Abstract
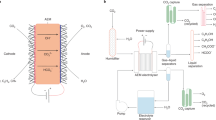
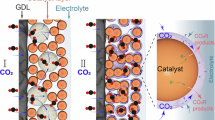
Electrochemical CO2 reduction (CO2R) using renewable electricity is a key pathway toward synthesizing fuels and chemicals. In this study, multi-physics modeling is used to interpret experimental data obtained for CO2R to CO using Ag catalysts in a membrane electrode assembly. The one-dimensional model is validated using measured CO2 crossover and product formation rates. The kinetics of CO formation are described by Marcus–Hush–Chidsey kinetics, which enables accurate prediction of the experimental data by accounting for the reorganization of the solvent during CO2R. The results show how the performance is dictated by competing phenomena including ion formation and transport, CO2 solubility, and water management. The model shows that increasing the ion-exchange capacity of the membrane and surface area of the catalyst increases CO formation rates by >100 mA cm–2 without negatively impacting CO2 utilization. Here we provide insights into how to manage the trade-off between productivity and CO2 utilization in CO2 electrolyzers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The experimental data are available as an excel file in the Supplementary Information.
References
Izadi, P. & Harnisch, F. Microbial vertical bar electrochemical CO2 reduction: to integrate or not to integrate? Joule 6, 935–940 (2022).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Somoza-Tornos, A., Guerra, O. J., Crow, A. M., Smith, W. A. & Hodge, B. M. Process modeling, techno-economic assessment, and life cycle assessment of the electrochemical reduction of CO2: a review. Iscience 24, 102813 (2021).
Salvatore, D. & Berlinguette, C. P. Voltage matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 215–220 (2020).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Endrodi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolysers. Nat. Energy 6, 439–448 (2021).
Kutz, R. B. et al. Sustainion imidazolium-functionalized polymers for carbon dioxide electrolysis. Energy Technol. Ger. 5, 929–936 (2017).
Jeng, E. & Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 5, 1768–1775 (2020).
Blommaert, M. A., Subramanian, S., Yang, K., Smith, W. A. & Vermaas, D. A. High indirect energy consumption in AEM-based CO2 electrolyzers demonstrates the potential of bipolar membranes. ACS Appl. Mater. Interfaces 14, 557–563 (2022).
da Cunha, S. & Resasco, J. Maximizing single-pass conversion does not result in practical readiness for CO2 reduction electrolyzers. Nat. Commun. 14, 5513 (2023).
Xie, K. et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products. Nat. Commun. 13, 3609 (2022).
Disch, J., Ingenhoven, S. & Vierrath, S. Bipolar membrane with porous anion exchange layer for efficient and long-term stable electrochemical reduction of CO2 to CO. Adv. Energy Mater. 13, 2301614 (2023).
Lees, E. W., Mowbray, B. A. W., Parlane, F. G. L. & Berlinguette, C. P. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 7, 55–64 (2022).
Salvatore, D. A. et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 6, 339–348 (2021).
Weng, L. C., Bell, A. T. & Weber, A. Z. Towards membrane–electrode assembly systems for CO2 reduction: a modeling study. Energ. Environ. Sci. 12, 1950–1968 (2019).
Blake, J. W., Padding, J. T. & Haverkort, J. W. Analytical modelling of CO2 reduction in gas-diffusion electrode catalyst layers. Electrochim. Acta 393, 138987 (2021).
Weng, L. C., Bell, A. T. & Weber, A. Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018).
Loffelholz M. et al Modeling electrochemical CO2 reduction at silver gas diffusion electrodes using a TFFA approach. Chem. Eng. J. 435, 134920 (2022).
Romiluyi, O., Danilovic, N., Bell, A. T. & Weber, A. Z. Membrane-electrode assembly design parameters for optimal CO2 reduction. Electrochem. Sci. Adv. 3, e2100186 (2023).
Brown, S. M. et al Electron transfer limitation in carbon dioxide reduction revealed by data-driven Tafel analysis. Preprint at https://doi.org/10.26434/chemrxiv.13244906.v1 (2020).
Corpus, K. R. M. et al. Coupling covariance matrix adaptation with continuum modeling for determination of kinetic parameters associated with electrochemical CO2 reduction. Joule https://doi.org/10.1016/j.joule.2023.05.007 (2023).
Henstridge, M. C., Laborda, E., Rees, N. V. & Compton, R. G. Marcus–Hush–Chidsey theory of electron transfer applied to voltammetry: a review. Electrochim. Acta 84, 12–20 (2012).
Bazant, M. Z. Unified quantum theory of electrochemical kinetics by coupled ion-electron transfer. Faraday Discuss. 246, 60–124 (2023).
Reddy, P. N., Reddy, M. H. P., Pierson, J. F. & Uthanna, S. Characterization of silver oxide films formed by reactive RF sputtering at different substrate temperatures. ISRN Opt. 2014, 684317 (2014).
Vass, A. et al. Local chemical environment governs anode processes in CO2 electrolyzers. ACS Energy Lett. 6, 3801–3808 (2021).
Wheeler, D. G. et al. Quantification of water transport in a CO2 electrolyzer. Energ. Environ. Sci. 13, 5126–5134 (2020).
Petrovick, J. G. et al. Electrochemical measurement of water transport numbers in anion-exchange membranes. J. Electrochem. Soc. 170, 114519 (2023).
Dunwell, M. et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 139, 3774–3783 (2017).
Hashiba, H. et al. Effects of electrolyte buffer capacity on surface reactant species and the reaction rate of CO in electrochemical CO2 reduction. J. Phys. Chem. C 122, 3719–3726 (2018).
Luo X. Y., Kushner D. I., Kusoglu A. Anion exchange membranes: the effect of reinforcement in water and electrolyte. J. Membrane Sci. https://doi.org/10.1016/j.memsci.2023.121945 (2023).
Andersen, M. B. et al. Current-induced membrane discharge. Phys. Rev. Lett. 109, 108301 (2012).
Zenyuk, I. V., Das, P. K. & Weber, A. Z. Understanding impacts of catalyst-layer thickness on fuel-cell performance via mathematical modeling. J. Electrochem. Soc. 163, F691–F703 (2016).
Bui, J. C. et al. Analysis of bipolar membranes for electrochemical CO2 capture from air and oceanwater. Energ. Environ. Sci. 16, 5076–5095 (2023).
Weng, L. C., Bell, A. T. & Weber, A. Z. A systematic analysis of Cu-based membrane-electrode assemblies for CO2 reduction through multiphysics simulation. Energ. Environ. Sci. 13, 3592–3606 (2020).
Khan, S. U. M. & Bockris, J. O. Electronic states in solution and charge-transfer. J. Phys. Chem. US 87, 2599–2603 (1983).
Zeng, Y., Smith, R. B., Bai, P. & Bazant, M. Z. Simple formula for Marcus–Hush–Chidsey kinetics. J. Electroanal. Chem. 735, 77–83 (2014).
Smith, R. B. & Bazant, M. Z. Multiphase porous electrode theory. J. Electrochem. Soc. 164, E3291 (2017).
Singh, M. R., Goodpaster, J. D., Weber, A. Z., Head-Gordon, M. & Bell, A. T. Mechanistic insights into electrochemical reduction of CO2 over Ag using density functional theory and transport models. Proc. Natl Acad. Sci. USA 114, E8812–E8821 (2017).
Peng, J., Roy, A. L., Greenbaum, S. G. & Zawodzinski, T. A. Effect of CO2 absorption on ion and water mobility in an anion exchange membrane. J. Power Sources 380, 64–75 (2018).
Fuller, E. N., Schettle, P. D. & Giddings, J. C. A new method for prediction of binary gas-phase diffusion coeffecients. Ind. Eng. Chem. 58, 19-+ (1966).
Yang, Z. M. et al. Modeling and upscaling analysis of gas diffusion electrode-based electrochemical carbon dioxide reduction systems. ACS Sustain. Chem. Eng. 9, 351–361 (2021).
Weisenberger, S. & Schumpe, A. Estimation of gas solubilities in salt solutions at temperatures from 273 K to 363 K. Aiche J. 42, 298–300 (1996).
Petrovick, J. G. et al. Electrochemical measurement of water transport numbers in anion-exchange membranes. J. Electrochem. Soc. 170, 114519 (2023).
Acknowledgements
The authors acknowledge A. J. King for graphic design and interpretation of the CO2R reorganization energy, and J. G. Petrovick for assistance with implementing the electro-osmotic coefficients in the model. Transmission electron microscopy and powder X-ray diffraction were performed by D. Lee in H. Zheng’s group, supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (BES), Materials Sciences and Engineering Division under contract no. DE-AC02-05-CH11231 within the in situ TEM programme (KC22ZH). The authors gratefully acknowledge Lawrence Berkeley National Laboratory’s Laboratory Directed Research and Development (LDRD) grant for funding. This material is also partially based upon work performed by support from the DOE EERE Bioenergy Technologies Office under contract no. DE-AC02-05CH11231 (A.Z.W). E.W.L. acknowledges support from the National Science and Engineering Research Council (NSERC) postdoctoral fellowship. J.C.B. was supported in part by a fellowship award under contract FA9550-21-F-0003 through the National Defense Science and Engineering Graduate (NDSEG) Fellowship Program, sponsored by the Army Research Office (ARO).
Author information
Authors and Affiliations
Contributions
E.W.L. conceived the study, developed the model and wrote the initial manuscript draft. J.C.B. helped implement the Marcus–Hush–Chidsey kinetics and provided modeling support. O.R. performed all the experiments in the study. A.T.B. and A.Z.W. supervised the study, contributed to theory and model development, helped analyze the experimental data and managed the project. All authors contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Haotian Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–23, model parameters and nomenclature.
Supplementary Data.
Experimental CO and H2 partial current densities and CO2 crossover measurements.
Source data
Source Data Fig. 2
Data in Fig. 2 of main text.
Source Data Fig. 3
Data in Fig. 3 of main text.
Source Data Fig. 4
Data in Fig. 4 of main text.
Source Data Fig. 5
Data in Fig. 5 of main text.
Source Data Fig. 6
Data in Fig. 6 of main text.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lees, E.W., Bui, J.C., Romiluyi, O. et al. Exploring CO2 reduction and crossover in membrane electrode assemblies. Nat Chem Eng 1, 340–353 (2024). https://doi.org/10.1038/s44286-024-00062-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-024-00062-0
This article is cited by
-
Using pseudo-steady-state operation to redefine stability in CO2 electrolysis
Nature Chemical Engineering (2025)
-
One year of Nature Chemical Engineering
Nature Chemical Engineering (2025)
-
Catalysts for electrochemical CO2 conversion: material sustainability perspective
npj Materials Sustainability (2025)
-
Energy-efficient CO(2) conversion to multicarbon products at high rates on CuGa bimetallic catalyst
Nature Communications (2024)
-
Solvent reorganization model takes the lead
Nature Chemical Engineering (2024)