Abstract
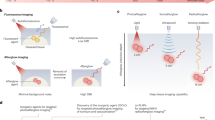
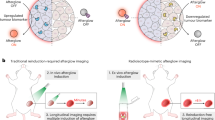
Afterglow luminescence imaging relies on the detection of photons from chemical or lattice defects after cessation of irradiation, enabling autofluorescence-free biomedical imaging with a higher signal-to-background ratio compared to fluorescence imaging. In particular, organic afterglow probes benefit from biocompatibility and can be designed with diverse molecular architectures and for various irradiation sources, including light, ultrasound and X-rays. In this Review, we first introduce the mechanisms governing afterglow emission. We then examine design strategies for organic afterglow probes, outlining strategies to improve their afterglow performance, particularly afterglow intensity, extended emission wavelengths, responsivity and diverse excitation sources, to allow bioimaging with high sensitivity and specificity in deep tissues. Finally, we highlight key biomedical applications in disease diagnosis and therapy and provide an overview of remaining challenges and opportunities of organic afterglow imaging.
Key points
-
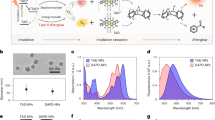
Organic afterglow luminescence is a process that converts external excitation energy into storable chemical energy, which is then slowly released as light after irradiation ceases.
-
Organic afterglow luminescence probes typically include an afterglow initiator that generates reactive oxygen species upon irradiation, an afterglow substrate that reacts with reactive oxygen species to form a chemical defect and a relay unit that emits the afterglow.
-
Organic probes can be optimized to extend the afterglow wavelength, enhance brightness and allow activation by specific biomarkers.
-
Organic afterglow luminescence can be applied to various biomedical applications, including cancer diagnosis and treatment, inflammation imaging, and image-guided therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weissleder, R. Molecular imaging in cancer. Science 312, 1168–1171 (2006).
Rubin, G. D. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 273, S45–S74 (2014).
Withers, P. J. et al. X-ray computed tomography. Nat. Rev. Methods Primers 1, 18 (2021).
Terreno, E., Castelli, D. D., Viale, A. & Aime, S. Challenges for molecular magnetic resonance imaging. Chem. Rev. 110, 3019–3042 (2010).
Moser, E., Stadlbauer, A., Windischberger, C., Quick, H. H. & Ladd, M. E. Magnetic resonance imaging methodology. Eur. J. Nucl. Med. Mol. Imaging 36, 30–41 (2009).
Zhou, J. et al. Fluorescent diagnostic probes in neurodegenerative diseases. Adv. Mater. 32, 2001945 (2020).
Sharma, A. et al. Theranostic fluorescent probes. Chem. Rev. 124, 2699–2804 (2024).
Fujita, K. & Urano, Y. Activity-based fluorescence diagnostics for cancer. Chem. Rev. 124, 4021–4078 (2024).
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 10, 507–518 (2013).
Hernot, S., van Manen, L., Debie, P., Mieog, J. S. D. & Vahrmeijer, A. L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 20, e354–e367 (2019).
Bakhtiar, N., Jaleel, F., Moosa, F. A., Qureshi, N. A. & Jawaid, M. Sentinel lymph node identification by blue dye in patients with breast carcinoma. Pak. J. Med. Sci. 32, 448–451 (2016).
Ishizawa, T. et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 115, 2491–2504 (2009).
Croce, A. C. & Bottiroli, G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur. J. Histochem. 58, 320–337 (2014).
del Rosal, B. & Benayas, A. Strategies to overcome autofluorescence in nanoprobe-driven in vivo fluorescence imaging. Small Methods 2, 1800075 (2018).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
McCapra, F. Chemical mechanisms in bioluminescence. Acc. Chem. Res. 9, 201–208 (1976).
Badr, C. E. & Tannous, B. A. Bioluminescence imaging: progress and applications. Trends Biotechnol. 29, 624–633 (2011).
Blau, R., Shelef, O., Shabat, D. & Satchi-Fainaro, R. Chemiluminescent probes in cancer biology. Nat. Rev. Bioeng. 1, 648–664 (2023).
Dragulescu-Andrasi, A., Chan, C. T., De, A., Massoud, T. F. & Gambhir, S. S. Bioluminescence resonance energy transfer (BRET) imaging of protein–protein interactions within deep tissues of living subjects. Proc. Natl. Acad. Sci. USA 108, 12060–12065 (2011).
Buckley, S. M. K. et al. In vivo bioimaging with tissue-specific transcription factor activated luciferase reporters. Sci. Rep. 5, 11842 (2015).
Yang, M. et al. Chemiluminescence for bioimaging and therapeutics: recent advances and challenges. Chem. Soc. Rev. 49, 6800–6815 (2020).
Tannous, R. et al. Spirostrain-accelerated chemiexcitation of dioxetanes yields unprecedented detection sensitivity in chemiluminescence bioassays. ACS Cent. Sci. 10, 28–42 (2024).
David, M. et al. Chemiexcitation acceleration of 1,2-dioxetanes by spiro-fused six-member rings with electron-withdrawing motifs. Angew. Chem. Int. Ed. 63, e202410057 (2024).
Xiang, H., Cheng, J., Ma, X., Zhou, X. & Chruma, J. J. Near-infrared phosphorescence: materials and applications. Chem. Soc. Rev. 42, 6128–6185 (2013).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Liu, Y., Li, C., Ren, Z., Yan, S. & Bryce, M. R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 3, 18020 (2018).
Zhen, X. et al. Ultralong phosphorescence of water-soluble organic nanoparticles for in vivo afterglow imaging. Adv. Mater. 29, 1606665 (2017).
Kabe, R. & Adachi, C. Organic long persistent luminescence. Nature 550, 384–387 (2017).
Nishimura, N., Lin, Z., Jinnai, K., Kabe, R. & Adachi, C. Many exciplex systems exhibit organic long-persistent luminescence. Adv. Funct. Mater. 30, 2000795 (2020).
Chen, L.-J., Yang, C.-X. & Yan, X.-P. Liposome-coated persistent luminescence nanoparticles as luminescence trackable drug carrier for chemotherapy. Anal. Chem. 89, 6936–6939 (2017).
Sun, S.-K. et al. Turning solid into gel for high-efficient persistent luminescence-sensitized photodynamic therapy. Biomaterials 218, 119328 (2019).
le Masne de Chermont, Q. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 104, 9266–9271 (2007).
Liang, L. et al. Controlling persistent luminescence in nanocrystalline phosphors. Nat. Mater. 22, 289–304 (2023).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Palner, M., Pu, K., Shao, S. & Rao, J. Semiconducting polymer nanoparticles with persistent near-infrared luminescence for in vivo optical imaging. Angew. Chem. Int. Ed. 54, 11477–11480 (2015).
Wang, Y. et al. Cyclic amplification of the afterglow luminescent nanoreporter enables the prediction of anti-cancer efficiency. Angew. Chem. Int. Ed. 60, 19779–19789 (2021).
Liao, S. et al. A novel afterglow nanoreporter for monitoring cancer therapy. Theranostics 12, 6883–6897 (2022).
Zhen, X., Xie, C. & Pu, K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angew. Chem. Int. Ed. 57, 3938–3942 (2018).
Lyu, Y. et al. Near-infrared afterglow semiconducting nano-polycomplexes for the multiplex differentiation of cancer exosomes. Angew. Chem. Int. Ed. 58, 4983–4987 (2019).
Jiang, Y. et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
Ni, X. et al. Near-Infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Liu, Y. et al. Significantly enhanced afterglow brightness via intramolecular energy transfer. ACS Mater. Lett. 3, 713–720 (2021).
Zheng, X. et al. Organic nanoparticles with persistent luminescence for in vivo afterglow imaging-guided photodynamic therapy. Chem. Eur. J. 27, 6911–6916 (2021).
Chen, W. et al. Near-Infrared afterglow luminescence of chlorin nanoparticles for ultrasensitive in vivo imaging. J. Am. Chem. Soc. 144, 6719–6726 (2022).
Duan, X. et al. Activatable persistent luminescence from porphyrin derivatives and supramolecular probes with imaging-modality transformable characteristics for improved biological applications. Angew. Chem. Int. Ed. 61, e202116174 (2022).
Zhu, J. et al. A self-sustaining near-infrared afterglow chemiluminophore for high-contrast activatable imaging. Angew. Chem. Int. Ed. 63, e202318545 (2024).
Yang, L. et al. A highly bright near-infrared afterglow luminophore for activatable ultrasensitive in vivo imaging. Angew. Chem. Int. Ed. 63, e202313117 (2024).
Wang, Y. et al. Enhancing fractionated cancer therapy: a triple-anthracene photosensitizer unleashes long-persistent photodynamic and luminous efficacy. J. Am. Chem. Soc. 146, 6252–6265 (2024).
Wang, Y. et al. In vivo ultrasound-induced luminescence molecular imaging. Nat. Photonics 18, 334–343 (2024).
Wang, Y. et al. Ultrabright and ultrafast afterglow imaging in vivo via nanoparticles made of trianthracene derivatives. Nat. Biomed. Eng. 9, 656–670 (2025).
Xu, C. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics. Nat. Biomed. Eng. 7, 298–312 (2023).
Huang, J. et al. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat. Mater. 22, 1421–1429 (2023).
Xu, C. et al. A cascade X-ray energy converting approach toward radio-afterglow cancer theranostics. Nat. Nanotechnol. 20, 286–295 (2024).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
Anjong, T. F. et al. Multifunction-harnessed afterglow nanosensor for molecular imaging of acute kidney injury in vivo. Small 18, 2200245 (2022).
Chen, C. et al. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Liu, Y. et al. Ratiometric afterglow luminescent nanoplatform enables reliable quantification and molecular imaging. Nat. Commun. 13, 2216 (2022).
Gao, Z., Zhang, Y., Liu, Q. & Ding, D. Mechanism and design of organic afterglow luminescent probes for cancer theranostics. Med. Mat. 1, 27–39 (2024).
Shen, H. et al. Organic afterglow nanoparticles in bioapplications. Chem. Eur. J. 29, e202301209 (2023).
Wang, X. & Pu, K. Molecular substrates for the construction of afterglow imaging probes in disease diagnosis and treatment. Chem. Soc. Rev. 52, 4549–4566 (2023).
Li, Z., Liu, H. & Zhang, X.-B. Reactive oxygen species-mediated organic long-persistent luminophores light up biomedicine: from two-component separated nano-systems to integrated uni-luminophores. Chem. Soc. Rev. 53, 11207–11227 (2024).
Qu, R., Jiang, X. & Zhen, X. Light/X-ray/ultrasound activated delayed photon emission of organic molecular probes for optical imaging: mechanisms, design strategies, and biomedical applications. Chem. Soc. Rev. 53, 10970–11003 (2024).
Zhu, J., Zhao, L., An, W. & Miao, Q. Recent advances and design strategies for organic afterglow agents to enhance autofluorescence-free imaging performance. Chem. Soc. Rev. 54, 1429–1452 (2025).
Jiang, K. et al. Triple-mode emission of carbon dots: applications for advanced anti-counterfeiting. Angew. Chem. Int. Ed. 55, 7231–7235 (2016).
Alam, P. et al. Organic long-persistent luminescence from a single-component aggregate. J. Am. Chem. Soc. 144, 3050–3062 (2022).
Jinnai, K., Kabe, R., Lin, Z. & Adachi, C. Organic long-persistent luminescence stimulated by visible light in p-type systems based on organic photoredox catalyst dopants. Nat. Mater. 21, 338–344 (2022).
Li, W. et al. Organic long-persistent luminescence from a thermally activated delayed fluorescence compound. Adv. Mater. 32, 2003911 (2020).
Lin, C. et al. Charge trapping for controllable persistent luminescence in organics. Nat. Photonics 18, 350–356 (2024).
Li, Z. et al. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 137, 5304–5307 (2015).
Lécuyer, T. et al. Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics 6, 2488–2524 (2016).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Pham, T. C., Nguyen, V.-N., Choi, Y., Lee, S. & Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 121, 13454–13619 (2021).
Chen, D. et al. Type I photosensitizers revitalizing photodynamic oncotherapy. Small 17, 2006742 (2021).
Xie, C., Zhen, X., Miao, Q., Lyu, Y. & Pu, K. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumors. Adv. Mater. 30, 1801331 (2018).
Su, X. et al. Enhanced blue afterglow through molecular fusion for bio-applications. Angew. Chem. Int. Ed. 61, e202201630 (2022).
Cui, D., Xie, C., Li, J., Lyu, Y. & Pu, K. Semiconducting photosensitizer-incorporated copolymers as near-infrared afterglow nanoagents for tumor imaging. Adv. Healthc. Mater. 7, 1800329 (2018).
Xu, Y. et al. An aggregation-induced emission dye-powered afterglow luminogen for tumor imaging. Chem. Sci. 11, 419–428 (2020).
Ma, G. et al. Rechargeable afterglow nanotorches for in vivo tracing of cell-based microrobots. Angew. Chem. Int. Ed. 63, e202400658 (2024).
Yang, J. et al. Turn-on chemiluminescence probes and dual-amplification of signal for detection of amyloid beta species in vivo. Nat. Commun. 11, 4052 (2020).
Zhang, J. et al. In vivo three-dimensional brain imaging with chemiluminescence probes in Alzheimer’s disease models. Proc. Natl. Acad. Sci. USA 120, e2310131120 (2023).
Lei, L. et al. Noninvasive imaging of tumor glycolysis and chemotherapeutic resistance via de novo design of molecular afterglow scaffold. J. Am. Chem. Soc. 145, 24386–24400 (2023).
Zheng, G. et al. Photooxidation triggered ultralong afterglow in carbon nanodots. Nat. Commun. 15, 2365 (2024).
Gutkin, S. et al. Boosting chemiexcitation of phenoxy-1,2-dioxetanes through 7-norbornyl and homocubanyl spirofusion. JACS Au 4, 3558–3566 (2024).
Shelef, O. et al. Biocompatible flash chemiluminescent assay enabled by sterically hindered spiro-strained-oxetanyl-1,2-dioxetane. Chem. Eur. J. 30, e202402981 (2024).
Reguero, M., Bernardi, F., Bottoni, A., Olivucci, M. & Robb, M. A. Chemiluminescent decomposition of 1,2-dioxetanes: an MC-SCF/MP2 study with VB analysis. J. Am. Chem. Soc. 113, 1566–1572 (1991).
Vacher, M. et al. Chemi- and bioluminescence of cyclic peroxides. Chem. Rev. 118, 6927–6974 (2018).
Koo, J.-Y. & Schuster, G. B. Chemically initiated electron exchange luminescence. A new chemiluminescent reaction path for organic peroxides. J. Am. Chem. Soc. 99, 6107–6109 (1977).
Schuster, G. B. Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism. Acc. Chem. Res. 12, 366–373 (1979).
Isobe, H., Takano, Y., Okumura, M., Kuramitsu, S. & Yamaguchi, K. Mechanistic insights in charge-transfer-induced luminescence of 1,2-dioxetanones with a substituent of low oxidation potential. J. Am. Chem. Soc. 127, 8667–8679 (2005).
Catalani, L. H. & Wilson, T. Electron transfer and chemiluminescence. Two inefficient systems: 1,4-dimethoxy-9,10-diphenylanthracene peroxide and diphenoyl peroxide. J. Am. Chem. Soc. 111, 2633–2639 (1989).
Matsumoto, M., Sakuma, T. & Watanabe, N. Synthesis of bicyclic dioxetanes bearing a 3-hydroxy-4-isoxazolylphenyl moiety: new CIEEL-active dioxetanes emitting light with remarkable high-efficiency in aqueous medium. Tetrahedron Lett. 43, 8955–8958 (2002).
Green, O. et al. Opening a gateway for chemiluminescence cell imaging: distinctive methodology for design of bright chemiluminescent dioxetane probes. ACS Cent. Sci. 3, 349–358 (2017).
Wu, L. et al. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 49, 5110–5139 (2020).
He, S., Xie, C., Jiang, Y. & Pu, K. An organic afterglow protheranostic nanoassembly. Adv. Mater. 31, 1902672 (2019).
Zhang, Y. et al. Molecular engineering of a self-sustaining modular afterglow scaffold for in vivo activatable imaging. Angew. Chem. Int. Ed. 64, e202500801 (2025).
Jares-Erijman, E. A. & Jovin, T. M. FRET imaging. Nat. Biotechnol. 21, 1387–1395 (2003).
Wang, C. & Li, Z. Molecular conformation and packing: their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 1, 2174–2194 (2017).
Borisov, S. M. & Wolfbeis, O. S. Optical biosensors. Chem. Rev. 108, 423–461 (2008).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Xu, C. et al. Activatable sonoafterglow nanoprobes for T-cell imaging. Adv. Mater. 35, 2211651 (2023).
Niu, H. et al. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 52, 2322–2357 (2023).
Zeng, W. et al. An activatable afterglow/MRI bimodal nanoprobe with fast response to H₂S for in vivo imaging of acute hepatitis. Angew. Chem. Int. Ed. 61, e202111759 (2022).
Huang, W. et al. Ratiometric afterglow luminescent imaging of matrix metalloproteinase-2 activity via an energy diversion process. Angew. Chem. Int. Ed. 63, e202404244 (2024).
Yue, R. et al. Imaging-guided companion diagnostics in radiotherapy by monitoring APE1 activity with afterglow and MRI imaging. Nat. Commun. 15, 6349 (2024).
Samanta, P. K. & Misra, R. Intramolecular charge transfer for optical applications. J. Appl. Phys. 133, 020901 (2023).
Schaap, A. P. & Gagnon, S. D. Chemiluminescence from a phenoxide-substituted 1,2-dioxetane: a model for firefly bioluminescence. J. Am. Chem. Soc. 104, 3504–3506 (1982).
Wei, X. et al. Leveraging long-distance singlet-oxygen transfer for bienzyme-locked afterglow imaging of intratumoral granule enzymes. J. Am. Chem. Soc. 146, 17393–17403 (2024).
Shelef, O. et al. Enzymatic activity profiling using an ultrasensitive array of chemiluminescent probes for bacterial classification and characterization. J. Am. Chem. Soc. 146, 5263–5273 (2024).
Liu, P. et al. Mechanically triggered bright chemiluminescence from polymers by exploiting a synergy between masked 2-furylcarbinol mechanophores and 1,2-dioxetane chemiluminophores. J. Am. Chem. Soc. 146, 22151–22156 (2024).
Peukert, C. et al. Enzyme-activated, chemiluminescent siderophore-dioxetane probes enable the selective and highly sensitive detection of bacterial pathogens. Angew. Chem. Int. Ed. 61, e202201423 (2022).
Liu, J., Huang, J., Wei, X., Cheng, P. & Pu, K. Near-infrared chemiluminescence imaging of chemotherapy-induced peripheral neuropathy. Adv. Mater. 36, 2310605 (2024).
Skovsen, E., Snyder, J. W., Lambert, J. D. C. & Ogilby, P. R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 109, 8570–8573 (2005).
Midden, W. R. & Wang, S. Y. Singlet oxygen generation for solution kinetics: clean and simple. J. Am. Chem. Soc. 105, 4129–4135 (1983).
Jiang, Y. et al. Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 15, 2124 (2024).
Cheng, P. & Pu, K. Enzyme-responsive, multi-lock optical probes for molecular imaging and disease theranostics. Chem. Soc. Rev. 53, 10171–10188 (2024).
Zhang, P. et al. And-logic strategy for accurate analysis of Alzheimer’s disease via fluorescent probe lighted up by two specific biomarkers. Anal. Chem. 93, 11337–11345 (2021).
Zhou, H. et al. A tumor-microenvironment-activatable molecular pro-theranostic agent for photodynamic and immunotherapy of cancer. Adv. Mater. 35, 2211485 (2023).
Wei, P. et al. Deformylation reaction-based probe for in vivo imaging of HOCl. Chem. Sci. 9, 495–501 (2018).
Wu, R. et al. Ultrasound-activated NIR chemiluminescence for deep tissue and tumor foci imaging. Anal. Chem. 95, 11219–11226 (2023).
Mitragotri, S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 4, 255–260 (2005).
Martinoli, C. et al. Sonography of entrapment neuropathies in the upper limb (wrist excluded). J. Clin. Ultrasound. 32, 438–450 (2004).
de Almeida e Borges, V. F., Diniz, A. L. D., Cotrim, H. P., Rocha, H. L. O. G. & Andrade, N. B. Sonographic hepatorenal ratio: a noninvasive method to diagnose nonalcoholic steatosis. J. Clin. Ultrasound. 41, 18–25 (2013).
Goddi, A. et al. Vector flow imaging techniques: an innovative ultrasonographic technique for the study of blood flow. J. Clin. Ultrasound. 45, 582–588 (2017).
Flannigan, D. J. & Suslick, K. S. Plasma formation and temperature measurement during single-bubble cavitation. Nature 434, 52–55 (2005).
Wang, Y. et al. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat. Commun. 12, 3508 (2021).
Momose, A. X-ray phase imaging reaching clinical uses. Phys. Med. 79, 93–102 (2020).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Nguyen, L. N. M. et al. The mechanisms of nanoparticle delivery to solid tumours. Nat. Rev. Bioeng. 2, 201–213 (2024).
Lyle, A. N. & Taylor, W. R. The pathophysiological basis of vascular disease. Lab. Invest. 99, 284–289 (2019).
Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935 (2007).
Kass, G. E. N. Mitochondrial involvement in drug-induced hepatic injury. Chem. Biol. Interact. 163, 145–159 (2006).
Pashayan, N. & Pharoah, P. D. P. The challenge of early detection in cancer. Science 368, 589–590 (2020).
Richards, M. A., Westcombe, A. M., Love, S. B., Littlejohns, P. & Ramirez, A. J. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 353, 1119–1126 (1999).
Richards, M. A., Hiom, S. & Hamilton, W. Diagnosing cancer earlier: what progress is being made? Br. J. Cancer 128, 441–442 (2023).
Andrade, R. J. et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 5, 58 (2019).
Wang, X. & Chen, X. Clinical characteristics of 162 patients with drug-induced liver and/or kidney injury. BioMed. Res. Int. 2020, 3930921 (2020).
Ramachandran, A. & Jaeschke, H. Acetaminophen hepatotoxicity. Semin. Liver Dis. 39, 221–234 (2019).
Wang, S. et al. Fluorescence imaging of pathophysiological microenvironments. Chem. Soc. Rev. 50, 8887–8902 (2021).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Chen, S. et al. Macrophages in immunoregulation and therapeutics. Sig. Transduct. Target. Ther. 8, 207 (2023).
Szabó, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Lord, S. J., Rajotte, R. V., Korbutt, G. S. & Bleackley, R. C. Granzyme B: a natural born killer. Immunol. Rev. 193, 31–38 (2003).
Gao, Z. et al. An activatable near-infrared afterglow theranostic prodrug with self-sustainable magnification effect of immunogenic cell death. Angew. Chem. Int. Ed. 61, e202209793 (2022).
Hao, L. et al. Engineering light-initiated afterglow lateral flow immunoassay for infectious disease diagnostics. Biosens. Bioelectron. 212, 114411 (2022).
Chen, W. et al. O₂-relevant afterglow luminescence of chlorin nanoparticles for discriminative detection and isotopic analysis of H₂O and D₂O. Anal. Chem. 95, 5340–5345 (2023).
Yuan, H. et al. Afterglow amplification for fast and sensitive detection of porphyria in whole blood. ACS Appl. Mater. 13, 27991–27998 (2021).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Baskaran, R., Lee, J. & Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 22, 25 (2018).
Dougherty, T. J. et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 38, 2628–2635 (1978).
Agostinis, P. et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 61, 250–281 (2011).
Ran, C. & Pu, K. Molecularly generated light and its biomedical applications. Angew. Chem. Int. Ed. 63, e202314468 (2024).
Xu, X. G., Bednarz, B. & Paganetti, H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys. Med. Biol. 53, R193–R241 (2008).
He, S., Song, J., Qu, J. & Cheng, Z. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 47, 4258–4278 (2018).
Wen, K. et al. Achieving efficient NIR-II type-I photosensitizers for photodynamic/photothermal therapy upon regulating chalcogen elements. Adv. Mater. 34, 2108146 (2022).
Wang, S. et al. Beyond traditional light: NIR-II light-activated photosensitizers for cancer therapy. J. Mater. Chem. B. 11, 8315–8326 (2023).
Wang, X. et al. Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy. Nat. Commun. 13, 5091 (2022).
Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photonics 15, 187–192 (2021).
Gan, N. et al. Organic phosphorescent scintillation from copolymers by X-ray irradiation. Nat. Commun. 13, 3995 (2022).
Shi, L. et al. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 13, 10748–10764 (2021).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Gao, Y., Joshi, M., Zhao, Z. & Mitragotri, S. PEGylated therapeutics in the clinic. Bioeng. Transl. Med. 9, e10600 (2024).
Obaid, G. et al. Engineering photodynamics for treatment, priming and imaging. Nat. Rev. Bioeng. 2, 752–769 (2024).
Kennedy, J. C., Pottier, R. H. & Pross, D. C. Photodynamic therapy with endogenous protoporphyrin: IX: basic principles and present clinical experience. J. Photoch. Photobio. B. 6, 143–148 (1990).
Liu, J., Guo, M. & Chen, C. Nano-bio interactions: a major principle in the dynamic biological processes of nano-assemblies. Adv. Drug Deliv. Rev. 186, 114318 (2022).
Stater, E. P., Sonay, A. Y., Hart, C. & Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16, 1180–1194 (2021).
Poon, W. et al. Elimination pathways of nanoparticles. ACS Nano 13, 5785–5798 (2019).
Llop, J. & Lammers, T. Nanoparticles for cancer diagnosis, radionuclide therapy and theranostics. ACS Nano 15, 16974–16981 (2021).
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3, 358–374 (2018).
Zhong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Sig. Transduct. Target. Ther. 6, 201 (2021).
Li, Z. et al. Superoxide anion-mediated afterglow mechanism-based water-soluble zwitterion dye achieving renal-failure mice detection. J. Am. Chem. Soc. 145, 26736–26746 (2023).
Liu, Y., Teng, L., Lou, X.-F., Zhang, X.-B. & Song, G. “Four-in-one” design of a hemicyanine-based modular scaffold for high-contrast activatable molecular afterglow imaging. J. Am. Chem. Soc. 145, 5134–5144 (2023).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Salehi Farid, A. et al. CD45-PET is a robust, non-invasive tool for imaging inflammation. Nature 639, 214–224 (2025).
Woo, X. Y. et al. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat. Genet. 53, 86–99 (2021).
Allen, T. M. et al. Humanized immune system mouse models: progress, challenges and opportunities. Nat. Immunol. 20, 770–774 (2019).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Wang, X., Yuan, W., Xu, M., Su, X. & Li, F. Visualization of acute inflammation through a macrophage-camouflaged afterglow nanocomplex. ACS Appl. Mater. 14, 259–267 (2022).
Lin, Y. et al. Highly photoreactive semiconducting polymers with cascade intramolecular singlet oxygen and energy transfer for cancer-specific afterglow theranostics. J. Am. Chem. Soc. 147, 2597–2606 (2025).
Pei, Y. et al. Chemical energy lights up Europium-based ultra-bright afterglow for bioanalysis application. Angew. Chem. Int. Ed. 64, e202423791 (2025).
Agrahari, V. & Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 12, 819–823 (2017).
Ildikó, C., Ruba, I., Orsolya, J.-L. & Edina, P. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 28, 7461–7476 (2021).
Verbeek, F. P. et al. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: a first in human experience. J. Urol. 190, 574–579 (2013).
Shakeri-Zadeh, A. & Bulte, J. W. M. Imaging-guided precision hyperthermia with magnetic nanoparticles. Nat. Rev. Bioeng. 3, 245–260 (2025).
FDA. Drug products, including biological products, that contain nanomaterials guidance for industry; https://www.fda.gov/media/157812/download (2022).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22274107) and the Outstanding Youth Fund of Jiangsu Province (BK20230009).
Author information
Authors and Affiliations
Contributions
L.Z. researched data and contributed to the discussion of content and writing. Q.M. researched data and contributed to the discussion of content, writing, and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Citation diversity statement
The authors acknowledge that research authored by scholars from historically excluded groups are systematically under-cited. Every attempt has been made to reference relevant research in a manner that is equitable in terms of racial, ethnic, gender and geographical representation.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Xiaoyuan Chen, Chongzhao Ran, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Miao, Q. Organic afterglow luminescence for disease diagnosis and treatment. Nat Rev Bioeng 3, 955–975 (2025). https://doi.org/10.1038/s44222-025-00343-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00343-0