Abstract
Endurance exercise promotes adaptive growth and improved function of myocytes, which is supported by increased mitochondrial activity. In skeletal muscle, these benefits are in part transcriptionally coordinated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). The importance of PGC-1α to exercise-induced adaptations in the heart has been unclear. Here we show that deleting PGC-1α specifically in cardiomyocytes prevents the expected benefits from exercise training and instead leads to heart failure after just 6 weeks of training. Consistent with this, in humans, rare genetic variants in PPARGC1A, which encodes PGC-1α, are associated with increased risk of heart failure. In this model, we identify growth differentiation factor 15 (GDF15) as a key heart-secreted mediator that contributes to this dysfunction. Blocking cardiac Gdf15 expression improves cardiac performance and exercise capacity in these mice. Finally, in human heart tissue, lower cardiomyocyte PPARGC1A expression is associated with higher GDF15 expression and reduced cardiomyocyte density. These findings uncover a crucial role for cardiomyocyte PGC-1α in enabling healthy cardiac adaptation to exercise in part through suppression of GDF15.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Human DNA sequencing data are available from the UK Biobank (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access/). Analysis in this paper was approved by the Mass General Brigham Institutional Review Board (protocol 2013P001840; UK Biobank application 7089). Researchers with institutional review board approval may apply directly to the UK Biobank for data access. snRNA-seq data from human nonfailing, HCM and DCM hearts were downloaded from the The Broad Institute Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/). Bulk heart RNA-seq data have been deposited with the NCBI Gene Expression Omnibus under accession number GSE302867. Source data are provided with this paper. All other data related to the findings of this study are available in the Article and Supplementary Information.
Code availability
This study did not generate any custom code for data analysis. Standard analysis tools and software were used as detailed in Methods.
References
Virani, S. S. et al. Heart Disease and Stroke Statistics-2021 update: a report from the American Heart Association. Circulation 143, e254–e743 (2021).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390 (2019).
Contrepois, K. et al. Molecular choreography of acute exercise. Cell 181, 1112–1130 (2020).
Schmitz, K. H. et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer. J. Clin. 69, 468–484 (2019).
Myers, J. et al. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 346, 793–801 (2002).
Vega, R. B., Konhilas, J. P., Kelly, D. P. & Leinwand, L. A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 25, 1012–1026 (2017).
Khetarpal, S. A., Li, H., Lerchenmüller, C., Rhee, J. & Rosenzweig, A. Molecular mediators of the cardiac benefits of exercise. Circ. Res. 137, 163–183 (2025).
Hawley, J. A., Lundby, C., Cotter, J. D. & Burke, L. M. Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab. 27, 962–976 (2018).
Ashcroft, S. P., Stocks, B., Egan, B. & Zierath, J. R. Exercise induces tissue-specific adaptations to enhance cardiometabolic health. Cell Metab. 36, 278–300 (2024).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Lin, J. et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Jannig, P. R., Dumesic, P. A., Spiegelman, B. M. & Ruas, J. L. SnapShot: regulation and biology of PGC-1α. Cell 185, 1444–1444.e1 (2022).
Furrer, R. et al. Molecular control of endurance training adaptation in male mouse skeletal muscle. Nat. Metab. 5, 2020–2035 (2023).
Zechner, C. et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 12, 633–642 (2010).
Lin, J. et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119, 121–135 (2004).
Mittenbühler, M. J. et al. Isolation of extracellular fluids reveals novel secreted bioactive proteins from muscle and fat tissues. Cell Metab. 35, 535–549 (2023).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Hoffmann, C. & Weigert, C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb. Perspect. Med. 7, a029793 (2017).
Rao, R. R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Chow, L. S. et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 18, 273–289 (2022).
Ruas, J. L. et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151, 1319–1331 (2012).
Bernardo, B. C., Weeks, K. L., Pretorius, L. & McMullen, J. R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128, 191–227 (2010).
Arany, Z. et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl Acad. Sci. USA 103, 10086–10091 (2006).
Leone, T. C. et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101 (2005).
Patten, I. S. & Arany, Z. PGC-1 coactivators in the cardiovascular system. Trends Endocrinol. Metab. 23, 90–97 (2012).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Chung, H. K. et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 216, 149–165 (2017).
Patten, I. S. et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485, 333–338 (2012).
Gill, J. F. et al. Peroxisome proliferator-activated receptor γ coactivator 1α regulates mitochondrial calcium homeostasis, sarcoplasmic reticulum stress, and cell death to mitigate skeletal muscle aging. Aging Cell 18, e12993 (2019).
Boström, P. et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143, 1072–1083 (2010).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Howe, D. G. et al. Model organism data evolving in support of translational medicine. Lab Anim. 47, 277–289 (2018).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
Chaffin, M. et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 608, 174–180 (2022).
Vujic, A. et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 9, 1659 (2018).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Wang, D. et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 17, 592–607 (2021).
Suriben, R. et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 26, 1264–1270 (2020).
Xu, J. et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 98, 342–350 (2006).
Takaoka, M. et al. GDF15 antagonism limits severe heart failure and prevents cardiac cachexia. Cardiovasc. Res. 120, 2249–2260 (2024).
Flaherty, S. E. et al. GDF15 neutralization ameliorates muscle atrophy and exercise intolerance in a mouse model of mitochondrial myopathy. J. Cachexia Sarcopenia Muscle 16, e13715 (2025).
Mihaylov, S. R. et al. The master energy homeostasis regulator PGC-1α exhibits an mRNA nuclear export function. Nat. Commun. 14, 5496 (2023).
Lau, K. H., Waldhart, A. N., Dykstra, H., Avequin, T. & Wu, N. PPARγ and C/EBPα response to acute cold stress in brown adipose tissue. iScience 26, 105848 (2023).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Patel, S. et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 29, 707–718 (2019).
Wong, Y. L. et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. eLife 8, e42940 (2019).
Asundi, J. et al. GDF15 is a dynamic biomarker of the integrated stress response in the central nervous system. CNS Neurosci. Ther. 30, e14600 (2024).
Wang, D. et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 619, 143–150 (2023).
Fejzo, M. et al. GDF15 linked to maternal risk of nausea and vomiting during pregnancy. Nature 625, 760–767 (2024).
Coll, A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020).
Kempf, T. et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 98, 351–360 (2006).
Kempf, T. et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 17, 581–588 (2011).
Xu, X. et al. Growth differentiation factor (GDF)-15 blocks norepinephrine-induced myocardial hypertrophy via a novel pathway involving inhibition of epidermal growth factor receptor transactivation. J. Biol. Chem. 289, 10084–10094 (2014).
Chan, J. S. F. et al. Growth differentiation factor 15 alleviates diastolic dysfunction in mice with experimental diabetic cardiomyopathy. Cell Rep. 43, 114573 (2024).
Ozcan, M. et al. Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity. Cell Metab. 35, 928–942 (2023).
Sigvardsen, C. M., Richter, M. M., Engelbeen, S., Kleinert, M. & Richter, E. A. GDF15 is still a mystery hormone. Trends Endocrinol. Metab. 36, 591–601 (2025).
Emmerson, P. J. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219 (2017).
Rochette, L., Zeller, M., Cottin, Y. & Vergely, C. Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol. Metab. 31, 939–951 (2020).
Mullican, S. E. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 23, 1150–1157 (2017).
Weng, J.-H. et al. Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat. Metab. 3, 513–522 (2021).
Olsen, O. E., Skjærvik, A., Størdal, B. F., Sundan, A. & Holien, T. TGF-β contamination of purified recombinant GDF15. PLoS ONE 12, e0187349 (2017).
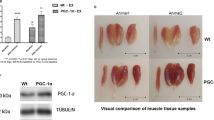
Kärkkäinen, O. et al. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc. Res. 115, 107–118 (2019).
Naumenko, N. et al. PGC-1α deficiency reveals sex-specific links between cardiac energy metabolism and EC-coupling during development of heart failure in mice. Cardiovasc. Res. 118, 1520–1534 (2022).
Latorre-Muro, P. et al. A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation. Cell Metab. 33, 598–614 (2021).
Wieckowski, M. R., Giorgi, C., Lebiedzinska, M., Duszynski, J. & Pinton, P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 4, 1582–1590 (2009).
Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 28, 656–666 (2018).
Li, H. et al. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation https://doi.org/10.1161/CIRCULATIONAHA.121.056850 (2022).
Lerchenmüller, C. et al. Restoration of cardiomyogenesis in aged mouse hearts by voluntary exercise. Circulation 146, 412–426 (2022).
Lu, J. F. et al. GDF15 is a major determinant of ketogenic diet-induced weight loss. Cell Metab. 35, 2165–2182 (2023).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
McLaren, W. et al. The Ensembl variant effect predictor. Genome Biol. 17, 122 (2016).
Acknowledgements
This work was supported by NIH grants R01 DK119117 to B.M.S., NIH grants R01 AG061034 and R35 HL155318 and American Heart Association (AHA) grants 23MERIT1038415 and SFRN (https://doi.org/10.58275/AHA.24SFRNPCN1284382.pc.gr.194135 and https://doi.org/10.58275/AHA.24SFRNCCN1276092.pc.gr.194131) to A.R., NIH grant K08 HL177169, a Burroughs Wellcome Fund Career Award for Medical Scientists and John S. LaDue Memorial Fellowship from Harvard Medical School to S.A.K., AHA grants 20CDA35310184 and 24SCEFIA1253853 and NIH grants R01HL171201 and R21AG077040 to H.L., NIH grant K08HL140200 and R03HL177119 to J.R., a Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Walter Benjamin Programme (530157297) grant to S.P., NIH grant T32HL160522 to J.L., a fellowship from Hope Funds for Cancer Research (HFCR-20-03-01-02) to H.-G.S., a DFG grant (Projektnummer 461079553) to M.J.M., NIH grant 1T32GM145407-01 to T.V., Damon Runyon Cancer Research Foundation Fellowship (DRG 120-17) and NIH grant K99 DK125722 to P.A.D., NIH grants HL163172 and K08 HL145019 to A.A., NIH grants K76AG064328 and R01HL170058 and the Yeatts Fund for Innovative Research to J.D.R., AHA Innovation Project Award 23IPA1054031 and NIH grants R01AG086369 and R01DK136640 to P.P. We acknowledge the participants of the UK BioBank for their participation; J. W. Wu from the College of Animal Science and Technology, Northwest A&F University, Yangling, Shaanxi, China for generously providing Gdf15-luciferase reporter DNA constructs; Y. Iwamoto (Center for Systems Biology, Massachusetts General Hospital) for help with sample staining; and J. Lako, A. Kohli and J. Danielson for administrative support. We thank all members of the Spiegelman, Rosenzweig, Puigserver, Banks and Chouchani laboratories for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.A.K.: conceptualization, investigation, formal analysis, writing, funding. H.L.: conceptualization, investigation. T.V.: investigation, formal analysis. J.R.: conceptualization. S.C.: formal analysis. C.C.: investigation. Y.S.: investigation. S.P.: investigation. J.L.: investigation. A.L.S.: investigation. K.A.B.: investigation. L.G.: formal analysis. M.J.M.: conceptualization, investigation. M.J.K.: investigation. A.V.-C.: investigation. C.C.: investigation. J.M.N.-P.: investigation. C.W.: investigation. N.E.H.: conceptualization. H.-G.S.: conceptualization. S.J.J.: formal analysis. K.J.B.: formal analysis. A.K.: investigation, formal analysis. R.F.: investigation. D.B.: investigation. P.T.E.: resources. M.N.: resources. J.A.P.: investigation. S.P.G.: supervision, resources. A.A.: conceptualization, supervision, resources. P.A.D.: conceptualization, resources. K.A.G.: formal analysis, resources, supervision. P.P.: conceptualization, supervision. J.D.R.: conceptualization, supervision, resources. B.M.S.: supervision, conceptualization, writing, resources, funding. A.R.: supervision, conceptualization, writing, resources, funding.
Corresponding authors
Ethics declarations
Competing interests
B.M.S. is an inventor on patent WO2015051007A1; the patent applicant is Dana Farber Cancer Institute. Inventors include B.M.S. and Christiane D. Wrann (not affiliated with this study). The application number is WO2015051007A1. Patent is currently active. No aspect of the current paper is covered in the patent application. A.A. is an inventor on patent US20210163495A1; the patent applicant is The General Hospital Corporation. Inventors include A.A. and several investigators unaffiliated with this study (R. T. Peterson, Y. Liu, B. Zheng, Y. Wang). The application number is US20210163495A1. The patent is currently active. No aspect of this paper is covered in the patent application. J.D.R. and A.R. are inventors on patent US11834508B2; patent applicants are Norvartis AG and Beth Israel Deaconess Medical Center. Inventors include J.D.R., A.R. and D. J. Glass (not affiliated with this study). The application number is US11834508B2. The patent is currently active. No aspect of the current study is covered in the patent application. In addition, we note the additional following competing interests. B.M.S. is an academic cofounder and consultant for Aevum Therapeutics, all unrelated to this current work. J.R. is a consultant for Takeda Neurosciences, unrelated to the current work. S.P. is currently employed by the Novartis Institute of Biomedical Research. J.D.R. has received research support from Amgen, Genentech and Keros, all unrelated to this current work. A.R. is a scientific founder of Thryv Therapeutics, unrelated to this current work. M.N. has received funds or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis and Pfizer, as well as consulting fees from Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, Verseau Therapeutics and Bitterroot, all unrelated to the current work. A.A. reports on the Board of Directors of Corventum. The other authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Sam Lockhart, Gregory Steinberg and Rong Tian for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Acute treadmill exercise heart gene expression timecourse.
a, Ppargc1a mRNA expression in the indicated tissues in WT and KO mice. b, Experimental design for the acute treadmill exercise timecourse experiment. Created with BioRender.com. c–h, Relative mRNA expression of the indicated genes in the heart at the indicated timepoints following a single 45-minute bout of treadmill exercise. For (a), statistical significance was assessed by two-sided two-way ANOVA followed by Sidak’s multiple comparisons test (two-sided). Exact P values: 0.041 (heart), 1 (liver), 1 (kidney), 0.65 (gastrocnemius), 0.79 (iWAT). Data are presented as mean±s.e.m. For (a), n = 5 biologically independent mice per group. For (c–h), n = 4 biologically independent mice per group. Significance symbols: * P < 0.05; N.S., not significant. For all data in this figure, a single experiment was performed without a separate biological replication cohort. Mouse strain and age: for (a), mice studied were 10 week old male mice of the indicated strain (WT vs. KO) and n per group as above. For (c-h), all mice used were C57BL/6 mice from The Jackson Laboratory (age relative to initiation of the experiment) of the indicated n per group.
Extended Data Fig. 2 Exercise tolerance and cardiac function in female WT and KO mice after endurance training.
a, Treadmill exhaustion test results showing maximum velocity and distance run before exhaustion in 14-week-old female WT and KO mice pre- and post-training. b, Representative M-mode echocardiographic images at rest in WT and KO mice from (a) before and after 6 weeks of voluntary wheel running. c, Echocardiographic measurements in WT and KO mice from (a), including fractional shortening (FS), left ventricular internal diameter at diastole (LVIDd), interventricular septal thickness at diastole (IVSd), and left ventricular posterior wall thickness at diastole (LVPWd). d, Heart weight normalized to tibia length in sedentary vs exercise-trained WT and KO mice. Data are shown as mean ± s.e.m. For (a), (c), and (d), statistical significance was assessed by two-sided two-way ANOVA followed by Sidak’s multiple comparisons test. For a-b, n = 5 mice per group (WT and KO pre-training), and n = 4 mice per group (WT and KO post-training). For (c), n = 4 mice/group. All data points represent individual mice as biological replicates. For echocardiographic quantifications in (c), single measurements were made per mouse. Exact P values: a, Max treadmill velocity: 0.95 (pre), 0.000072 (post); a, Max distance: 0.94 (pre), 0.00041 (post); c, FS: 0.42 (pre), 8.1e-9 (post); c, LVIDd: 0.78 (pre), 6.8e-8 (post); c, IVSd: 0.16 (pre), 0.000028 (post); c, LVPWd: 1.0 (pre), 0.00070 (post); d, Heart weight/tibia length: 0.31 (sedentary), 0.00068 (trained). Significance symbols: *P < 0.05, **P < 0.01, **P < 0.001; N.S., not significant. For all data in this figure, a single experiment was performed without a separate biological replication cohort. Mouse strain and age: all data in this experiment was derived from 14 week old female mice (age relative to initiation of the experiment) of the indicated strains (WT vs. KO as defined above) and n per group.
Extended Data Fig. 3 Fat and muscle wasting and energy expenditure in sedentary vs exercise-trained WT and KO mice.
a, Average daily voluntary wheel running over 7 days (n = 14 mice/group). b, Inguinal white adipose tissue (iWAT) and gastrocnemius weights normalized to body weight (n = 10 mice/group for WT_Sed and KO_Sed groups, n = 14 mice for WT_Ex group, n = 12 mice for KO_Ex group). c, Relative gene expression in iWAT and gastrocnemius normalized to Rplp0 (iWAT: WT_Sed – n = 4 mice, KO_Sed – n = 5 mice, WT_Ex – n = 6 mice, KO_Ex – n = 4 mice; Gastrocnemius: n = 5 mice/group for WT_Sed and KO_Sed, n = 7 mice/group for WT_Ex and KO_Ex). d, Indirect calorimetry–based energy expenditure in mice maintained at 22 °C in metabolic cages after 3 days of acclimatization (Day 1 of recording) and then again 14 days later (Day 15). Sample size was as follows: Day 1- n = 8/group for WT, n = 6/group for KO; Day 15- n = 7/group for WT, n = 6/group for KO. No correlation was found between energy expenditure and genotype or weight at either Day 1 or Day 15 timepoints (Supplementary Fig. 1a). e, Food intake over the same durations from the same mice in (d). No correlation was found between food intake and genotype or weight at either Day 1 or Day 15 timepoints (Supplementary Fig. 1b). f, Cumulative food intake over the time course in (d-e). Sample sizes: n = 7/group for WT, n = 6/group for KO. g, Body weights at beginning (day 1) and end (day 15) of metabolic cage housing with wheel access Sample size was as follows: Day 1- n = 8/group for WT, n = 6/group for KO; Day 15- n = 7/group for WT, n = 6/group for KO. Data are presented as mean±s.e.m. Each dot indicates a biologically independent animal. Statistical tests: a: unpaired two-tailed t-test, b–c, g: two-sided two-way ANOVA with Sidak’s multiple comparisons, d–f: unpaired two-tailed t-test of AUC. Exact P values: a: 0.74 b: 0.099 (iWAT), 0.00015 (gastrocnemius) c (iWAT genes): Ppargc1a: 1, Ucp1: 6.6e-11, Prdm16: 1, Elovl3: 1, Dio2: 1, Cidea: 1, Phospho1: 1, Adipoq: 1 c (gastrocnemius genes): Myh1: 0.79, Myh2: 0.21, Myh4: 0.80, Myh7: 1 d: 0.044 (Day 1), 0.10 (Day 15) e: 0.15 (Day 1), 0.25 (Day 15) f: 0.44 g: 0.76 (Day 1), 0.91 (Day 15) Significance symbols: *P < 0.05, **P < 0.01, ***P < 0.001, N.S., not significant. For all data in this figure, a single experiment was performed without a separate biological replication cohort. Mouse strain and age: all data in this experiment was derived from 10 week old male mice (age relative to initiation of the experiment) of the indicated strains (WT vs. KO as defined above) and n per group.
Extended Data Fig. 4 Heart failure phenotyping in WT vs. KO mice.
a, Top 10 Gene Ontology (GO) Biological Process terms for downregulated (top) and upregulated (bottom) genes (adjusted P < 0.05) from RNA-seq data corresponding to comparisons in Fig. 2e–f (KO_Sed vs WT_Sed on left, KO_Ex vs WT_Ex on right). Each term shown passed FDR < 0.05 and is colored by enrichment score. b, Heatmap of representative Hallmark inflammatory response genes from the RNA-seq dataset; all genes shown passed adjusted P < 0.05 (n = 5 mice/group). c, Immune cell frequency estimates from deconvolution of bulk heart RNA-seq using the mMCP-counter (left) and Digital Cell Quantification (DCQ) (right) tools (n = 5 mice/group). d, Representative images (left) and quantification (right) of CD3⁺/DAPI⁺ T cells per high-power field (HPF) in frozen heart sections from indicated groups. n = 3/group (WT_Sed and KO_Sed), n = 6/group (WT_Ex and KO_Ex). e, Heatmap of relative RNA-seq expression of selected extracellular matrix (ECM)–related genes, all of which passed adjusted P < 0.05. n = 5 mice/group. f, Immunoblot of fibrosis-related proteins from heart lysates (n = 3 mice/group). g, Representative images (left) and quantification (right) of TUNEL⁺/DAPI⁺/cTnT⁺ nuclei per HPF. Green: cardiac troponin; blue: DAPI; pink: TUNEL⁺ nuclei; arrows indicate examples of TUNEL⁺ cells. N = 4 mice/group. h, Immunoblot of cleaved PARP9, cleaved caspase-3, and tubulin (loading control) in heart lysates (n = 3 per group). For (c), (d), and (g), data are shown as mean±s.e.m. For (a) enrichment scores are plotted for the comparison of the indicated group for the indicated Gene Ontology term (Webgestalt, described in the Methods). For (b) and (e), each square in the heatmap represents the relative expression of an individual gene from a single mouse with the color intensity normalized to the average expression of that gene from the WT_Sed group, as determined from DESeq2-processed RNA-seq data. For (c), (d), and (g), statistical significance was assessed by two-sided two-way ANOVA followed by Sidak’s multiple comparisons test. For RNA-seq analyses in (a), (b), and (e), DESeq2 was used with Benjamini-Hochberg correction (FDR < 0.05). No statistical tests were performed for immunoblots in (f) or (h). Exact P values: c, mMCP-counter (WT_Ex vs KO_Ex): T cell = 0.91, CD8⁺ T cell = 0.73, NK cell = 0.94, B cell = 0.99, Memory B cell = 1, Monocyte/Macrophage = 0.0028, Monocyte = 0.32, Stem Cell = 1, Mast Cell = 0.39, Eosinophil = 0.82, Neutrophil = 0.97. DCQ (WT_Ex vs KO_Ex): B cell = 0.98, B cell naïve = 0.29, Myeloid dendritic cell = 0.088, Plasmacytoid dendritic cell = 0.81, Granulocyte-monocyte progenitor = 1, Macrophage = 0.0020, Stem cell = 0.59, Monocyte = 0.0039, NK cell = 1, T cell NK = 0.69, T cell immature = 0.038, T cell CD4⁺ memory = 1, T cell CD8⁺ effector memory = 0.036, T cell CD8⁺ memory = 0.92, T cell CD8⁺ naïve = 1, γδT cell = 1. d, CD3⁺ T cells per HPF (WT_Ex vs KO_Ex) = 0.0000056 g, TUNEL⁺ nuclei per HPF (WT_Ex vs KO_Ex) = 1. Significance symbols: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; N.S., not significant. n refers to biologically independent animals. For (d) and (g), dots in bar graphs show quantification for an individual mouse, with 10 images used for each mouse. For the remainder of the figure, measurements were made once per biological sample. For all data in this figure, a single experiment was performed without a separate biological replication cohort. Mouse strain and age: all data in this experiment was derived from 10 week old male mice (age relative to initiation of the experiment) of the indicated strains (WT vs. KO as defined above) and n per group.
Extended Data Fig. 5 Cell types in hearts of sedentary and exercise-trained WT and KO mice.
a, Representative images (left) and quantification (right) of Ki67⁺/PCM1⁺/DAPI⁺ cardiomyocyte nuclei per high-power field (HPF) in WT_Ex and KO_Ex hearts (n = 4 mice/group for WT_Sed and KO_Sed groups; n = 6 mice/group for WT_Ex and KO_Ex groups). b, Representative images of freshly isolated rod-shaped and rounded cardiomyocytes from WT_Ex and KO_Ex mice (left). Quantification of cardiomyocyte cross-sectional area from 100 representative cells per mouse (right) (n = 4 mice/group). c, Histogram showing distribution of cardiomyocyte size from (b). d, Flow cytometric depiction of live cell sorting by DAPI exclusion from WT_Ex and KO_Ex hearts. e, Flow cytometry gating for CD31⁺, CD45⁺, and CD31⁻CD45⁻ populations from WT_Ex and KO_Ex hearts. Flow cytometry gating strategy is provided in Supplementary Fig. 2. f, Quantitative RT-PCR for cardiomyocyte markers (Nppa, Nppb, Myh6, Myh7) and non-cardiomyocyte gene markers (Tgfb1, Col1a1) in sorted cell populations from WT_Ex and KO_Ex hearts (n = 5 mice/group for WT and n = 4 mice/group for KO for CD45 + , CD45-/CD31- and CD31+ cells, n = 4 mice/group for both groups for CM). g, Gdf15 mRNA levels in heart, liver, kidney, gastrocnemius, and inguinal white adipose tissue (iWAT) from sedentary WT and KO mice (n = 5 mice/group for both groups). h, Plasma corticosterone concentrations from sedentary WT and KO mice (n = 5 mice/group for both groups). Data in (a-b), (g), and (h) are presented as mean ± S.E.M.; each data point represents an individual mouse. For (f), RT-qPCR data represent relative gene expression normalized to housekeeping gene(s); statistical comparisons were performed for each gene individually. Statistical tests and exact P values: a, Two-sided two-way ANOVA with Sidak’s post hoc test: P = 0.00035 (WT_Ex vs. KO_Ex). b, Unpaired two-tailed t-test: P = 0.0028. f, Unpaired two-tailed t-test: Nppa (CMs): P = 4.2 × 10⁻¹⁰, Nppb (CMs): P = 3.6 × 10⁻⁷, Myh6 (CMs): P = 2.4 × 10⁻⁷, Myh7 (CMs): P = 2.4 × 10⁻⁷, Tgfb1 (CD45⁻/CD31⁻): P = 0.0048, Tgfb1 (CD31⁺): P = 0.0066, Col1a1 (CD45⁻/CD31⁻): P = 8.3 × 10⁻⁷. G, Unpaired two-tailed t-test: Heart: P = 1.3 × 10⁻¹⁵, Liver: P = 1.0, Kidney: P = 1.0, Gastrocnemius: P = 0.96, iWAT: P = 0.79. h, Unpaired two-tailed t-test: P = 0.22. Significance symbols: **P < 0.01, ***P < 0.001, ****P < 0.0001; N.S., not significant. n refers to biologically independent animals. For (a), dots in bar graphs show quantification for an individual mouse, with 10 images used for each mouse. For (b), dots in bar graphs show average quantification for an individual mouse, with 100 cardiomyocytes used for each mouse. For (f-h), measurements were made once per biological sample. For all data in this figure, a single experiment was performed without a separate biological replication cohort. Mouse strain and age: all data in this experiment was derived from 10 week old male mice (age relative to initiation of the experiment) of the indicated strains (WT vs. KO as defined above) and n per group.
Extended Data Fig. 6 Characterization of mouse heart extracellular fluid (EF).
a, Protein concentration in heart EF and gastrocnemius EF relative to plasma in WT male mice (10 weeks old, heart EF – n = 7 mice, gastrocnemius EF n = 5 mice, serum – n = 7 mice). b, Silver stain of 10 μg total protein from the indicated samples separated by SDS–PAGE. c, Silver stain of heart tissue lysate, EF, and plasma (10 μg per sample) from WT male mice (10 weeks old) collected 1 hour after acute treadmill exercise to exhaustion (Ex) or from sedentary controls (Sed). d, Immunoblot of mitochondrial oxidative phosphorylation proteins and a nuclear marker (Lamin A/C) from 50 μg of protein from the indicated samples. In (b–d), each lane represents an individual biologically independent sample (mouse). No statistical tests were performed. For all data in this figure, a separate biological replication cohort was studied. Mouse strain and age: all data in this experiment was derived from 12 week old male C57BL/6 mice obtained from The Jackson Laboratory (age relative to initiation of the experiment) with the indicated n per group.
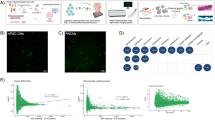
Extended Data Fig. 7 Regulation of Gdf15 by PGC-1α in cardiomyocytes.
a, Immunoblot for FLAG, PGC-1α, and tubulin from LPS853 liposarcoma cell lysates after infection with Ad.FLAG–PGC-1α at the indicated multiplicity of infection (MOI) for 72 h (n = 1 immunoblot per condition; experiment performed once without a separate biological replicate experiment). b, ChIP–qPCR analysis of FLAG–PGC-1α enrichment relative to IgG control at the promoters of Esrra and Fndc5 in NRVMs infected with Ad.FLAG–PGC-1α at MOI 100 for 72 h (n = 3 technical replicates from one chromatin IP, experiment independently repeated once). c, UCSC genome browser snapshots showing PGC-1α ChIP–seq peaks at the promoters of ESRRA and GDF15 in HEK293 cells from the indicated published study. d, UCSC genome browser snapshots showing PGC-1α ChIP–seq peaks at the promoters of Esrra and Gdf15 from mouse brown adipose tissue after 4 h cold exposure, from the indicated published study. e, Gene set enrichment analysis (GSEA) of the integrated stress response (ISR) gene set using transcriptomics from NRVMs treated with Ad.GFP or Ad.PGC-1α. Gene sets are shown if FDR < 0.05. Experiment performed in one biological cohort without a replication cohort. f, GSEA of ISR gene set from transcriptomic comparisons of mouse hearts WT_Sed, KO_Sed, WT_Ex and KO_Ex mice. Gene sets are shown if FDR < 0.05. n = 5 mice/group. Experiment performed in one biological cohort without a replication cohort. g, mRNA expression of ISR-related genes (Ppargc1a, Bnip3, Map1lc3b) in NRVMs treated with Ad.GFP or Ad.PGC-1α with or without tunicamycin and/or 2BAct (n = 3 biologically independent wells per group, experiment independently repeated once in a separate biological replication cohort). Data in (b) and (g) are shown as mean ± s.e.m. Statistical significance in (b) and (g) was assessed using unpaired two-tailed t-tests (b) or two-sided one-way ANOVA followed by Sidak’s multiple comparisons test (g). No statistical tests were performed in (a), (c), or (d). All GSEA results shown in (e-f) passed FDR < 0.05. Measurements were performed once per sample in all panels. Significance symbols: *P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; N.S., not significant. n refers to biologically independent replicates except for (b) where n refers to technical replicates as stated above. Exact P values: b, Esrra: 0.023 (primer pair 3), 0.026 (primer pair 5), 0.026 (primer pair 8); Fndc5: 0.014 (primer pair 6). g, Ppargc1a: 0.77 (Ad.GFP vs. Ad.GFP+Tunicamycin), 0.18 (Ad.GFP+Tunicamycin+2BAct), 0.99 (Ad.PGC-1α vs. Ad.PGC-1α+Tunicamycin), 0.69 (Ad.PGC-1α vs. Ad.PGC-1α+Tunicamycin+2BAct); Bnip3: 0.0000025 (Ad.GFP vs. Ad.GFP+Tunicamycin), 0.0020 (Ad.GFP+Tunicamycin+2BAct), 0.0085 (Ad.PGC-1α vs. Ad.PGC-1α+Tunicamycin), 0.050 (Ad.PGC-1α vs. Ad.PGC-1α+Tunicamycin+2BAct); Map1lc3b: 0.00051 (Ad.GFP vs. Ad.GFP+Tunicamycin), 0.084 (Ad.GFP+Tunicamycin+2BAct), 0.0096 (Ad.PGC-1α vs. Ad.PGC-1α+Tunicamycin), 0.73 (Ad.PGC-1α vs. Ad.PGC 1α+Tunicamycin+2BAct). Mouse strain and age: data from mouse tissues in (f) was derived from 10 week old male mice (age relative to initiation of the experiment) of the indicated strains (WT vs. KO as defined above) and n per group.
Extended Data Fig. 8 GDF15 treatment and GFRAL expression in the heart.
a, Representative images (left) and quantification (right) of NRVMs stained with sarcomeric α-actinin after treatment with PBS or recombinant human GDF15 (Sino Biological 10936-H01Y, 100 ng/mL) for 48 hours in serum-free media (n = 3 biologically independent wells per condition, separate biological replication experiment performed once). b, Gdf15 mRNA expression (left) and GDF15 protein levels in conditioned media (right) from NRVMs transduced with Ad.GFP or Ad.Gdf15 at a multiplicity of infection (MOI) of 50 for 72 hours (n = 3 biologically independent wells per condition, separate biological replication experiment performed once). c, Representative images (left) and quantification (right) of sarcomeric α-actinin staining of NRVMs from (b) to assess cardiomyocyte size (n = 3 biologically independent wells per condition, separate biological replication experiment performed once). d, Relative mRNA expression of Gfral, Glp1r (AP/NTS marker), and Satb2 (cortical neuron marker) in mouse area postrema/nucleus tractus solitarius (AP/NTS, n = 3 independent mice), cortex (n = 4 independent mice), and heart (n = 4 independent mice). Primer sequences are listed in Supplementary Table 4. Experiment performed in one set of mice without a separate biological replication experiment. Created with BioRender.com. e, Relative GFRAL expression across individual cardiac cell types from human hearts using single-nucleus RNA sequencing (snRNA-seq) (n = 16 independent non-failing [NF] heart samples, N = 15 hypertrophic cardiomyopathy [HCM] samples, n = 11 independent dilated cardiomyopathy [DCM] samples). Experiment performed in one cohort without a separate biological replication cohort. f, Relative expression of GFRAL, GDF15, and PPARGC1A across all nuclei from the same snRNA-seq dataset in (e). Experiment performed in one cohort without a separate biological replication cohort. Data in (a)–(c) are shown as mean±s.e.m.Box plots in (e-f) display boxes extending from 25th to 75th percentile of the distribution of the values, with whiskers extending to minimum and maximum values. For (a)–(c), statistical significance was assessed using unpaired two-tailed t-tests. For (d)–(f), statistical significance was assessed using two-sided one-way ANOVA followed by Sidak’s multiple comparisons test (d), or two-sided two-way ANOVA with Sidak’s post-test (e–f). Exact P values: a, 0.038; b, Gdf15 mRNA: 0.038, GDF15 protein: 0.000013; c, 0.0051; d, Glp1r: 0.000089 (AP/NTS vs. cortex), 0.000074 (AP/NTS vs. heart); Gfral: 0.0044 (AP/NTS vs. cortex), 0.0017 (AP/NTS vs. heart); Satb2: 0.0016 (AP/NTS vs. cortex), 1 (AP/NTS vs. heart); e, GFRAL in fibroblast I: 1.1e–15 (NF vs. DCM), 0.00032 (NF vs. HCM); f, PPARGC1A: 1 (NF vs. DCM), 1 (NF vs. HCM). Significance symbols: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; N.S., not significant. n refers to biologically independent samples. Measurements in (a)–(c) represent the average value per well from 30-35 cells per well. For (d), each data point represents an individual mouse tissue sample. For (e-f), each data point reflects one biological replicate (human donor). Representative microscopy images in (a) and (c) are from experiments independently repeated twice with similar results. Mouse strain and age: data in (d) was derived from 10 week old female C57BL/6 mice obtained from The Jackson Laboratory (age relative to initiation of the experiment) of the indicated n per group.
Extended Data Fig. 9 Cardiac phenotyping in GDF15 overexpression and Gdf15 knockdown.
a, Gdf15 mRNA expression in tissues of WT mice treated with AAV9-GFP (n = 10 mice/group) or AAV9-GDF15 (n = 8 mice/group). b, Daily average voluntary wheel running over 7 days in groups from (a) (n = 5 mice/group). c, Representative images (left) and quantification (center) of freshly isolated cardiomyocyte area from mice in (a) (n = 4 mice per group, data points show average calculations from 100 cells per mouse), (right) cardiomyocyte cross-sectional area size distribution plot. d, Representative images (left) and quantification (right) of Ki67⁺/PCM1⁺/DAPI⁺ cardiomyocyte nuclei per high-power field (HPF) in heart sections (n = 4 mice/group for both groups, 10 images per mouse). e, Representative images (left) and quantification (right) of CD68⁺/PCM1⁺/DAPI⁺ cardiomyocyte nuclei per HPF in heart sections (AAV9-GFP: n = 4 mice/group, AAV9-Gdf15: n = 5 mice/group, 10 images per mouse). f, Representative Masson’s trichrome-stained heart sections (left) and quantification (right) of trichrome⁺ area as percentage of HPF area (AAV9-GFP: n = 4 mice/group, AAV9-Gdf15: n = 5 mice/group, 10 images per mouse). g, Plasma corticosterone levels measured by ELISA after 5 weeks of exercise (n = 5 mice per group). h, Gdf15 mRNA expression in tissues of WT and KO mice treated with AAV9-shScr or AAV9-shGdf15 (n = 5 mice per group). i, Daily average voluntary wheel running over 7 days in mice from (h) (n = 5 mice per group). j, Representative images (left) and quantification (right) of Ki67⁺/PCM1⁺/DAPI⁺ cardiomyocyte nuclei per HPF (n = 4 mice/group, averaged from 10 images per mouse). k, Representative images (left) and quantification (right) of CD68⁺/PCM1⁺/DAPI⁺ cardiomyocyte nuclei per HPF (n = 4 mice/group, averaged from 10 images per mouse). l, Masson’s trichrome-stained sections (left) and quantification (right) of trichrome⁺ area as percentage of HPF (n = 4 mice per group, 10 HPFs averaged per mouse). m, Plasma corticosterone levels by ELISA after 3 weeks of exercise training (n = 5 mice per group). Data are presented as mean±s.e.m. Dots in quantification graphs reflect individual biological replicates. For panels a–g, statistical significance was assessed by unpaired two-tailed t-test. For panels h–m, two-sided two-way ANOVA followed by Sidak’s multiple comparisons test was used. No statistical test was performed for the cardiomyocyte size distribution plot in (c). Exact P values: a: 0.045 (heart), 0.000013 (liver), 0.99 (kidney), 0.0013 (gastrocnemius), 0.82 (iWAT) b: 0.24 c: 0.0023 d: 0.13 e: 0.08 f: 0.0081 g: 0.98 h: 1 (WT_shScr vs. WT_shGdf15), 5.5e-11 (KO_shScr vs. KO_shGdf15) i: 1 (WT_shScr vs. WT_shGdf15), 0.73 (KO_shScr vs. KO_shGdf15) j: 0.96 (KO_shScr vs. KO_shGdf15) k: 0.045 (KO_shScr vs. KO_shGdf15) l: 0.0095 (KO_shScr vs. KO_shGdf15) m: 0.97 (KO_shScr vs. KO_shGdf15). Significance symbols: *P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001; N.S., not significant. n refers to biologically independent animals. Data in this figure was performed in one biological cohort of the indicated n for each panel, without a separate biological replication cohort. Mouse strain and age: data in (a-g) was derived from 10 week old male C57BL/6 mice obtained from The Jackson Laboratory (age relative to initiation of the experiment) with the indicated AAV treatment groups and n indicated as above. Data in (h-m) was derived from 10 week old male mice of the indicated strains (WT vs. KO as indicated above and indicated AAV treatment groups) and n per group.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Tables 1–4
Supplementary Table 1: Exercise test and echocardiographic measures at rest and at peak stress before and after 6 weeks of endurance training in male WT versus KO mice. Supplementary Table 2: Characteristics of UK Biobank participants with rare PPARGC1A coding variants and all-cause heart failure. Supplementary Table 3: Exercise test and echocardiographic measures in WT and KO mice treated with AAV9-shRNA-scramble versus AAV9-shRNA-Gdf15. Supplementary Table 4: Primers for RT–qPCR.
Supplementary Data
Source data for Supplementary Figs. 1 and 2.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed and uncropped blots.
Source Data Extended Data Fig. 6
Unprocessed and uncropped blots and gels.
Source Data Extended Data Fig. 7
Unprocessed and uncropped blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khetarpal, S.A., Li, H., Vitale, T. et al. Cardiac adaptation to endurance exercise training requires suppression of GDF15 via PGC-1α. Nat Cardiovasc Res (2025). https://doi.org/10.1038/s44161-025-00712-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44161-025-00712-3
This article is cited by
-
Interaction of PGC-1α and GDF15 in the stressed heart
Nature Cardiovascular Research (2025)