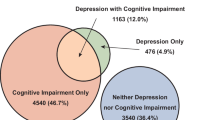
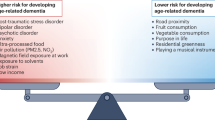
Abstract
Loneliness is one critical risk factor for cognitive health. Here we combined data from ongoing aging studies and the published literature and provide the largest meta-analysis on the association between loneliness and dementia (k = 21 samples, N = 608,561) and cognitive impairment (k = 16, N = 103,387). Loneliness increased the risk for all-cause dementia (hazard ratio (HR) 1.306, 95% confidence interval (CI) 1.197–1.426), Alzheimer’s disease (HR 1.393, 95% CI 1.290–1.504; k = 5), vascular dementia (HR 1.735, 95% CI 1.483–2.029; k = 3) and cognitive impairment (HR 1.150, 95% CI 1.113–1.189). The associations persisted when models controlled for depression, social isolation and/or other modifiable risk factors for dementia. The large heterogeneity across studies was partly due to differences in loneliness measures and ascertainment of cognitive status. The results underscore the importance to further examine the type or sources of loneliness and cognitive symptoms to develop effective interventions that reduce the risk of dementia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The present study includes a coordinated analysis of data from eight public cohort studies: HRS, https://hrs.isr.umich.edu/data-products; ELSA, https://www.elsa-project.ac.uk/accessing-elsa-data; SHARE, https://share-eric.eu/data/data-access; TILDA, https://www.icpsr.umich.edu/web/ICPSR/series/726; MHAS, https://www.mhasweb.org/DataProducts/Home.aspx; KLoSA, https://survey.keis.or.kr/eng/myinfo/login.jsp; CHARLS, https://charls.charlsdata.com/pages/data/111/en.html; and HILDA, https://melbourneinstitute.unimelb.edu.au/hilda/for-data-users. Our access to the data does not allow for data redistribution. Individual researchers can access data from each of these studies after registration at each study data portal; we described each cohort in detail in the Supplementary Information. Data for the meta-analysis are available in the Open Science Framework repository for social sciences (https://doi.org/10.17605/OSF.IO/TFPHS).
Code availability
The R script that supports meta-analytic results is available via the Open Science Framework repository for social sciences at https://doi.org/10.17605/OSF.IO/TFPHS.
References
National Academies of Sciences, Engineering and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System (National Academies Press, 2020).
Office of the Surgeon General. Our epidemic of loneliness and isolation: The US Surgeon General’s Advisory on the healing effects of social connection and community (US Department of Health and Human Services, 2023).
Hawkley, L. C. & Cacioppo, J. T. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218–227 (2010).
Cacioppo, J. T. et al. Loneliness within a nomological net: an evolutionary perspective. J. Res. Pers. 40, 1054–1085 (2006).
Wilson, R. S. et al. Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry. 64, 234–240 (2007).
Luchetti, M. et al. Loneliness is associated with risk of cognitive impairment in the survey of health, ageing and retirement in Europe. Int. J. Geriatr. Psychiatry. 35, 794–801 (2020).
Sundström, A., Adolfsson, A. N., Nordin, M. & Adolfsson, R. Loneliness increases the risk of all-cause dementia and Alzheimer’s disease. J. Gerontol. B 75, 919–926 (2020).
Sutin, A. R., Stephan, Y., Luchetti, M. & Terracciano, A. Loneliness and risk of dementia. J. Gerontol. B 75, 1414–1422 (2020).
Sutin, A. R. et al. Loneliness and risk of all-cause, Alzheimer’s, vascular, and frontotemporal dementia: a prospective study of 492,322 individuals over 15 years. Int. Psychogeriatr. 35, 283–292 (2023).
Salinas, J. et al. Association of loneliness with 10-year dementia risk and early markers of vulnerability for neurocognitive decline. Neurology. 98, e1337–e1348 (2022).
Goldberg, T. E., Choi, J., Lee, S., Gurland, B. & Devanand, D. P. Effects of restriction of activities and social isolation on risk of dementia in the community. Int. Psychogeriatr. 33, 1207–1215 (2021).
Huang, Y., Zhu, X., Liu, X. & Li, J. The effects of loneliness, social isolation, and associated gender differences on the risk of developing cognitive impairment for Chinese oldest old. Aging Ment. Health 27, 1360–1367 (2023).
Joyce, J. et al. Social isolation, social support, and loneliness and their relationship with cognitive health and dementia. Int. J. Geriat. Psychiatry. 37, gps.5644 (2022).
Zhou, Z., Wang, P. & Fang, Y. Loneliness and the risk of dementia among older Chinese adults: gender differences. Aging. Ment. Health 22, 519–525 (2018).
Lara, E. et al. Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Res. Rev. 52, 7–16 (2019).
Lazzari, C. & Rabottini, M. COVID-19, loneliness, social isolation and risk of dementia in older people: a systematic review and meta-analysis of the relevant literature. Int. J. Psychiatry Clin. Pract. 26, 196–207 (2022).
Piolatto, M. et al. The effect of social relationships on cognitive decline in older adults: an updated systematic review and meta-analysis of longitudinal cohort studies. BMC Public Health 22, 278 (2022).
Wang, S., Molassiotis, A., Guo, C., Leung, I. S. H. & Leung, A. Y. M. Association between social integration and risk of dementia: a systematic review and meta‐analysis of longitudinal studies. J. Am. Geriatr. Soc. 71, 632–645 (2023).
Qiao, L. et al. Association between loneliness and dementia risk: a systematic review and meta-analysis of cohort studies. Front. Hum. Neurosci. 16, 899814 (2022).
Mahalingam, G. et al. Social connections and risk of incident mild cognitive impairment, dementia, and mortality in 13 longitudinal cohort studies of ageing. Alzheimers Dem. 9, 5114–5128 (2023).
Dabiri, S., Mwendwa, D. T. & Campbell, A. Psychological and neurobiological mechanisms involved in the relationship between loneliness and cognitive decline in older adults. Brain Behav. Immun. 116, 10–21 (2024).
Petersen, R. C. et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90, 126–135 (2018).
Willroth, E. C. & Atherton, O. E. Best laid plans: a guide to reporting preregistration deviations. Adv. Methods Pract. Psychol. Sci. https://doi.org/10.1177/25152459231213802 (2024).
Luo, M. Social Isolation, loneliness, and depressive symptoms: a twelve-year population study of temporal dynamics. J. Gerontol. B 78, 280–290 (2023).
Kim, A. J., Beam, C. R., Greenberg, N. E. & Burke, S. L. Health factors as potential mediators of the longitudinal effect of loneliness on general cognitive ability. Am. J. Geriatr. Psychiatry 28, 1272–1283 (2020).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012 (2000).
Dubois, B., Von Arnim, C. A. F., Burnie, N., Bozeat, S. & Cummings, J. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 15, 175 (2023).
Freak-Poli, R. et al. Loneliness, not social support, is associated with cognitive decline and dementia across two longitudinal population-based cohorts. J. Alzheimers Dis. 85, 295–308 (2022).
Shibata, M. et al. Emotional loneliness is associated with a risk of dementia in a general Japanese older population: the Hisayama study. J. Gerontol. B 76, 1756–1766 (2021).
Holwerda, T. J. et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam study of the elderly (AMSTEL). J. Neurol. Neurosurg. Psychiatry 85, 135–142 (2014).
Chen, R. et al. Incident dementia in a defined older Chinese population. PLoS ONE 6, e24817 (2011).
Lobo, A. et al. Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer’s type. Neurotox. Res. 14, 263–272 (2008).
Bickel, H. & Cooper, B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol. Med. 24, 179–192 (1994).
Rolandi, E. et al. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: a population-based study. Alzheimers Res. Ther. 12, 94 (2020).
He, Y. L., Zhang, X. K. & Zhang, M. Y. Psychosocial risk factors for Alzheimeras disease. Hong Kong J. Psychiatry 10, 2 (2000).
Zhou, Z. et al. The association between loneliness and cognitive impairment among older men and women in China: a nationwide longitudinal study. Int. J. Environ. Res. Public Health 16, 2877 (2019).
Wei, K. et al. Living arrangement modifies the associations of loneliness with adverse health outcomes in older adults: evidence from the CLHLS. BMC Geriatr. 22, 59 (2022).
Rawtaer, I. et al. Psychosocial risk and protective factors and incident mild cognitive impairment and dementia in community dwelling elderly: findings from the Singapore longitudinal ageing study. J. Alzheimers Dis. 57, 603–611 (2017).
Wang, H. et al. Longitudinal analysis of the impact of loneliness on cognitive function over a 20-year follow-up. Aging Ment. Health 24, 1815–1821 (2020).
Terracciano, A., Luchetti, M., Karakose, S., Stephan, Y. & Sutin, A. R. Loneliness and risk of Parkinson disease. JAMA Neurol. 80, 1138–1144 (2023).
Wang, F. et al. A systematic review and meta-analysis of 90 cohort studies of social isolation, loneliness, and mortality. Nat. Hum. Behav. 7, 1307–1319 (2023).
Yan, S. et al. Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl. Psychiatry 10, 112 (2020).
Zhong, G., Wang, Y., Zhang, Y., Guo, J. J. & Zhao, Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 10, e0118333 (2015).
Aschwanden, D. et al. Is personality associated with dementia risk? A meta-analytic investigation. Ageing Res. Rev. 67, 101269 (2021).
Buecker, S., Maes, M., Denissen, J. J. A. & Luhmann, M. Loneliness and the big five personality traits: a meta-analysis. Eur J. Pers. 34, 8–28 (2020).
Perissinotto, C. M., Stijacic Cenzer, I. & Covinsky, K. E. Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med. 172, 1078–1083 (2012).
Cachón-Alonso, L., Hakulinen, C., Jokela, M., Komulainen, K. & Elovainio, M. Loneliness and cognitive function in older adults: longitudinal analysis in 15 countries. Psychol. Aging 38, 778–789 (2023).
Rafnsson, S. B., Orrell, M., d’Orsi, E., Hogervorst, E. & Steptoe, A. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English longitudinal study of ageing. J. Gerontol. B 75, 114–124 (2020).
Ferreira, R. G., Brandão, M. P. & Cardoso, M. F. An update of the profile of older adults with dementia in Europe: findings from SHARE. Aging Ment. Health 24, 374–381 (2020).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
IntHout, J., Ioannidis, J. P. A., Borm, G. F. & Goeman, J. J. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J. Clin. Epidemiol. 68, 860–869 (2015).
McHugh Power, J., Tang, J., Kenny, R. A., Lawlor, B. A. & Kee, F. Mediating the relationship between loneliness and cognitive function: the role of depressive and anxiety symptoms. Aging Ment. Health 24, 1071–1078 (2020).
Cacioppo, J. T., Hawkley, L. C. & Thisted, R. A. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago health, aging, and social relations study. Psychol. Aging 25, 453–463 (2010).
O’Rourke, H. M., Collins, L. & Sidani, S. Interventions to address social connectedness and loneliness for older adults: a scoping review. BMC Geriatr. 18, 214 (2018).
Joshi, P. et al. Social connections as determinants of cognitive health and as targets for social interventions in persons with or at risk of Alzheimer’s disease and related disorders: a scoping review. Int. Psychogeriatr. 36, 92–118 (2024).
Hawkley, L. C., Thisted, R. A. & Cacioppo, J. T. Loneliness predicts reduced physical activity: cross-sectional and longitudinal analyses. Health Psychol. 28, 354–363 (2009).
Christiansen, J., Larsen, F. B. & Lasgaard, M. Do stress, health behavior, and sleep mediate the association between loneliness and adverse health conditions among older people? Soc. Sci. Med. 152, 80–86 (2016).
Shiovitz-Ezra, S. & Parag, O. Does loneliness ‘get under the skin’? Associations of loneliness with subsequent change in inflammatory and metabolic markers. Aging Ment. Health 23, 1358–1366 (2019).
Paul, E., Bu, F. & Fancourt, D. Loneliness and risk for cardiovascular disease: mechanisms and future directions. Curr. Cardiol. Rep. 23, 68 (2021).
Maharani, A., Pendleton, N. & Leroi, I. Hearing Impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English longitudinal study on ageing. Am. J. Geriatr. Psychiatry 27, 1348–1356 (2019).
Akhter‐Khan, S. C. et al. Associations of loneliness with risk of Alzheimer’s disease dementia in the Framingham heart study. Alzheimers Dem. 17, 1619–1627 (2021).
Li, Y. et al. Eight-year trajectories of late-life loneliness and incident dementia: a nationally representative cohort study. Am. J. Geriatr. Psychiatry. 31, 475–486 (2023).
Akhter-Khan, S. C., Prina, M., Wong, G. H.-Y., Mayston, R. & Li, L. Understanding and addressing older adults’ loneliness: the social relationship expectations framework. Perspect. Psychol. Sci. 18, 762–777 (2023).
McHugh Power, J. E. et al. Lonely SARTs: loneliness and sustained attention in the Irish longitudinal study of aging. Neuropsychol. Dev. Cogn. B 27, 197–206 (2020).
Yang, R. et al. Loneliness as a mediator of the impact of social isolation on cognitive functioning of Chinese older adults. Age Ageing 49, 599–604 (2020).
Yu, B., Steptoe, A., Chen, Y. & Jia, X. Social isolation, rather than loneliness, is associated with cognitive decline in older adults: the China health and retirement longitudinal study. Psychol. Med. 51, 2414–2421 (2021).
Hughes, M. E., Waite, L. J., Hawkley, L. C. & Cacioppo, J. T. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res. Aging 26, 655–672 (2004).
Smith, J., Ryan, L. H., Fisher, G. G., Sonnega, A. & Weir, D. R. HRS Psychosocial and Lifestyle Questionnaire 2006–2016 (Survey Research Center, Institute for Social Research, 2017).
Radloff, L. S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol. Meas. 1, 385–401 (1977).
Lewinsohn, P. M., Seeley, J. R., Roberts, R. E. & Allen, N. B. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12, 277–287 (1997).
Irwin, M., Artin, K. H. & Oxman, M. N. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch. Intern. Med. 159, 1701–1704 (1999).
Crimmins, E. M., Kim, J. K., Langa, K. M. & Weir, D. R. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J. Gerontol. B 66B, i162–i171 (2011).
Quinn, T. J. et al. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within community dwelling populations. Cochrane Database Syst. Rev. 7, CD010079 (2014).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Bassuk, S. S., Wypij, D. & Berkmann, L. F. Cognitive impairment and mortality in the community-dwelling elderly. Am. J. Epidemiol. 151, 676–688 (2000).
Creavin, S. T. et al. Mini-mental state examination (MMSE) for the detection of Alzheimer’s dementia and other dementias in asymptomatic and previously clinically unevaluated people aged over 65 years in community and primary care populations. Cochrane Database Syst. Rev. 2016, CD011145 (2016).
Mejia-Arango, S. et al. Effect of demographic and health dynamics on cognitive status in Mexico between 2001 and 2015: evidence from the Mexican health and aging study. Geriatrics. 6, 63 (2021).
Lugo-Palacios, D. G. & Gannon, B. Health care utilisation amongst older adults with sensory and cognitive impairments in Europe. Health Econ. Rev. 7, 44 (2017).
Giné-Garriga, M. et al. Is loneliness a predictor of the modern geriatric giants? Analysis from the survey of health, ageing, and retirement in Europe. Maturitas 144, 93–101 (2021).
Gontié, R. et al. Relationship between physical activity and incidence of dementia in people aged 50 and over in Europe. Aging Ment. Health 27, 1429–1435 (2023).
Aschwanden, D., Sutin, A. R., Luchetti, M., Stephan, Y. & Terracciano, A. Personality and dementia risk in England and Australia. GeroPsych. 33, 197–208 (2020).
Hosking, D. E. & Anstey, K. J. The economics of cognitive impairment: volunteering and cognitive function in the HILDA survey. Gerontology 62, 536–540 (2016).
Zhang, K. & Zhang, W. Adverse childhood experiences and mild cognitive impairment in later life: exploring rural/urban and gender differences using CHARLS. J. Appl. Gerontol. 41, 1454–1464 (2022).
Karim, J., Weisz, R., Bibi, Z. & ur Rehman, S. Validation of the eight-item Center for Epidemiologic Studies Depression Scale (CES-D) among older adults. Curr. Psychol. 34, 681–692 (2015).
Kim, H., Park, S.-M., Jang, S.-N. & Kwon, S. Depressive symptoms, chronic medical illness, and health care utilization: findings from the Korean longitudinal study of ageing (KLoSA). Int. Psychogeriatr. 23, 1285–1293 (2011).
Ware, J. E. & Gandek, B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) project. J. Clin. Epidemiol. 51, 903–912 (1998).
Castro-Costa, E. et al. Ascertaining late-life depressive symptoms in Europe: an evaluation of the survey version of the EURO-D scale in 10 nations. The SHARE project. Int. J. Methods Psychiatr. Res. 17, 12–29 (2008).
Tabachnick, B. G. & Fidell, L. S. Using Multivariate Statistics (Pearson, 2013).
Viechtbauer, W. Conducting meta-analyses in R with the metafor Package. J. Stat. Soft. 36, 1–48 (2010).
Stanley, T. D. & Doucouliagos, H. Meta-regression approximations to reduce publication selection bias. Res. Synth. Methods 5, 60–78 (2014).
Simonsohn, U., Nelson, L. D. & Simmons, J. P. P-curve: a key to the file-drawer. J. Exp. Psychol. Gen. 143, 534–547 (2014).
Simonsohn, U., Simmons, J. P. & Nelson, L. D. Better P-curves: making P-curve analysis more robust to errors, fraud, and ambitious P-hacking, a reply to Ulrich and Miller (2015). J. Exp. Psychol. Gen. 144, 1146–1152 (2015).
Acknowledgements
The work reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (grant nos. R01AG074573 and RF1AG053297 to A.R.S. and R01AG068093 to A.T.). The funders had no role in the study design, analysis, decision to publish or preparation of the manuscript. We further thank all participants, national and international agencies that support each cohort study analyzed in this work: HRS sponsored by the US National Institute on Aging (grant number NIA U01AG009740) and coordinated by the University of Michigan; ELSA sponsored by the US National Institute on Aging (grant number NIA R01AG017644) and the UK Government Departments coordinated by the National Institute for Health and Care Research; SHARE funded by the European Commission and Horizon 2020, and supported by the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the US National Institute on Aging and various national funding sources (www.share-project.org); TILDA based in Trinity College Dublin, for which data are hosted by the Irish Social Science Data Archive and the Inter-university Consortium for Political and Social Research based in the University of Michigan; MHAS supported by the US National Institute on Aging (grant no. NIA R01AG018016) with the collaborative effort from the University of Texas Medical Branch, the Instituto Nacional de Estadística y Geografía (Mexico), the University of Wisconsin, the Instituto Nacional de Geriatría (Mexico) and the Instituto Nacional de Salud Pública (Mexico); KLoSA, for which data are cured by the Korea Employment Information Service; CHARLS supported by the US National Institute on Aging (grant number NIA R01AG037031), the Natural Science Foundation of China, the World Bank and Peking University; HILDA, funded by Australian Government Department of Social Services and managed by the Melbourne Institute of Applied Economic and Social Research. For each study, data were collected with the informed consent of participants. All procedures, materials and participant compensations were approved by the institutional review boards of their respective institutions.
Author information
Authors and Affiliations
Contributions
M.L. and A.R.S. conceived the project. M.L. and D.A. carried out the literature review and selection of published articles with supervision from A.R.S. M.L. performed all analyses with input from D.A., A.T. and A.R.S. M.L. drafted the first version of the manuscript. D.A., A.A.S., X.Z., P.S.O., Y.S., A.T. and A.R.S. provided inputs on the interpretation of results and critical revisions to the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Patrick Lao, Eduardo Zimmer and the other, anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Forest plot of fully adjusted models.
The figure shows supplementary analyses with studies that accounted for depression, social isolation, and/or modifiable clinical factors for dementia, panel a (18 samples, 559,890 participants) and cognitive impairment, panel b (16 samples, 81,709 participants). Effect sizes for the individual studies and average random-effects (RE) model are displayed in hazard ratios (HR) with 95% confidence intervals (95% CI). Circle sizes and lines represent the weight of each study and 95% CI limits. Upper-level 95% CIs that exceeded 4.0 are not shown. Study abbreviations: H70 Study = Gothenberg H70 Birth Cohort Study; LEILA = Leipzig Longitudinal Study of the Aged; LRGSTUA = Neuroprotective Model for Healthy Longevity among Malaysian Older Adults Towards Using Ageing; RS = Rotterdam Study; SNAC-K = Swedish National Study on Aging and Care in Kungsholmen; Zhou (2019) included results for male and female (M / F) individuals. Selected cohorts: HRS = Health and Retirement Study; ELSA = English Longitudinal Study of Ageing; SHARE = Study of Health, Ageing and Retirement in Europe; TILDA = The Irish Longitudinal Study on Ageing; MHAS = Mexican Health and Aging Study; KLoSA = Korean Longitudinal Study of Aging; CHARLS = China Health and Retirement Longitudinal Study; and HILDA = Household, Income and Labour Dynamics in Australia. Data Extraction File includes the complete list of control variables within each study: https://osf.io/tfphs/.
Extended Data Fig. 2 Funnel plots to evaluate publication bias.
The figure shows funnel plots for the meta-analysis concerning risk of dementia (panel a) and cognitive impairment (panel b). It represents log-transformed hazard ratios and standard errors used in R metafor to run the analysis. The close dots indicate the observed studies and the open dots indicate the missing studies imputed by the trill-and-fill method.
Supplementary information
Supplementary Information
Supplementary Table 1. Preregistration deviations. Supplementary Note 1. Description of the individual cohort studies. Supplementary Table 2. Loneliness measures across individual cohort studies. Supplementary Table 3. Classification of cognitive status across individual cohort studies. Supplementary Table 4. Selected control variables across individual cohort studies. Supplementary Table 5. MOOSE checklist for meta-analyses of observational studies. Supplementary Table 6. Final decision and reasons for exclusion of the screened full-text articles. Supplementary Note 2. Supplementary analysis to evaluate publication bias.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luchetti, M., Aschwanden, D., Sesker, A.A. et al. A meta-analysis of loneliness and risk of dementia using longitudinal data from >600,000 individuals. Nat. Mental Health 2, 1350–1361 (2024). https://doi.org/10.1038/s44220-024-00328-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-024-00328-9
This article is cited by
-
The relationship between retirement, social isolation and loneliness: A longitudinal analysis using the English Longitudinal Study of Ageing
BMC Public Health (2025)
-
REMINDER program: a randomized controlled trial protocol of a neuropsychological intervention for lifestyle modification in older adults at risk of dementia
BMC Geriatrics (2025)