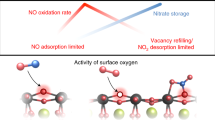
Abstract
Understanding Pd effects on NOx storage and release is crucial for designing passive NOx adsorber (PNA) to control NOx emissions during diesel cold-starts. Herein, we report two oxidation states of Pd species on CexZr1-xO2 regulated by metal-support interaction. Pdδ+ (0 < δ < 2) in Pd/Ce0.25Zr0.75O2 exhibits a high affinity for O2 adsorption, which promotes the oxidation of adsorbed NO to nitrates at 100 °C. These nitrates are thermally unstable due to electron transfer from the Pd atom to the N-O bond, facilitating the decomposition of nitrates to NO2 above 200 °C. In contrast, Pd2+ in Pd/Ce0.75Zr0.25O2 prefer to NO adsorption. A large amount of adsorbed NO and nitrites accumulate on Pd2+ and Ce4+ results in high levels of NO release below 200 °C. For the potential application in PNA, Pd/Ce0.25Zr0.75O2 is recommended due to its proper NOx release temperature as well as better water and SO2 resistance.
Similar content being viewed by others
Introduction
Increasingly stringent emission legislations pose new challenges for emission control of NOx from diesel engines. Despite the successful application of Cu-SSZ-13 in selective catalytic reduction (SCR) technology in exhaust aftertreatment to reduce NOx emissions, inadequate SCR performance at low temperature (below 200 °C) leads to upwards of 80% NOx release during the cold-start period until the exhaust reaches the light-off temperature of SCR reaction1,2. In addition, the temperature of urea injection usually needs to be above 180 °C in SCR system3,4. To address this issue, passive NOx adsorber (PNA) is designed to trap NOx below 200 °C and release them once the SCR catalysts are functioning normally.
Pd-exchanged zeolites are widely studied as PNA materials due to its high Pd dispersion and specific pore structure for NOx capture. However, high concentration of CO leads to severe deterioration of NOx adsorption in zeolites because of Pd sintering and hydrocarbon poisoning5. CeO2-based materials are potential PNA materials and it has good NOx storage capacity with abundant defects and Ce-O sites on surface6,7,8. Besides, it presents superior oxygen storage capacity and both acidic and basic sites for the adsorption and activation of gaseous molecules, making it excellent support for the Pd loading in vehicle exhaust control catalysts such as diesel oxidation catalysts or gasoline three-way catalysts9,10,11,12. Pd/CeO2 activates the lattice oxygen in surrounding interfacial zone because of metal-support interaction (MSI) mediates the unsaturated coordination environment of square planar [PdO4], obviously enhancing redox property of CeO213,14,15,16. Zr leads to change crystal asymmetry and electronic structure of CeO2 due to a smaller cation radius of Zr4+ to Ce4+ and more covalency of Zr-O than Ce-O bonds17,18. Ce-rich Pd/CexZr1-xO2 presents excellent redox ability for soot or toluene oxidations19,20, but Ce-poor Pd/CexZr1-xO2 is usually used in CO2 hydrogenations21,22. The addition of Pd in CeO2 or CexZr1-xO2 in PNA materials is considered to promote low-temperature desorption of NOx (<350 °C)23,24. Nonetheless, the roles of Pd and CexZr1-xO2 on NOx storage and release behaviors remain unclear.
The adsorption of gaseous molecules and their reaction pathways are largely influenced by the charge transfer between metal oxides and adsorbed molecules. This transfer can be tailored by the metal-support interaction (MSI), which promotes charge redistribution of Pd or Pt by tuning the d-band structure25,26,27. Consequently, the valence state and coordination of noble metals result in different effects on molecules adsorption, the energy barrier, and the rate-limiting step. For example, the electron-rich Pt with low oxidation states induced by MSI facilitates electrophilic O2 transformation to nucleophilic oxygen species, while electron-depleted Pt with high oxidation state is conducive to dehydrogenation reactions and acetone oxidation28,29,30. In terms of PNA materials design, the adsorbed NOx, nitrites and nitrates formation as well as decomposition could be regulated by the MSI between Pd and supports. Understanding the relationship between NOx adsorption strength and MSI is necessary to realize stable NOx storage and prompt release at different operation stages.
Herein, we present a simple method to control the intensity of MSI between Pd and CexZr1-xO2 solid solution by varying the molar ratios of Ce to Zr. Among the 5 prepared samples, we choose two, the Pd2+/Ce0.75Zr0.25O2 and Pdδ+/Ce0.25Zr0.75O2 (denoted as Pd/0.75Ce and Pd/0.25Ce), for the further investigation. The high-resolution transmission electron microscopy (HRTEM), X-ray absorption fine spectroscopy (XAFS), X-ray photoelectronic spectroscopy (XPS) and CO titration confirm the valence and coordination of Pd and chemical structure of supports. In situ diffuse reflectance infrared Fourier transform spectrum (DRIFTS), near ambient pressure XPS (NAP-XPS) and density functional theory (DFT) calculations elucidate the reaction pathways and charge transfer processes. The results demonstrate that relatively strong MSI of Pd/0.25Ce presents excellent PNA performance with facile nitrate formation and suitable NO2 desorption temperatures. This pivotal discovery offers a comprehensive insight into NOx storage and release mechanism on Pd-based oxides and vitally illuminates the path for advanced development of PNA materials.
Results and Discussion
Textural properties and chemical structures
Details of the sample synthesis, the PNA performance evaluations (Supplementary Fig. S1), physiochemical characterizations, and DFT calculations (Supplementary Fig. S2) are presented in the supplementary information. An in-depth exploration on the morphology, composition, and crystal structure of Pd/0.25Ce and Pd/0.75Ce catalysts was studied via HRTEM (Fig. 1a, Supplementary Fig. S3 and S4), energy-dispersive X-ray (EDX) mapping (Supplementary Fig. S5), and X-ray diffraction (XRD) patterns (Fig. 1b). These results suggest that the two catalysts both show a face-centered cubic fluorite-like phase (PDF#97-016-9029), and the (111) facet is the principal exposed plane. Compared to Pd/0.75Ce, the diffraction peak of Pd/0.25Ce shifts slightly to higher degrees and the interplanar spacing of (111) facet contracts (Fig. 1b), confirming a lattice contraction by the replacement of Ce4+ (0.97 Å) with Zr4+ (0.84 Å)18,31. This observation is further corroborated by optimized unit cells of Ce0.75Zr0.25O2 and Ce0.25Zr0.75O2 models in DFT calculations (Supplementary Fig. S2), in which the Ce0.25Zr0.75O2 model manifests smaller lattice constants than the Ce0.75Zr0.25O2. Besides, XRD patterns reveal a peak broadening of Pd/0.75Ce and Pd/0.25Ce compared to Pd/CeO2, indicating that Zr addition leads to a decrease in particle size according to Scherrer equation. The HRTEM images show that average particle sizes of Pd/0.75Ce and Pd/0.25Ce are within 6 and 7 nm, which is consistent to the results calculated from XRD (Supplementary Table S1). Raman spectra (Supplementary Fig. S6) further confirm that the Pd/0.75Ce exhibits the cubic fluorite-like structure with F2g mode at 469 cm−1 and the Pd/0.25Ce demonstrate a pseudo-cubic structure between cubic fluorite-like phase CeO2 and tetragonal ZrO2 due to the appearance of bands at 136, 248, 312, and 616 cm−1 7,14. Besides, the N2 physisorption results (Supplementary Fig. S7) show that the BET specific surface area of Pd/0.75Ce with micropore is 64.269 m2·g−1 and that of Pd/0.25Ce is 27.341 m2·g−1 with slit-structured mesopores.
a High-resolution transmission electron microscopy (HRTEM) images and particle sizes distribution. b X-ray diffraction (XRD) patterns. c Normalized X-ray absorption fine spectroscopy (XAFS) of O K-edge. d In situ diffuse reflectance infrared Fourier transform spectrum (DRIFTS) of CO adsorption at 40 °C (1% CO+99% N2, a total flow rate of 50 mL·min-1). e Fourier-transformed extended X-ray absorption fine structure (EXAFS) spectra of Pd K-edge for Pd/0.75Ce, Pd/0.25Ce, Pd foil and PdO in the R-space. f Hydrogen temperature programmed reduction (H2-TPR) profiles.
The O K-edge soft-XAFS was employed to probe the covalency between oxygen and metals (Fig. 1c). Three feature peaks labeled as 4f0, d-eg, and d-t2g are indicative of the excitation traces from O 1 s to Ce 4 f, Ce 5d-eg/Zr 4d-eg, and Ce 5d-t2g/Zr 4d-t2g, respectively32. Compared with the Pd/0.75Ce, the energy shift of O 2p-Ce 4 f in Pd/0.25Ce to higher energy is related to the occupancy of Ce 4 f orbitals, suggesting the electrons transfer from O to Ce. To discern surface O species, O 1 s XPS (Supplementary Fig. S8) results present a higher percentage of surface oxygen (O2-, O22- or O-) is yielded on the Pd/0.25Ce, suggesting that the adsorption-activation of O2 is promoted derived from the unsaturated sites of Pd/0.25Ce33. Furthermore, the lattice oxygen (O2-) peak of Pd/0.25Ce shifts to a higher binding energy, indicating a decrease in electron cloud density around O2- due to electron transfer from O2- to Ce. To confirm these results, both the Ce L3-edge (Supplementary Fig. S9) and M4,5-edge (Supplementary Fig. S10) XAFS spectra exhibit a lower oxidation state of Ce in Pd/0.25Ce relative to Pd/0.75Ce, which is primarily due to lower extent delocalization of f-electrons. However, the XPS spectra of Zr 3 d (Supplementary Fig. S11) do not show any notable shift in the two catalysts, suggesting that Zr as a structural promoter induces the formation of pseudo-cubic phase in the Pd/0.25Ce, which disturbs the electronic structure of lattice and facilitates the charge redistribution between Ce and O2-34.
To understand the oxidation state of Pd, in situ DRIFTS of CO adsorption was performed (Fig. 1d). The peaks at 2149 and 2099 cm−1 can be attributed to the vibrations of linear CO on Pd linked with support through two surface-bridged oxygens (Pd2+) and Pd linked with support by a surface-bridged oxygen (Pdδ+, 0 < δ < 2), respectively35,36. The bands in 1873 − 1916 cm−1 represent the tri-coordinated CO adsorptions (with a CO/Pd ratio of 1:3) and isolated-bridged CO adsorptions (CO/Pd ratio of 1:2) on Pd0 37,38. At the initial stage of CO adsorption, only Pd2+ is detected on the Pd/0.75Ce, while both Pd2+ and Pdδ+ are observed on the Pd/0.25Ce. This suggests that the self-reduction of Pd on the Pd/0.25Ce may be triggered by the charge transfer from the pseudo-cubic structure. As the adsorption time increases, the peak of Pd2+ diminishes and the peak of Pdδ+ increases on the Pd/0.25Ce, whereas Pd2+ continues to dominate on the Pd/0.75Ce. The detection of weak bands of Pd0 clusters on the Pd/0.25Ce can be attributed to the CO-assisted Pd migration39,40. Comparatively, the Pd/0.75Ce presents more stable Pd2+ with less Pd migration. These findings indicate that Pd is more prone to reduction on the Pd/0.25Ce. Additionally, Supplementary Fig. S12 exhibits that the Pd K-edge X-ray absorption near edge structure (XANES) spectrum of Pd/0.25Ce shifts closer to that of Pd foil compared to Pd/0.75Ce, and the binding energy of Pd 3 d on the Pd/0.25Ce is lower than that on Pd/0.75Ce according to the XPS results. All these results demonstrate lower oxidation of Pd on Pd/0.25Ce vs. Pd/0.75Ce, confirming the formation of Pdδ+ on the Pd/0.25Ce.
The coordination environment of Pd was investigated by the extended X-ray absorption fine structure (EXAFS) of Pd K-edge (Fig. 1e). The first shell peak centered at 1.53 Å is attributed to the Pd-O bond similar as PdO reference, while the second shell peak at 2.55 Å is assigned to the Pd-Pd bond for Pd clusters because it is too short in distance relative to common Pd-Pd bond for PdO reference. Compared with the Pd/0.75Ce, the coordination numbers (CNs) of Pd-O bond on the Pd/0.25Ce are smaller (Supplementary Table S2), which is attributed to the formation of low-coordinated Pdδ+ cations rather than 5-fold coordinated Pd2+. Notably, Fig. 1d exhibits that Pd could connect to support via two oxygens, which could be caused by the extraction of ligand oxygen of 5-fold coordinated Pd2+ by CO. The CNs of Pd-Pd bond are calculated to be 2.5 for the Pd/0.75Ce and 4.0 for the Pd/0.25Ce, respectively (Supplementary Table S2). This suggests that large Pd clusters may be present on the Pd/0.25Ce. The contour plots of wavelet transform of EXAFS results (Supplementary Fig. S13) exhibit two distinct regions at (~1.53 Å, ~5 Å−1) and (~2.55 Å, ~9 Å−1), corresponding to the Pd-O and Pd-Pd bonds, respectively. The weaker Pd-O bond on the Pd/0.25Ce confirms the formation of Pdδ+ with lower oxygen ligands.
Hydrogen temperature programmed reduction (H2-TPR) measurement was applied to evaluate the strength of MSI between Pd species and supports via measuring reducibility (Fig. 1f). The peak at approximately 80 °C is attributed to the reduction of PdO41, and the sharp peak at 97 °C originates from the Pd-O-Ce interface reduction6. The broad peaks above 250 °C are attributed to the reduction of O2- on supports. For the Pd/0.25Ce, smaller peaks of PdO reduction at 77 °C could be ascribed to the low oxidation state of reducible Pd species on the surface. The broadening peak at 97 °C and the appearance of weak peak at 143 °C indicate that the Pd/0.25Ce presents stronger MSI than the Pd/0.75Ce resulting in surface lattice reduction via H2 spillover. Further, charge transfer was investigated by the charge density difference and Bader charge based on DFT calculations (Supplementary Fig. S14), which highlights that more electrons tend to accumulate around Pd on the Pd/0.25Ce. The above results illustrate that the Pdδ+ formation is originated from the charge transfer from Ce0.25Zr0.75O2 to Pd.
Performance of NOx storage and release
The protocol of performance evaluation is illustrated in Supplementary Fig. S1. The curves of NOx adsorption at 100 °C and temperature-programmed desorption (TPD) in 100 − 550 °C are displayed in Supplementary Fig. S15. The negative peaks below 210 ppm represent the NO storage process, while the positive peaks above 210 ppm correspond to the NOx release process. The temperature of NOx desorption decreases as the decline of Ce content. Moreover, the Pd/0.75Ce and Pd/0.25Ce exhibit higher NOx storage capacities than the other samples under the wet condition (Supplementary Fig. S16). Figure 2a, b display different NOx concentrations of Pd/0.75Ce and Pd/0.25Ce under the dry and wet conditions. At the adsorption stage, the Pd/0.75Ce shows a decline in NOx storage under the wet conditions, whereas the Pd/0.25Ce demonstrates better water resistance. At 100 °C, the adsorption capacity of Pd/0.25Ce reaches 85.8 μmol· gcat−1, outperforming Pd/0.75Ce, which has an adsorption capacity of 56.8 μmol· gcat−1 in the first cycle (Fig. 2c). Although the Pd/0.75Ce achieves a NOx adsorption of 63.5 μmol· gcat−1 above 100 °C, approximately 56.0% of this adsorption takes place around 300 °C, a temperature at which the commercial SCR catalyst is already active. Additionally, the Pd/0.25Ce exhibits a deeper NOx uptake peak compared to Pd/0.75Ce, corresponding to a higher NOx storage efficiency (NSE) (Fig. 2d). Whereas, at the desorption stage, the Pd/0.75Ce displays a dual-peak pattern and the Pd/0.25Ce shows a single-peak pattern (Fig. 2a, b) regardless of the dry or wet conditions. The concentration curves of NO, NO2, CO, and CO2 during the performance evaluation are displayed in Supplementary Fig. S17 and S18. The first desorption peak below 200 °C of Pd/0.75Ce is due to the NO release, which is inhibited on the Pd/0.25Ce. The NOx desorption amount is shown in Supplementary Fig. S19, demonstrating that more NO release from the Pd/0.75Ce. Above 200 °C, NO2 is rapidly released from the Pd/0.25Ce, while the release is relatively slow on the Pd/0.75Ce (Supplementary Fig. S17 and S18). We speculate that the Pd/0.25Ce promotes the oxidation of adsorbed NO to more stable forms such as nitrites or nitrates which could decomposition to NO2 above 200 °C. To confirm the NO2 release behaviors, NO + O2-TPD was performed and the results (Supplementary Fig. S20) show that the NO2 release from Pd/0.25Ce (68.6 μmol·gcat−1) is higher than that from Pd/0.75Ce (47.3 μmol·gcat−1). We also calculated the SBET normalized reaction rates for NO oxidation (Supplementary Fig. S21). Larger reaction rate for the Pd/0.25Ce below 200 °C indicates its stronger oxidation ability. These results indicate that the Pd/0.25Ce favors the transformation of adsorbed NO to NO2. The different NOx storage and release behaviors of Pd/0.75Ce and Pd/0.25Ce could be attributed to the effects of chemical properties of Pd and supports, which will be discussed in the following sections.
NOx concentration of (a) Pd/0.75Ce and (b) Pd/0.25Ce under the dry and wet condition. c The calculated NOx adsorption amount. d NOx storage efficiency (NSE) and (e) NOx desorption efficiency (NDE) of Pd/0.75Ce and Pd/0.25Ce. Reaction conditions: 210 ppm NO, 2100 ppm CO, 10 vol% O2, 300 ppm C3H6 (when used), 5 vol% H2O (when used), balanced with N2 at a flow rate of 200 mL· min-1.
Furthermore, effects of other gas components on NOx storage and release performance were also examined. For H2O, the concentration curves (Supplementary Fig. S22) indicate that more H2O adsorbs on the Pd/0.75Ce compared to the Pd/0.25Ce, likely due to the higher hydrophilicity. A H2O desorption peak is observed around 350 °C on Pd/0.75Ce, which aligns with the second NOx adsorption peak in Fig. 2a. This suggests that H2O desorption reveals NO adsorption sites. We also investigated the impact of H2O on the NOx storage and release of Pd/0.25Ce, finding that NO adsorption decreases from 88.4 to 77.9 μmol· gcat−1 and NSE also declines with increasing the H2O concentration (Supplementary Fig. S23). However, a high concentration of H2O primarily impacts NO adsorption at low temperatures, with minimal effect on high-temperature NOx release, as the NOx desorption curves remain nearly identical in both 5 vol% and 8 vol% H2O conditions. Figure 2d, e and Supplementary Fig. S24 illustrates the effect of hydrocarbon (C3H6) on the PNA performance. Compared to the NO + CO atmosphere, the addition of C3H6 facilitates the NO adsorption at 100 °C. The adsorption amount of Pd/0.75Ce and Pd/0.25Ce are 140.10 and 88.11 μmol· gcat−1 in the presence of C3H6, which could be attributed to the improvement in hydrophobicity. The NOx desorption efficiency (NDE) has a decrease for both the Pd/0.75Ce and Pd/0.25Ce. This is due to N2O formation at the beginning of C3H6 oxidation, which is also observed in our previous works42,43. Finally, the impacts of SO2 on NOx storage capacity were performed (Supplementary Fig. S25). The 5-minute NSE of Pd/0.25Ce reaches 54.87% while that of Pd/0.75Ce is 49.00%, demonstrating that the Pd/0.25Ce exhibits a better SO2 resistance than the Pd/0.75Ce. All the results suggest that the Pd/0.25Ce is more suitable for low-temperature NOx adsorption under realistic exhaust conditions.
Evolutions of N-contained species
The evolutions of surface species during NOx storage and release processes were explored by in situ DRIFTS. The assignments of surface nitrites and nitrates are listed in Supplementary Table S3. For the Pd/0.75Ce (Fig. 3a), bidentate nitrite (1220−1160 and 1300 cm−1), nitro-nitrite (1202 and 1525 cm−1) and anionic nitrosyl NO- (1167 cm−1) are detected at the initial stages of NO and O2 introduction, followed by the trivial generation of monodentate nitrate (1009, 1245, and 1539 cm−1) and monodentate nitrite (1068 and 1450 cm−1) and nitro (1345 and 1406 cm−1) species. As the adsorption process proceeds, the occurrent of sharp peaks in 1540–1620 cm−1 indicates the formation of stable chelating and bridging-bidentate nitrates on the Pd/0.75Ce. With increasing the temperature up to 500 °C, a rapid diminution of peak at 1167 cm−1 and bands in 1300 − 1500 cm−1 are observed. This phenomenon is attributed to two factors. First, these nitrites decompose into gaseous NO, aligning with the NO desorption peak below 200 °C (Fig. 2a and Supplementary Fig. S17). Second, nitrites transform into nitrates, which is confirmed by the increased peaks in the 1540 − 1620 cm−1 range (chelating and bridging bidentate nitrates). This transformation derives from oxidation of nitrites by active lattice oxygen motivated by increasing temperature according to Mars-van-Krevelen (MvK) mechanism44. These formed nitrates are stable on the Pd/0.75Ce and cannot decompose easily to NOx below 400 °C.
In situ diffuse reflectance infrared Fourier transform spectrum (DRIFTS) in 20 min of NO + O2 adsorption and subsequent desorption for (a) Pd/0.75Ce and (b) Pd/0.25Ce between 100 and 500 °C. c In situ near ambient pressure X-ray photoelectronic spectroscopy (NAP-XPS) of N 1 s at 100 °C and 300 °C in NO atmosphere. d Binding energy shifts of O 1 s based on in situ NAP-XPS between 100 and 400 °C.
Figure 3b presents the DRIFTS spectra for the Pd/0.25Ce. Similar to Pd/0.75Ce, the Pd/0.25Ce initially exhibits a broad peak at 1179 cm−1 during NO and O2 adsorption, suggesting the formations of bidentate nitrite and anionic nitrosyl NO-. However, no band is detected in 1300–1500 cm−1, suggesting that monodentate nitrite and nitro compounds are suppressed on the Pd/0.25Ce due to the lack of Ce4+-O sites and a lower Ce oxidation state. New peaks at 1003, 1210–1240, and 1575 cm−1 can be attributed to chelating bidentate nitrates, and weak shoulders at 1250 and 1500 cm−1 is due to monodentate nitrate. Moreover, peak at 1179 cm−1 start to decrease and peak at 1220 cm−1 gradually increases after 6 min, indicating the conversion of NO- to bidentate nitrite. Meanwhile, two peaks at 1560 and 1619 cm−1 also increase, which demonstrates the formation of chelating bidentate and bridging bidentate nitrates. This observation implies the oxidation pathway of NO(g) → NO- → NO2- → NO3- at 100 °C. With increasing the temperatures, both chelating and bridging bidentate nitrates undergo gradual decomposition to NO2 and disappear at 450 °C. In contrast, these nitrates remain stable on the Pd/0.75Ce at the same temperature. These results indicate that the two catalysts exhibit distinct NOx storage and release behaviors, differing in both the distribution of nitrite or nitrate species and their thermal stability.
To further investigate the mechanism of NOx storage and release, DRIFTS of NO adsorption without O2 was performed (Supplementary Fig. S26). Bridging bidentate (1615, 1220, and 1000 cm−1) and monodentate nitrate (1530, 1290, and 1000 cm−1) are rapidly generated on the Pd/0.75Ce in the beginning of NO adsorption and then nitrosyl (NO-) is detected at 1170 cm−1. A sharp peak of nitrosyl can be observed on the Pd/0.25Ce and other peaks assigned to nitrates are very weak. These results indicate that the NO- of Pd/0.25Ce cannot be oxidized to nitrites and nitrates in the absence of O2. The O 1 s spectra (Supplementary Fig. S8) reveal that the Pd/0.25Ce provides more surface oxygen than the Pd/0.75Ce, which do not seem to promote NO oxidation in the absence of O2. This can be explained by more EPR-active O2- (attributable to an unpaired electron, Supplementary Fig. S27) bonded to oxygen vacancies on Pd/0.75Ce. It was proposed that superoxide (O2-) is an active oxygen for NO oxidation since peroxide (O22-) is regarded as an spectator for this process8. This implies that surface oxygen species are mainly O22- or O- on the Pd/0.25Ce. Therefore, nitrates formation and NO2 release on the Pd/0.25Ce relies on the presence of O2, which is related to Langmuir-Hinshelwood (L-H) mechanism.
In situ NAP-XPS spectra of N 1 s in NO were used to further identify the chemisorbed nitrogenous species. The peaks in Fig. 3c were deconvoluted using the Gaussian-Lorentzian line shape and the attribution of peaks is based on previous works45,46. More NO(g) on the Pd/0.75Ce is associated with a large amount of surface Ce4+ cations as adsorption sites. An abundance of metal-NO3- on the Pd/0.75Ce originates from NO oxidation by O2- on oxygen vacancy. The percentage of atomic N at 398.5 eV in Pd/0.25Ce is higher than that in Pd/0.75Ce, which results from NO- decomposition due to injection of electrons from Pdδ+ into the N-O antibonding orbital. Upon heating to 300 °C, more metal-NO2 formed on Pd/0.25Ce due to the oxidation of metal-NO and atomic N and metal-NO3- decomposition. However, considerable percentage of metal-NO3- species are still existed on Pd/0.75Ce, indicating they are thermally stable at this temperature. Supplementary Fig. S28 displays in situ NAP-XPS spectra of the adsorption of NO or NO + O2 at 100 − 400 °C for 30 min, and Fig. 3d exhibits the corresponding shifts of the binding energy of O 1 s spectra. During NO adsorption at 100 °C, a noticeable shift is observed on both catalyst, assigned to the formation of surface metal-NO/NO2/NO3 species47. With increasing the temperatures, the shift continues to rise in NO atmosphere at 200 °C, potentially due to atomic N oxidation. The similar energy shift on the Pd/0.75Ce at 300 °C in the NO and NO + O2 is attributed to the enhanced mobility of lattice oxygen, which promotes the oxidation of NO to nitrate. An obvious increase of energy shift on the Pd/0.25Ce during NO + O2 atmosphere at 100 °C, indicating that the presence of O2 facilitates nitrates formation. The energy shift on the Pd/0.25Ce decreased under NO + O2 atmosphere at 400 °C, while that on the Pd/0.75Ce remains unchanged, suggesting that metal-NO3 species are more stable on Pd/0.75Ce.
In situ Raman spectra of NO + O2 adsorption (Supplementary Fig. S29) display that a redshift of F2g (469 cm−1) mode by 1.44 cm−1 on the Pd/0.75Ce during NO + O2 adsorption at 100 °C. This shift is indicative of the creation of oxygen vacancies, thereby corroborating the participation of Ce4+-O sites in the transformation of NO into nitrite/nitrate species8,40. Furthermore, no phase reconstruction is detected on both catalysts, signifying that their framework are stable.
The effects of H2O on N-contained species formation were investigated by in situ DRIFTS as shown in Supplementary Fig. S30. Water induces more ionic nitrites and ionic nitrates formation on Pd/0.75Ce, indicating that hydroxyl groups could react with NO to generate HNO2 which is further oxidized to form HNO3. The surface hydroxyl groups are exhibited in Supplementary Fig. S31. Water addition leads to the increment of peak at 3688 cm−1 on the Pd/0.75Ce, indicating the transformation of adsorbed water to bridging hydroxyl groups48. However, this phenomenon is minimized on the Pd/0.25Ce. The formation of hydroxyl groups such as Pd-O(H)-Ce suppress interfacial oxygen reactivity for nitrates formation. The influences of CO on the NOx storage and release processes were also examined (Supplementary Fig. S32). New peaks around 2163 cm−1 on both catalysts could be assigned to Pd2+(CO)(NO) or surface hydroxyl groups, such as Pd2+(OH)(CO)23,49. However, this peak is not detected within the in situ DRIFTS spectra of CO adsorption (Fig. 1d), indicating the new peak exclusively represents the Pd2+(CO)(NO). As the temperature rises, the intensities of peaks associated with chelating and bridging nitrates diminish in the presence of CO, implying a positive influence of CO on the decomposition of nitrates. This effect is attributed to the generation of local ‘hot spots’ during CO oxidation as an exothermic reaction50.
DFT calculations
The calculated DFT models are denoted as the Pd/0.25Ce (111) and Pd/0.75Ce (111) slab models, respectively. As illustrated in Fig. 4a, adsorbed oxygen is more prone to activation and reacts with adsorbed NO to yield nitrates on the Pd/0.25Ce (111). The formation energies of chelating (che) and bridging (bri) nitrates of Pd/0.25Ce (111) are lower at -1.66 and -1.99 eV, respectively compared to Pd/0.75Ce (111) (-1.24 and -1.04 eV). To understand the interaction between Pd and nitrate species in the conversion to NO2, we studied the electronic structures of che and bri nitrates on the optimized models. The charge density difference (Fig. 4a and Supplementary Fig. S33) reveals that the charge enriched on che and bri nitrates and the charge lost on Pd, leading to the electron transfer from Pd to nitrates. A noticeable decrease is detected in the charge density of the N-O bond attached to Pd compared to other N-O bonds. The Bader charge of Pd on the Pd/0.25Ce (111) is smaller than that on the Pd/0.75Ce (111), suggesting that more electron transfer from Pd to nitrates occurs on Pd/0.25Ce (111), which facilitates the N-O breakage in nitrate species. Consequently, both the che and bri nitrates of Pd/0.25Ce (111) are relatively less stable compared to Pd/0.75Ce (111).
a Free energy diagrams of nitrates evolution and difference charge density of che and bri nitrates. b Crystal orbital Hamilton populations (COHP) of Pd-O (Nitrates) bonds of Pd/0.75Ce (111) and Pd/0.25Ce (111) in bri and che nitrates. c Adsorption energies of O2 and NO on Pd sites. d Schematic overview of NOx storage and release processes on the Pd/0.75Ce and Pd/0.25Ce catalysts.
The project density of states (PDOS) and crystal orbital Hamilton populations (COHP) further elucidated the bonding features of Pd to nitrates. A stronger hybridization is observed between the O 2p orbitals of nitrates and Pd 4 d orbitals in the valence band of Pd/0.75Ce (111) (Supplementary Fig. S34). However, the top of O 2p and Pd 4 d orbitals mix in the valence band corresponding to antibonding state (Fig. 4b and Supplementary Table S4). Less antibonding portions below the Fermi level implies more stable bonding, i.e., a more stable Pd-O (nitrates) bond forms on the Pd/0.25Ce (111). Similarly, the -ICOHP values of bri (1.001) and che (1.105) nitrates on the Pd/0.25Ce (111) are higher than those on the Pd/0.75Ce (111) (0.868 and 0.303 a.u., respectively). Such enhancement of Pd-O (nitrates) bond promotes the breakage of N-O bond in nitrates to yield NO2 on the Pd/0.25Ce (111). Correspondingly, the energy differences in the decomposition of che and bri nitrates (Fig. 4a) to NO2 on the Pd/0.25Ce (111) (0.68 and 0.62 eV) are lower than those on the Pd/0.75Ce (111). This was also consistent with in situ NAP-XPS results in Fig. 3c, which shows that more -NO2 species are detected on the Pd/0.25Ce at 300 °C. The similar decomposition patterns of monodentate nitrate are also shown in Supplementary Fig. S35. Therefore, the strong orbitals hybridization between the O 2p orbitals of nitrates and Pd 4 d orbitals leads to more stable Pd-O bonds of Pd/0.25Ce, promoting the breakage of N-O bonds.
To further reveal the process of NO transformation, adsorption energies of O2 and NO were calculated (Fig. 4c). Notably, the Pd/0.25Ce (111) demonstrates a higher affinity for gaseous O2 adsorption due to the presence of Pdδ+, which provides adequate adsorbed O2 for the transformation of adsorbed NO into nitrates. Conversely, the Pd/0.75Ce (111) prefers to adsorb NO and large amounts of adsorbed NO and nitrite species cannot be effectively oxidized due to a limitation of oxygen supplementation at low temperatures, resulting in premature NO release during desorption stage. A schematic overview of Pd-assisted NOx storage and release mechanism is depicted in Fig. 4d. During NOx storage, Pd2+ and Ce4+ sites on the Pd/0.75Ce prefer to NO adsorption and part of adsorbed NO could be oxidized to nitrates by superoxide (O2-) (Eq. 1). Other adsorbed NO accumulates on the surface and needs to be further oxidized by lattice oxygen at high temperature following the MvK mechanism. (Eq. 2) Pdδ+ on the Pd/0.25Ce exhibits high affinity for O2 adsorption and activation (Eq. 3), which facilitates the formation of nitrates from adsorbed NO via the L-H mechanism (Eq. 4, M represents Pd or Ce sites). During NOx release, nitrites on the Pd/0.75Ce either decompose to NO or oxidize to form stable nitrates. In contrast, nitrates on the Pd/0.25Ce decompose into NO2 due to the electron transfer from Pdδ+ to the nitrates.
Conclusion
This work provides insights into the role of Pd oxidation states in NOx storage and release behaviors on CexZr1-xO2 supports. The Pd/0.25Ce shows a lower Pd valence than the Pd/0.75Ce due to more electrons transfer from Ce0.25Zr0.75O2 support to Pd. The PNA performance exhibits that the Pd/0.25Ce displays a suitable NOx release temperature with a singular peak around 314 °C and good resistance to H2O and SO2. In situ spectroscopies and DFT results reveal that Pdδ+ of Pd/0.25Ce easily forms nitrates due to its high affinity for O2 adsorption which accelerates adsorbed NO oxidation via L-H mechanism, while Pd2+ and Ce4+ of Pd/0.75Ce prefer to form nitrites as a result of higher O2 but lower NO adsorption energies. In the NOx desorption process, nitrates on the Pd/0.25Ce decompose into NO2 due to electrons transfer from Pdδ+ to the nitrates. In contrast, nitrites either decompose to NO below 200 °C or gradually oxidize to stable nitrates by lattice oxygen at high temperature on the Pd/0.75Ce. This work could shed light on the strategic design of Pd-based PNA materials for NOx control of diesel exhaust aftertreatment.
Methods
Samples preparation
A series of CexZr1-xO2 supports with different Ce/Zr ratios were synthesized by sol-gel method. A certain amount (total metal atoms are 0.04 mol) of Ce(NO3)3·6H2O and Zr(NO3)4 were added into the deionized water to prepare the aqueous solution of 100 mL named A (stirred at 80 °C to dissolve the Zr salt). 0.10 mol citric acid and 0.1 mol ethylene glycol were taken to configure aqueous solution of 100 mL denoted B. Solutions A and B were mixed and stirred in a water bath at 80 °C until gelation, then dried overnight in a drying oven at 110 °C. The obtained solid was ground into a powder and placed in a muffle furnace for calcination. The calcination process involved a heating rate of 5 °C min−1, reaching a temperature of 300 °C and holding for 1 h, followed by further heating to 550 °C with a 5 h calcination. These obtained samples were denoted as xCe (x = 0, 0.25, 0.50, 0.75, 1.00, for example, 0.25Ce represents Ce0.25Zr0.75O2).
The equal volume impregnation method was employed to load the above supports with palladium. First, the water absorption volume of the carrier was measured. Specifically, deionized water was added dropwise to submerge 1 g of support for 30 min, followed by wiping the free water on the surface with absorbent paper and then weighing, and calculating the difference in mass between the before and after. The Pd(OAc)2 impregnation solution (dissolved with concentrated ammonia) was configured according to the water absorption volume, then added to the support and mixed to form a thick paste, left for 24 h at room temperature. Finally, the dry paste was transferred to a drying oven at 80 °C for 12 h, ground into a powder and then roasted at 500 °C for 4 h in a muffle furnace heated at a rate of 5 °C min−1. The obtained catalyst was denoted as Pd/xCe. The loadings of Pd in the Pd/0.25Ce and Pd/0.75Ce according to the ICP experiments were 0.41 and 0.42 wt%, respectively.
PNA performance evaluation
PNA performance evaluation were carried out in experimental devices as shown in Supplementary Fig. S1. Before the experiments, samples of 120 mg were pretreated at 500 °C for 30 min under 10 vol% O2 flow (N2 as the balance gas) to remove adsorbed impurities. At the beginning of the experiment, the gas was switched from the bypass to the pretreated samples which were exposed to gas mixtures that consisted of 210 ppm NO + 2100 ppm CO + 10 vol% O2 balance N2 with flow rate of 200 mL· min−1 at 100 °C for the isothermal adsorption of NOx. The duration of the adsorption process is 10 min. For NOx desorption, feed gas remained unchanged and the reactor was heated to 550 °C at a temperature rise rate of 10 °C min−1. The entire test protocol was shown in the Supplementary Fig. S1. H2O resistance tests were performed with the same experimental procedures as above and 5 vol% H2O was added into the feeding gas during adsorption and desorption. The outlet NOx concentration was detected using the MultiGas 2030 FTIR gas analyzer (MKS Instruments, USA).
The amount of NOx adsorption or desorption was calculated from an integration of the adsorption/desorption profile according to the following equation1.
where \({Q}_{{{NO}}_{x}}\) (μmol· g−1) corresponds to the amount of NOx adsorption/desorption. F (mL· min−1) represents the gas flow. Fin (ppm) is the initial NOx and Fout (ppm) is the outlet NOx. The mcat (g) represents the weight of sample, and t (min) represents the duration of adsorption process.
The error bars were calculated by standard deviation according to the following equation.
where S represents the standard deviation and n represents the number of tests (all tests are repeated three times as shown in Supplementary Fig. S16). \(\overline{{Q}_{{{NO}}_{x}}}\) (μmol· g−1) corresponds to the mean value of \({Q}_{{{NO}}_{x}}\).
NOx storage efficiency (NSE) and NOx desorption efficiency (NDE) were calculated by following equations2.
where tads represents the NOx storage time. t(T0) is the start time of desorption stage. t(T) is the end time of desorption stage.
The NO conversion (X) and SBET normalized reaction rates (rS, mol·m-2·s−1) were calculated by the following equations.
Characterizations
X-ray diffraction (XRD) results were obtained by a Rigaku X-ray diffractometer (D/max-2200) fitted with a Cu Kα source (λ = 0.15405 nm, 40 kV and 200 mA). The Raman spectra were recorded by Renishaw confocal Raman spectrometer (RM2000) with a 532 nm laser. Transmission electron microscope (TEM) and high-Resolution transmission electron microscopy (HRTEM) images were obtained from JEM F200 electron field emission transmission electron microscope (JEOL, Japan). Inductively Coupled plasma-optical emission spectroscopy (ICP-OES) measurements were carried out on IRIS Intrepid II ICP spectrometer (Thermo Scientific, USA). N2 adsorption-desorption curves were carried out on Belsorp max II (MicrotracBEL Corp.). X-ray photoelectron spectroscopy (XPS) results were obtained by a multifunctional imaging electron spectrometer (VG, ESCALAB 250XI, Thermo Scientific, Surrey) with Al Kα (hν = 1486.6 eV) radiation. Electron paramagnetic resonance (EPR) was carried out on Bruker A300 at room temperature using amplitude modulation of 10 G and frequency modulation of 100 kHz. Hydrogen temperature programmed reduction (H2-TPR) was carried through a Micromeritics Autochem II 2920 apparatus. Temperature programmed desorption of NO + O2 (NO + O2-TPD) was performed on MKS MultiGas 2030 HG. The soft X-ray absorption spectra (XAS) at O K-edge and Ce L/M-edge, X-ray absorption fine structure (XAFS) spectra including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) at Pd K-edge were collected at the Singapore Synchrotron Light Source (SSLS) center.
In situ diffuse reflaxions infrared fourier transformations spectroscopy (In situ DRIFTS) spectra of gas molecule adsorption were determined on a FTIR spectrometer (Nicolet IS-50) equipped with a reaction cell (Harrick). All samples were pretreated under O2 + N2 at 350 °C for 30 min with a total gas flow of 50 mL· min−1. All of the spectra were recorded by accumulating 16 scans with a resolution of 4 cm−1. (1) For CO adsorption, the reactor was cooled to 40 °C and the background was recorded in a pure N2 gas flow and then 1000 ppm CO with the balance of N2 were introduced into the reaction cell and the spectra were collected every minute. (2) For NOx adsorption and desorption, the background spectra were sequentially collected in a pure N2 gas flow at 450, 400, 350, 300, 250, 200, 150, and 100 °C. For the adsorption stage, the spectra were collected every minute and the adsorption stage lasted 20 min in gas mixture atmosphere (1000 ppm NO + 10 vol. % O2 + 1000 ppm CO (when used) + 3 vol% H2O (when used)). During the desorption stage, only N2 was present in the gas flow and the spectra were recorded at temperatures of 150, 200, 250, 300, 350, 400, and 450 °C, respectively.
In situ near-ambient pressure X-ray photoelectron spectroscopy (in situ NAP-XPS) measurements were carried out on a SPECS NAP-XPS system. All spectra were obtained using monochromatized Al Kα irradiation (1486.6 eV) generated by an Al anode (SPECS XR-50) and an excitation source power fixed at 50 W. XPS measurements at pressures up to 20 mbar is possible owing to a differential pumping system, which separates the electron analyzer (SPECS Phoibos NAP-150) from the reaction area. The samples were heated up to 350 °C under vacuum and held for 30 min, then cooled down to 100 °C to test the full and fine spectra, and the fine spectra were tested after passing 2 mbar NO or 1 mbar NO + 1 mbar O2 and holding the temperature at 100, 200, 300, and 400 °C for 30 min, respectively.
Computational details
All DFT + U calculations in this paper were carried out using the Vienna Ab-initio Simulation Package (VASP). Core electrons were described by the projected-augmented wave (PAW) pseudopotentials. The Perdew-Burke-Ernzerhof (PBE) functional with the generalized gradient approximation (GGA) was used as exchange-correlation potential. The plane-wave cutoff energy was set to 500 eV. A value of Ueff = 4.5 eV was adopted to account for the strong on-site Coulomb repulsion between localized Ce 4f electrons. The Brillouin zone integration was sampled using the Monkhorst-Pack scheme with a k-point mesh of 5 × 5 × 5 for all bulk CexZr1-xO2 (x = 0.25, 0.75) structures and 1 × 1 × 1 for surface slab models of CexZr1-xO2(111). The convergence criteria of the force and energy were set as 0.02 eV Å−1 and 10-5 eV, respectively. Cubic fluorite-type CexZr1-xO2 bulk structures were constructed via replacing Ce atoms by Zr atoms in the face-centered cubic (fcc) fluorite-type CeO2 unit cell with space group Fm3m and bulk relaxation results are shown in Supplementary Fig. S2. The (2 × 2) surface slab models for CexZr1-xO2 were constructed with a vacuum region of 15 Å along the z-axis normal in which all surface slab models were terminated by a plane of oxygen atoms. In all relaxation calculations, the bottom two rows of the slabs were kept fixed. The position of the Pd atom anchored at the slab surface were determined by calculating the energy change of the Pd located at four hollow sites based on the symmetry of slab, in which the position corresponding to the lowest energy was used for subsequent calculations.
The adsorption energy (Eads) was calculated as
where Emolecule +slab represents the total energy of adsorbed molecule and the corresponding slab, Emolecule is the energy of the isolated molecule, and Eslab is the energy of the clean slab.
Data availability
The data that support the findings of this study are included in the published article and its Supplementary Information. Source data are provided with this manuscript and is available from the corresponding author upon request. The data of DFT calculation have been deposited in the FigShare database: https://doi.org/10.6084/m9.figshare.26788690.v1.
References
Chen, H.-Y. et al. Cold Start Concept (CSCTM): a novel catalyst for cold start emission control. SAE Int. J. Fuels Lubr. 6, 372–381 (2013).
Li, J., Meng, X. & Xiao, F.-S. Zeolites for control of NO emissions: opportunities and challenges. Chem. Catalysis 2, 253–261 (2022).
Gu, Y. & Epling, W. S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. A Gen. 570, 1–14 (2019).
Chen, Z. et al. Charge shielding effect of sodium ions in improving the hydrophobicity and performance of Co/Na‐SSZ‐13 zeolite for passive NOx adsorber. Adv. Funct. Mater. 2310682 (2024).
Ryou, Y. et al. Effect of reduction treatments (H2 vs. CO) on the NO adsorption ability and the physicochemical properties of Pd/SSZ-13 passive NOx adsorber for cold start application. Appl. Catal. A Gen. 569, 28–34 (2019).
Wang, Z. et al. Metal-support interaction induced atomic dispersion and redispersion of Pd on CeO2 for passive NOx adsorption. Chem. Eng. J. 470, 144080 (2023).
Ji, Y. et al. Pt- and Pd-promoted CeO2–ZrO2 for passive NOx adsorber applications. Ind. Eng. Chem. Res. 56, 111–125 (2017).
Filtschew, A. & Hess, C. Unravelling the mechanism of NO and NO2 storage in ceria: The role of defects and Ce-O surface sites. Appl. Catal. B Environ. Energy 237, 1066–1081 (2018).
Zhang, L. et al. Improved Pd/CeO2 catalysts for low-temperature NO reduction: Activation of CeO2 lattice oxygen by Fe doping. ACS Catal. 11, 5614–5627 (2021).
Xu, J. et al. Single-atom Rh on high-index CeO2 facet for highly enhanced catalytic CO oxidation. Angew. Chem. Int. Ed. e202302877 (2023).
Chen, S. et al. Elucidation of active sites for CH4 catalytic oxidation over Pd/CeO2 via tailoring metal-support interactions. ACS Catal 11, 5666–5677 (2021).
Khivantsev, K. et al. Single Ru(II) ions on ceria as a highly active catalyst for abatement of NO. J. Am. Chem. Soc. 145, 5029–5040 (2023).
Kim, Y. et al. Surface density dependent catalytic activity of single palladium atoms supported on ceria. Angew. Chem. Int. Ed. 60, 22769–22775 (2021).
Si, R., Zhang, Y.-W., Li, S.-J., Lin, B.-X. & Yan, C.-H. Urea-based hydrothermally derived homogeneous nanostructured Ce1-xZrxO2 (x = 0 − 0.8) solid solutions: A strong correlation between oxygen storage capacity and lattice strain. J. Phys. Chem. B 108, 12481–12488 (2004).
Li, Z. et al. (2023) Evolution hydrothermal aging resistance mechanism study of zirconium and manganese doped CeO2 catalysts in soot catalytic combustion based on low Miller indices crystal surface effect. J. Rare Earths S1002072123001515 https://doi.org/10.1016/j.jre.2023.06.001
Parastaev, A. et al. Boosting CO2 hydrogenation via size-dependent metal-support interactions in cobalt/ceria-based catalysts. Nat. Catal. 3, 526–533 (2020).
Weck, P. F., Juan, P.-A., Dingreville, R. & Kim, E. Density functional analysis of fluorite-structured (Ce, Zr)O2/CeO2 interfaces. J. Phys. Chem. C 121, 14678–14687 (2017).
Rodriguez, J. A. et al. Physical and chemical properties of Ce1−xZrxO2 nanoparticles and Ce1−xZrxO2(111) surfaces: synchrotron-based studies. J. Mol. Catal. A Chem. 228, 11–19 (2005).
Atribak, I., Bueno-López, A. & García-García, A. Combined removal of diesel soot particulates and NO over CeO2–ZrO2 mixed oxides. J. Catal. 259, 123–132 (2008).
Shah, P. M., Day, A. N., Davies, T. E., Morgan, D. J. & Taylor, S. H. Mechanochemical preparation of ceria-zirconia catalysts for the total oxidation of propane and naphthalene volatile organic compounds. Appl. Catal. B Environ. Energy 253, 331–340 (2019).
Silva, L. P. C., Terra, L. E., Coutinho, A. C. S. L. S. & Passos, F. B. Sour water–gas shift reaction over Pt/CeZrO2 catalysts. J. Catal. 341, 1–12 (2016).
Pokrovski, K., Rhodes, M. & Bell, A. Effects of cerium incorporation into zirconia on the activity of Cu/ZrO2 for methanol synthesis via CO hydrogenation. J. Catal. 235, 368–377 (2005).
Ji, Y. et al. Pd-promoted WO3-ZrO2 for low temperature NOx storage. Appl. Catal. B Environ. and Energy 264, 118499 (2020).
Jones, S., Ji, Y., Bueno-Lopez, A., Song, Y. & Crocker, M. CeO2-M2O3 passive NOx adsorbers for cold start applications. Emiss. Control Sci. Technol. 3, 59–72 (2017).
Bruix, A. et al. A new type of strong metal-support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J. Am. Chem. Soc. 134, 8968–8974 (2012).
Wang, L. et al. Electron donation promotes the dual activation of lattice oxygen and molecular oxygen: the Pt-Pd/CeO2 catalyst efficiently catalyzes toluene. J. Catal. 428, 115133 (2023).
Li, Y., Zhang, Y., Qian, K. & Huang, W. Metal–support interactions in metal/oxide catalysts and oxide-metal interactions in oxide/metal inverse catalysts. ACS Catal. 12, 1268–1287 (2022).
Jiang, Z. et al. Modulating the electronic metal‐support interactions in single‐atom Pt1 − CuO catalyst for boosting acetone oxidation. Angew. Chem. Int. Ed. 61, e202200763 (2022).
Li, J. et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal-support interactions. J. Am. Chem. Soc. 141, 14515–14519 (2019).
Xiao, M., Yu, X., Guo, Y. & Ge, M. Boosting toluene combustion by tuning electronic metal-support interactions in in situ grown Pt@Co3O4 Catalysts. Environ. Sci. Technol. 56, 1376–1385 (2022).
Chen, H.-T. & Chang, J.-G. Oxygen vacancy formation and migration in Ce1−xZrxO2 catalyst: A DFT + U calculation. J. Chem. Phys 132, 214702 (2010).
Rodriguez, J. A. et al. Properties of CeO2 and Ce1-xZrxO2 nanoparticles: X-ray absorption near-edge spectroscopy, density functional, and time-resolved X-ray diffraction studies. J. Phys. Chem. B 107, 3535–3543 (2003).
Li, Y. et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nat. Commun. 14, 7149 (2023).
Yamamoto, T. et al. Origin and dynamics of oxygen storage/release in a Pt/ordered CeO2–ZrO2 catalyst studied by time-resolved XAFS analysis. Angew. Chem. Int. Ed. 119, 9413–9416 (2007).
Spezzati, G. et al. Atomically dispersed Pd–O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Muravev, V. et al. Interface dynamics of Pd–CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 4, 469–478 (2021).
Chen, B.-R. et al. Morphology and CO oxidation activity of Pd nanoparticles on SrTiO3 nanopolyhedra. ACS Catal. 8, 4751–4760 (2018).
Xing, F., Jeon, J., Toyao, T., Shimizu, K. & Furukawa, S. A Cu–Pd single-atom alloy catalyst for highly efficient NO reduction. Chem. Sci. 10, 8292–8298 (2019).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Jiang, D. et al. Dynamic and reversible transformations of subnanometre-sized palladium on ceria for efficient methane removal. Nat. Catal. 6, 618–627 (2023).
Jiang, F. et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 10, 11493–11509 (2020).
Wang, H. et al. Influence of oxygen vacancy-induced coordination change on Pd/CeO2 for NO reduction. Environ. Sci. Technol. 58, 2133–2143 (2024).
Wang, H. et al. Influence mechanism of Pd, Pt, Rh/γ-Al2O3 on NH3 formation for NO reduction by CO. Appl. Catal. B Environ. Energy 352, 124009 (2024).
Yang, Q. et al. Surface tailoring on SrMnO3@SmMn2O5 for boosting the performance in diesel oxidation catalyst. Appl. Catal. B Environ. Energy 320, 121993 (2023).
Hwang, J. et al. Regulating oxygen activity of perovskites to promote NOx oxidation and reduction kinetics. Nat. Catal. 4, 663–673 (2021).
Yang, Q. et al. Synergistic effects of a CeO2/SmMn2O5–H diesel oxidation catalyst induced by acid-selective dissolution drive the catalytic oxidation reaction. ACS Appl. Mater. Interfaces 14, 2860–2870 (2022).
Haubrich, J., Quiller, R. G., Benz, L., Liu, Z. & Friend, C. M. In situ ambient pressure studies of the chemistry of NO2 and water on rutile TiO2(110). Langmuir 26, 2445–2451 (2010).
Wang, F. et al. Identification of direct anchoring sites for monoatomic dispersion of precious metals (Pt, Pd, Ag) on CeO2 support. Angew. Chem. Int. Ed. 63, e202318492 (2024).
Chen, D. et al. Unravelling phosphorus-induced deactivation of Pd-SSZ-13 for passive NOx adsorption and CO oxidation. ACS Catal. 11, 13891–13901 (2021).
Khivantsev, K. et al. Palladium/ferrierite versus palladium/SSZ‐13 passive NOx adsorbers: adsorbate‐controlled location of atomically dispersed palladium(II) in ferrierite determines high activity and stability. Angew. Chem. Int. Ed. 61, e202107554 (2022).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (22276104 and T2341002).
Author information
Authors and Affiliations
Contributions
C.G.: conceptualization, investigation, methodology, data curation, formal analysis, software, visualization, writing—original draft, review and editing. H.W.: investigation, methodology, formal analysis, software, writing—review and editing. B.Z.: in situ NAP-XPS measurements, data curation, formal analysis. B.W.: formal analysis, software, visualization, writing—review and editing. R.W.: formal analysis, software, writing—review and editing. Y.L.: methodology, formal analysis, writing—review and editing. D.W.: methodology, formal analysis, writing—review and editing. Y.P.: conceptualization, formal analysis, supervision, writing—review and editing, funding acquisition, project administration. J.L.: conceptualization, supervision, funding acquisition, project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Engineering thanks Niko Kinnunen, Melanie Hazlett and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, C., Wang, H., Zhou, B. et al. Palladium-assisted NOx storage and release on CexZr1-xO2 for passive NOx adsorber in diesel exhaust aftertreatment. Commun Eng 3, 164 (2024). https://doi.org/10.1038/s44172-024-00311-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44172-024-00311-3