Abstract
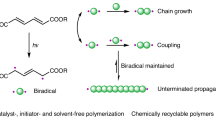
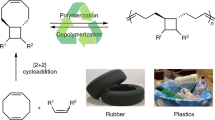
Currently, there are few examples of circularly recyclable polymers with all-carbon backbones, probably owing to the challenge of using selective C–C bond cleavage to efficiently produce monomers in recycling processes. Here we demonstrate a series of biologically sourced polymuconate polymers synthesized via simple free-radical polymerization that exhibit intrinsically weakened C–C bonds and controlled chemical recycling to monomers. Modifying the side chains and copolymerization ratios allows a wide range of mechanical property tuning, achieving performances comparable to those of commercial plastics such as polystyrene, polymethyl methacrylate and polybutadiene. Techno-economic analysis and life cycle assessment for production at a scale of 100 kilotons per year show that the materials are currently slightly more expensive and environmentally intensive compared with conventional rubbers. However, use of recycled materials via depolymerization can greatly decrease the cost and environmental impacts of polymuconate production (for example, down to US$1.59 per kilogram) to outperform its commercial counterparts.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are included in the article and its Supplementary Information. Source data are provided with this paper.
References
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Hong, M. & Chen, E. Y. X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706 (2017).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Cywar, R. M., Rorrer, N. A., Hoyt, C. B., Beckham, G. T. & Chen, E. Y. X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 7, 83–103 (2022).
Bachmann, M. et al. Towards circular plastics within planetary boundaries. Nat. Sustain. 6, 599–610 (2023).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Tennakoon, A. et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 3, 893–901 (2020).
Rorrer, J. E., Troyano-Valls, C., Beckham, G. T. & Román-Leshkov, Y. Hydrogenolysis of polypropylene and mixed polyolefin plastic waste over Ru/C to produce liquid alkanes. ACS Sustain. Chem. Eng. 9, 11661–11666 (2021).
Dong, Q. et al. Depolymerization of plastics by means of electrified spatiotemporal heating. Nature 616, 488–494 (2023).
Zhu, J. B., Watson, E. M., Tang, J. & Chen, E. Y. X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Lillie, L. M., Tolman, W. B. & Reineke, T. M. Degradable and renewably-sourced poly(ester-thioethers) by photo-initiated thiol-ene polymerization. Polym. Chem. 9, 3272–3278 (2018).
Christensen, P. R., Scheuermann, A. M., Loeffler, K. E. & Helms, B. A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 11, 442–448 (2019).
Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Zhang, Z., Shi, C., Scoti, M., Tang, X. & Chen, E. Y. X. Alternating isotactic polyhydroxyalkanoates via site- and stereoselective polymerization of unsymmetrical diolides. J. Am. Chem. Soc. 144, 20016–20024 (2022).
Cywar, R. M. et al. Redesigned hybrid nylons with optical clarity and chemical recyclability. J. Am. Chem. Soc. 144, 5366–5376 (2022).
Zhang, X., Guo, W., Zhang, C. & Zhang, X. A recyclable polyester library from reversible alternating copolymerization of aldehyde and cyclic anhydride. Nat. Commun. 14, 5423 (2023).
Zhang, Z. et al. Strong and tough supramolecular covalent adaptable networks with room-temperature closed-loop recyclability. Adv. Mater., https://doi.org/10.1002/adma.202208619 (2023).
Monfette, S. & Fogg, D. E. Equilibrium ring-closing metathesis. Chem. Rev. 109, 3783–3816 (2009).
Sathe, D. et al. Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 13, 743–750 (2021).
Bywater, S. & Black, P. E. Thermal depolymerization of polymethyl methacrylate and poly-α-methylstyrene in solution in various solvents. J. Phys. Chem. 69, 2967–2970 (1965).
Ye, L., Liu, M., Huang, Y., Zhang, Z. & Yang, J. Effects of molecular weight on thermal degradation of poly(α-methyl styrene) in nitrogen. J. Macromol. Sci. Part B 54, 1479–1494 (2015).
Allen, F. H. et al. Tables of bond lengths determined by x-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. https://doi.org/10.1039/P298700000S1 (1987).
Zavitsas, A. A. The relation between bond lengths and dissociation energies of carbon-carbon bonds. J. Phys. Chem. A 107, 897–898 (2003).
Luo, X. et al. Circularly recyclable polymers featuring topochemically weakened carbon–carbon bonds. J. Am. Chem. Soc. 144, 16588–16597 (2022).
Marsh, R. D. L. & Blanshard, J. M. V. The application of polymer crystal growth theory to the kinetics of formation of the B-amylose polymorph in a 50% wheat-starch gel. Carbohydr. Polym. 9, 301–317 (1988).
Matsumoto, A. Stereospecific polymerization of 1,3-diene monomers in the crystalline state. Prog. React. Kinet. Mech. 26, 59–110 (2001).
Matsumoto, A. Polymer structure control based on crystal engineering for materials design.Polymer J. 35, 93–121 (2003).
Mon, Y. & Matsumoto, A. Molecular stacking and photoreactions of fluorine-substituted benzyl muconates in the crystals. Cryst. Growth Des. 7, 377–385 (2007).
Biradha, K. & Santra, R. Crystal engineering of topochemical solid state reactionsns. Chem. Soc. Rev. 42, 950–967 (2013).
Agbolaghi, S., Abbaspoor, S. & Abbasi, F. A comprehensive review on polymer single crystals—from fundamental concepts to applications. Prog. Polym. Sci. 81, 22–79 (2018).
Saeed, M. H. et al. Recent advances in the polymer dispersed liquid crystal composite and its applications. Molecules 25, 1–22 (2020).
Hema, K. et al. Topochemical polymerizations for the solid-state synthesis of organic polymers. Chem. Soc. Rev. 50, 4062–4099 (2021).
Zia, K. M., Akram, N., Tabasum, S., Noreen, A. & Akbar, M. U. in Processing Technology for Bio-Based Polymers (eds Zia, K. M. et al.) 29–61 (Elsevier, 2021); https://doi.org/10.1016/B978-0-323-85772-7.00005-7
Morris, B. A. in The Science and Technology of Flexible Packaging 2nd edn (ed. Morris, B. A.) Ch. 4, 85–138 (William Andrew Publishing, 2022); https://doi.org/10.1016/B978-0-323-85435-1.00017-X
Itoh, T., Nomura, S. & Uno, T. Topochemical polymerization of 7,7,8,8-tetrakis(methoxycarbonyl)quinodimethane. Angew. Chem. Int. Ed. 41, 4306–4309 (2002).
Matsumoto, A. et al. Crystal engineering for topochemical polymerization of muconic esters using halogen–halogen and CH/π interactions as weak intermolecular interactions. J. Am. Chem. Soc. 124, 8891–8902 (2002).
Matsumoto, A., Tanaka, T. & Oka, K. Stereospecific radical polymerization of substituted benzyl muconates in the solid state under topochemical control. Synthesis https://doi.org/10.1055/s-2005-865320 (2005).
Matsumoto, A., Furukawa, D., Mori, Y., Tanaka, T. & Oka, K. Change in crystal structure and polymerization reactivity for the solid-state polymerization of muconic esters. Cryst. Growth Des. 7, 1078–1085 (2007).
Dou, L. et al. Single-crystal linear polymers through visible light-triggered topochemical quantitative polymerization. Science 343, 272–277 (2014).
Wei, Z. et al. Side-chain control of topochemical polymer single crystals with tunable elastic modulus. Angew. Chem. Int. Ed. 61, e202213840 (2022).
Quintens, G., Vrijsen, J. H., Adriaensens, P., Vanderzande, D. & Junkers, T. Muconic acid esters as bio-based acrylate mimics. Polym. Chem. 10, 5555–5563 (2019).
Dardé, T., Diomar, É., Schultze, X. & Taton, D. An expedient route to bio-based polyacrylate alternatives with inherent post-chemical modification and degradation capabilities by organic catalysis for polymerization of muconate esters. Angew. Chem. Int. Ed. 63, e202411249 (2024).
Li, T. et al. Chain flexibility and glass transition temperatures of poly(n-alkyl (meth)acrylate)s: implications of tacticity and chain dynamics. Polymer 213, 123207 (2021).
Hendrich, M. & Vana, P. Tuning the mechanical properties of multiblock copolymers generated by polyfunctional RAFT agents. Macromol. Mater. Eng. 302, 1700018 (2017).
Draths, K. M. & Frost, J. W. Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 116, 399–400 (1994).
Vardon, D. R. et al. Adipic acid production from lignin. Energy Environ. Sci. 8, 617–628 (2015).
Shanks, B. H. & Keeling, P. L. Bioprivileged molecules: creating value from biomass. Green Chem. 19, 3177–3185 (2017).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 378, 207–211 (2022).
Settle, A. E. et al. Iodine-catalyzed isomerization of dimethyl muconate. ChemSusChem 11, 1768–1780 (2018).
Nicholson, S. R. et al. The critical role of process analysis in chemical recycling and upcycling of waste plastics. Annu. Rev. Chem. Biomol. Eng. 13, 301–324 (2022).
Mokwatlo, S. C. et al. Bioprocess development and scale-up for cis,cis-muconic acid production from glucose and xylose by Pseudomonas putida. Green Chem. 26, 10152–10167 (2024).
Nicholson, S. R., Rorrer, N. A., Carpenter, A. C. & Beckham, G. T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 5, 673–686 (2021).
Taylor, B. PureCycle Ironton plant up and running. recyclingtoday https://www.recyclingtoday.com/news/purecycle-polyproylene-plastic-recycling-ohio-facility-maintenance-running/#:~:text=At%20full%20capacity%20%2Cpure%20Cycle%20expects,of%20PP%20resin%20per%20year (2023).
Kingsport, Tennessee, site. Eastman https://www.eastman.com/en/sustainability/environmental/circularity/site-locations/tennessee-site#:~:text=Located at Eastman’sKingsport%2CTenn,recyclingfacilitiesintheworld (2024).
Singh, A. et al. Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate). Joule 5, 2479–2503 (2021).
Milbrandt, A., Coney, K., Badgett, A. & Beckham, G. T. Quantification and evaluation of plastic waste in the United States. Resour. Conserv. Recycl. 183, 1–16 (2022).
Frisch, M. J. et al. Gaussian 16 (Gaussian, 2016).
Haynes, W. M. CRC Handbook of Chemistry and Physics (CRC Press, 2016); https://doi.org/10.1201/9781315380476
Grimme, S. Exploration of chemical compound, conformer, and reaction space with meta-dynamics simulations based on tight-binding quantum chemical calculations. J. Chem. Theory Comput. 15, 2847–2862 (2019).
Lin, Y.-S., Li, G.-D., Mao, S.-P. & Chai, J.-D. Long-range corrected hybrid density functionals with improved dispersion corrections. J. Chem. Theory Comput. 9, 263–272 (2013).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Zhao, Q. & Savoie, B. M. Algorithmic explorations of unimolecular and bimolecular reaction spaces. Angew. Chem. Int. Ed. 61, e202210693 (2022).
ISO 14851 Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer (International Organization for Standardization, 2019).
ASTM D5988 Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil (ASTM International, 2018).
Hanes, R. J. & Carpenter, A. Evaluating opportunities to improve material and energy impacts in commodity supply chains. Environ. Syst. Decis. 37, 6–12 (2017).
U.S. Life Cycle Inventory Database (National Renewable Energy Laboratory, 2022).
Acknowledgements
This work is supported primarily by Ralph W. and Grace M. Showalter Research Trust and Charles Davidson Rising Star Startup Fund of Purdue University. The work performed by L.A.O. and B.M.S. was supported by the Office of Naval Research through the Energetic Materials Program (MURI grant number N00014-21-1-2476, program manager C. Stoltz). This work was authored in part by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the US Department of Energy (DOE) under contract no. DE-AC36-08GO28308. Funding was provided to A.A., J.S.D., G.T.B., S.X. and U.D.M. by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office (AMMTO) and Bioenergy Technologies Office (BETO). This work was performed as part of the BOTTLE Consortium and was supported by AMMTO and BETO under contract no. DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC and under contract no. DE-AC02-06CH11357 with the Argonne National Laboratory, operated by UChicago Argonne, LLC.. Funding for B.C.K. and G.T.B. was provided by the US Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office (BETO) for the Agile BioFoundry. The views expressed in the Article do not necessarily represent the views of the DOE or the US Government. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US Government purposes. We thank I. Burch and L. Franquilino for the help with synthesis and mechanical testing; respectively. We thank C. Mokwatlo, C. Kneucker, C. Singer, V. Sànchez i Nogué, R. Lyons, M. Baker, D. Salvachúa, Y. Chen, L. Stanley, J. Miscall and T. Uekert for production and purification of the muconic acid.
Author information
Authors and Affiliations
Contributions
The project was designed by L.D. Q.H. and X.L. contributed equally to this work. Q.H. and X.L. carried out material synthesis, characterized recyclability and depolymerization kinetics, and conducted ultrasonication processing and hot solvent processing. L.A.O. conducted calculations on carbon–carbon bond dissociation energies and other simulations. Q.H. carried out mechanical tests of the polymer materials. Q.H. carried out the 3D-printing demonstration of the extruded polymer filaments and injection molding of the toy sample. A.A., J.S.D. and B.C.K. conducted TEA and LCA. S.X. performed the biodegradation test of polymer samples. T.H. and D.M. helped with material synthesis and depolymerization study. L.D., Q.H. and X.L. wrote the paper with contributions from all authors. P.W., Z.W., C.L., B.B., J.M., M.U.-D., G.T.B. and B.M.S. discussed the data interpretation and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Joshua Worch, Wei Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Q., Luo, X., Ogunfowora, L.A. et al. Scalable, biologically sourced depolymerizable polydienes with intrinsically weakened carbon–carbon bonds. Nat Chem Eng 2, 130–141 (2025). https://doi.org/10.1038/s44286-025-00183-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00183-0
This article is cited by
-
Recyclable polyolefin-like materials with weakened all-carbon backbones
Nature Chemical Engineering (2025)