Abstract
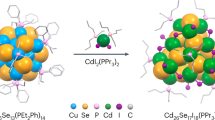
Precision engineering of semiconductor nanomaterials remains difficult. Here we present a programmable approach to synthesizing a library of atomically precise semiconductor nanoclusters via cation exchange. Using a universal metal–ligand coordination complex to program cations and surface ligands, and a Cu26Se13 template cluster to control the anion lattice, we synthesize a homologous series of Zn14Se13, Cd14Se13 and Hg14Se13 clusters, all featuring a pair of prominent absorption peaks. The shared A14B13 framework with icosahedral anion packing and tetrahedral cation bonding serves as a blueprint for constructing chiral semiconductor nanostructures. Theoretical calculations reveal atom-like frontier orbitals, including triply degenerate P-type orbitals for holes and singlet S-type orbitals for electrons. The doublet absorptions originate from the spin–orbit splitting of the P → S transition. The precise and programmable synthesis is expected to enable atomic-level control over the optical, electronic and spin properties of semiconductor artificial atoms and their assemblies.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2345658 (1), 2345659 (2), 2345655 (3), 2345652 (4), 2345653 (5), 2345657 (6), 2345656 (7) and 2345654 (8). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/. All other relevant data generated and analysed during this study, which include experimental, spectroscopic, crystallographic and computational data, are included in this article and its Supplementary Information.
References
Efros, A. L. & Brus, L. E. Nanocrystal quantum dots: from discovery to modern development. ACS Nano 15, 6192–6210 (2021).
Murray, C. B., Norris, D. J. & Bawendi, M. G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Shevchenko, E. V., Talapin, D. V., Kotov, N. A., O’Brien, S. & Murray, C. B. Structural diversity in binary nanoparticle superlattices. Nature 439, 55–59 (2006).
Boles, M. A., Engel, M. & Talapin, D. V. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 116, 11220–11289 (2016).
Kagan, C. R., Lifshitz, E., Sargent, E. H. & Talapin, D. V. Building devices from colloidal quantum dots. Science 353, 885–892 (2016).
Kagan, C. R., Bassett, L. C., Murray, C. B. & Thompson, S. M. Colloidal quantum dots as platforms for quantum information science. Chem. Rev. 121, 3186–3233 (2021).
Hanrath, T. Colloidal nanocrystal quantum dot assemblies as artificial solids. J. Vac. Sci. Technol. A 30, 030802 (2012).
Boneschanscher, M. P. et al. Long-range orientation and atomic attachment of nanocrystals in 2D honeycomb superlattices. Science 344, 1377–1380 (2014).
Rainò, G. et al. Superfluorescence from lead halide perovskite quantum dot superlattices. Nature 563, 671–675 (2018).
Bloom, B. P., Graff, B. M., Ghosh, S., Beratan, D. N. & Waldeck, D. H. Chirality control of electron transfer in quantum dot assemblies. J. Am. Chem. Soc. 139, 9038–9043 (2017).
Abelson, A. et al. Collective topo-epitaxy in the self-assembly of a 3D quantum dot superlattice. Nat. Mater. 19, 49–55 (2020).
Kagan, C. R. & Murray, C. B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 10, 1013–1026 (2015).
Kim, Y.-H. et al. Chiral-induced spin selectivity enables a room-temperature spin light-emitting diode. Science 371, 1129–1133 (2021).
Ugras, T. J. et al. Transforming achiral semiconductors into chiral domains with exceptional circular dichroism. Science 387, eado7201 (2025).
Zeng, C., Chen, Y., Kirschbaum, K., Lambright, K. J. & Jin, R. Emergence of hierarchical structural complexities in nanoparticles and their assembly. Science 354, 1580–1584 (2016).
Owen, J. The coordination chemistry of nanocrystal surfaces. Science 347, 615–616 (2015).
Yin, Y. & Alivisatos, A. P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437, 664–670 (2005).
Herron, N., Calabrese, J. C., Farneth, W. E. & Wang, Y. Crystal structure and optical properties of Cd32S14(SC6H5)36·DMF4, a cluster with a 15 angstrom CdS core. Science 259, 1426–1428 (1993).
Vossmeyer, T. et al. A ‘double-diamond superlattice’ built up of Cd17S4(SCH2CH2OH)26 clusters. Science 267, 1476–1479 (1995).
Zheng, N., Bu, X., Lu, H., Zhang, Q. & Feng, P. Crystalline superlattices from single-sized quantum dots. J. Am. Chem. Soc. 127, 11963–11965 (2005).
Soloviev, V. N., Eichhöfer, A., Fenske, D. & Banin, U. Size-dependent optical spectroscopy of a homologous series of CdSe cluster molecules. J. Am. Chem. Soc. 123, 2354–2364 (2001).
Zhang, J., Bu, X., Feng, P. & Wu, T. Metal chalcogenide supertetrahedral clusters: synthetic control over assembly, dispersibility, and their functional applications. Acc. Chem. Res. 53, 2261–2272 (2020).
Zhang, J., Feng, P., Bu, X. & Wu, T. Atomically precise metal chalcogenide supertetrahedral clusters: frameworks to molecules, and structure to function. Natl Sci. Rev. 9, nwab076 (2022).
Gary, D. C. et al. Single-crystal and electronic structure of a 1.3 nm indium phosphide nanocluster. J. Am. Chem. Soc. 138, 1510–1513 (2016).
Sandeno, S. F. et al. Ligand steric profile tunes the reactivity of indium phosphide clusters. J. Am. Chem. Soc. 146, 3102–3113 (2024).
Sandeno, S. F. et al. Synthesis and single crystal X-ray diffraction structure of an indium arsenide nanocluster. ACS Cent. Sci. 10, 744–751 (2024).
Smith, A. M., Lane, L. A. & Nie, S. Mapping the spatial distribution of charge carriers in quantum-confined heterostructures. Nat. Commun. 5, 4506 (2014).
Ithurria, S. et al. Colloidal nanoplatelets with two-dimensional electronic structure. Nat. Mater. 10, 936–941 (2011).
Ekimov, A. I. et al. Absorption and intensity-dependent photoluminescence measurements on CdSe quantum dots: assignment of the first electronic transitions. J. Opt. Soc. Am. B 10, 100–107 (1993).
Del Ben, M., Havenith, R. W. A., Broer, R. & Stener, M. Density functional study on the morphology and photoabsorption of CdSe nanoclusters. J. Phys. Chem. C 115, 16782–16796 (2011).
Voznyy, O., Mokkath, J. H., Jain, A., Sargent, E. H. & Schwingenschlögl, U. Computational study of magic-size CdSe clusters with complementary passivation by carboxylic and amine ligands. J. Phys. Chem. C 120, 10015–10019 (2016).
Ma, F., Abboud, K. A. & Zeng, C. Precision synthesis of a CdSe semiconductor nanocluster via cation exchange. Nat. Synth. 2, 949–959 (2023).
Son, D. H., Hughes, S. M., Yin, Y. & Alivisatos, A. P. Cation exchange reactions in ionic nanocrystals. Science 306, 1009–1012 (2004).
De Trizio, L. & Manna, L. Forging colloidal nanostructures via cation exchange reactions. Chem. Rev. 116, 10852–10887 (2016).
Fenton, J. L., Steimle, B. C. & Schaak, R. E. Tunable intraparticle frameworks for creating complex heterostructured nanoparticle libraries. Science 360, 513–517 (2018).
Htoon, S. & Ladd, M. F. C. Crystallographic studies of dihalogenotetramethylethylenediamine complexes of zinc, cadmium, and mercury. IV: general crystallography and molecular geometry. J. Cryst. Mol. Struct. 6, 55–58 (1976).
Pearson, R. G. Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem. 27, 734–740 (1988).
Paoletti, P. Formation of metal complexes with ethylenediamine: a critical survey of equilibrium constants, enthalpy and entropy values. Pure Appl. Chem. 56, 491–522 (1984).
Deveson, A., Dehnen, S. & Fenske, D. Syntheses and structures of four new copper(I)–selenium clusters: size dependence of the cluster on the reaction conditions. J. Chem. Soc. Dalton Trans. 4491–4498 (1997).
Bootharaju, M. S. et al. Structure of a subnanometer-sized semiconductor Cd14Se13 cluster. Chem 8, 2978–2989 (2022).
Wang, Y. et al. Isolation of the magic-size CdSe nanoclusters [(CdSe)13(n-octylamine)13] and [(CdSe)13(oleylamine)13]. Angew. Chem. Int. Ed. 51, 6154–6157 (2012).
Gréboval, C. et al. Mercury chalcogenide quantum dots: material perspective for device integration. Chem. Rev. 121, 3627–3700 (2021).
Taniguchi, S., Green, M. & Lim, T. The room-temperature synthesis of anisotropic CdHgTe quantum dot alloys: a “molecular welding” effect. J. Am. Chem. Soc. 133, 3328–3331 (2011).
Lee, W. & Smith, A. M. Interdiffusion-enhanced cation exchange for HgSe and HgCdSe nanocrystals with infrared bandgaps. Nat. Synth. 3, 1243–1254 (2024).
Dean, J. A. Lange’s Handbook of Chemistry 15th edn (McGraw-Hill, 1999).
Khadka, C. B., Eichhöfer, A., Weigend, F. & Corrigan, J. F. Zinc chalcogenolate complexes as precursors to ZnE and Mn/ZnE (E = S, Se) clusters. Inorg. Chem. 51, 2747–2756 (2012).
Pasteur, L. Recherches sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et les sens de la polarisation rotatoire. Ann. Chim. Phys. Sér. 24, 442–459 (1848).
Gal, J. Pasteur and the art of chirality. Nat. Chem. 9, 604–605 (2017).
Walsh, M. P., Barclay, J. A., Begg, C. S., Xuan, J. & Kitching, M. O. Conglomerate crystallization in the Cambridge Structural Database (2020–2021). Cryst. Growth Des. 23, 2837–2844 (2023).
Walsh, M. P. et al. Identifying a hidden conglomerate chiral pool in the CSD. JACS Au 2, 2235–2250 (2022).
Williamson, C. B. et al. Chemically reversible isomerization of inorganic clusters. Science 363, 731–735 (2019).
Zhu, M., Aikens, C. M., Hollander, F. J., Schatz, G. C. & Jin, R. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 130, 5883–5885 (2008).
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Zeng, C. Precision at the nanoscale: on the structure and property evolution of gold nanoclusters. Pure Appl. Chem. 90, 1409–1428 (2018).
Gawlik, K.-U., Kipp, L., Skibowski, M., Orłowski, N. & Manzke, R. HgSe: metal or semiconductor? Phys. Rev. Lett. 78, 3165–3168 (1997).
Madelung, O. in Semiconductors: Data Handbook (ed. Madelung, O.) 173–244 (Springer, 2004).
Zhou, Y. et al. Isolation of amine derivatives of (ZnSe)34 and (CdTe)34. Spectroscopic comparisons of the (II–VI)13 and (II–VI)34 magic-size nanoclusters. Inorg. Chem. 58, 1815–1825 (2019).
Wang, Y., Zhou, Y., Zhang, Y. & Buhro, W. E. Magic-size II–VI nanoclusters as synthons for flat colloidal nanocrystals. Inorg. Chem. 54, 1165–1177 (2015).
Heath, R. J. Covalency in semiconductor quantum dots. Chem. Soc. Rev. 27, 65–71 (1998).
Alivisatos, A. P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. B 100, 13226–13239 (1996).
Hoffmann, R. How chemistry and physics meet in the solid state. Angew. Chem. Int. Ed. 26, 846–878 (1987).
Ekimov, A. I. & Onushchenko, A. A. Quantum size effect in three-dimensional microscopic semiconductor crystals. JETP Lett. 6, 345–349 (1981).
Cardona, M. Fundamental reflectivity spectrum of semiconductors with zinc-blende structure. J. Appl. Phys. 32, 2151–2155 (1961).
Kasuya, A. et al. Ultra-stable nanoparticles of CdSe revealed from mass spectrometry. Nat. Mater. 3, 99–102 (2004).
Htoon, S. & Ladd, M. F. C. Diiodo-(N,N,N′,N′-tetramethylethylenediamine)zinc(II), C6H16I2N2Zn. J. Cryst. Mol. Struct. 4, 357–360 (1974).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Pollak, P. & Weigend, F. Segmented contracted error-consistent basis sets of double- and triple-ζ valence quality for one- and two-component relativistic all-electron calculations. J. Chem. Theory Comput. 13, 3696–3705 (2017).
Zheng, J., Xu, X. & Truhlar, D. G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 128, 295–305 (2011).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Perdew, J. P., Burke, K. & Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 54, 16533–16539 (1996).
Acknowledgements
We thank the University of Florida start-up research fund for supporting the experimental work. We thank the National Science Foundation for funding the X-ray diffractometer through grant number CHE-1828064. Theoretical work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science and Los Alamos National Laboratory Institutional Computing Program. Los Alamos National Laboratory, an affirmative action equal opportunity employer, is managed by Triad National Security, LLC for the US Department of Energy’s NNSA, under contract 89233218CNA000001.
Author information
Authors and Affiliations
Contributions
F.M., S.A.I. and C.Z. conceived of the idea. F.M. performed the synthesis and characterization. F.M. and L.M.D. solved the crystal structures. S.A.I. performed theoretical analysis involving the synthesized structures and ESI-MS characterization. F.M., S.A.I. and C.Z. wrote the paper. C.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
C.Z. and F.M. declare a pending patent application on the precision synthesis of semiconductor nanoclusters via cation exchange (US2430202). S.A.I. and L.M.D. declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Paul Raithby, Tao Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of three MI2(tmeda) complexes (M = Zn, Cd, Hg).
a, 1H NMR of the free tmeda ligand and three complexes in CDCl3. The satellite peaks in CdI2(tmeda) correspond to the coupling between 1H and 113Cd (J = 4 Hz). b, TGA measurements of the complexes.
Extended Data Fig. 2 Synthesis of II–VI nanoclusters from Cu26Se13 template cluster and MI2(tmeda) cation carriers (M = Zn, Cd, Hg).
a, Reaction with ZnI2(tmeda). b, Reaction with CdI2(tmeda). Excess PPr3 was added to increase complex solubility in toluene. c, Reaction with HgI2. Excess tmeda was added to stabilize the HgSe cluster. Left: Photos showing initial colour changes upon injection of the Cu26Se13 cluster solution (0.1 mL) into the cation solution (0.9 mL). Right: In situ UV-Vis spectroscopy monitoring of the diluted reaction mixture. The absorption spectra were recorded every 10 minutes in the first two hours and every hour for the next 14 hours. The first scan was measured around two minutes after mixing the reagents.
Extended Data Fig. 3 Crystal structures of resulting II–VI clusters and their packing in unit cells.
a, [Zn14Se13(tmeda)6]2+ cluster with co-crystallized [BPh4]‒ counterions and CH2Cl2 solvent molecules. Each unit cell contains two clusters and four counterions with balanced charges. b, Cd14Se13(tmeda)6I2 cluster co-crystallized with one CdI2(tmeda) complex and several toluene molecules. c, Hg14Se13(tmeda)6I2 cluster. Top: individual cluster; middle: top view of unit cell; bottom: side view of unit cell. The cations are drawn in space-filling mode to highlight the orientation of clusters in unit cells. The dark and light green colours in (b) indicate the left and right Cd14Se13 isomers.
Extended Data Fig. 4 Comparison of Se13 anion lattices of the template cluster and three product clusters.
Top: peripheral Se–Se distances in the icosahedron; middle: radial Se–Se distances in the icosahedron; bottom: tabulated Se–Se distances in clusters and bulk lattices.
Extended Data Fig. 5 Average metal-selenium and metal–ligand distances in the product clusters 1-3.
Top: Scheme for cation positions in A14B13 skeleton; bottom: tabulated M–Se and M–L distances of clusters and bulk lattices. The distance between non-bonded atoms is shown in grey.
Extended Data Fig. 6 Solvent-induced structural variations of Cd14Se13 and Zn14Se13 clusters and their UV-vis spectra comparison.
a, Crystal structures of [Zn14Se13(tmeda)6(CH3CN)]2+ (7) crystallized from CH3CN. b, Crystal structures of Cd14Se13(tmeda)6I2 cluster (8) synthesized from CH2Cl2. c–e, Comparison of absorption spectra of Zn14Se13 (c), Cd14Se13 (d), and Hg14Se13 (e) clusters.
Extended Data Fig. 7 A14B13 skeleton as a blueprint for constructing semiconductor nanoclusters.
a, Packing of anions in the bulk face-centred cubic (FCC) lattice and in the icosahedral cluster lattice. b, Bonding of cations in the zincblende lattice and in the A14B13 cluster. c, A14B13 skeleton identified in reported structures. Top: inorganic core oriented along a Cn rotation axis. Middle: B13 icosahedral anion framework highlighted with shading; Bottom: A14 cation coordination shown in space-filling mode. The clusters are reoriented to visualize similar cation positions. Redrawn from the CIF files of the references24,25,26,32.
Extended Data Fig. 8 Visualizing the wavefunctions of II14VI13 artificial atoms via Kohn–Sham orbital analysis.
a, [Zn14Se13(tmeda)6]2+. b, Hg14Se13I2(tmeda)6.
Extended Data Fig. 9 Comparison of calculated TDDFT spectra of clusters 1-3.
a, Without spin–orbit coupling. b, With spin–orbit coupling.
Extended Data Fig. 10 TDDFT structures and spectra.
Comparison of TDDFT structures and spectra of a series of CdSe clusters modified based on Cd14Se13(tmeda)6I2.
Supplementary information
Supplementary Video 1
Kohn–Sham orbitals of [Zn14Se13(tmeda)6]2+ (1).
Supplementary Video 2
Kohn–Sham orbitals of Cd14Se13(tmeda)6I2 (2).
Supplementary Video 3
Kohn–Sham orbitals of Hg14Se13(tmeda)6I2 (3).
Supplementary Data 1
[Zn14Se13(tmeda)6][BPh4]2 (1).
Supplementary Data 2
Cd14Se13(tmeda)6I2 (2).
Supplementary Data 3
Hg14Se13(tmeda)6I2 (3).
Supplementary Data 4
Zn14Se13(tmeda)6Cl2 (4).
Supplementary Data 5
[Cd14Se13(tmeda)6Cl2]n (5).
Supplementary Data 6
[Hg14Se13(tmeda)6Cl2]n (6).
Supplementary Data 7
[Zn14Se13(tmeda)6(CH3CN)][BPh4]2 (7).
Supplementary Data 8
Cd14Se13(tmeda)6I8×0.25 (8).
Source data
Source Data Fig. 1
Source data for UV-vis spectra of clusters in reaction mixture and crystal solution.
Source Data Fig. 6
Source data for energy plots.
Source Data Fig. 7
Source data for energy levels and theoretical spectra.
Source Data Extended Data Fig./Table 1
Source data for NMR and TGA of MI2(tmeda) complexes.
Source Data Extended Data Fig./Table 2
Source data for in situ UV-vis spectra of cation exchange.
Source Data Extended Data Fig./Table 6
Source data for UV-vis spectra of eight clusters.
Source Data Extended Data Fig./Table 9
Source data for theoretical spectrum comparison.
Source Data Extended Data Fig./Table 10
Source data for theoretical spectra of various CdSe clusters.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, F., Ivanov, S.A., Dobrzycki, L.M. et al. Programmable synthesis of atomically precise semiconductor artificial atoms. Nat. Synth 4, 1258–1269 (2025). https://doi.org/10.1038/s44160-025-00823-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00823-6