Abstract
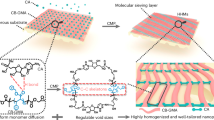
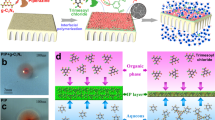
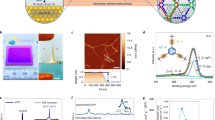
Mixed-dimensional membranes are promising candidates for efficient water purification. Integrating a conventional flat two-dimensional (2D) membrane with structures of different dimensionalities is expected to create additional water transport sites. However, organizing the membrane building blocks into a mixed-dimensional hierarchy capable of facilitating rapid water transfer, while also enabling large-scale, cost-effective manufacturing, remains a significant challenge. Here we report the discovery of rapid self-organization of large-area mixed-dimensional polyamide membranes with an intriguing hierarchical structure consisting of one-dimensional nanotubes on a 2D nanofilm under room temperature using only two types of small molecules at an oil–water interface. The resulting architecture with one-dimensional nanotubes on a 2D nanofilm offers a substantially increased available area for water transport per projected area, enabling energy-efficient nanofiltration membranes with outstanding water–salt separation performance that well surpasses most state of the art membranes. Control experiments, coupled with molecular dynamic simulations, reveal that the two types of molecular monomers self-organize into a 2D nanopore network during the incipient reaction stage and then capillarity within these nanopores drives the upwards polymerization of these nanotubes. Our findings provide valuable insights into how the interplay of interfacial physical and chemical interactions organizes molecular seeds into large-scale, complex hierarchical nanostructures under ambient conditions. This opens new opportunities for developing scalable, mixed-dimensional water purification membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Michalak, A. M. et al. The frontiers of water and sanitation. Nat. Water 1, 10–18 (2023).
Shannon, M. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Chaplin-Kramer, R. et al. Global modeling of nature’s contributions to people. Science 366, 255–258 (2019).
Giorno, L. Membranes that filter and destroy pollutants. Nat. Nanotechnol. 17, 334–335 (2022).
Wang, K. et al. Tailored design of nanofiltration membranes for water treatment based on synthesis–property–performance relationships. Chem. Soc. Rev. 51, 672–719 (2022).
Mohammad, A. W. et al. Nanofiltration membranes review: recent advances and future prospects. Desalination 356, 226–254 (2015).
Fane, A. G., Wang, R. & Hu, M. X. Synthetic membranes for water purification: status and future. Angew. Chem. Int. Ed. 54, 3368–3386 (2015).
Wang, R., He, R., He, T., Elimelech, M. & Lin, S. Performance metrics for nanofiltration-based selective separation for resource extraction and recovery. Nat. Water 1, 291–300 (2023).
Cadotte, J. E. Interfacially synthesized reverse osmosis membrane. US patent 4277344A (1979).
Gohil, J. M. & Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: potential for water treatment and desalination. Sep. Purif. Technol. 181, 159–182 (2017).
Dai, R. et al. Nanovehicle-assisted monomer shuttling enables highly permeable and selective nanofiltration membranes for water purification. Nat. Water 1, 281–290 (2023).
Yang, Z., Guo, H. & Tang, C. Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 590, 117297 (2019).
Fang, W., Shi, L. & Wang, R. Mixed polyamide-based composite nanofiltration hollow fiber membranes with improved low-pressure water softening capability. J. Membr. Sci. 468, 52–61 (2014).
Peng, H. et al. Quaternization-spiro design of chlorine-resistant and high-permeance lithium separation membranes. Nat. Commun. 14, 5483 (2023).
Jiang, C., Tian, L., Hou, Y. & Niu, Q. J. Nanofiltration membranes with enhanced microporosity and inner-pore interconnectivity for water treatment: excellent balance between permeability and selectivity. J. Membr. Sci. 586, 192–201 (2019).
Shi, L. et al. The tailoring of nanofiltration membrane structure for mono/divalent anions separation via precisely adjusting the reaction site distance. J. Membr. Sci. 668, 121252 (2023).
Liu, S.-H. et al. Sub-8 nm networked cage nanofilm with tunable nanofluidic channels for adaptive sieving. Nat. Commun. 15, 2478 (2024).
Tang, Y.-J., Xu, Z.-L., Xue, S.-M., Wei, Y.-M. & Yang, H. A chlorine-tolerant nanofiltration membrane prepared by the mixed diamine monomers of PIP and BHTTM. J. Membr. Sci. 498, 374–384 (2016).
Pendergast, M. M. & Hoek, E. M. V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 4, 1946–1971 (2011).
Ji, Y.-L. et al. Roll-to-roll fabrication of large-area metal–organic framework-based membranes for high-performance aqueous separations. Nat. Water 2, 183–192 (2024).
Liu, C. et al. Interfacial polymerization at the alkane/ionic liquid interface. Angew. Chem. Int. Ed. 60, 14636–14643 (2021).
Tan, Z., Chen, S., Peng, X., Zhang, L. & Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 360, 518–521 (2018).
Wu, M.-B. et al. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 515, 238–244 (2016).
Wang, Z. et al. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 9, 2004 (2018).
Zhang, Y. et al. Ice-confined synthesis of highly ionized 3D-quasilayered polyamide nanofiltration membranes. Science 382, 202–206 (2023).
Jiang, C. et al. Thin-film composite membranes with aqueous template-induced surface nanostructures for enhanced nanofiltration. J. Membr. Sci. 589, 117244 (2019).
Liu, Y., Coppens, M.-O. & Jiang, Z. Mixed-dimensional membranes: chemistry and structure–property relationships. Chem. Soc. Rev. 50, 11747–11765 (2021).
Han, S. et al. Microporous organic nanotube assisted design of high performance nanofiltration membranes. Nat. Commun. 13, 7954 (2022).
Gherasim, C. V., Luelf, T., Roth, H. & Wessling, M. Dual-charged hollow fiber membranes for low-pressure nanofiltration based on polyelectrolyte complexes: one-step fabrication with tailored functionalities. ACS Appl. Mater. Interfaces 8, 19145–19157 (2016).
Liu, S., Wu, C., Hung, W.-S., Lu, X. & Lee, K.-R. One-step constructed ultrathin Janus polyamide nanofilms with opposite charges for highly efficient nanofiltration. J. Mater. Chem. A 5, 22988–22996 (2017).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Ding, Y. et al. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed. 56, 1–6 (2017).
Yang, H. et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 10, 2101 (2019).
Karan, S., Jiang, Z. & Livingston, A. G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Jiang, Z. et al. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 609, 58–64 (2022).
Lu, X. & Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: history, current efforts and future directions. Chem. Soc. Rev. 50, 6290–6307 (2021).
Zhang, F., Fan, J.-B. & Wang, S. Interfacial polymerization: from chemistry to functional materials. Angew. Chem. Int. Ed. 59, 21840–21856 (2020).
Freger, V. Kinetics of film formation by interfacial polycondensation. Langmuir 21, 1884–1894 (2005).
Wang, S. et al. Polyamide membrane with nanocluster assembly structure for desalination. J. Membr. Sci. 628, 119230 (2021).
Shen, Q. et al. When self-assembly meets interfacial polymerization. Sci. Adv. 9, eadf6122 (2023).
Liu, C., Zhu, C.-Y., Zhang, C., Yang, H.-C. & Xu, Z.-K. Thermodynamic and kinetic understanding for managing the controllability of interfacial polymerization. Prog. Polym. Sci. 152, 101815 (2024).
Yuan, F. et al. Visualization of the formation of interfacially polymerized film by an optical contact angle measuring device. J. Phys. Chem. C 116, 11496–11506 (2012).
Siegal, M. P., Kaatz, F. H., Graham, W. R., Santiago, J. J. & Spiegel, J. Formation of epitaxial yttrium silicide on (111) silicon. J. Appl. Phys. 66, 2999–3006 (1989).
Keavney, D. J., Fullerton, E. E. & Bader, S. D. Perpendicular conductance and magnetic coupling in epitaxial Fe/MgO/Fe (100) trilayers. J. Appl. Phys. 81, 795–798 (1997).
Dixon, H. H. & Philos, J. J. On the ascent of sap. Philos. Trans. R. Soc. B 186, 563–576 (1895).
Freger, V. Nanoscale heterogeneity of polyamide membranes formed by interfacial polymerization. Langmuir 19, 4791–4797 (2003).
Yang, F., Zhang, S., Yang, D. & Jian, X. Preparation and characterization of polypiperazine amide/PPESK hollow fiber composite nanofiltration membrane. J. Membr. Sci. 301, 85–92 (2007).
Brown, H. R. The theory of the rise of sap in trees: some historical and conceptual remarks. Phys. Perspect. 15, 320–358 (2013).
Honschoten, J. W., Brunets, N. & Tas, N. R. Capillarity at the nanoscale. Chem. Soc. Rev. 39, 1096–1114 (2010).
Yang, X., Du, Y., Zhang, X., He, A. & Xu, Z.-K. Nanofiltration membrane with a mussel-inspired interlayer for improved permeation performance. Langmuir 33, 2318–2324 (2017).
Chen, H. et al. Free-volume depth profile of polymeric membranes studied by positron annihilation spectroscopy: layer structure from interfacial polymerization. Macromolecules 40, 7542–7557 (2007).
Acknowledgements
We thank H. Jia, W. Li, X. F. Li, J. D. Li, L. R. Liang, Y. N. Li, Y. Li and J. F. Xu for technical assistance. This work was supported by the National Natural Science Foundation of China (grant nos. 51978466 (to C.W.) and 52170047 (to C.W.)), National Key R&D Programme of China (2021YFC330010003-2 to C.W.) and the research funding provided by Cangzhou Institute of Tiangong University (grant no. TGCYY-F-0102 to C.W.). C.W., S.-H.L., B.X., J.S., L.S. and X.L. are inventors on patent application 201910669603.3 submitted by Tiangong University, which covers polyamide membranes with nanotube surface. We thank the Analytical and Testing Centre of Tiangong University for membrane structure analysis (for example, XPS, FESEM and TEM) work. We also thank Z. Jiang from Queen Mary University of London for a productive suggestion.
Author information
Authors and Affiliations
Contributions
S.-H.L., C.W. and X.L. designed the experiments and analysed the data. S.-H.L. performed the experiments. W.S. conducted the simulation analysis. W.-S.H. and K.-R.L. performed the PAS analysis. S.-H.L., L.S., B.X., J.S. and Z.S. performed the SEM tests. S.G. contributed to data analysis. All authors discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Jiangnan Shen, Lin Zhang and Yatao Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Discussion and Tables 1–2.
Supplementary Video 1
Top view of simulation of nanopore network formation during the initial reaction stage of interfacial polycondensation.
Supplementary Video 2
Side view of simulation of nanopore network formation during the initial reaction stage of interfacial polycondensation.
Supplementary Video 3
Capillary flow process in PPA nanopore with a diameter of 9 nm and nanopore height of 9 nm.
Supplementary Video 4
Capillary flow process in PPA nanopore with a diameter of 9 nm and nanopore height of 18 nm.
Supplementary Video 5
Capillary flow process in PPA nanopore with a diameter of 18 nm and nanopore height of 9 nm.
Supplementary Video 6
Capillary flow process in PPA nanopore with a diameter of 30 nm and nanopore height of 9 nm.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, SH., Shi, W., Hung, WS. et al. Interfacial self-organization of large-area mixed-dimensional polyamide membranes for rapid aqueous nanofiltration. Nat Water 2, 1238–1248 (2024). https://doi.org/10.1038/s44221-024-00348-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-024-00348-w
This article is cited by
-
Nanofiltration membranes with mixed-dimensional structures
Nature Water (2024)