Abstract
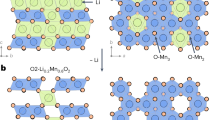
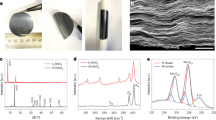
When nanoclusters crystallize, weak supramolecular interactions lead to the formation of independent monomers (zero-dimensional molecular nodes), while strong intermolecular coordination promotes omnidirectional crystal packing into three-dimensional superstructures. However, achieving two-dimensional (2D) crystalline assembly, such as 2D grid networks, is more challenging. Here we achieve the 2D crystalline assembly of atomically precise metal nanoclusters by establishing intermolecular interactions between the peripheral carboxyl groups of cluster-based nodes and introduced alkali metal cations, forming an alkali metal-dependent and solvent-dependent 2D crystalline assembly. We also observe that the intercluster or interlayer alkali metal cations are mobile, allowing the materials to serve as cation sponges. The high ionic mobility of the cluster-based crystalline materials renders them efficient electrolytes for Li-ion batteries. Overall, these 2D assemblies of atomically precise crystalline nanoclusters provide a versatile platform for ion conduction and energy transmission.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are included within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2370566 (Ag29-PPh3), 2370567 (Ag29-dimer), 2370568 (Ag29-Li), 2370781 (Ag29-COOLi-DMF), 2106345 (Ag29-COONa-DMF), 2370782 (Ag29-COOK-DMF), 2370587 (Ag29-COORb-DMF), 2370586 (Ag29-COOCs-DMF), 2370588 (Ag29-COOLi-MeOH), 2370591 (Ag29-COONa-MeOH), 2370592 (Ag29-COOK-MeOH), 2370593 (Ag29-COORb-MeOH) and 2370590 (Ag29-COOCs-MeOH). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Pepinsky, R. Crystal engineering: new concept in crystallography. Phys. Rev. 100, 971 (1955).
Desiraju, G. R. Crystal Engineering: The Design Of Organic Solids. (Elsevier, 1989).
Disa, A. S., Nova, T. F. & Cavalleri, A. Engineering crystal structures with light. Nat. Phys. 17, 1087–1092 (2021).
Mukherjee, A., Tothadi, S. & Desiraju, G. R. Halogen bonds in crystal engineering: like hydrogen bonds yet different. Acc. Chem. Res. 47, 2514–2524 (2014).
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Aakeroy, C. B. & Seddon, K. R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 22, 397–407 (1993).
Moulton, B. & Zaworotko, M. J. From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem. Rev. 101, 1629–1658 (2001).
Braga, D., Grepioni, F. & Desiraju, G. R. Crystal engineering and organometallic architecture. Chem. Rev. 98, 1375–1405 (1998).
Yao, Q. et al. Counterion-assisted shaping of nanocluster supracrystals. Angew. Chem. Int. Ed. 54, 184–189 (2015).
Alhilaly, M. J. et al. Assembly of atomically precise silver nanoclusters into nanocluster-based frameworks. J. Am. Chem. Soc. 141, 9585–9592 (2019).
Wei, X. et al. Hierarchical structural complexity in atomically precise nanocluster frameworks. Natl Sci. Rev. 8, nwaa077 (2021).
Nonappa & Ikkala, O. Hydrogen bonding directed colloidal self-assembly of nanoparticles into 2D crystals, capsids, and supracolloidal assemblies. Adv. Funct. Mater. 28, 1704328 (2018).
Deng, G., Teo, B. K. & Zheng, N. Assembly of chiral cluster-based metal–organic frameworks and the chirality memory effect during their disassembly. J. Am. Chem. Soc. 143, 10214–10220 (2021).
Nakatani, R. et al. Unlocking the potential of an atom-precise Ag12 cluster assembled material as a highly efficient SERS sensor for the detection of Hg2+ ions. ACS Mater. Lett. 6, 438–445 (2024).
Li, H. et al. Optical activity from anisotropic-nanocluster-assembled supercrystals in achiral crystallographic point groups. J. Am. Chem. Soc. 144, 4845–4852 (2022).
Manzeli, S. et al. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2, 17033 (2017).
Matus, M. F. & Häkkinen, H. Understanding ligand-protected noble metal nanoclusters at work. Nat. Rev. Mater. 8, 372–389 (2023).
Chakraborty, I. & Pradeep, T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271 (2017).
Kenzler, S. & Schnepf, A. Metalloid gold clusters – past, current and future aspects. Chem. Sci. 12, 3116–3129 (2021).
Shi, W.-Q. et al. Near-unity NIR phosphorescent quantum yield from a room-temperature solvated metal nanocluster. Science 383, 326–330 (2024).
Albright, E. L. et al. N-Heterocyclic carbene-stabilized atomically precise metal nanoclusters. J. Am. Chem. Soc. 146, 5759–5780 (2024).
Zhao, J. et al. Anchoring of metal complexes on Au25 nanocluster for enhanced photocoupled electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 63, e202316649 (2024).
Shen, H. et al. N-Heterocyclic carbene-stabilized gold nanoclusters with organometallic motifs for promoting catalysis. J. Am. Chem. Soc. 144, 10844–10853 (2022).
Zhao, H. et al. Assembly of air-stable copper(I) alkynide nanoclusters assisted by tripodal polydentate phosphoramide ligands. Nat. Synth. 3, 517–526 (2024).
Kang, X. & Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 48, 2422–2457 (2019).
Wang, X. et al. Ligand-protected metal nanoclusters as low-loss, highly polarized emitters for optical waveguides. Science 381, 784–790 (2023).
Li, H. et al. Fluorescence resonance energy transfer in atomically precise metal nanoclusters by cocrystallization-induced spatial confinement. Nat. Commun. 15, 5351 (2024).
Huang, R.-W. et al. Tandem silver cluster isomerism and mixed linkers to modulate the photoluminescence of cluster-assembled materials. Angew. Chem. Int. Ed. 57, 8560–8566 (2018).
Hou, Y. et al. Synergistic multiple bonds induced dynamic self-assembly of silver nanoclusters into lamellar frameworks with tailored luminescence. Chem. Mater. 34, 8013–8021 (2022).
Kang, X. & Zhu, M. Intra-cluster growth meets inter-cluster assembly: the molecular and supramolecular chemistry of atomically precise nanoclusters. Coord. Chem. Rev. 394, 1–38 (2019).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Tan, C. et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117, 6225–6331 (2017).
Novoselov, K. S. et al. 2D materials and van der Waals heterostructures. Science 353, aac9439 (2016).
Huang, B. et al. Recycling of lithium-ion batteries: recent advances and perspectives. J. Power Sources 399, 274–286 (2018).
Zhang, C. et al. Anion-sorbent composite separators for high-rate lithium-ion batteries. Adv. Mater. 31, 1808338 (2019).
Li, J. et al. From materials to cell: state-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 122, 903–956 (2022).
Degen, F. et al. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 8, 1284–1295 (2023).
AbdulHalim, L. G. et al. Ag29(BDT)12(TPP)4: a tetravalent nanocluster. J. Am. Chem. Soc. 137, 11970–11975 (2015).
Furukawa, H. et al. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Yuan, S. et al. Stable metal–organic frameworks: design, synthesis, and applications. Adv. Mater. 30, 1704303 (2018).
Lu, W. et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 43, 5561–5593 (2014).
Xie, X.-Y. et al. The origin of the photoluminescence enhancement of gold-doped silver nanoclusters: the importance of relativistic effects and heteronuclear gold–silver bonds. Angew. Chem. Int. Ed. 57, 9965–9969 (2018).
Zhang, J. et al. Evolution of self-assembled ZnTe magic-sized nanoclusters. J. Am. Chem. Soc. 137, 742–749 (2015).
Döllefeld, H., Weller, H. & Eychmüller, A. Semiconductor nanocrystal assemblies: experimental pitfalls and a simple model of particle−particle interaction. J. Phys. Chem. B 106, 5604–5608 (2002).
Dong, X.-Y. et al. A flexible fluorescent SCC-MOF for switchable molecule identification and temperature display. Chem. Mater. 30, 2160–2167 (2018).
Fang, H. & Jena, P. Kinetic effects of anion clusters on the interfacial stability between solid-state electrolyte and metal anode. Phys. Rev. X 2, 043013 (2023).
Fang, H. & Jena, P. Argyrodite-type advanced lithium conductors and transport mechanisms beyond paddle-wheel effect. Nat. Commun. 13, 2078 (2022).
Jena, P., Fang, H. & Sun, Q. Cluster-based materials for energy harvesting and storage. In Superatoms: Principles, Synthesis and Applications (eds Jena, P. & Sun, Q.) 277–316 (John Wiley & Sons, 2022).
Kim, S. et al. A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 10, 1081 (2019).
Huo, H. et al. A flexible electron-blocking interfacial shield for dendrite-free solid lithium metal batteries. Nat. Commun. 12, 176 (2021).
Lee, D. et al. Shear force effect of the dry process on cathode contact coverage in all-solid-state batteries. Nat. Commun. 15, 4763 (2024).
Bruce, P. G., Evans, J. & Vincent, C. A. Conductivity and transference number measurements on polymer electrolytes. Solid State Ion. 28, 918–922 (1988).
Xu, J. et al. High-energy lithium-ion batteries: recent progress and a promising future in applications. Energy Environ. Mater. 6, e12450 (2023).
Leverick, G. & Shao-Horn, Y. Controlling electrolyte properties and redox reactions using solvation and implications in battery functions: a mini-review. Adv. Energy Mater. 13, 2204094 (2023).
Wang, Y. et al. Fluorine chemistry in rechargeable batteries: challenges, progress, and perspectives. Chem. Rev. 124, 3494–3589 (2024).
Sankar, T. K., Abhilash & Meshram, P. Environmental impact assessment in the entire life cycle of lithium‑ion batteries. Rev. Environ. Contam. Toxicol. 262, 5 (2024).
Acknowledgements
We acknowledge the financial support of the NSFC (22371003, 22101001, 22471001, U24A20480 and 22473017), the Ministry of Education, Natural Science Foundation of Anhui Province (2408085Y006), the University Synergy Innovation Program of Anhui Province (GXXT-2020-053) and the Scientific Research Program of Universities in Anhui Province (2022AH030009).
Author information
Authors and Affiliations
Contributions
X.K. and M.Z. conceived the initial idea. X.W. designed the research. X.W. and H.L. performed materials preparation, structural determination and characterization. L.Z., X.S. and Z.S. conducted the electrochemical performance tests. Q.T. and Z.H carried out the theoretical calculations. X.W., X.K., Q.T., L.Z. and M.Z. wrote the paper and all authors commented on it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Puru Jena and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quantitative estimation of the activation energy.
(a) Arrhenius plots of the Ag29-COOLi and Ag29-Li electrolytes. (b and c) Chronoamperometric profiles for extracting the electronic conductivity.
Supplementary information
Supplementary Information
Supplementary Figs. 1–78, Tables 1–13, Experiment and Characterization, ORTEP view of Ag29 nanoclusters system structure, Explanations for A and B alerts in Ag29 nanoclusters system structure.
Supplementary Data 1
Crystallographic data for Ag29-PPh3.
Supplementary Data 2
Crystallographic data for Ag29-dimer.
Supplementary Data 3
Crystallographic data for Ag29-Li.
Supplementary Data 4
Crystallographic data for Ag29-COOLi-DMF.
Supplementary Data 5
Crystallographic data for Ag29-COONa-DMF.
Supplementary Data 6
Crystallographic data for Ag29-COOK-DMF.
Supplementary Data 7
Crystallographic data for Ag29-COORb-DMF.
Supplementary Data 8
Crystallographic data for Ag29-COOCs-DMF.
Supplementary Data 9
Crystallographic data for Ag29-COOLi-MeOH.
Supplementary Data 10
Crystallographic data for Ag29-COONa-MeOH.
Supplementary Data 11
Crystallographic data for Ag29-COOK-MeOH.
Supplementary Data 12
Crystallographic data for Ag29-COORb-MeOH.
Supplementary Data 13
Crystallographic data for Ag29-COOCs-MeOH.
Source data
Source Data Fig. 4
NMR, DFT.
Source Data Fig. 5
EIS, LSV, Coulombic efficiency.
Source Data Extended Data Fig. 1
Arrhenius plots, chronoamperometric profiles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Huang, Z., Sun, X. et al. Atomically defined two-dimensional assembled nanoclusters for Li-ion batteries. Nat. Synth 4, 1349–1358 (2025). https://doi.org/10.1038/s44160-025-00852-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00852-1