Abstract
Nanofiltration membranes with confined nanopores are vital for energy-efficient molecular and ionic sieving towards sustainable ecosystems. However, the production of contemporary nanofiltration membranes still relies on hazardous petrochemical-based chemicals, raising serious water contamination concerns and complicating after-usage disposal. This phenomenon contradicts the sustainability of membranes derived from green chemistry principles, emphasizing not only their eco-friendly application but also their preparation and end of life. Here we report the synthesis of a sustainable nanofiltration membrane (SNFM) with superior performance for water treatment and an inherent natural soil degradation mechanism through a safer approach utilizing integrated low-hazard chemicals. Experiments and simulations confirmed that our SNFM can be fabricated in an environmentally friendly manner and decomposed by natural soil microorganisms, contributing to its distinctive eco-friendliness. Notably, the SNFM demonstrated both exceptional water permeance and molecular and ionic sieving capability, outperforming commercial and state-of-the-art membranes. This approach establishes a new paradigm for next-generation water recycling and sustainable chemical processes.
Similar content being viewed by others
Main
Nanofiltration (NF) membranes with well-defined nanopores can effectively purify water and separate organic compounds through confined molecular and ionic sieving to unlock eco-efficient water treatment by enabling water resource recycling and recovery, energy efficiency and environmental sustainability, aligning with the principles of green sustainable development1,2. Contemporary NF membranes rely on highly hazardous petrochemical-based and non-biodegradable chemicals, contributing to unsustainable development through severe water pollution, risks to human health and challenges in waste disposal. On the basis of the third (less hazardous chemical syntheses), sixth (design for energy efficiency) and tenth (design for degradation) principles of green chemistry, the sustainability of membranes encompasses all stages, from preparation and operation to end of life3,4. However, NF membranes still focus mainly on energy-efficient applications, while the preparation and end-of-life phases remain untouched because confined nanopores are extremely difficult to form via the current synthesis methods and explored materials.
A direct method to improve the safer preparation of NF membranes is to replace the hazardous polymers, monomers and solvents used in conventional membrane preparation protocols with more benign alternatives5. To achieve these goals, low-hazard solvents, such as TamiSolve6, PolarClean7, triethyl phosphate8, methyl lactate8, methyl sesamol9 and γ-valerolactone10, can be considered for the safer preparation of NF membranes. Coincidentally, algal biomass11, allylated gallic acid12, maltitol13 and lignin alkali14 have been proposed as low-hazard monomers for synthesizing dense layers in a safer manner. However, despite these advances, the reliance on certain hazardous solvents and monomers in these so-called safer preparation processes remains inevitable because of the strict requirements for achieving well-defined sieving nanopores, which necessitate a delicate balance between chemical hazard and synthesis methods15. This underscores the urgent need for the development of safer, more sustainable alternatives.
Furthermore, the sustainable nature of NF membranes at the end of life also needs to be addressed because the environmental impact of waste membranes is a growing concern, as 73,000 tonnes of waste NF membranes are generated globally each year16. Twelve per cent of this waste is incinerated, releasing CO2, whereas 80% is discarded in landfills. The waste NF membranes in landfills ultimately break down into non-biodegradable microplastics that contaminate water and penetrate the main food chain, causing significant threats to ecosystems and human health17. To address this, biodegradable and recyclable materials, such as cellulose18, interpenetrating polymer networks19 and dynamic covalently crosslinked nanofibres20,21, have been used to facilitate the end of life of the membrane. However, degradation strategies relying on proteases or hazardous reagents are not compatible with practical applications and green chemistry principles. Consequently, there is a strong consensus that NF membranes should not only be fabricated via safer methods but also be designed for natural degradation, aligning with the sustainable development of the membrane.
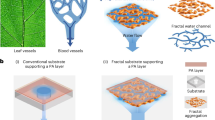
In this work, we synthesized a sustainable NF membrane (SNFM) with inherent natural disposability for efficient water treatment through a safer preparation process using low-hazard chemicals (Fig. 1). A series of experimental and simulation results confirmed that the SNFM was fabricated using low-hazard solvents, monomers and polymers in a greener manner and is biodegradable by soil microorganisms. Most importantly, the synthesized SNFM exhibited ultrafast water transport, excellent confined molecular and ionic sieving capabilities, superior antifouling ability and operational stability, outperforming commercial and state-of-the-art membranes. Our strategy provides great potential for the use of low-hazard solvents, monomers, polymers and natural microorganisms for safer fabrication, energy-efficient application and degradation of NF membranes, significantly advancing the development of real sustainable membrane technology.
Results
Synthesis of the SNFM
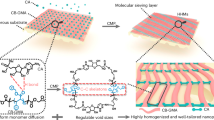
Our SNFM was synergistically designed on the basis of monomers, solvents and polymers for realizing both sustainability and superior confined molecular and ionic separation performance. We chose polylactic acid (PLA), Tween 80 (T80) and dimethyl sulfoxide (DMSO) as the substrate material, porogen and solvent, respectively, to prepare substrates via the non-solvent-induced phase separation (NIPs) method. After that, the SNFM was synthesized via interfacial polymerization (IP) on the PLA substrate. Typically, the PLA substrate was immersed in an aqueous solution with xylitol and dopamine (DA) and then put into a 1,3,5-benzenetricarbonyl chloride (TMC)/oleic acid (OA) solution at room temperature. The hydroxyl and amino groups in xylitol and DA underwent nucleophilic addition and Schiff base reactions with acid chloride groups at the water–OA interface, respectively, which induced the formation of a selective layer. The specific reaction mechanism is illustrated in Supplementary Fig. 1. For comparative analysis, a traditional composite membrane (TCM) was also prepared via NIPs and conventional IP methods. Frequently used polyacrylonitrile (PAN), polyethylene glycol (PEG), dimethylformamide (DMF), hexane, m-phenylenediamine (MPD) and terephthaloyl chloride (TPC) were used as substrate, porogen, solvents and monomers, respectively.
Fourier transform infrared and X-ray photoelectron spectroscopy were used to investigate the chemical compositions of the TCM and SNFM (Supplementary Figs. 2 and 3). Compared with the porous substrates, the TCM and SNFM presented strong new –C = O–NH– and –NH– telescopic vibration peaks at 1,713 cm−1 and 3,502 cm−1, indicating the occurrence of a Schiff base reaction22 (Supplementary Fig. 2). Furthermore, the SNFM presented a new peak at 1,756 cm−1, attributed to the –COO– telescopic vibration, which indicated the occurrence of a nucleophilic addition reaction22. Coincidentally, the TCM and SNFM presented peaks at 400.1 and 400.5 eV attributed to –NH– and tertiary amines, respectively23. Moreover, the characteristic peak of –COO– at 288.7 eV was also observed in the spectrum of the SNFM24 (Supplementary Fig. 3 and Supplementary Table 1). These results further confirmed that TCM and SNFM were synthesized.
Cross-sectional transmission electron microscopy images revealed that the SNFM presented a selective layer thickness of 45 nm, smaller than that of the TCM (64 nm), which was consistent with the results obtained from ellipsometry (Supplementary Figs. 7 and 8). More interestingly, the SNFM presented dense and irregular stripe structures covering the surface, resulting in a larger surface roughness of 7.8 ± 0.5 nm, which was different from that of the TCM with a smooth surface (Fig. 2a and Supplementary Figs. 9 and 10). During the IP diffusion process, the formation of a dense and irregular stripe structure is attributed to molecular motion. A system with DA and xylitol in water and TMC in OA (the SNFM system) was built via molecular dynamics (MD) simulations to investigate the formation mechanism of the irregular stripe structure (Fig. 2b). For comparison, individual systems of xylitol (the SNFM-XY system) and DA (the SNFM-DA system) were also subjected to the same simulation (Supplementary Fig. 11). As shown in Fig. 2c, the SNFM system exhibited consistent increases (∆N) of 21 and 14 for xylitol and DA at the water–OA interface, respectively, which were greater than those of the SNFM-XY (16) and SNFM-DA (4) systems (Supplementary Fig. 12). Coincidentally, the mean square displacement in the z direction exhibited higher self-diffusion coefficients of 1.5 × 10−6 m2 s−1 and 1.1 × 10−6 m2 s−1 for xylitol and DA, respectively, than those of the SNFM-XY and SNFM-DA systems (Supplementary Fig. 13). Furthermore, the total amounts of xylitol and DA that ultimately diffused to the interface in the SNFM system were 21 and 19, respectively, which surpassed those observed in the SNFM-XY and SNFM-DA systems (Supplementary Fig. 14). These results demonstrated that both DA and xylitol tended to diffuse towards the water–OA interface. This phenomenon is attributed to the strong interaction between xylitol and DA induced by H-bonds, resulting in one of the molecules diffusing into the interface and promoting the diffusion of the other molecules into the interface, which can increase the reaction intensity and form a dense selective layer (Fig. 2c and Supplementary Fig. 15). Interestingly, the phenolic hydroxyl and amine groups on DA and the alcoholic hydroxyl groups on xylitol presented 28.2, 19.1 and 25.1 kcal mol−1 energy barriers to react with TMC, respectively, which was conducive to the formation of competitive reactions between molecules, resulting in the formation of irregular stripe structures (Fig. 2d and Supplementary Fig. 16).
a, Surface SEM images of the SNFM (magnification 50,000×). b, Snapshots from MD simulations of the SNFM synthesis process. c, The number of hydrogen bonds between DA and xylitol molecules (top) and their increased number (ΔN) curve at the interface (bottom) in the SNFM simulation system. d, Gibbs free energy of the reaction between DA, xylitol and TMC in the SNFM synthesis process. e, LCA of the TCM and SNFM systems. LT, land transformation. The TCM system also had biogenic and LT impacts, but they were too small to be seen here (the specific data are presented in Supplementary Table 4). f, Growth images of E. coli in the blank and SNFM groups.
To investigate the safer preparation of the membranes, TCM and SNFM were subjected to a life cycle assessment (LCA) on the basis of their raw chemicals. Compared with the TCM, the SNFM demonstrated a markedly greater biogenic and land transformation emission profile, which indicated the intensive use of biomass and extensive land-use change25 (Fig. 2e and Supplementary Table 4). Interestingly, this significant impact was mitigated by the sensitivity analysis of the LCA of biomass chemicals. For example, OA prepared via different methods exhibited different CO2 emissions, which was mainly because the environmental impact of OA largely depended on their feedstock, with conventional sources such as palm and soybean often linked to deforestation and ecological degradation26,27 (Supplementary Fig. 17 and Supplementary Table 5). More importantly, the SNFM system presented overall low CO2 emissions, which implied that the raw chemicals of the SNFM had very minimal environmental impact (Fig. 2e). This result is attributed to substantially greater CO2 uptake by the SNFM system, which is driven by the use of fast-growing, carbon-sequestering biomass and land management practices that increase carbon removal from the atmosphere, resulting in substantial negative emissions and positioning SNFM as a strong carbon sink28.
To further investigate the safety of the raw chemicals, the hazard of the TCM (TCM group) and SNFM (SNFM group) raw chemicals was analysed and compared via pictograms of chemicals from the Material Safety Data Sheet (Supplementary Tables 6–9). Among the TCM group, DMF is a common example of an environmental and health hazard and is classified as the most hazardous solvent29,30. Meanwhile, MPD, which has three manifestations—hazard, health and environmental hazards—is suboptimal. Furthermore, hexane is also an undesired chemical that is applied as a non-polar solvent. Inhaling large amounts of hexane can cause numbness and muscle weakness in the limbs31,32. Interestingly, for the SNFM group, DMSO, DA and xylitol had no pictograms, and T80, OA and TMC were somewhat irritant and corrosive33,34. More importantly, the PLA polymer is less hazardous than the traditional PAN polymer because of its renewable plant resources and industrially compostable degradation31. These results demonstrated that the SNFM was fabricated in a safer manner. Furthermore, Escherichia coli, a significant bacterium present in the environment and mammalian intestines, was also selected as a probe and cultured in Luria–Bertani broth (LB) medium supplemented with these chemicals (TCM and SNFM groups) to further explore the hazard of the raw materials35. Compared with the blank group (Fig. 2f), where pure LB medium without any supplements was used, almost no E. coli was detected (with bare white points) in the TCM group (Supplementary Fig. 18), and the growth rate of E. coli was close to −100%, indicating a strong damaging effect on E. coli (Supplementary Fig. 19). Interestingly, E. coli grew more vigorously (with more white points) in the SNFM group than in the blank group, which indicated that the raw materials of the SNFM did not harm E. coli (Fig. 2f). Specifically, the quantity of surviving E. coli was greater in the SNFM group than in the blank and TCM group (Supplementary Fig. 19). In addition, E. coli also demonstrated significant positive growth, with growth rates ranging from 55.8% to 418.3% in LB medium supplemented with SNFM raw materials (Supplementary Fig. 19). For example, the growth rate of E. coli in LB medium supplemented with T80 was 392.4% for the chemicals in the substrates. Meanwhile, the growth rate reached as high as 418.3% in a xylitol environment for the raw materials in the active layer. These results clearly demonstrate that the chemicals used for the SNFM are significantly less hazardous than those used for the TCM. Overall, SNFM can be fabricated in a safer manner by utilizing raw materials that are less hazardous than conventional materials.
SNFM degradation ability
Various commercial enzymes, including porcine trypsin, esterase and proteinase K, were used to explore the degradation of TCM and SNFM. For better comparison, the commercial GC-NF5001 membrane was also subjected to the same degradation experiment. Unlike GC-NF5001, which remained unchanged, a virtually transparent TCM and the absence of SNFM were observed in the proteinase K solution, indicating membrane degradation based on snapshot observations (Supplementary Figs. 20–22). Furthermore, the surfaces of the SNFM in the porcine trypsin and esterase solutions also became looser and even cracked after 5 days of degradation, indicating that the SNFM can be enzymatically hydrolysed (Supplementary Figs. 23–25). More importantly, the SNFM exhibited degradation rates of 99.4%, 9.4% and 3.9% in proteinase K, porcine trypsin and esterase solutions, respectively, higher than those of the GC-NF5001 (approximately 0%) and TCM (16.9%, 0.6% and 0.3%), which further confirmed the superior degradability of the SNFM (Supplementary Fig. 26). Furthermore, TCM and SNFM, with potential degradability indicated by enzymatic degradation experiments, were buried in soil to further investigate their actual degradation properties. We found that, while the TCM remained largely unchanged (Supplementary Fig. 27), traces of the SNFM gradually decreased with increasing burial time (Fig. 3a). After being buried for 6 months, the presence of SNFM was hardly observable, confirming their degradation properties (Fig. 3a).
a, Images of SNFM after 0, 3 and 6 months of natural soil degradation. b, SEM images of the SNFM surfaces degraded by soil microorganisms at 3 (left) and 6 (right) months (Magnification: 50,000×). c, Degradation rates of the SNFM based on soil microorganisms. Inset: snapshot of SNFM degradation by soil microorganisms at 0, 3 and 6 months. d, Per cent community abundance in the original soil and SNFM soil. e, Community abundance of different functional species in the original soil and SNFM soil. f, The abundance of enzymes in the original soil and SNFM soil. g, Degradation products of the SNFM. h, Effects of SNFM degradation products on the growth of E. coli. Inset: growth images of E. coli in a solution with SNFM degradation products. Error bars in h represent the s.d. (n = 3, n derived from different experimental units), and data are presented as mean values ± s.d.
To better track the soil degradation of the SNFM, soil microorganisms were extracted and used in inorganic salt medium to degrade the SNFM36 (Fig. 3b,c). Similarly, an inorganic salt medium with the TCM was also selected as a control group. After 6 months of degradation, the TCM remained essentially unchanged (Supplementary Fig. 29). However, the shape of the SNFM changed from complete to powder as the degradation time increased (Fig. 3c). Interestingly, scanning electron microscopy (SEM) images of the SNFM degraded by the soil microorganisms revealed gradually increasing voids as the degradation time increased (Fig. 3b and Supplementary Fig. 30), which was different from the TCM control group with maintained structures (Supplementary Fig. 31). These results confirmed that SNFM had the potential to be disposed of naturally again. More importantly, the degradation rate of the SNFM reached 90% after 6 months, which was greater than that of the TCM (8%), confirming the excellent degradation properties of the SNFM (Fig. 3c).
To further explore the degradation mechanism of the SNFM, the biomes of the soil degradation solutions with SNFM as the only carbon source (SNFM soil) were analy ed. For comparison, the original soil solution (original soil) was selected as the control group, and biome analysis was performed. Compared with those in the control group with 384 species, the number of species in the SNFM soil decreased to 150 (Supplementary Fig. 32). Furthermore, the Shannon and Simpson indices were greater, but the Chao estimator was lower than that of the original soil (Supplementary Table 10), again supporting a decrease in the number of species in the SNFM soil. These results indicate that SNFM serves as a carbon source to induce the selective survival of microorganisms, mainly because microorganisms that cannot degrade SNFM will gradually be eliminated due to a lack of carbon sources to maintain survival. To further clarify the species names, the bacterial genera and their abundance in the communities were determined. As shown in Fig. 3d, the abundance of the Delftia (0.50) and Tissierella (0.14) genera in the SNFM soil community was much greater than that in the control group (correspondence between colour and genus names is shown in Supplementary Table 11). In addition, the significance test of differences between groups revealed great differences between the Delftia (−50%) and Tissierella (−15%) genera in the two samples (Supplementary Fig. 33). These results indicated that the Delftia and Tissierella genera strongly contributed to the degradation of SNFM, which may be related to their functions. Delftia and Tissierella are common ester-degrading bacteria, so they can destroy the –COO– bond in PLA substrates and selective layers37,38. To further explore the degradation routes of the SNFM, FAPROTAX (Functional Annotation of Prokaryotic Taxa) prediction, characterizing community metabolic functions, was performed (Supplementary Table 12). The abundance of plastic-degrading functional bacteria was 19-fold greater in the SNFM soil community than that in the control group because SNFM was a typical plastic material (Fig. 3e). Interestingly, the abundance of fermentation functional bacteria was 1.8-fold greater in the SNFM soil community than in the original soil community, but the abundances of chemoheterotrophic and aerobic chemoheterotrophic functional bacteria exhibited the opposite trend (Fig. 3e). This result indicated that the degradation of SNFM occurred mainly through the metabolic process of fermentation39,40. However, enzymes are often involved in metabolic processes. Therefore, KEGG (Kyoto Encyclopedia of Genes and Genomes) prediction was performed to explore changes in enzyme abundance during fermentation. We found that the abundance of acetyl-CoA associated with fatty acids increased dramatically in the SNFM soil community, indicating fat fermentation41,42 (Fig. 3f). Therefore, we believe that Delftia and Tissierella bacteria use SNFM as a substrate to produce acetyl-CoA through fat fermentation and then convert it into energy for survival, which leads to the continuous degradation of SNFM.
To investigate the environmental impact of SNFM degradation products, we conducted liquid chromatography‒mass spectrometry analysis and E. coli toxicity assays (Supplementary Fig. 34). As shown in Fig. 3g, the main degradation products of SNFM included lactic acid, PLA oligomers (dimers and trimers) and catechol, which were generated primarily from the breakdown of PLA and polyesters, consistent with the degradation mechanism described above. More importantly, the growth of E. coli in the SNFM degradation solution, with a growth rate of 577%, reached 8.9 × 108 colony-forming units (CFU) ml−1, which was greater than that of the blank group (1.3 × 108 CFU ml−1) (Fig. 3h). These results indicated that the degradation products of SNFM are low hazard.
The superior confined molecular and ionic sieving of the SNFM
The SNFM with excellent Congo Red (CR) rejection of up to 99.9% presented a water permeance of 100.7 l m−2 h−1 bar−1, which was 212% and 928% greater than that of the TCM and commercial GC-NF5001 selected owing to similar pore sizes, respectively (Fig. 4a and Supplementary Fig. 39). The excellent performance of the SNFM occurred because the irregular stripe surface structure can enhance water‒membrane interactions, improve the water diffusion coefficient and reduce both the water mass transfer resistance and energy barriers, as shown in Supplementary Figs. 40–44. Moreover, the dense selective layer can prevent CR molecules from passing through nanopores, as shown by the pore size distribution (Supplementary Fig. 39).
a, Pure water permeance and CR rejection of commercial GC-NF5001, TCM and SNFM. b, Performance of commercial GC-NF5001, TCM and SNFM in the presence of various active aqueous molecules. c, Comparison of the separation performance of commercial GC-NF5001, TCM and SNFM with state-of-the-art membranes: MPCM (molecularly porous cross-linked membranes)43, GO-TBO (graphene oxide-toluidine blue O)44, QL-COF (4-carboxyl-quinoline linked covalent organic frameworks)45, PGO (porous graphene oxide)46, ZIF-8/GO (zeolitic imidazolate framework-8/graphene oxide)47, TFCM (thin-film composite membranes)24, MOA (self-assembled multiblock oligomer amines)48 and salt-mediated polyamide49 for removing CR from water. d, Permeance and separation factor of commercial GC-NF5001, TCM and SNFM for CR and salts. e, Effects of different CR concentrations in aqueous CR/NaCl solution on membrane performance. f, Operational stability of commercial GC-NF5001, TCM and SNFM in a CR/NaCl aqueous solution. Inset: a schematic diagram of the SNFM inhibiting pollutant adsorption. Error bars in a, b and d–f represent the s.d. (n = 3, n derived from different experimental units), and data are presented as mean values ± s.d.
Furthermore, the performance of GC-NF5001, TCM and SNFM for the aqueous treatment of different active molecules was evaluated (the structures are presented in Supplementary Table 14). As shown in Fig. 4b, the SNFM exhibited high solution permeance and excellent rejection (above 99%) for CR, indocyanine green (IG) and Evans blue (EB) molecules and was superior to the GC-NF5001 and TCM. Interestingly, as the molecular weight of the active molecules increased, the solution permeance gradually decreased, which was attributed to the strong osmotic pressure caused by large active molecules. Coincidentally, the SNFM exhibited a rejection of more than 99% for IG aqueous solutions (35–200 ppm) of different concentrations (Supplementary Fig. 45). Finally, the performance of the SNFM was superior to that of commercial (Supplementary Table 15) and state-of-the-art membranes24,43,44,45,46,47,48,49 (Fig. 4c).
Considering that real dye wastewater usually contains salts, the performance of GC-NF5001, TCM and SNFM in dye and salt solutions was examined. As shown in Fig. 4d, the SNFM exhibited 96.9 l m−2 h−1 bar−1 solution permeance and a 244.5 CR/NaCl separation factor, which were far superior to those of the TCM (29.8 l m−2 h−1 bar−1, 81.8) and GC-NF5001 (9.1 l m−2 h−1 bar−1, 122.4). This phenomenon was attributed mainly to the excellent rejection of SNFM for CR in salt solutions, as shown in Supplementary Fig. 46. Interestingly, in monovalent anion systems, the SNFM demonstrated a greater separation factor than did those in divalent anion solutions, which was related to the Donnan effect. The negatively charged surface of the SNFM, as demonstrated by the zeta potential (Supplementary Fig. 47), had stronger electrostatic repulsion towards divalent anions, resulting in greater rejection. Furthermore, the SNFM exhibited excellent solution permeances (80.7–96.9 l m−2 h−1 bar−1) and separation factors (242.9–244.5) in CR/NaCl with various CR concentrations, indicating the potential of the SNFM for treating high-concentration molecular/ionic solutions (Fig. 4e). In addition to separation performance, membrane stability, which affects the cost of molecular/ionic treatment, is very important for commercial applications. The SNFM showed stable water permeance and CR/NaCl separation factors as the solution pH increased from 4 to 9, indicating the structural stability of the membrane and highlighting its potential for application in complex environments (Supplementary Fig. 49). During continuous operation for 14 days, the CR/NaCl solution permeance of the SNFM was maintained at 92.9 l m−2 h−1 bar−1, which was 1,091% and 431% greater than that of the GC-NF5001 and TCM, respectively (Fig. 4f). It also exhibited a persistent high separation factor of 244.2. This occurred because the surface can slow the clogging of pores in membranes caused by the adhesion of pollutants because of its excellent antifouling ability. As shown in Supplementary Fig. 51, the flux recovery ratio of our SNFM after bovine albumin pollution cycling was still 95%, which was attributed to the surface hydrophilicity of the SNFM. Therefore, the SNFM surface can form a hydration layer to inhibit the adhesion of pollutants50 (Fig. 4f).
Discussion
In summary, an SNFM with a greener life cycle for highly efficient water treatment was successfully developed through a systematic design approach, from raw materials to synergistic fabrication and synthesis methods. LCA and E. coli survival experiments demonstrated that SNFM can be prepared more safely, using raw materials with lower environmental impact and hazard than those used in TCM, as indicated by lower CO₂ emissions and enhanced E. coli growth. Coincidentally, the enzyme and soil degradation analyses also implied that SNFM can be degraded by proteinase K within 5 days and by the Delftia and Tissierella genera within 6 months, whereas both commercial GC-NF5001 and TCM showed no degradability, indicating the sustainability of the membranes. More importantly, the SNFM exhibited a high water permeance of 100.7 l m−2 h−1 bar−1 (928% and 212% higher than that of GC-NF5001 and TCM), excellent rejection, a high molecular and ionic separation factor, superior antifouling ability and operational stability, and superior commercial and state-of-the-art membranes. This membrane-based sustainable strategy provides a new route and enriches the chemical box for designing next-generation membranes for diverse applications.
Methods
Study design
Research hypotheses
This study is based on the hypothesis that NF membranes constructed via a safer fabrication strategy using low-hazard raw materials can simultaneously achieve natural degradability and excellent separation performance, thereby promoting the sustainable development of membrane technology.
Research objectives:
-
(1)
To fabricate the SNFM using low-hazard monomers, solvents and supports, and to compare them with the TCM prepared from conventional raw materials.
-
(2)
To verify the safer preparation of SNFM, the environmental impact and hazard of the raw materials in SNFM and TCM were evaluated and compared through LCA and E. coli growth characterization.
-
(3)
To investigate the degradability of the SNFM, its degradation behaviour was compared with that of commercial GC-NF5001 membranes and TCM in both enzymatic and soil microbial environments.
-
(4)
To investigate and compare the performance of the SNFM, GC-NF5001 and TCM in terms of water permeance, molecular rejection and molecular and ionic separation factors, the energy efficiency application potential of the SNFM was validated.
PLA substrate preparation
An 18 wt% PLA/DMSO solution with 2.5 wt% T80 was introduced into a three-necked flask and swirled at 95 °C for 12 h. Then, this homogeneous solution was defoamed at 120 °C for 8 h. Next, this solution was poured onto a preheated glass plate and smoothed with a metal spatula. Finally, the glass plate with the solution was soaked in water to prepare the PLA membranes via the NIPs method.
SNFM preparation
First, the PLA substrate was soaked in 20 ml of aqueous solution with 2 wt% xylitol and 2 wt% DA for 30 min in a shaker to induce monomer loading on the substrate. Then, the support with the monomers was removed, and the mixture was washed with plenty of water. Filter paper was used to remove excess water from the membrane surface. Finally, the dried membrane was soaked in 20 ml of OA solution with 0.05 wt% TMC for 4 min to prepare a SNFM. Notably, the SNFM was stored in deionized water for 8 h before testing to stabilize its structure.
TCM preparation
An 18 wt% PAN/DMF solution containing 2 wt% PEG-800 was cast on a glass plate with a stainless-steel scraper. After phase conversion by immersion in water, PAN membranes were fabricated. The TCM was prepared through IP as follows: the PAN membrane was immersed in a water phase containing 5 wt% MPD for 20 min. Then, the excess amine monomer was removed by washing the membrane surface and placing it on a glass plate to dry the surface. After that, the membrane was soaked with 0.1 wt% TPC/hexane solution for polymerization. The membrane was immersed in water at 50 °C for 20 min for heat stabilization to avoid shrinkage of the selective layer and the support and then stored in deionized water (8 h) for testing.
LCA
This LCA study was conducted following the guidelines outlined in ISO 14044 standards. To compile the life cycle inventory and perform impact assessments, SimaPro 9.0 software was utilized under an educational licence. The analysis adopted a global perspective to represent the large-scale commercialization of the membrane market. Similarly, the global electricity mix was incorporated into the simulation to model the electricity generation system throughout the assessment.
E. coli survival test of the raw materials
First, the LB medium (10 g of peptone, 5 g of yeast extract and 10 g of sodium chloride) was autoclaved at 121 °C for 30 min. Sterilized LB medium was used to culture E. coli at 37 °C for 1 day. Ten millilitres of sterilized LB medium with 1 g of the chemicals used in the preparation of the SNFM was placed in a 15-ml centrifuge tube, and 0.1 ml of E. coli culture medium was added to each tube. This mixture was subsequently placed in an incubator at 37 °C and incubated with E. coli for 10 h. Next, 0.1 ml of the culture mixture was diluted 101 times by adding 0.9 ml of sterilized ultrapure water. This process was repeated five times, and the sample was diluted 105 times. Then, 0.1 ml of the diluted solution was spread on LB solid medium. After even coating, the Petri dish was inverted at 37 °C overnight. Finally, the number of E. coli was counted. For comparison, some materials used to prepare TCM were subjected to the same test to explore their hazard.
Enzyme degradation of membranes
The membranes were soaked in 10 ml of 500 ppm enzyme (porcine trypsin, esterase and proteinase K) aqueous solution. The solution was subsequently placed in a water bath at 37 °C for degradation. The duration of the degradation cycle was 5 days, and samples were taken every other day for SEM analysis. The degradation rate was calculated via equation (1).
where mo and m1 are the original weight and the weights of the membranes after degradation for a period of time, respectively.
Soil microbial degradation of the membranes
Ten grams of soil around the School of Environment, Harbin Institute of Technology, was added to 500 ml of sterilized deionized water. After the soil naturally settled, 10 ml of the bacterial suspension was removed, placed in sterilized LB medium and incubated at 35 °C for 10 h to rapidly amplify the microorganisms in the soil. One millilitre of the above culture medium was added to 200 ml of inorganic salt medium supplemented with SNFM (the preparation method of the medium is described in Supplementary Information sections 1.3.1 and 1.3.2). This culture medium was placed in a biochemical incubator at 35 °C to test the biological degradation of the SNFM. SEM was performed every month to observe the degradation of the SNFM, and the membrane was further weighed to calculate the degradation rate of the SNFM. An inorganic salt medium with the above culture medium and TCM was selected as the blank group.
Characterization and performance evaluation
The relevant experimental methods are described in Supplementary Methods sections 1.1, 1.2 and 1.4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are included within the Article and its Supplementary Information. Source data are provided with this paper.
Code availability
All codes are available in the Article or its Supplementary Information.
References
Uliana, A. A. et al. Ion-capture electrodialysis using multifunctional adsorptive membranes. Science 372, 296–299 (2021).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Amara, Z. et al. Applying green chemistry to the photochemical route to artemisinin. Nat. Chem. 7, 489–495 (2015).
Li, B., Wang, S., Loh, X. J., Li, Z. & Chung, T.-S. Closed-loop recyclable membranes enabled by covalent adaptable networks for water purification. Proc. Natl Acad. Sci. USA 120, e2301009120 (2023).
Wen, Y. et al. Metal–organic framework enables ultraselective polyamide membrane for desalination and water reuse. Sci. Adv. 8, eabm4149 (2022).
Overmans, S. et al. Microalgal-mediated circular reuse of polymer membrane manufacturing wastewater. Sustain. Sci. Technol. 2, 014002 (2025).
Talukder, M. E. et al. Eco-friendly synthesis of porous and charged polyethersulfone membrane for improved protein separation efficiency. Results Eng. 25, 104422 (2025).
Kim, S. et al. Sustainable fabrication of solvent resistant biodegradable cellulose membranes using green solvents. Chem. Eng. J. 494, 153201 (2024).
Dargo, G. et al. MeSesamol, a bio-based and versatile polar aprotic solvent for organic synthesis and depolymerization. Chem. Eng. J. 471, 144365 (2023).
Zou, D., Nunes, S. P., Vankelecom, I. F., Figoli, A. & Lee, Y. M. Recent advances in polymer membranes employing non-toxic solvents and materials. Green Chem. 23, 9815–9843 (2021).
Yang, C., Cavalcante, J., de Freitas, B. B., Lauersen, K. J. & Szekely, G. Crude algal biomass for the generation of thin-film composite solvent-resistant nanofiltration membranes. Chem. Eng. J. 470, 144153 (2023).
Alhazmi, B. et al. Naturally derived allylated gallic acid for interfacially polymerized membranes. ACS Sustain. Chem. Eng. 10, 13585–13594 (2022).
Zhu, X. et al. Green maltitol-based nanofiltration membranes featuring high permeance and excellent anti-fouling performance for dye desalination. Desalination 586, 117851 (2024).
Zhan, S. et al. Green lignin‐based polyester nanofiltration membranes with ethanol and chlorine resistance. J. Appl. Polym. Sci. 139, 51427 (2022).
Zhang, Y. et al. Ice-confined synthesis of highly ionized 3D-quasilayered polyamide nanofiltration membranes. Science 382, 202–206 (2023).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Alammar, A., Hardian, R. & Szekely, G. Upcycling agricultural waste into membranes: from date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 24, 365–374 (2022).
Hardian, R., Alammar, A., Holtzl, T. & Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose–chitosan biopolymer blends. J. Membr. Sci. 658, 120743 (2022).
Cavalcante, J. et al. Biobased interpenetrating polymer network membranes for sustainable molecular sieving. ACS Nano 18, 7433–7443 (2024).
Wang, S. et al. In-situ forming dynamic covalently crosslinked nanofibers with one-pot closed-loop recyclability. Nat. Commun. 14, 1182 (2023).
Li, B. et al. Closed-loop recyclable dynamic covalent crosslinked nanofibrous membranes for efficient oil/water separation. J. Membr. Sci. 693, 122378 (2024).
Zhao, G., Gao, H., Qu, Z., Fan, H. & Meng, H. Anhydrous interfacial polymerization of sub-1 Å sieving polyamide membrane. Nat. Commun. 14, 7624 (2023).
Zeng, W. et al. A general strategy for recycling polyester wastes into carboxylic acids and hydrocarbons. Nat. Commun. 15, 160 (2024).
Bai, Y. et al. Microstructure optimization of bioderived polyester nanofilms for antibiotic desalination via nanofiltration. Sci. Adv. 9, eadg6134 (2023).
Hong, C. et al. Global and regional drivers of land-use emissions in 1961–2017. Nature 589, 554–561 (2021).
Pikula, K. et al. The advances and limitations in biodiesel production: feedstocks, oil extraction methods, production, and environmental life cycle assessment. Green Chem. Lett. Rev. 13, 275–294 (2020).
Meijaard, E. et al. The environmental impacts of palm oil in context. Nat. Plants 6, 1418–1426 (2020).
Benefits and Costs of Shifts to Biomass Crops: Producer and Public Perspectives (BIOCAP, 2006).
Liu, C. et al. An action potential initiation mechanism in distal axons for the control of dopamine release. Science 375, 1378–1385 (2022).
Grove, J. C. et al. Dopamine subsystems that track internal states. Nature 608, 374–380 (2022).
Wang, M. et al. Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation. Nat. Nanotechnol. 18, 403–411 (2023).
Cheng, X. et al. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil–water separation. Sci. Adv. 9, eadh8195 (2023).
Castillo-Quan, J. I. et al. An antisteatosis response regulated by oleic acid through lipid droplet–mediated ERAD enhancement. Sci. Adv. 9, eadc8917 (2023).
Lin, L. et al. Oleic acid availability impacts thymocyte preprogramming and subsequent peripheral Treg cell differentiation. Nat. Immunol. 25, 54–65 (2024).
Vasilyev, N., Liu, M. M. J., Epshtein, V., Shamovsky, I. & Nudler, E. General transcription factor from Escherichia coli with a distinct mechanism of action. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-023-01154-w (2024).
Hu, Z. et al. Nutrient-induced acidification modulates soil biodiversity–function relationships. Nat. Commun. 15, 2858 (2024).
Huang, W. et al. Delftia tsuruhatensis TC1 symbiont suppresses malaria transmission by anopheline mosquitoes. Science 381, 533–540 (2023).
Wylensek, D. et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 11, 6389 (2020).
Senne de Oliveira Lino, F., Bajic, D., Vila, J. C. C., Sánchez, A. & Sommer, M. O. A. Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 12, 1498 (2021).
Moraïs, S. et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science 383, eadj9223 (2024).
Yeudall, S. et al. Macrophage acetyl-CoA carboxylase regulates acute inflammation through control of glucose and lipid metabolism. Sci. Adv. 8, eabq1984 (2022).
Zhang, J. et al. PARylated PDHE1α generates acetyl-CoA for local chromatin acetylation and DNA damage repair. Nat. Struct. Mol. Biol. 30, 1719–1734 (2023).
Huang, T. et al. Molecularly-porous ultrathin membranes for highly selective organic solvent nanofiltration. Nat. Commun. 11, 5882 (2020).
Wang, Z. et al. Graphene oxide nanofiltration membranes for desalination under realistic conditions. Nat. Sustain. 4, 402–408 (2021).
Yang, Y. et al. Constructing chemical stable 4-carboxyl-quinoline linked covalent organic frameworks via Doebner reaction for nanofiltration. Nat. Commun. 13, 2615 (2022).
Liu, H., Huang, X., Wang, Y., Kuang, B. & Li, W. Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation. Nat. Commun. 15, 164 (2024).
Zhang, W. H. et al. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotechnol. 16, 337–343 (2021).
Li, S. et al. Hydrophobic polyamide nanofilms provide rapid transport for crude oil separation. Science 377, 1555–1561 (2022).
Shen, L. et al. Polyamide-based membranes with structural homogeneity for ultrafast molecular sieving. Nat. Commun. 13, 500 (2022).
Wang, D. et al. Design of robust superhydrophobic surfaces. Nature 582, 55–59 (2020).
Acknowledgements
This work was supported by the National Key R&D Program (grant no. 2023YFE0127000, L.S.), National Natural Science Foundation of China (grant nos. 92475205, L.S., 22208072, Y.Z., and 524B2037 J.H.), the Open Research Fund of Suzhou Laboratory (grant no. SZLAB-1308-2024-ZD007, L.S.), the State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology, grant no. 2024DX02, L.S.), the National Key Research and Development Program of China (grant no. 2024YFB3815400, L.S.), the Fundamental Research Funds for the Central Universities of Ministry of Education (grant nos. HIT.OCEF.2024007, Y.Z., and HIT.DZJJ.2024018 L.S.), the Open Project of State Key Laboratory of Urban-rural Water Resources and Environment, Harbin Institute of Technology (grant no. ZD202504, Y.Z.), the National Natural Science Foundation of China under the Basic Science Center Program for ‘Space Robot Intelligent Manipulation’ (grant no. T2388101, Y.Z.) and Jiangsu Future Membrane Technology Innovation Center (grant no. BM2021804, L.S.). J.H. acknowledges the Department of Chemical and Biological Engineering at Monash University for hosting his visiting research. C.H.L. and L.F. acknowledge financial support from EPSRC (grant no. EP/X042286/1, C.H.L.).
Funding
Open access funding provided by Monash University.
Author information
Authors and Affiliations
Contributions
L.S., J.H., M.Y., Y.Z., L.F., C.H.L. and H.W. designed the experiments. J.H. carried out the material synthesis, material characterization and performance measurements. L.F. and C.H.L. performed LCA. M.Y. and S.Q. performed conducted the soil microbial degradation experiment. J.H., M.Y., L.F., Y.Z., J. G., S.Q., L.S., C.H.L. and H.W. performed the data analysis. J.H. wrote the manuscript, and J.H., M.Y., Y.Z., J.G., L.F., S.Q., C.H.L., L.S. and H.W. discussed the results and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Shadi Hasan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–52 and Tables 1–16.
Source data
Source Data Fig. 2
Preparation of SNFM and hazard of the raw materials, source data.
Source Data Fig. 3
Degradation mechanism and products of the SNFM, source data.
Source Data Fig. 4
Performance of the SNFM, source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Yuan, M., Zhang, Y. et al. Sustainable nanofiltration membranes enable ultrafast water purification. Nat Water 3, 1048–1056 (2025). https://doi.org/10.1038/s44221-025-00492-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00492-x
This article is cited by
-
Green separation membranes for water sustainability
Science China Materials (2025)