d, for example through crystallization prior to use.
Reaction conditions: (i) G1 (1 equiv.), bisphenol A (5 equiv.), DBU (5 equiv.), LiBr (2 equiv.), THF, r.t., 30 min; (ii) G6 (1 equiv.), 1d (3 equiv.), LiBr (4 equiv.), DBU (1.5 equiv.), THF, r.t., 2 h; (iii) G7 (1 equiv.), G4 (1.1 equiv.), DBU (1.5 equiv.), LiBr (2 equiv.), THF, r.t., 2 h. THF, tetrahydrofuran; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; tol, p-tolyl.
Sequence-controlled sulfonimidate polymers
To further expand the utility of the dormant property of the fluorous-tag phenol part on the oligomer chain, we finally aimed to induce SuPhenEx polymerization of sequence-defined oligomers to obtain sequence-controlled tail-to-head-type polymers. As a proof-of-concept (Fig. 7a), trimer D1 (10 mg) in 100 µl anhydrous acetonitrile/anhydrous N,N-dimethylformamide (DMF) (1:1) was polymerized at 80 °C for 48 h in the presence of DBU (1.1 equiv.) as the base. Polymer P1 was obtained as a white powder in a nearly quantitative yield (number-average molecular weight: Mn = 62 kDa; polydispersity index: Đ = 1.36) (Supplementary Section 7). To demonstrate that more complicated polymers could be made in this fashion, a tetramer with four different units was employed in which the reacting phenol was sterically hindered by an ortho-methyl moiety (tetramer D4, 10 mg). Even for such a polymerization, P2 was obtained as a yellow–white powder with a nearly quantitative yield (Mn = 28 kDa; Đ = 1.62) (Supplementary Section 7). To also demonstrate that sequence-controlled tail-to-head polymerization could proceed in an enantiospecific manner, an oxidized trimer (S, Racemic)-D5 was designed and utilized as the monomer (Fig. 7c). This retained only the chirality of the sulfur atom as the SuPhenEx reactive site, while intentionally making the other chiral sulfur atom, which was not involved in the reaction, racemic, to eliminate its influence on any chiroptical measurements. When G1 was exposed to a solution of KF in THF, the fluoride anion would attack in a racemizing fluoride exchange onto the end site of the sulfur centre. This racemization process was monitored by chiral HPLC and the results demonstrated that equilibrium could be reached after 2 h to obtain (S, Racemic)-J2 (chiral HPLC figures in Supplementary Section 7). After that, (S, Racemic)-J2 was substituted by 2a and subsequently treated with H2O2 to obtain D5 (15 mg, 11 µmol) with a yield of 48% over three steps. The polymer P3 was prepared under the same conditions as P1 and obtained as a white powder with a yield of 80% (Mn = 39 kDa; Đ = 1.6) (Supplementary Section 7). From the results of circular dichroism (CD) measurements (Fig. 8b), an obvious absorption peak opposite to that of D5 was observed, demonstrating that the head-to-tail polymerization indeed takes place with inversion of the configuration of the central S(VI) moiety.
a, Polymer P1 from D1. b, Polymer P2 from D4. c, Polymer (R, Racemic)-P3 from (S, Racemic)-D5. d, Polymer (R,R)-P4 from (S,S)-D6. Reaction conditions: (i) Oxidized monomer (D1, D4 or D5, 1 equiv.), DBU (1.1 equiv.), MeCN/DMF (1:1), 80 °C, 48 h; (ii) G1 (1 equiv.), KF (2.2 equiv.), 18-crown-6 (3.3 equiv.), THF, r.t., 2 h; (iii) J1 (1 equiv.), bisphenol A (5 equiv.), THF, r.t., 2 h; (iv) Monomers (J2 or K1, 1 equiv.), ammonium molybdate tetrahydrate (0.1 equiv.), 30% H2O2 (18 equiv.), THF, r.t., 2 h; (v) 1d (1 equiv.), 4-(methylthio)phenol (2.2 equiv.), LiBr (3 equiv.), DBU (2.2 equiv.), THF, r.t., 6 h; (vi) D6 (1 equiv.), 2a (1 equiv.), DBU (2.2 equiv.), MeCN/DMF, 80 °C, 2 days. SuPhenEx: sulfur–phenolate exchange reaction; THF, tetrahydrofuran; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DMF, N,N-dimethylformamide; tol, p-tolyl.
a, Ultraviolet–visible absorption spectra of D5 and polymer P3. b, CD spectra of D5 and polymer P3. c, Ultraviolet–visible absorption spectra of D6 and polymer P4. d, CD spectra of D6 and polymer P4. e, CD spectra of (S,S)-P4 (black line; data from ref. 40) and (R,R)-P4 (red line).
In the aforementioned head-to-tail chiral polymerization, only one sulfur atom served as the SuPhenEx reactive site involved in the polymerization, thereby inverting the configuration of only one sulfur(VI) centre during the polymerization. We next aimed to investigate the enantiospecificity of the polymerization with two sulfur atoms as SuPhenEx reactive sites to further increase the tunability of the polymerization. (S,S)-K1 (Fig. 7d) was synthesized by a SuFEx reaction of 1d (60 mg, 0.105 mmol, 1 equiv., >99% d.e.) and 4-(methylthio)phenol (33 mg, 0.235 mmol, 2.2 equiv.) and was obtained with a yield of 84% (72 mg, 0.089 mmol) and virtually unchanged >99% enantiomeric excess (e.e.). This shows a promising tendency that with the higher enantiopurity of starting materials, the high enantiopurity of the products could be obtained. After an oxidation reaction using H2O2, (S,S)-D6 (10 mg, 11 µmol, 1 equiv., >99% e.e.) reacted with 2a (3 mg, 13 µmol, 1.1 equiv.) to produce polymer P4 as a white powder (8 mg, 97%, Mn = 37 kDa; Đ = 1.6) (Supplementary Section 7). CD measurements (Fig. 8d), clearly show an absorption peak opposite to that of D6, demonstrating that the symmetric polymerization takes place with a double stereoinversion. It is worth noting that the combination of SuFEx and SuPhenEx, allows to obtain a chiral polymer and its enantiomeric polymer by using only one configuration of a chiral di-SF monomer (prepared by a four-step synthesis), which efficiently shortens the overall synthetic efforts. We combined the reported CD curve (S,S)-P4 (ref. 40) and our currently prepared (R,R)-P4 in one figure (Fig. 8e). These CD data show opposite absorption peaks, confirming the success of such an approach. Moreover, the success of this polymerization demonstrates that the SuPhenEx reaction also worked for the sulfonyl-linked phenol caps with other alkyl groups (here CH3), and is, as hypothesized, not limited to the perfluoro tag used above. Therefore this readily and greatly expands the series of potential substrates for caps on the oligomer chain. Moreover, chiral SuFEx-based polymers with control over the configuration of each S(VI) centre can be easily obtained in this manner, paving the way to polymer synthesis with excellent control over the stereocentres in sequence-controlled polymers.
Conclusions
A highly flexible synthetic strategy was developed for the stepwise iterative synthesis of oligosulfonimidates via SuFEx and SuPhenEx reactions. The whole sequence, including the cap, two types of building block and the configuration of each sulfur centre can be fine tuned in great detail, both regarding the sequence and the chirality of each sulfur atom. As a key feature we use here a perfluoro-tagged phenol as a dual-purpose cap: it not only greatly simplifies purification processes but also possesses dormant characteristics as it can be turned from a stable end cap to an excellent leaving group by a simple oxidation step that does not harm the rest of the oligomer. By leveraging this dormant feature, a sequence-defined thirteen-mer (MW = 4.50 kDa) was successfully synthesized through a convergent method and sequence-controlled sulfonimidate polymers were synthesized by a tail-to-head-type polymerization strategy. Finally, chiral oligosulfonimidates and polysulfonimidates with different configurations can be obtained by adjusting the configurations of the monomers and the method of polymerization. We expect that these synthetic methods will further pave the way for digital data storage and thus are a significant contribution to materials science41.
Methods
General procedure for purifying products by FSPE
Fluorous silica gel (40 g)29,30 was packed into a column and treated with 100 ml DMF, followed by 200 ml 80% MeOH/H2O. Subsequently, the concentrated reaction mixture (maximum 2–3 g per loading) was dissolved in 1–5 ml dichloromethane (DCM) and loaded onto the column. Initially, 80% MeOH/H2O was used to wash out non-fluorous tag compounds (for example monomers taken in excess). Subsequently THF or ACN were employed to wash out any fluorous-tagged compounds. If needed, the product was further purified by normal-phase flash chromatography.
General procedure for chain growth via reaction with a di-SF monomer
An oligomer with phenol-reactive sites (see details below) and DBU (1.5 equiv.) were placed in an oven-dried one-dram vial, dissolved in DCM or THF (see details in the Supplementary Information) and the reaction mixture was stirred for 10 min. Afterwards, the mixture was added with a syringe pump at a rate of 0.05 ml min−1 to another 15 ml glass vial containing the di-SF monomer (1a–c, 5 equiv.) in DCM. The reaction mixture was stirred for 30 min to 2 h and the reaction progress was monitored by TLC. Then, the mixture was quenched with water, the organic phase was extracted and the aqueous phase was further extracted with DCM three times. The resulting organic phases were combined and the solvent was evaporated under reduced pressure. The residue was purified by FSPE and the product was further purified by normal-phase flash chromatography.
General procedure for chain growth via reaction with a diphenol monomer
In an oven-dried one-dram vial, a bisphenol monomer (5 equiv.) and DBU (5 equiv.) were dissolved in THF and the reaction mixture was stirred for 10 min. Afterwards, the oligomer with an SF-reactive site (see details in the Supplementary Information) in THF was added with a syringe pump at a rate of 0.05 ml min−1. The reaction mixture was stirred for 30 min to 2 h and the reaction progress was monitored by TLC. After completion, the reaction mixture was evaporated under reduced pressure and the residue was purified by FSPE. The product was further purified by normal-phase flash chromatography.
General procedure for the oxidation of sulfur on the fluorous tag
In a one-dram vial, an oligomer containing a fluorous tag (A3, A5 or A7, 1 equiv., see details below) was dissolved in THF (0.17 M). Subsequently, ethanol (1/3 volume of THF) was added to the mixture, followed by the dropwise addition of ammonium molybdate tetrahydrate (0.1 equiv.) in 30% hydrogen peroxide (18 equiv.). The reaction mixture was stirred for 2 h and TLC was used to monitor the reaction progress. After that, water was added to quench the reaction mixture and DCM was used to extract the aqueous layer three times. The resulting organic layers were combined and evaporated under reduced pressure. Formed products (D1–3) were obtained as white solids and were further used without additional purification.
General procedure for the substitution of the fluorous tag with phenol
In an oven-dried one-dram vial, oxidized intermediate (D1–3, 1 equiv., see details in the Supplementary Information) and phenol (10 equiv.) were dissolved in THF (0.15 M). Subsequently, DBU (4 equiv.) in THF (0.5 M) was added to the reaction mixture. The reaction mixture was stirred at room temperature for 2 h and TLC was used to monitor the progress of the reaction. After that, the solvent was removed under reduced pressure. The crude product was purified by normal-phase flash chromatography to yield products E1–E3 as white solids.
General procedure for polymerization by SuPhenEx reactions
The polymerization was performed inside an argon-filled glovebox (MBRAUN MB 20 G-LMF gas purifier with H2O values of <0.1 ppm). In a 2 ml Biotage microwave glass vial equipped with a magnetic stir bar, the oxidized oligomer (D1, D4 or D5, 1 equiv.) was dissolved in a mixture of 0.05 ml anhydrous acetonitrile and 0.05 ml anhydrous DMF with vigorous stirring. Then, DBU (1.1 equiv.) was added and the reaction mixture was stirred at 80 °C for 48 h. After that, 0.1 ml DMF was added. The vial was shaken to facilitate dissolution and the resulting solution was slowly poured into 50 ml MeOH while continuously stirring. Precipitation occurred and stirring was continued for an additional 10 min. After allowing the formed precipitate to settle for 10 min, the methanol layer was removed and a minimal amount of DMF was added to redissolve the precipitate. This cycle of precipitation, sedimentation and dissolution was repeated four times. The resulting white powder/fibrous material was transferred into a 4 ml glass vial and dried at 50 °C under reduced pressure for a minimum of 12 h.
Data availability
All relevant data generated and analysed during this study, which include experimental and spectroscopic data, are included in this Article and its Supplementary Information.
References
Lutz, J.-F. Sequence-Controlled Polymers (Wiley-VCH, 2018).
Lutz, J.-F. Sequence-controlled polymerizations: the next Holy Grail in polymer science? Polym. Chem. 1, 55–62 (2010).
Ceze, L., Nivala, J. & Strauss, K. Molecular digital data storage using DNA. Nat. Rev. Genet. 20, 456–466 (2019).
Church, G. M., Gao, Y. & Kosuri, S. Next-generation digital information storage in DNA. Science 337, 1628 (2012).
Prathap, K. J. & Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 51, 11096–11099 (2015).
Mendes, A. C., Baran, E. T., Reis, R. L. & Azevedo, H. S. Self‐assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 5, 582–612 (2013).
Nam, K. T. et al. Free-floating ultrathin two-dimensional crystals from sequence-specific peptoid polymers. Nat. Mater. 9, 454–460 (2010).
Sun, J. et al. Self-assembly of crystalline nanotubes from monodisperse amphiphilic diblock copolypeptoid tiles. Proc. Natl Acad. Sci. USA 113, 3954–3959 (2016).
Singh, Y., Dolphin, G. T., Razkin, J. & Dumy, P. Synthetic peptide templates for molecular recognition: recent advances and applications. ChemBioChem. 7, 1298–1314 (2006).
Shi, Q., Deng, Z., Hou, M., Hu, X. & Liu, S. Engineering precise sequence-defined polymers for advanced functions. Prog. Polym. Sci. 141, 101677 (2023).
Röder, B., Frühwirth, K., Vogl, C., Wagner, M. & Rossmanith, P. Impact of long-term storage on stability of standard DNA for nucleic acid-based methods. J. Clin. Microbiol. 48, 4260–4262 (2010).
Yu, L. et al. Digital synthetic polymers for information storage. Chem. Soc. Rev. 52, 1529–1548 (2023).
Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149–2154 (1963).
Barnes, J. C. et al. Iterative exponential growth of stereo- and sequence-controlled polymers. Nat. Chem. 7, 810–815 (2015).
He, W., Wang, S., Li, M., Wang, X. & Tao, Y. Iterative synthesis of stereo‐ and sequence‐defined polymers via acid‐orthogonal deprotection chemistry. Angew. Chem. 134, e202112439 (2022).
Kolb, H. C., Finn, M. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Geng, Z., Shin, J. J., Xi, Y. & Hawker, C. J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 59, 963–1042 (2021).
Ghosal, K., Bhattacharyya, S. K., Mishra, V. & Zuilhof, H. Click chemistry for biofunctional polymers: from observing to steering cell behavior. Chem. Rev. 124, 13216–13300 (2024).
Pfeifer, S., Zarafshani, Z., Badi, N. & Lutz, J.-F. Liquid-phase synthesis of block copolymers containing sequence-ordered segments. J. Am. Chem. 131, 9195–9197 (2009).
Yang, C., Flynn, J. P. & Niu, J. Facile synthesis of sequence‐regulated synthetic polymers using orthogonal SuFEx and CuAAC click reactions. Angew. Chem. Int. Ed. 57, 16194–16199 (2018).
Li, Z. et al. Protecting-group-free iterative exponential growth method for synthesizing sequence-defined polymers. ACS Macro Lett. 10, 223–230 (2021).
Huang, Z. et al. Discrete and stereospecific oligomers prepared by sequential and alternating single unit monomer insertion. J. Am. Chem. Soc. 140, 13392–13406 (2018).
Mertens, C. et al. Stereocontrolled, multi-functional sequence-defined oligomers through automated synthesis. Polym. Chem. 11, 4271–4280 (2020).
Liang, D. D. et al. Silicon‐free SuFEx reactions of sulfonimidoyl fluorides: scope, enantioselectivity, and mechanism. Angew. Chem. 132, 7564–7570 (2020).
Rowan, S. J., Cantrill, S. J., Cousins, G. R., Sanders, J. K. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Chao, Y. et al. Sulfur–phenolate exchange: SuFEx‐derived dynamic covalent reactions and degradation of SuFEx polymers. Angew. Chem. Int. Ed. 61, e202207456 (2022).
Kanasty, R. L. et al. Sequence‐defined oligomers from hydroxyproline building blocks for parallel synthesis applications. Angew. Chem. Int. Ed. 55, 9529–9533 (2016).
Porel, M. & Alabi, C. A. Sequence-defined polymers via orthogonal allyl acrylamide building blocks. J. Am. Chem. Soc. 136, 13162–13165 (2014).
Porel, M., Thornlow, D. N., Phan, N. N. & Alabi, C. A. Sequence-defined bioactive macrocycles via an acid-catalysed cascade reaction. Nat. Chem. 8, 590–596 (2016).
Curran, D. P. Fluorous reverse phase silia gel. A new tool for preparative separations in synthetic organic and organofluorine chemistry. Synlett. 9, 1488–1496 (2001).
Curran, D. P., Hadida, S. & He, M. Thermal allylations of aldehydes with a fluorous allylstannane. Separation of organic and fluorous products by solid phase extraction with fluorous reverse phase silica gel. J. Org. Chem. 62, 6714–6715 (1997).
Aksakal, R., Mertens, C., Soete, M., Badi, N. & Du Prez, F. Applications of discrete synthetic macromolecules in life and materials science: recent and future trends. Adv. Sci. 8, 2004038 (2021).
Yang, C. et al. Tetra-(tetraalkylammonium) octamolybdate catalysts for selective oxidation of sulfides to sulfoxides with hydrogen peroxide. Green Chem. 11, 1401–1405 (2009).
Herle, B., Holstein, P. M. & Echavarren, A. M. Stereoselective cis-vinylcyclopropanation via a gold(I)-catalyzed retro-Buchner reaction under mild conditions. ACS Catal. 7, 3668–3675 (2017).
Van den Boom, A. F., Subramaniam, M. & Zuilhof, H. Sulfur-phenolate exchange as a fluorine-free approach to S(VI) exchange chemistry on sulfonyl moieties. Org. Lett. 24, 8621–8626 (2022).
Van den Boom, A. F. & Zuilhof, H. Sulfur-phenolate exchange as a mild, fast, and high-yielding method toward the synthesis of sulfonamides. Org. Lett. 25, 788–793 (2023).
Hansch, C., Leo, A. & Taft, R. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Kim, M. P. et al. Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers. Nat. Commun. 15, 3381 (2024).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Liang, D. D. et al. Configurationally chiral SuFEx‐based polymers. Angew. Chem. Int. Ed. 61, e202116158 (2022).
Yang, C., Wu, K. B., Deng, Y., Yuan, J. & Niu, J. Geared toward applications: a perspective on functional sequence-controlled polymers. ACS Macro Lett. 10, 243–257 (2021).
Acknowledgements
We thank Y. Ma (Hunan Normal University), K. Namitharan, S. Kouwenberg and A. van Haandel (Wageningen University) for stimulating interactions. We acknowledge the Chinese Scholarship Council (CSC; scholarship to Y.H.), Wageningen University and the Top-Talent Program of Zhejiang Province (Jiaxing University to H.Z.) for funding.
Author information
Authors and Affiliations
Contributions
F.M.M. and H.Z. conceived and designed the experiments. B.C., F.M.M. and H.Z. supervised the project. Y.H. designed and performed all small molecule syntheses and was helped by M.S. for some monomer syntheses. S.P.P. performed the polymerizations, X-ray photoelectron spectroscopy, gel permeation chromatography and CD measurements. H.Z. performed the theoretical calculations. Y.H., F.M.M. and H.Z. analysed all the data. Y.H. wrote the first draft of the manuscript and F.M.M. and H.Z. revised the manuscript. All authors discussed the results and commented on and agreed with the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Sung You Hong, Hannes Houck and Youhua Tao for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–13, Figs. 1–109 and experimental details.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Y., Pujari, S.P., Subramaniam, M. et al. Synthesis of chiral sequence-defined oligomers via sulfur–fluoride and sulfur–phenolate exchange reactions. Nat. Synth 4, 1106–1117 (2025). https://doi.org/10.1038/s44160-025-00805-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00805-8
This article is cited by
-
Unravelling emergence of chirality in click-chemistry polymers down to the single-chain level
Nature Communications (2025)