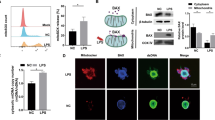
Abstract
Platelets have emerged as key inflammatory cells implicated in the pathology of sepsis, but their contributions to rapid clinical deterioration and dysregulated inflammation have not been defined. Here, we show that the incidence of thrombocytopathy and inflammatory cytokine release was significantly increased in patients with severe sepsis. Platelet proteomic analysis revealed significant upregulation of gasdermin D (GSDMD). Using platelet-specific Gsdmd-deficient mice, we demonstrated a requirement for GSDMD in triggering platelet pyroptosis in cecal ligation and puncture (CLP)-induced sepsis. GSDMD-dependent platelet pyroptosis was induced by high levels of S100A8/A9 targeting toll-like receptor 4 (TLR4). Pyroptotic platelet-derived oxidized mitochondrial DNA (ox-mtDNA) potentially promoted neutrophil extracellular trap (NET) formation, which contributed to platelet pyroptosis by releasing S100A8/A9, forming a positive feedback loop that led to the excessive release of inflammatory cytokines. Both pharmacological inhibition using Paquinimod and genetic ablation of the S100A8/A9–TLR4 signaling axis improved survival in mice with CLP-induced sepsis by suppressing platelet pyroptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The primary and processed proteomics data reported in this paper have been deposited in the OMIX, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (https://ngdc.cncb.ac.cn/omix: accession no. OMIX001255 (https://ngdc.cncb.ac.cn/omix/release/OMIX001255). Source data are provided with this paper. All other data supporting the finding in this study are included in the main article and associated files.
References
Weiss, S. L. et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 46, 10–67 (2020).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020).
Kaukonen, K. M., Bailey, M., Pilcher, D., Cooper, D. J. & Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 372, 1629–1638 (2015).
Vardon-Bounes, F. et al. Platelets are critical key players in sepsis. Int. J. Mol. Sci. 20, 3494 (2019).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Jorgensen, I. & Miao, E. A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265, 130–142 (2015).
Cornelius, D. C. et al. NLRP3 inflammasome activation in platelets in response to sepsis. Physiol. Rep. 7, e14073 (2019).
Wang, S. et al. Reduced intracellular antioxidant capacity in platelets contributes to primary immune thrombocytopenia via ROS-NLRP3-caspase-1 pathway. Thromb. Res. 199, 1–9 (2021).
Neuwirt, E. et al. NLRP3 as a sensor of metabolism gone awry. Curr. Opin. Biotechnol. 68, 300–309 (2021).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Kaplan, M. J. & Radic, M. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189, 2689–2695 (2012).
Sreejit, G. et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 141, 1080–1094 (2020).
Nagareddy, P. R. et al. NETosis is required for S100A8/A9-induced granulopoiesis after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 40, 2805–2807 (2020).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Dubois, C. et al. High plasma level of S100A8/S100A9 and S100A12 at admission indicates a higher risk of death in septic shock patients. Sci. Rep. 9, 15660 (2019).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 e36 (2018).
Gao, S., Yang, Y., Fu, Y., Guo, W. & Liu, G. Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am. J. Emerg. Med. 33, 1278–1282 (2015).
Boyd, J. H., Kan, B., Roberts, H., Wang, Y. & Walley, K. R. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ. Res. 102, 1239–1246 (2008).
Wang, Y. et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Invest. 124, 2160–2171 (2014).
Bjork, P. et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 7, e97 (2009).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Wu, X. et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 9, 171 (2018).
Claushuis, T. A. et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 127, 3062–3072 (2016).
Kraakman, M. J. et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Invest. 127, 2133–2147 (2017).
Plunkett, A. & Tong, J. Sepsis in children. BMJ 350, h3017 (2015).
Zhaolin, Z., Guohua, L., Shiyuan, W. & Zuo, W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 52, e12563 (2019).
Carestia, A., Davis, R. P., Davis, L. & Jenne, C. N. Inhibition of immunothrombosis does not affect pathogen capture and does not promote bacterial dissemination in a mouse model of sepsis. Platelets 31, 925–931 (2020).
Rolfes, V. et al. Platelets fuel the inflammasome activation of innate immune cells. Cell Rep. 31, 107615 (2020).
Guo, Q. et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe 29, 222–235 e224 (2021).
Dubois, C. et al. Top-down and bottom-up proteomics of circulating S100A8/S100A9 in plasma of septic shock patients. J. Proteome Res. 19, 914–925 (2020).
Pirr, S. et al. S100A8/A9 is the first predictive marker for neonatal sepsis. Clin. Transl. Med. 11, e338 (2021).
Hottz, E. D. et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 122, 3405–3414 (2013).
Assinger, A., Schrottmaier, W. C., Salzmann, M. & Rayes, J. Platelets in sepsis: an update on experimental models and clinical data. Front. Immunol. 10, 1687 (2019).
Xu, D. F. et al. Elevated angiotensin II induces platelet apoptosis through promoting oxidative stress in an AT1R-dependent manner during sepsis. J. Cell. Mol. Med. 25, 4124–4135 (2021).
Carestia, A., Kaufman, T. & Schattner, M. Platelets: new bricks in the building of neutrophil extracellular traps. Front. Immunol. 7, 271 (2016).
Jiao, Y. et al. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit. Care 24, 380 (2020).
Palacios-Acedo, A. L. et al. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front. Immunol. 10, 1805 (2019).
Chen, Z. et al. Review: The emerging role of neutrophil extracellular traps in sepsis and sepsis-associated thrombosis. Front. Cell. Infect. Microbiol. 11, 653228 (2021).
Cecconi, M., Evans, L., Levy, M. & Rhodes, A. Sepsis and septic shock. Lancet 392, 75–87 (2018).
Boros, F. & Vecsei, L. Progress in the development of kynurenine and quinoline-3-carboxamide-derived drugs. Expert Opin. Investig. Drugs 29, 1223–1247 (2020).
Bengtsson, A. A. et al. Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus. Arthritis Rheum. 64, 1579–1588 (2012).
Liao, Y. L. et al. S100A9 upregulation contributes to learning and memory impairments by promoting microglia M1 polarization in sepsis survivor mice. Inflammation 44, 307–320 (2021).
Goldstein, B., Giroir, B. & Randolph, A., International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8 (2005).
Mathias, B., Mira, J. C. & Larson, S. D. Pediatric sepsis. Curr. Opin. Pediatr. 28, 380–387 (2016).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254 (1976).
Ma, J. et al. Quantitative proteomics analysis of young and elderly skin with DIA mass spectrometry reveals new skin aging-related proteins. Aging (Albany NY) 12, 13529–13554 (2020).
Zhu, W. et al. Proteomic characterization and comparison of ram (Ovis aries) and buck (Capra hircus) spermatozoa proteome using a data independent acquisition mass spectometry (DIA-MS) approach. PLoS ONE 15, e0228656 (2020).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Bruderer, R., Bernhardt, O. M., Gandhi, T. & Reiter, L. High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics 16, 2246–2256 (2016).
Choi, M. et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30, 2524–2526 (2014).
Zhang, Y. et al. Reduced platelet miR-223 induction in Kawasaki disease leads to severe coronary artery pathology through a miR-223/PDGFRbeta vascular smooth muscle cell axis. Circ. Res. 127, 855–873 (2020).
Angelou, A. et al. Platelet depletion/transfusion as a lethal factor in a colitis-associated cancer mouse model. Anticancer Res. 39, 2443–2446 (2019).
Salzmann, M. et al. Genetic platelet depletion is superior in platelet transfusion compared to current models. Haematologica 105, 1738–1749 (2020).
Xu, M. et al. GPIbα is required for platelet-mediated hepatic thrombopoietin generation. Blood 132, 622–634 (2018).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 (2007).
Chen, L. et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 9, 597 (2018).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant nos. 82100141, 82101655, 82022003, 81970437 and 81903605), Natural Science Foundation of Guangdong Province (Grant no. 2021A1515011304), Guangzhou Science and Technology Project (Grant nos. 202102020164 and 202102010151), Guangzhou Science and Post-doctoral Research Project (Grant no. 2180157 and 011302031), China Postdoctoral Science Foundation (2021M700934) and Guangzhou Women and Children’s Medical Center/Guangzhou Institute of Pediatrics (Grant nos. 3001076 and 3001149).
Author information
Authors and Affiliations
Contributions
M.S., J.H. and W.H.T. conceptualized the project. M.S. designed and performed most of the experiments. C.C., S.L., Z.Z., L.X., X.F., Q.L., Y.W., Y.L., Y.B., Y.Z., J.Q., M.G., M.Q. and L.S. performed, or helped in the interpretation and design of some key experiments. M.S., C.C. and W.H.T. wrote the original manuscript draft. P.W., M.L., X.L., W.L., F.C. and D.Z. recruited and provided patient care and clinical assessments. R.L., J.H. and W.H.T. reviewed and edited the manuscript. W.H.T., M.S., C.C. and Z.Z. acquired funding. W.H.T. and J.H. provided resources and supervised the study. All authors provided critical comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.H.T., M.S. and C.C. are named inventors on a technical patent under review related to the therapeutic evaluation of the inhibitors of platelets pyroptosis in sepsis (202210225079.2). All other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Koichi Yuki, Angel Garcia Alonso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identified purity of platelet, representative proteins related to different cell death signal pathways and platelet pyroptosis in sepsis.
a, Purified platelets were obtained from human. Purity of platelet preparation was determined by FACS analysis using FITC anti-human CD41a and PE anti-human CD45 (n = 3). b, Heatmap of representative proteins expression related to different cell deaths signal pathways in purified platelet samples from HS (n = 3) and severe sepsis (with or without septic shock) (n = 3) using high-throughput proteomics analysis. c, Bar graphs displaying the percentage of activations of caspase 1 in platelets from sepsis and severe sepsis (with or without septic shock) and HS using FACS (HS: n = 13, Sepsis: n = 10, Severe sepsis with or without septic shock: n = 13). Data was presented as mean ± s.d. Kruskall-Wallis test and Dunn’s multiple comparisons test for c. HS, healthy subjects; Severe sepsis, severe sepsis/septic shock; PLTs, platelets.
Extended Data Fig. 2 TEM images of platelets induced by apoptosis, autophagy or pyroptosis agonists and apoptosis in severe sepsis patients.
a, Platelets were induced to apoptosis (10 μM ABT-737 induces apoptosis), autophagy (10 μM FCCP induces autophagy) and pyroptosis (10 μg/ml LPS and 5 μM Nigericin induces pyroptosis). TEM imaging of different states in platelets. b, Representative lower and higher power TEM field demonstrating loss of platelet ultrastructure in severe sepsis (with or without septic shock) patients (n = 5), with reduced granules/organelles and increased vacuolation. Apoptosis, red arrowheads indicate shrinkage of cell membrane and apoptotic bodies in apoptosis of platelet. Scale bars: 1 μm and 500 nm. Severe sepsis, severe sepsis/septic shock; PLTs, platelets.
Extended Data Fig. 3 The expression and localization of NLRP3 and ASC in severe sepsis platelets or rhS100A8/A9-induced platelets.
a, b, Immunofluorescence analysis showing the co-localization of CD41 (green), ASC (red) and NLRP3 (blue) in platelets from severe sepsis (with or without septic shock) patients (a) (n = 8); and platelets treated with 1 μg/ml rhS100A8/A9 or 10 μM Paquinimod (b) (n = 6); purple indicates overlap. Scale bars: 5 μm and 1 μm. HS, healthy subjects; Severe sepsis, severe sepsis/septic shock; NLRP3, NOD-like receptors containing domain pyrin 3 inflammasome; ASC, adaptor-apoptosis-associated speck-like protein; Paq, Paquinimod.
Extended Data Fig. 4 The identification and classic functions of platelets from platelet-specific Gsdmd KO mice.
a, b, The Gsdmdfl/fl PF4-Cre mice were identified by PCR (a) and confirmed by western blot (b), respectively (n = 6). c, The platelet (isolated from mice) suspensions were incubated with 0.1 U/ml thrombin for 30 minutes. P selectin translocation to membrane was assessed by FACS after stimulation with thrombin. The representative plots were presented as the number of counts over the log of associated fluorescence (baseline refers to the group without thrombin). Quantification of data presented as percentage of platelet activation. Data are expressed as mean ± SD (Gsdmdfl/fl + HBSS, n = 5; Gsdmdfl/fl + Thrombin, n = 6; Gsdmdfl/fl PF4-Cre+Thrombin, n = 4). d, Tail bleeding times of mouse was measured with the tail dipped into warmed saline to assess haemostasis using a tail-guillotine. Data are expressed as mean ± SD (Gsdmdfl/fl mice, n = 7; Gsdmdfl/fl PF4-Cre mice, n = 6). One-way ANOVA and Tukey’s multiple comparisons test for c. Unpaired t test with two-tailed for d. MT, mutation; WT, wild type; Tg, transgene; Ctrl, control; GSDMD, Gasdermin D; PLTs, platelets; HBSS, hank’s balanced salt solution.
Extended Data Fig. 5 The levels of S100A8/A9 in sepsis patients/mice and the caspase 1 activity in platelets from the CLP or rmS100A8/A9-injected mice.
a-c, Boxplots displaying the level of heterodimer S100A8/A9 in plasma from (a) severe sepsis (with or without septic shock) patients (HS: n = 53, Severe sepsis: n = 51), (b) CLP-induced sepsis mice (Sham, n = 10, CLP: n = 20) and (c) LPS-induced sepsis mice (PBS, n = 8, LPS: n = 8) by ELISA. The boxes indicate the 25% quantile, median, and 75% quantile. d, In a mouse model, mice that were injected intravenously with rmS100A8/A9 (30 μg/kg) or normal saline (n = 4 mouse/group) for 6 hours. Another mouse model, mice were induced CLP for 6 hours. FACS analysis displaying the caspase 1 activity in platelets. Mann Whitney test with two-tailed for a-b. Unpaired t test with two-tailed for c. One-way ANOVA and Tukey’s multiple comparisons test for d. Data was presented as mean ± SD. HS, healthy subjects; Severe sepsis, severe sepsis/septic shock; Sham, sham-operated mice; CLP, CLP-induced sepsis mice; PBS, PBS-injected mice; LPS, LPS-injected mice.
Extended Data Fig. 6 Putative receptors for S100A8/A9-induced platelet pyroptosis.
The platelet (isolated from HS) suspensions were incubated in the presence of neutralizing monoclonal antibodies (20 μg/ml) against control IgG, CD36, RAGE, or TLR4, and then treated with 1 μg/ml rmS100A8/A9 for 4 hours. a, FACS analysis displaying the caspase 1 activity in human platelets after stimulation. The quantified results are shown on the below. b, P selectin translocation to membrane (CD62P) was assessed by flow cytometry after stimulation. The quantified results are shown on the below. Data was presented as mean ± SD, n = 4. One-way ANOVA and Tukey’s multiple comparisons test for a, b. HS, healthy subjects; TLR4, toll-like receptor 4; RAGE, advanced glycation end products.
Extended Data Fig. 7 NLRP3 inflammasome and caspase 1 activity of platelets in mice transfused with Tlr4-/- or WT platelets.
a, In vitro, FACS analysis displaying the caspase 1 activity in platelets (Tlr4-/- or WT) treated with 1 μg/ml rmS100A8/A9 for 4 hours (n = 5). The quantified results are shown on the right. b-f, In vivo, a total of 1.2 ×107 purified platelets (volume: 200 μl, concentration: 6 ×1010 platelets/L) from Tlr4-/- or WT mice were intravenously transfused to mT/mG: PF4-Cre mouse. (b) After platelet depletion, platelet counts in mice were assessed at 0, 2, 4, 6, 8, 24 and 26 hours using a hematology analyzer (n = 3). (c) Platelet counts in mice before and after transfused with WT or Tlr4-/- platelets were detected at 0, 0.5, 2, 4 and 6 hours using a hematology analyzer (n = 3). (d) The percentages of transfused Tlr4-/- platelets in total platelets of mice were detected at 0, 0.5, 2, 4 and 6 hours using FACS analysis (n = 3). (e) Caspase 1 activity was measured using FACS analysis (n = 6). (f) The association of ASC and NLRP3 inflammasome in murine platelets was measured by immunofluorescence analysis. Platelets were stained for CD41 (green), ASC (red) and NLRP3 (blue); scale bars: 5 μm and 1 μm; n = 6. Data was presented as mean ± SD. One-way ANOVA and Tukey’s multiple comparisons test for a. Two-way ANOVA test for c, d. Abbreviation is as follow: HBSS, hank’s balanced salt solution; Saline, mice transfused with normal saline; WT PLTs, LPS-injected mice transfused with WT platelets; Tlr4-/- PLTs, LPS-injected mice transfused with Tlr4-/- platelets. PBS, PBS-injected mice; LPS + saline, LPS-injected mice transfused with normal saline; LPS + WT PLTs, LPS-injected mice transfused with WT platelets; LPS + Tlr4-/- PLTs, LPS-injected mice transfused with Tlr4-/- platelets; LPS, lipopolysaccharide; NLRP3, NOD-like receptors containing domain pyrin 3 inflammasome; ASC, adaptor-apoptosis-associated speck-like protein.
Extended Data Fig. 8 The function of mitochondria in septic platelets and S100A8/A9-induced platelets.
a, In platelets from severe sepsis (with or without septic shock) patients, bar graphs displaying change of mitochondrial membrane potential (∆Ψm) by staining with 40 nM TMRM using FACS analysis (HS: n = 20, Severe sepsis: n = 25). b, c, In vitro, bar graphs displaying change of mitochondrial ∆Ψm (b) and ROS production (c) in platelets (Tlr4-/- or WT) treated with 1 μg/ml rmS100A8/A9 for 4 hours using FACS analysis (n = 5). d, e, In the LPS induced murine model, mice with platelets depletion were transfused with a total of 1.2 ×107 purified platelets (volume: 200 μl, concentration: 6 ×1010 platelets/L) from Tlr4-/- or WT mice (n = 6/group). After 6 hours, bar graphs displaying change of mitochondrial ∆Ψm (d) and ROS production (e) in platelets (Tlr4-/–or WT) using FACS analysis (n = 6). Data was presented as mean fluorescence ± SD. Unpaired t test with two-tailed for a. One-way ANOVA and Tukey’s multiple comparisons test for b-e. Abbreviation is as follow: HS, healthy subjects; Severe sepsis, severe sepsis/septic shock; HBSS, hank’s balanced salt Solution. PBS, PBS-injected mice; LPS + saline, LPS-injected mice transfused with normal saline; LPS + WT PLTs, LPS-injected mice transfused with WT platelets; LPS + Tlr4-/- PLTs, LPS-injected mice transfused with Tlr4-/- platelets; TMRM, tetramethylrhodamine methyl ester; ROS, reactive oxygen species.
Extended Data Fig. 9 The formation of NET with different treatments and the release of ox-mtDNA from S100A8/A9-induced platelets after MitoTempo treatment.
a, Representative immunofluorescence of platelets treated with rhS100A8/A9 alone or supernatants of rhS100A8/A9-induced platelets for 4 hours. Cells were stained with Hoechst for DNA (blue), anti-citrullinated H3 for PMNs or NETs (cyan); scale bars: 5 μm; n = 4. b-c, PMNs isolated from HS were incubated with PBS, 50 nM PMA, and resting platelets, 0.1 U/ml thrombin activated platelets or 1 μg/ml S100A8/A9-induced platelets for 4 hours. Representative immunofluorescence of NET formation treated with PMA, resting platelets, thrombin or S100A8/A9-induced platelets (b). Cells were stained with Hoechst for DNA (blue), anti-citrullinated H3 for PMNs or NETs (green), CD41 for platelet (red). (c) Quantification of MPO-DNA and dsDNA in the supernatant of NET formation using PicoGreen fluorescent dye and MPO-DNA-ELISA, respectively (n = 6). d-e, Purified platelets suspensions were treated with rhS100A8/A9 (1 μg/ml) and MitoTempo (5 mM) for 4 hours, and then 50 nM PMA, S100A8/A9-induced platelets or MitoTempo-S100A8/A9-induced platelets induced NET formation. (d) The levels of ox-mtDNA in supernatant of S100A8/A9-induced platelets were determined by General 8-OHdG ELISA Kit (n = 4). (e) Quantification of MPO-DNA and dsDNA (NETosis) in the supernatant of cells using PicoGreen fluorescent dye and MPO-DNA-ELISA, respectively (n = 3). Data was presented as mean ± SD. One-way ANOVA and Tukey’s multiple comparisons test for c-e. Abbreviation is as follow: HS, healthy subjects; PLTs, platelets; PMNs, polymorphonuclear neutrophils; PMA, phorbol myristate acetate; MPO, myeloperoxidase; dsDNA, double-stranded DNA; NET, neutrophil extracellular trap; ox-mtDNA, oxidized mitochondrial DNA.
Extended Data Fig. 10 Immunofluorescence of pyroptotic platelets in NETs and the change of platelet counts in Gsdmdfl/fl PF4-Cre mice by CLP.
a, PMNs (S100a9-/- or WT) were incubated with 50 nM PMA to induced NET formation for 4 hours, and then incubated with platelets for another 4 hours. Representative immunofluorescence of PMNs incubated with platelets. Cells were stained with Hoechst for DNA (blue), anti-MPO for PMNs or NETs (cyan), CD41 for platelet (red) and activated caspase 1 for pyroptosis (green); scale bars: 25 μm and 5 μm. b, In the CLP-induced sepsis model, platelet counts in Gsdmdfl/fl PF4-Cre mice and littermate control Gsdmdfl/fl mice were assessed at 0, 2, 4, and 6 hours using a hematology analyzer (n = 5). Data was presented as mean ± SD. Two-way ANOVA and Tukey’s multiple comparisons test for b. Abbreviation is as follow: PLT, platelet; NS, not statistically significant; PMNs, polymorphonuclear neutrophils; PMA, phorbol myristate acetate; MPO, myeloperoxidase; Sham, sham-operated mice; CLP, CLP-induced sepsis mice; GSDMD, Gasdermin D.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Supplementary Video 1
Gsdmd_flox_PLTs_video_1.
Supplementary Video 2
Gsdmd_KO_PLTs_video_2.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, M., Chen, C., Li, S. et al. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat Cardiovasc Res 1, 732–747 (2022). https://doi.org/10.1038/s44161-022-00108-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-022-00108-7
This article is cited by
-
Sepsis induces long-term reprogramming of human HSPCs and drives myeloid dysregulation in sepsis survivors
Journal of Inflammation (2026)
-
Dysregulation of neutrophil in sepsis: recent insights and advances
Cell Communication and Signaling (2025)
-
Extracellular vesicle-bound S100A8/A9 is differentially expressed in septic shock and prompts acute lung injury
Respiratory Research (2025)
-
Delaying pyroptosis with an AI-screened gasdermin D pore blocker mitigates inflammatory response
Nature Immunology (2025)
-
Single-Cell transcriptomic profiles of peripheral blood immune cells reveal early monocyte and platelet activation in the transition from high-risk states to clinical sepsis
Scientific Reports (2025)